Abstract
HMCES can covalently crosslink to abasic sites in single-stranded DNA at stalled replication forks to prevent genome instability. Here, we report crystal structures of the Human HMCES SRAP domain in complex with DNA-damage substrates, including HMCES crosslinked with an abasic site within a 3’ overhang DNA. HMCES interacts with both single-strand and duplex segments of DNA, with two independent duplex DNA interaction sites identified in the SRAP domain. The HMCES DNA-protein crosslink structure provides structural insights into a novel thiazolidine covalent interaction between the DNA abasic site and conserved Cys2 of HMCES. Collectively, our structures demonstrate the capacity for the SRAP domain to interact with variety of single-strand and double-strand containing DNA structures found in DNA-damage sites including 5’ and 3’ overhang DNAs and gapped DNAs with short single-strand segments.
Introduction
DNA bases are constantly damaged by factors such as reactive oxygen species (ROS), chemotoxic agents, ionizing radiation (IR) and UV radiation1, and are subject to physiological modification by enzymes such as AID, DNA methylases and the TETs2,3. These types of DNA alterations are primarily repaired by the base excision repair (BER) pathway, which is initiated by DNA glycosylases that recognize and cleave damaged or modified bases creating apurinic or apyrimidinic sites (AP sites) or abasic sites1. HMCES was recently reported to recognize and covalently crosslink to abasic sites in single-stranded DNA (ssDNA), generated by uracil DNA glycosylase (UDG) at stalled replication forks4. The authors suggested that these DNA-protein crosslink (DPC) intermediates prevented ssDNA breaks that may consequently occur upon cleavage by AP endonucleases, which could subsequently be repaired through error-prone pathways4.
Human HMCES has a highly conserved N-terminal SOS Response-Associated Peptidase domain (SRAPd) that is widely found in bacteria and eukaryotes, with a sporadic presence in certain bacteriophages and archaea5. Animal SRAP proteins have an additional C-terminal disordered extension with multiple copies of the PCNA-interacting motif (PIP)4,6. Gene-neighborhood analysis in prokaryotes identified SRAPd as a novel component of the bacterial SOS response, associated with multiple components of the DNA repair machinery5. The SRAPd contains a triad of predicted catalytic residues, namely Cys2, Glu127, and His210, which are highly conserved across SRAP domains from all the three superkingdoms of life and viruses. This triad is predicted to support autoproteolytic activity acting on the peptide bond N-terminal to Cys2 as all characterized SRAP proteins show a cleavage of residues N-terminal to this cysteine5. Moreover, Cys2 was recently shown to mediate the formation of the DPC via a covalent linkage to the deoxyribose at the abasic site of the DNA4. Here, we present the crystal structures of the human HMCES SRAP domain in DNA-free form and in complex with several 3’ overhang DNAs, including a DPC structure with an abasic site at 3’ overhang. Through structure analysis and mutagenesis experiments we delineate the key residues of HMCES that are involved in DNA-binding, revealing considerable flexibility in substrate recognition.
Results
Crystal Structures of the Human HMCES SRAP domain in Complex with 3’ Overhang DNA
To better understand the mechanism of HMCES association with DNA, we crystallized the human HMCES SRAPd (residues 2–270) in its DNA-free form (Apo_SRAPd) and in complex with several DNA-damage substrates containing 3’ overhangs of different lengths. The crystal structure of SRAPd in complex with duplex DNA containing a three-nucleotide overhang at the 3’ end (referred to here as SRAPd_3nt) revealed SRAPd binding to two DNA molecules: DNA-A interacts via the 3’ overhang, and another molecule (DNA-B) via the blunt-end (Fig. 1a). Both DNA interaction surfaces are highly conserved.
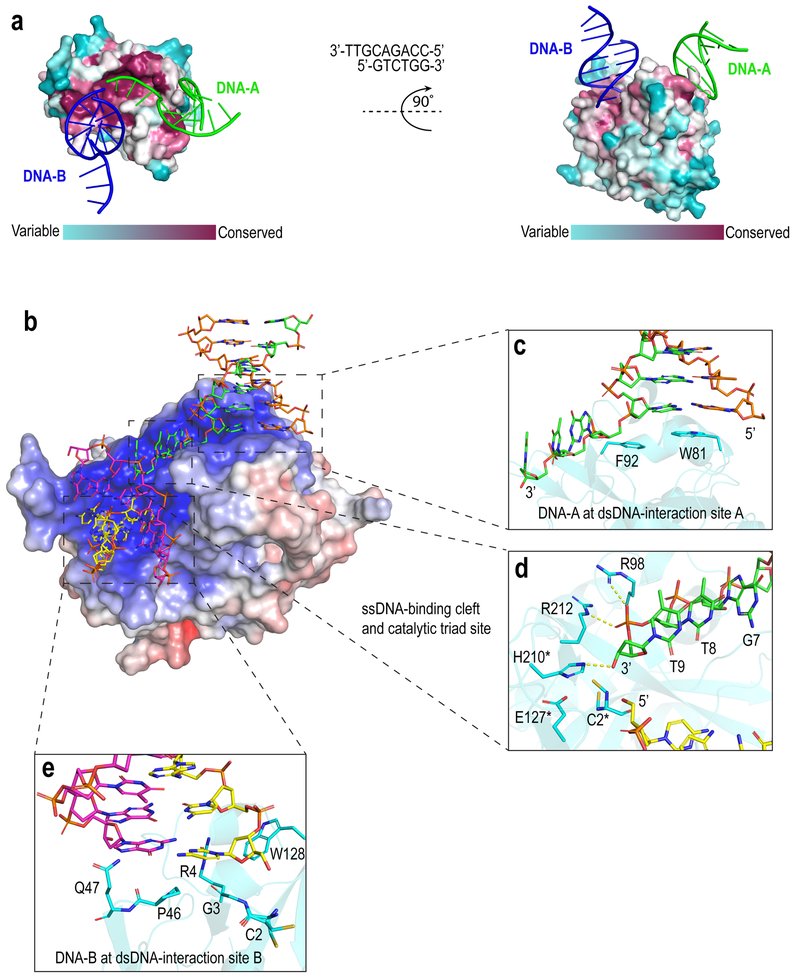
Figure 1. Interactions between SRAPd and 3’overhang DNA.
(a) Surface representation of SRAPd colored by degree of sequence conservation, bound to two symmetry-related DNA molecules shown in green (DNA-A) and blue (DNA-B). Evolutionary conservation was assessed by ConSurf web server13. The 3’ overhang DNA sequence that was used for co-crystallization is shown in the middle. (b) Electrostatic surface potential representation of SRAPd interacting with two symmetry-related DNA molecules: DNA-A in green and orange, and DNA-B in magenta and yellow. SRAPd surface color indicates electrostatic potential ranging from −7kT/e (red) to +7kT/e (blue). Electrostatic surface potentials were calculated using APBS14. (c) Close-up view of the SRAPd dsDNA-interaction site A in stacked conformation with the duplex segment of DNA-A. (d) Close-up view of the SRAPd catalytic triad site and ssDNA-binding cleft bound to the phosphate backbone of the single-strand segment of DNA-A. The catalytic triad residues as well as R98 and R212 in the ssDNA-binding cleft are shown as stick models in cyan. The ssDNA segment of DNA-A is colored green and DNA-B colored yellow. The catalytic triad residues are marked with asterisk. (e) Close-up view of the SRAPd dsDNA-interaction site B, which stacks with the blunt-end of DNA-B.
SRAPd interacts with the 3’ overhang of DNA-A through a hydrophobic shelf created by Trp81 and Phe92, which form π-stacking interactions with the duplex segment of DNA at the ssDNA-dsDNA junction and referred to here as dsDNA-interaction site A (Fig. 1b, c). At the same time, Arg106 inserts into the minor groove of dsDNA, further stabilizing the complex. In Apo_SRAPd, Arg106 is stacked onto Trp81 instead of the nucleobase (Fig. 2a). The ssDNA segment of the 3’ overhang is sharply bent by approximately 90 degrees and lies in a narrow, positively charged cleft directing it towards the catalytic triad. The ssDNA-binding cleft includes conserved Arg98 and Arg212, which form salt-bridges with the phosphate backbone of ssDNA (Fig. 1b, d). Alanine substitutions of either of these Arginine residues severely hinder ssDNA-binding (Supplementary Fig. 1), and are consistent with gel-shift assays reported by Mohni et al.4
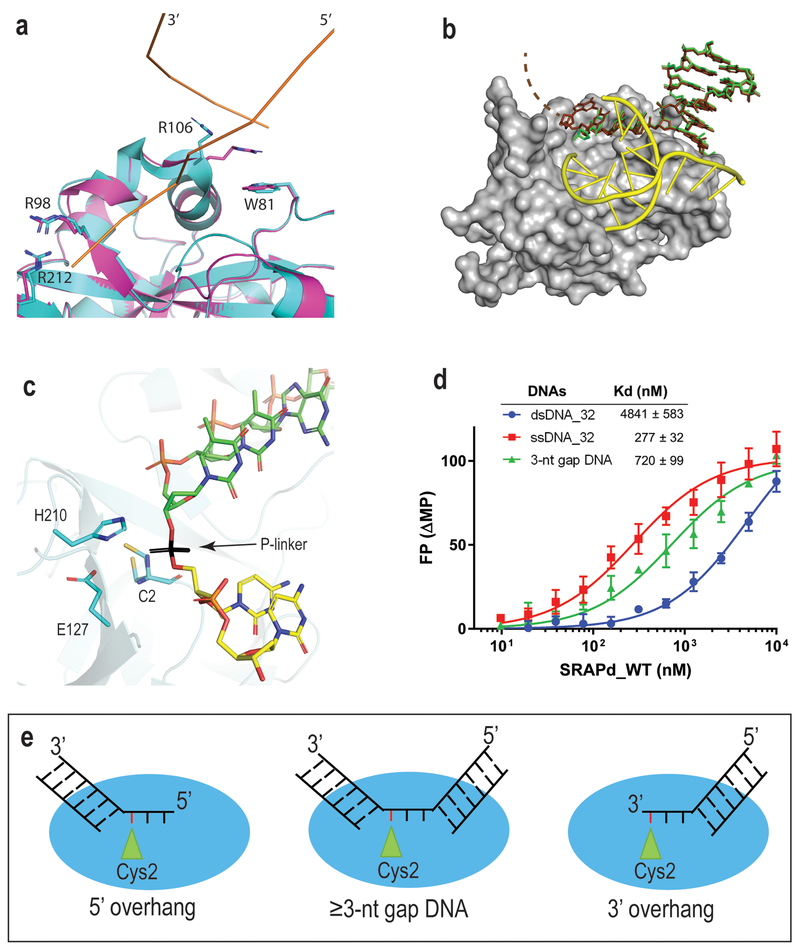
Figure 2. SRAPd interaction with Potential DNA-damage repair substrates.
(a) Crystal structure of Apo_SRAPd in magenta, superposed with SRAPd_3nt in cyan, at the dsDNA-interaction site A. DNA-A is shown as ribbon representation in orange. (b) Crystal structure of SRAPd_6nt in complex with six-nucleotide 3’ overhang. SRAPd is shown in surface representation in grey. The six-nucleotide overhang at the dsDNA-interaction site A is shown as a stick model in brown superposed with SRAPd_3nt three-nucleotide 3’ overhang, in green. The electron density for the last two nucleotides in SRAPd_6nt was not resolved and not modeled (indicated by dashed lines). DNA-B at the dsDNA-interaction site B is shown in yellow. (c) The 3’ and 5’ ends of two DNA molecules at the catalytic triad site of SRAPd_3nt are in close enough proximity to be linked by a phosphate group, shown in black color for the purposes of illustration. (d) Fluorescence polarization DNA-binding affinities of HMCES SRAPd to ssDNA, dsDNA, and dsDNA containing a three-nucleotide gap (3-nt gap DNA). Experiments were performed in triplicates and data are represented as mean +/− SD. (e) A model illustrating the potential DNA damage substrates that can be recognized by HMCES. Red line represents the abasic site in DNA. Source Data for panel d are available online.
The pocket housing the catalytic triad accommodates the 3’-hydroxyl of the ssDNA overhang (Fig. 1d). Interestingly, mutation of individual catalytic triad residues to alanine yielded SRAPd variants with higher affinity for ssDNA compared to wild-type (WT) protein, suggesting a role other than simply DNA binding (Supplementary Fig. 1).
The SRAPd_3nt structure also revealed that the blunt-end of DNA-B interacts with SRAPd via dsDNA-interaction site B, composed of residues Gly3, Arg4, Pro46, Asp47, Trp128 (Fig. 1e). This interaction surface represents a potential binding site for 5’ overhang DNA, as SRAPd was shown to bind both 5’ and 3’ overhangs with similar affinities4. This dsDNA-interaction site B accounts for the remaining residues, which are highly conserved in SRAP domains across all superkingdoms of life and phages5, suggesting that it is a universal functional feature of this domain. It is immediately adjacent to the catalytic triad and forms a contiguous, similarly charged surface with the ssDNA binding site (Fig. 1b). These features suggested that dsDNA-interaction site B may also be able to accommodate ssDNA extending from a longer 3’ overhang substrate bound to the dsDNA-interaction site A.
To address this question, we determined the crystal structure of SRAPd with DNA containing a six-nucleotide overhang at the 3’ end (referred to here as SRAPd_6nt). Although SRAPd has nearly 10-fold higher affinity for ssDNA compared to dsDNA (Fig. 2d), the longer 3’ overhang did not displace the blunt-end-interacting DNA-B from its dsDNA-interaction site B. Instead, the extra single strand bases protrude out of the catalytic triad pocket (Fig. 2b), and (Supplementary Fig. 2), suggesting that the dsDNA-interaction site B has specifically evolved to bind duplex DNA and may form the binding site for 5’ overhang DNA structures as well. Nevertheless, given that DNA is a mediator of the crystal lattice in this crystal form, we cannot entirely rule out that a longer ssDNA might occupy the dsDNA-interaction site B in the absence of a competing duplex DNA.
HMCES Prefers ssDNA and 3-Nucleotide Gap DNA over Intact dsDNA
In SRAPd_3nt, the distance between the 3’ end of DNA-A and the 5’ end of DNA-B at the catalytic triad is around 3.2Å, which is sufficient to accommodate a phosphate group linking the two substrates together (Fig. 2c). Consistent with our observations, the affinity of SRAPd to dsDNA with a 3-nucleotide gap is approximately 7-fold higher than intact dsDNA of the same sequence (Fig. 2d). These data suggest the potential for binding other types of gapped DNA structures that form during DNA repair (Fig. 2e), such as nucleotide excision repair intermediates. The Trp81Glu substitution at dsDNA-interaction site A, and Arg4Ala substitution at dsDNA-interaction site B, severely hindered SRAPd binding to 3-nt gap DNA, suggesting that both interaction sites are crucial for substrate DNA binding (Supplementary Fig. 1).
Crystal Structure of the Human HMCES SRAP domain Crosslinked to DNA Abasic site
To further investigate the covalent interaction of HMCES with a DNA abasic site, we crystallized the HMCES SRAP domain with 3’ overhang DNA (similar to that in SRAPd_6nt), having an abasic site at position 9 (AP9) of the longer strand (referred to here as SRAPd_DPC) (Fig. 3a). To generate a physiologically relevant, reactive aldehydic form of the abasic site capable of crosslinking with HMCES, we designed the 3’ overhang to have a deoxyuridine (dU) at position 9 of the longer DNA strand, which is predicted to bind immediately adjacent to the Cys2. We then treated this DNA with Uracil-DNA glycosylase (UDG) to generate AP9 DNA7. For the crosslink reaction to proceed between the AP9 and Cys2 of HMCES, the N-terminal methionine needs to be removed in order to expose the NH2 of Cys2. Mass spectrometry analysis of our purified C-terminally His-tagged SRAPd protein showed that the N-terminal methionine (Met1) was cleaved, either co-translationally, catalyzed by E.Coli Methionyl-aminopeptidase8 (MAP), or due to its predicted autopeptidase activity (Supplementary Fig. 3; Supplementary Table 1) yielding a catalytically active form of HMCES. Incubation of this active SRAPd with AP9 DNA yielded crosslinked SRAPd_DPC (Supplementary Fig. 3; Supplementary Table 1).
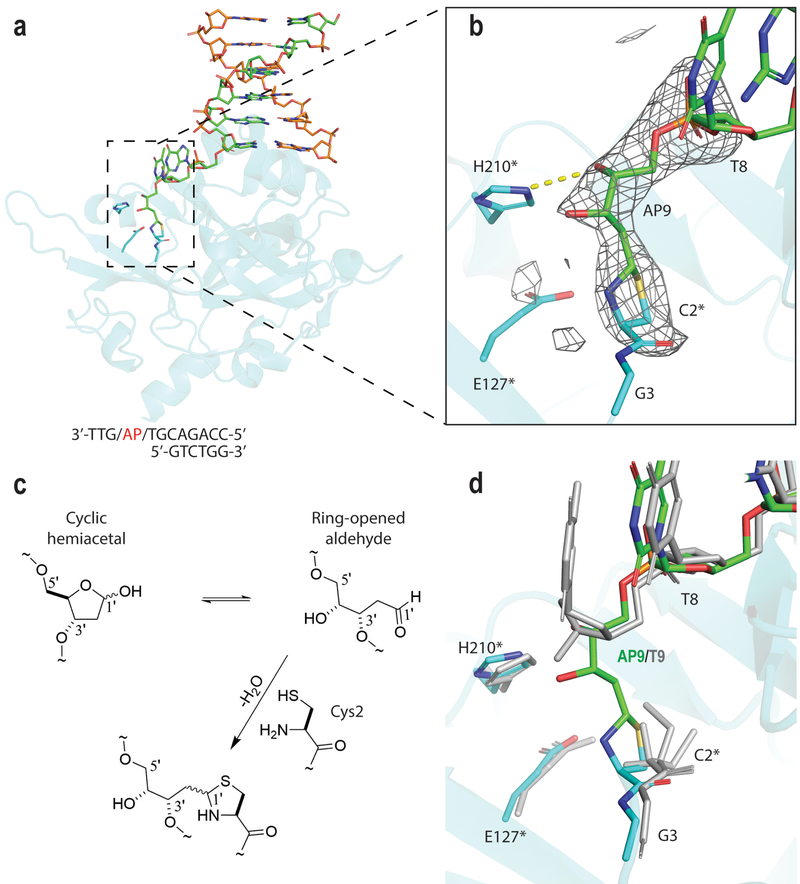
Figure 3. Crystal structure of the Human HMCES SRAPd crosslinked to a DNA abasic site.
(a) Overall structure of SRAPd_DPC. SRAPd is shown as cartoon representation in cyan, 3’ overhang DNA in green and orange. DNA-B at dsDNA-interaction site B is not shown for clarity. (b) The mFo-DFc electron density omit-map for the Cys2 crosslink with DNA abasic site (AP9) in the crystal structure of SRAPd_DPC, displayed as grey mesh and contoured at 3.0σ. The catalytic triad residues are marked with an asterisk. (c) Reaction scheme for formation of the covalent crosslink between Cys2 of HMCES and the ring-opened aldehyde form of abasic deoxyribose. (d) SRAPd_DPC structure in cyan (protein) and green (DNA), superposed with SRAPd_3nt in grey.
The SRAPd_DPC structure was refined to 2.2Å resolution and is isomorphous with both SRAPd_3nt and SRAPd_6nt structures. Continuous electron density was observed between Cys2 of HMCES and the AP9 confirming the covalent crosslink (Fig. 3a, b). The Cys2-AP9 crosslink was modeled at full occupancy, and shows an approximately 20% higher B-factor values compared to its surrounding residues (Table 1). The electron density map for the remaining three nucleotides downstream of the abasic site at the 3’ end was not resolved. Notably, the SRAPd_DPC structure, suggests a model in which the terminal Cys2 of HMCES reacts with the ring-opened aldehyde form of the abasic deoxyribose (AP9) to form a thiazolidine DNA-protein crosslink9 (Fig. 3a, b, c). This crosslink entails an approximately 120° rotation around AP9 γ10 and possible hydrogen bonding between the 4’-hydroxyl of AP9 and the Nτ position of His210 (Fig. 3b,d).
Table 1.
Data collection and refinement statistics
| Apo_SRAPd (PDB: 5KO9) |
SRAPd_3nt (PDB: 6OEB) |
SRAPd_6nt (PDB: 6OEA) |
SRAPd_DPC (PDB: 6OE7) |
|
|---|---|---|---|---|
| Data collectiona | ||||
| Space group | I 2 | I 2 | I 2 | I 2 |
| Cell dimensions | ||||
| a, b, c (Å) | 80.10, 44.74, 82.90 | 55.72, 51.15, 149.21 | 55.86, 52.06, 148.30 | 55.51, 51.53, 149.72 |
| α, β, γ (°) | 90.00, 107.15, 90.00 | 90.00, 92.76, 90.00 | 90.00, 93.11, 90.00 | 90.00, 92.72, 90.00 |
| Resolution (Å) | 48.37–1.5(1.53–1.50)b | 48.38–2.10(2.16–2.10) | 49.11–2.10(2.16–2.10) | 48.72–2.2(2.27–2.20) |
| Rmerge | 0.039 (0.61) | 0.069 (0.71) | 0.059 (0.74) | 0.045 (0.70) |
| I/σ(I) | 15.9 (1.9) | 10.2 (1.4) | 15.0 (2.2) | 12.3 (1.3) |
| CC1/2 | 0.667 | 0.814 | 0.926 | 0.818 |
| Completeness (%) | 97 (93.2) | 99.8 (99.7) | 99.9 (99.9) | 96.8 (98.0) |
| Redundancy | 3.8 (3.4) | 4.4 (4.3) | 6.6 (6.6) | 3.2 (3.4) |
| Refinement | ||||
| Resolution (Å) | 48.37–1.5 | 48.38–2.10 | 49.11–2.10 | 48.72–2.20 |
| No. reflections | 41504 | 24718 | 25066 | 20951 |
| Rwork / Rfree | 0.181/0.209 | 0.205/0.235 | 0.208/0.242 | 0.194/0.216 |
| No. atoms | 2292 | 2492 | 2457 | 2402 |
| Protein | 2062 | 2078 | 2056 | 2046 |
| DNA | - | 302 | 324 | 282 |
| Cys2-AP9 crosslink | - | - | - | 17 |
| Water | 186 | 77 | 46 | 33 |
| B factors | 25.3 | 50.8 | 60.5 | 62.5 |
| Protein | 24.6 | 50.4 | 59.6 | 62.4 |
| DNA | - | 53.7 | 66.7 | 62.7 |
| Cys2-AP9 crosslink | - | - | - | 72.9 |
| Water | 31.5 | 47.5 | 54.8 | 54.4 |
| R.m.s. deviations | ||||
| Bond lengths (Å) | 0.011 | 0.008 | 0.008 | 0.007 |
| Bond angles (°) | 1.499 | 1.526 | 1.563 | 1.509 |
A single crystal was used for all structures.
Values in parentheses are for highest-resolution shell.
Discussion
HMCES was recently reported to promote genome stability by shielding abasic sites from error-prone repair pathways at stalled replication forks. In particular, Mohni et al.4 showed that HMCES forms DPC intermediates with abasic sites in ssDNA generated by uracil-DNA glycosylase (UDG), which is a monofunctional glycosylase that cannot cleave ssDNA. However, other variants of damaged bases require the use of bifunctional glycosylases with both glycosylase and lyase activities, such as NEIL3, which is a single-strand specific glycosylase with a limited lyase activity able to cleave ssDNA 3’ to an abasic site to generate a 3’ overhang11. Our structures confirm this crosslink and reveal it to be a thiazolidine ring that involves both the sidechain sulfur and the NH2 of Cys2 that is exposed by the peptidolytic removal of N-terminal methionine. Cys2 along with Glu127, and His210 form a predicted catalytic triad that is situated in a pocket characteristic of SRAP domains5. Thus, our structure reveals that in addition to predicted autopeptidase activity this pocket is also required for the accommodation of the DPC. It also suggests that HMCES can recognize and covalently crosslink to DNA abasic sites at cleaved 3’ ends, which may shield them from further processing by exonucleases and regulate the choice between different DNA-repair pathways. The presence of two distinct dsDNA-interaction sites provides HMCES with flexibility to interact with abasic sites located at both 5’ and 3’ overhangs (Fig. 2e). Because HMCES binds ssDNA, 3’ and 5’ overhangs, and 3-nt gap DNA, it could be involved in a variety of DNA repair pathways other than at stalled replication forks. For example, it could potentially be involved in physiological genome rearrangements such as class switch recombination in lymphocytes.
Our SRAPd structures also shed light on other proposed activities of HMCES. Proteomics studies using dsDNA baits with modified cytosines identified HMCES as a reader for oxidized 5-methyl-Cytosines (oxi-mC) containing duplex DNA12. The SRAPd only contacts one base-pair at the ssDNA-dsDNA junction (Fig. 1b); hence SRAPd of HMCES could potentially recognize a single oxi-mC either at this junction or alternatively in single-strand regions. Taken together, our structures support an important role for HMCES in recognizing and sensing flapped and gapped DNA-damage products and describe, for the first time, the covalent interaction of HMCES with DNA abasic sites, revealing its broad substrate recognition spectrum.
Methods:
Protein expression and purification
Wild-type and mutant HMCES variants were subcloned into pNIC-CH vector by modifying the C-terminal tag with a TEV cleavable N-terminal His6-tag, and were expressed in E. coli Rosetta. The recombinant proteins were first purified by nickel-affinity chromatography and, after TEV cleavage of the His6-tag, by anion exchange and gel-filtration chromatography using S200 column. Purified SRAPd was concentrated to ~20 mg/mL in 20 mM Tris-HCl [pH 8.0], 150 mM NaCl, 2 mM tris(2-carboxyethyl)phosphine (TCEP). The sequences for all cloned constructs were verified by sequencing, and the corresponding molecular weight for all purified constructs were verified by liquid chromatography-mass spectrometry.
Crystallization and structural determination
Apo_SRAPd was crystallized using sitting drop vapor-diffusion method by mixing 1:1 ratio of protein and reservoir solution containing 0.1 M Bis-Tris Propane, 2% Tacsimate, 20% (w/v) PEG 3350. DNA used for co-crystallization was purchased from Integrated DNA Technologies, Inc. For SRAPd_DPC structure, a 12 residue ssDNA containing a deoxyuridine (dU) at position 9 (5’-CCAGACGTUGTT-3’) was first incubated with Uracil-DNA glycosylase (UDG) for 1 hour at 37°C, followed by heat treatment at 95°C for 10 min to inactivate the enzyme. Reaction products were immediately extracted with phenol chlorophorm to remove the UDG enzyme. Abasic site containing ssDNA was then annealed with the complementary strand (5’-GTCTTG-3’) by mixing equal amounts at 95°C followed by cooling to room temperature. The UDG enzyme was purchased from New England BioLabs (Cat # M0280L). SRAPd at 10 mg mL−1 was mixed, at a molar ratio of 1:1.2, with DNA containing site-specific abasic site and incubated for 0.5 h on ice. The mixture was then crystallized by setting 24-well vapor-diffusion sitting drops at room temperature, in a condition containing 20% (w/v) PEG 3350, 0.1 M KCl, 0.1 M Bis-Tris pH 5.5, 0.05 M MgCl2. Diffraction quality crystals were obtained by streak seeding the drops using Hampton research seeding tool (Cat # HR8–133) with previously generated SRAPd_6nt crystals and incubating them at 20°C for one week. SRAPd_DPC crystals were cryo-protected by using reservoir solution supplemented with 15% ethylene-glycol and cryo-cooled in liquid-nitrogen.
For SRAPd_3nt and SRAPd_6nt co-crystallization, purified SRAPd protein at 12 mg mL−1 was mixed, at a molar ratio of 1:1.2, with different 3’ overhang DNA prepared by annealing equimolar amounts of two oligonucleotides, 5’-CCAGACGTT-3’ and 5’-GTCTTG-3’ for DNA_3nt; 5’-GTCTTG-3’ and 5’-CCAGACGTTGTT-3’ for DNA_6nt, and incubated for 0.5 h on ice. The mixture was then crystallized using sitting drop vapor-diffusion method in a condition containing 25% (w/v) PEG 3350, 0.2 M ammonium sulphate, 0.1 M Hepes pH 7.5 for SRAPd_3nt; and 20% (w/v) PEG 3350, 0.1 M KCl, 0.1 M Bis-Tris pH 5.5, 0.05 M MgCl2 for SRAPd_6nt. Apo_SRAPd, SRAPd_3nt and SRAPd_6nt crystals were cryo-protected using reservoir solution supplemented with 20–30% glycerol and 20–30% ethylene-glycol, respectively, and cryo-cooled in liquid-nitrogen.
Diffraction data for the Apo_SRAPd and SRAPd_DPC was collected at the 19ID and 24ID-C beamlines of the Advanced Photon Source (APS), respectively. Diffraction data for SRAPd_3nt and SRAPd_6nt was collected at the 5.0.1 beamline of the Advanced Light Source (ALS) Berkeley Lab. Datasets were processed with XDS15 and merged with Aimless16,17. Initial phases for the Apo_SRAPd was obtained by molecular replacement with Phaser-MR18, using a combination of models (PDB ID: 2F20, 2BDV, 2ICU, 1ZN6) generated by FFAS19. Initial phases for the SRAPd_3nt were obtained by molecular replacement with Phaser-MR18, using the Apo_SRAPd (PDB ID: 5KO9) as a search model. Whereas, initial phases for SRAPd_6nt and SRAPd_DPC were obtained by Fourier transform using SRAPd_3nt structure as a starting model. Models were built with COOT20, and refined with refmac521. Structures were validated with Molprobity22. Data collection and refinement statistics are shown in (Table 1). Figures were generated with PyMOL (http://pymol.org).
Fluorescence-based DNA binding assay
All fluorescence polarization DNA binding assays were performed in a final volume of 20 μL in a buffer containing 20 mM Hepes, pH 7.4, 140 mM KCl, 5 mM NaCl, 0.1 mM Ethylenediaminetetraacetic acid (EDTA), 0.01% Triton X-100 and 0.2 mM TCEP in 384-well black polypropylene PCR plates. Fluorescence polarization (mP) measurements were performed at room temperature using a BioTek Synergy 4 (BioTek, Winooski, VT). The KD values were calculated by fitting the curves in GraphPad Prism 7.04 using nonlinear regression, one site-specific binding, equation Y=Bmax*X/(Kd + X). The sequences of 6-carboxyfluorescein (FAM)-labeled DNA oligonucleotides are listed in (Supplementary Table 2). All DNAs were purchased from Integrated DNA Technologies, Inc.
Liquid chromatography mass spectrometry analyses of HMCES
SRAPd was incubated with the abasic site containing 3’ overhang DNA (same as used in SRAPd_DPC co-crystallization) at 1:1.2 ratio in a buffer containing 150 mM NaCl, 20 mM Hepes pH 7.5, 10 mM MgCl2 at room temperature overnight. All LC-MS data were acquired according to the previously published protocol23, on an Agilent 6545 Q-TOF (Santa Clara, CA) equipped with a Dual Agilent Jet Stream ESI source coupled with an Agilent 1260 Infinity HPLC system (Santa Clara, CA). The analytical column utilized was a 300 StableBond Poroshell (Agilent, part number 883750–909) 2.1 × 100-mm-i.d. reversed-phase C3 (5 μm particle size). Mobile phase (A) consisted of 97% HPLC grade water with 0.5% formic acid and 2.5% ACN, while mobile phase (B) was 96% ACN with 0.5% formic acid and 3.5% HPLC grade water. A gradient profile was utilized at a flow rate of 500 μL/min. The mobile phase was held for 2 min at 5% B (with eluant going to waste) and then switched to the mass spectrometer from 2–6 min during which time solvent B increased from 5–95%. Two microliters of a 30μM solution of each sample was injected. Raw data files were analyzed by Agilent MassHunter BioConfirm software (vB.07.00). Mass spectra between 4 and 6 minutes were extracted, averaged and deconvoluted using the MaxEnt algorithm.
Supplementary Material
Acknowledgements
We are grateful to H. Wyatt for fruitful discussions, advice from P.J. Brown and W. Tempel on interpretation of DNA-protein crosslink chemistry, and S. Ackloo on mass-spectrometry data analysis. Special thanks to S. Duan for preparing the DNA abasic site digestion. We also thank U. Chinte, J. Chrzas, N. Duke and Z. Jin from SERCAT 22ID-D beamline for collecting the initial SRAPd_DPC datasets. This research used resources of the Advanced Light Source, which is a DOE Office of Science User Facility under contract no. DE-AC02–05CH11231. Results shown in this report are derived from work performed at Argonne National Laboratory, Structural Biology Center (SBC) at the Advanced Photon Source. SBC-CAT is operated by UChicago Argonne, LLC, for the U.S. Department of Energy, Office of Biological and Environmental Research under contract DE-AC02–06CH11357. This work is based on research conducted at the Advanced Photon Source on the Northeastern Collaborative Access Team beamlines, which are funded by the National Institute of General Medical Sciences from the NIH (P41 GM103403). The Pilatus 6M detector on beamline 24-ID-C is funded by a NIH Office of Research Infrastructure Programs High End Instrumentation grant (S10 RR029205).
The Structural Genomics Consortium is a registered charity (no: 1097737) that receives funds from AbbVie; Bayer Pharma AG; Boehringer Ingelheim; Canada Foundation for Innovation; Eshelman Institute for Innovation; Genome Canada through Ontario Genomics Institute [OGI-055]; Innovative Medicines Initiative (EU/EFPIA) [ULTRA-DD: 115766]; Janssen, Merck & Co.; Novartis Pharma AG; Ontario Ministry of Research Innovation and Science (MRIS); Pfizer, São Paulo Research Foundation-FAPESP, Takeda and the Wellcome Trust. This research is also supported by the Canadian Institutes of Health Research [FDN154328] and Natural Sciences and Engineering Research Council [RGPIN-2015–05939] to CHA, intramural funds of the National Library of Medicine, NIH, USA to LA, and the National Cancer Institute [R35 CA210043] to AR.
Footnotes
Competing Interests
The authors declare no competing financial interests.
Reporting Summary
Further information on experimental design is available in the Nature Research Reporting Summary linked to this article.
Data Availability
Coordinates and structure factors have been deposited in the Protein Data Bank under accession codes: 5KO9 for Apo_SRAPd, 6OEB for SRAPd_3nt, and 6OEA for SRAPd_6nt, 6OE7 for SRAPd_DPC. LC-MS data underlying Supplementary figure 3 have been deposited in Zenodo with DOI:10.5281/zenodo.2662532. Source data for figure 2d, and Supplementary figure 1 are available with the paper online.
References
- 1.Maynard S, Schurman SH, Harboe C, de Souza-Pinto NC & Bohr VA Base excision repair of oxidative DNA damage and association with cancer and aging. Carcinogenesis 30, 2–10 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nabel CS, Manning SA & Kohli RM The Curious Chemical Biology of Cytosine: Deamination, Methylation, and Oxidation as Modulators of Genomic Potential. ACS Chem. Biol 7, 20–30 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iyer LM, Zhang D, Maxwell Burroughs A & Aravind L Computational identification of novel biochemical systems involved in oxidation, glycosylation and other complex modifications of bases in DNA. Nucleic Acids Res. 41, 7635–7655 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohni KN et al. HMCES Maintains Genome Integrity by Shielding Abasic Sites in Single-Strand DNA. Cell (2018). doi: 10.1016/j.cell.2018.10.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aravind L, Anand S & Iyer LM Novel autoproteolytic and DNA-damage sensing components in the bacterial SOS response and oxidized methylcytosine-induced eukaryotic DNA demethylation systems. Biol. Direct 8, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Srivastava M et al. Replisome Dynamics and Their Functional Relevance upon DNA Damage through the PCNA Interactome. Cell Rep. 25, 3869–3883.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindahl T DNA N-glycosidases: properties of uracil-DNA glycosidase from Escherichia coli. J. Biol. Chem 252, 3286–3294 (1977). [PubMed] [Google Scholar]
- 8.Hirel PH, Schmitter MJ, Dessen P, Fayat G & Blanquet S Extent of N-terminal methionine excision from Escherichia coli proteins is governed by the side-chain length of the penultimate amino acid. Proc. Natl. Acad. Sci 86, 8247–8251 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts JC, Charyulu RL, Zera RT & Nagasawa HT Protection Against Acetaminophen Hepatotoxicity by Ribose-Cysteine (RibCys). Pharmacol. Toxicol 70, 281–285 (1992). [DOI] [PubMed] [Google Scholar]
- 10.Abbreviations and symbols for the description of conformations of polynucleotide chains (Recommendations 1982). Pure Appl. Chem 55, 1273–1280 (1983). [DOI] [PubMed] [Google Scholar]
- 11.Liu M et al. The mouse ortholog of NEIL3 is a functional DNA glycosylase in vitro and in vivo. Proc. Natl. Acad. Sci 107, 4925–4930 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spruijt CG et al. Dynamic Readers for 5-(Hydroxy)Methylcytosine and Its Oxidized Derivatives. Cell 152, 1146–1159 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Landau M et al. ConSurf 2005: the projection of evolutionary conservation scores of residues on protein structures. Nucleic Acids Res. 33, W299–W302 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jurrus E et al. Improvements to the APBS biomolecular solvation software suite: Improvements to the APBS Software Suite. Protein Sci. 27, 112–128 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.XDS Kabsch W.. Acta Crystallographica Section D Biological Crystallography 66, 125–132 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans PR & Murshudov GN How good are my data and what is the resolution? Acta Crystallographica Section D Biological Crystallography 69, 1204–1214 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winn MD et al. Overview of the CCP 4 suite and current developments. Acta Crystallographica Section D Biological Crystallography 67, 235–242 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCoy AJ et al. Phaser crystallographic software. Journal of Applied Crystallography 40, 658–674 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rychlewski L, Li W, Jaroszewski L & Godzik A Comparison of sequence profiles. Strategies for structural predictions using sequence information. Protein Science 9, 232–241 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emsley P, Lohkamp B, Scott WG & Cowtan K Features and development of Coot. Acta Crystallographica Section D Biological Crystallography 66, 486–501 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steiner RA, Lebedev AA & Murshudov GN Fisher’s information in maximum-likelihood macromolecular crystallographic refinement. Acta Crystallographica Section D Biological Crystallography 59, 2114–2124 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Williams CJ et al. MolProbity: More and better reference data for improved all-atom structure validation: PROTEIN SCIENCE.ORG. Protein Science 27, 293–315 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chalk R Mass Spectrometric Analysis of Proteins in Heterologous Gene Expression in E.coli (ed. Burgess-Brown NA.) 1586, 373–395 (Springer; New York, 2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.