Abstract
Cholinergic signaling in the cortex involves fast or transient signaling as well as a relatively slower neuromodulatory component. These two components of cholinergic activity mediate separate yet interacting aspects of cue detection and attentional control. The transient component appears to support the activation of cue-associated task or response sets, whereas the slower modulatory component stabilizes task-set and context representations, therefore potentially facilitating topdown control. Evidence from humans expressing genetic variants of the choline transporter as well as from patients with degenerating cholinergic systems support the hypothesis that attentional control capacities depend on levels of cholinergic neuromodulation. Deficits in cholinergic-attentional control impact diverse cognitive functions, including timing, working memory, and complex movement control.
Introduction:
The dual role of cholinergic signaling in cue detection and attentional control
As you walk into a grocery store to grab a bottle of wine on your way to a dinner party, you are confronted by a complex array of sights, sounds, and smells. The clerk hawking samples of local barbeque at a red and white table catches your eye for a moment, but you are focused on your mission and he barely enters your consciousness. Arriving at the wine section, out of habit you initially head to the area with your favorite hearty red, before remembering the menu. Scanning through the whites, you see what looks like a bottle of the Sauvignon Blanc your host had ordered – and raved about – the last time you were out for drinks together, but then realize it only looks similar. However, the correct bottle is on the same shelf as the imposter, so you grab it, and mission accomplished, now turn to your next task of finding the shortest checkout line.
Many of the cognitive processes used in this scenario (filtering, salience, goal-direction, selection) are discussed under the term “attention” and discussed by other papers in this special issue. Some of these would fall under the category of orienting sensory or attentional processes towards a salient stimulus. In the present review, we focus on the attentional process of cue detection - by which information about a stimulus enters the information-processing stream to activate an arbitrarily-associated (behavioral or computational) response. [1] Specifically, we discuss the evidence that cortical cholinergic signaling plays a critical role in activating (on a trial-level) and maintaining (over a longer period) the cortical ensembles representing cue-associated task and goal representations.
While distinct from orienting, the processes involved in cue detection also range from relatively automatic and “bottom-up”, to more controlled and “top-down” [2], depending on factors such as the saliency of the cue, the complexity of the associated response, and the strength of the cueresponse association. The strength of that association is frequently influenced by current representations of task context and goals. For example, you may have had the experience of sitting at a stoplight and absent-mindedly beginning to remove your foot from the brake when the left-turn signal in the next lane next turned green. Although such responses seemingly occur automatically and “without thinking”, the influence of task representations is demonstrated by your passenger’s feet remaining still. This “top-down” influence of prior experience and internally-represented goals is more explicit in situations where we are actively monitoring for cues, such as the street sign pointing to our destination.
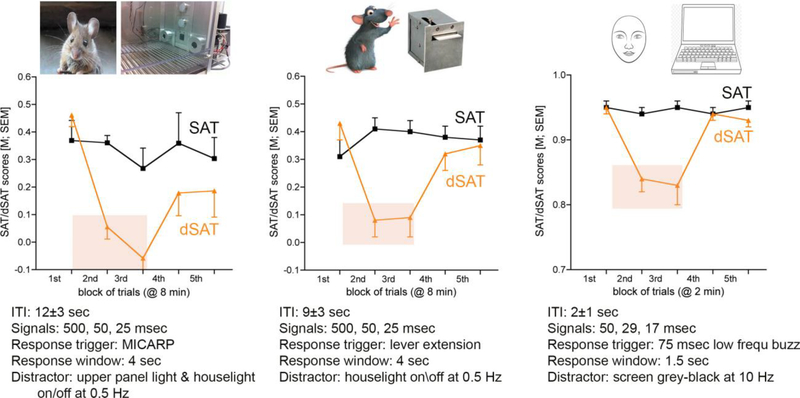
Most real-life situations and laboratory attention tasks involve both bottom-up and top-down attentional processes operating on multiple timescales. For example, we have used the Sustained Attention Task (SAT; Figure 1) in rodents and humans [3] to demonstrate different attentional-cholinergic contributions to bottom-up salience of the cue [2], but at different timescales the cholinergic system also contributes to successful activation of previously-learned, even habitual [4], response rules for cued and non-cued trials, and top-down mental computations that support task compliance over multiple trials and longer periods of time.
Figure 1.
Sustained Attention Task (SAT) performance, including the distractor version of this task (dSAT) of mice (left), rats (middle), and humans (right; some major task parameters are indicated below the individual task versions). The SAT consists of a semi-random sequence of signal and non-signal (or blank) trials. The intertrial interval (ITI) in all versions is variable, reducing the ability to time event onset. Following a signal or a non-signal event, a response window is opened either by extending two specialized nose-poke devices in the mouse version [‘MICARPs”; see 59], two retractable levers in the rat version [60], or by a low frequency buzz that activates to response keys on a computer keyboard in the human version [for details about signal presentation parameters see 3]. Subjects need to report a response in each trial within a defined period; a failure to do so is counted as an error of omission. Possible responses are hits (one response port or key) or misses (opposite response port or key) following signal events, or correct rejections or false alarms following non-signal events (reversed response port or key assignment.) A test session consists of 100–200 trials that are blocked post hoc (usually by time) to determine potential performance decrements. Hits and correct rejections are rewarded (water or pellets in rodents; symbol for later monetary reward in humans). Misses and false alarms have no further scheduled consequences and trigger, similar to hits and correct rejection responses, the next ITI. False alarms are relatively rare in all three species, and hits vary with signal duration. The ordinates in Fig. 1 depict the SAT score which combines the relative number of hits and correct rejections into one score that ranges from 0 (random response selection) to 1 (all responses are hits and correct rejections). Averaged over all signal durations, SAT scores (no distractor, black lines) generally are flat across sessions, with humans obviously performing robustly better than rodents. During dSAT sessions, the distractor, or perhaps more accurately the disruptor [this issue is discussed in 32], is turned on typically during trial blocks 2 and 3. In rodents, the distractor consists of the operant chamber houselight flashing on/off at 0.5 Hz. In humans, the screen alternates between silver and black at 10 Hz. As illustrated in Figure 1, the distractor suppresses performance in all three species, with rodents reaching chance level, followed by a relatively quick recovery of performance in block 4 and 5. In the presence of the distractor, humans adopt a more conservative and rodents a more liberal response bias [3], possibly also reflecting the qualitatively different capacity for top-down control in humans versus rodents. Our collective evidence indicates that cholinergic transients are necessary and sufficient to mediate hits, specifically in shift-hit trials. During the distractor, elevated levels of cholinergic neuromodulation predict the degree of post-distractor performance recovery and therefore is thought to mediate levels of topdown control designed to stabilize and recovery task performance (see main text).
Cholinergic transients mediate shifts from monitoring to detection
Early experiments on the effects of lesions of the rodent basal forebrain-cortical cholinergic projection system indicated that cholinergic activity is needed for cue detection. Specifically, removal of cholinergic projections to right frontal and parietal cortex permanently lowered hit rates. Correct rejection rates (CRs; correct responses on non-cued trials; see Figure 1 legend for a detailed description of SAT response categories) were not affected, indicating that the impairments were specific to cue detection and not a general performance decline [5]. There is some suggestion that CR rates are mediated by left-hemisphere processes, but this has not been examined as extensively ([6–8]; see [9,10] for similar results in humans).
The development of choline-sensitive biosensors [11] that allow trial-based recordings of cholinergic signaling significantly advanced understanding of its specific role in cue detection. Fast (second to subsecond scale) cholinergic signaling (“transients”) in right prefrontal cortex (rPFC) occurred when rats detected a cue. Importantly, these transients did not occur when the animal oriented to the cue but failed to initiate the associated response. Cholinergic transients were specifically associated with cue detection, rather than reward delivery or retrieval [12,13]. Furthermore, these transients only occur when there are long temporal delays between cues, or when a cue is preceded by an actual or perceived nonsignal trial.
These findings suggest that cholinergic transients mediate cue detection when such detection involves a task shift and activation of the cue-associated response set; i.e., a shift from monitoring to cue-directed behavior (“shift-hits”). In humans, shift-hits activate right frontal and right basal forebrain regions, and this greater activity correlates with faster response times, perhaps reflecting cholinergic signaling [13; additional evidence linking electrochemical recordings in rats with evidence from human fMRI studies is described in this reference]. Additional, causal evidence comes from studies in which the optogenetic generation of cholinergic transients induces the cue-associated response even when the cue has not been presented (false alarms), and suppression of endogenous transients leads to lesion-like decreases in hits [14, see also 15]. The task-shift function may explain why cholinergic responses have been associated with punishment and scale with “reinforcement surprise” [16], either of which would indicate that the currently-held task set is incorrect and should be shifted from.
Recordings of field potentials, via the electrodes used for recording transients, suggest that the underlying mechanism of this cholinergic transient-induced activation of the relevant task set includes cue-evoked high-frequency oscillations in prefrontal cortex and stimulation of postsynaptic muscarinic M1 receptors [17]. Moreover, cue-evoked transients force the synchronization of different populations of neurons oscillating at different frequencies (thetagamma coupling). Blocking postsynaptic effects of cholinergic transients disrupted this synchronization and reduced detection rates. Thus, cholinergic transients coordinate the mobilization and collaboration of multiple cortical networks, likely involving cholinergic effects on inhibitory interneurons [18] and extending synchronized activity across fronto-parietal and frontostriatal regions. The correlated activity of multiple neuronal populations coordinates the multiple cognitive operations required to utilize the information provided by the cue and execute the detection process [see also 19]. This is distinct from the within-area desynchronization associated with anticipatory attention (see discussion by [20]
Cholinergic neuromodulation and top-down attentional effort
Complementing the immediate (re)activation of dormant cue-response associations mediated by cholinergic transients, longer-term (tens to hundreds of seconds) cholinergic neuromodulation appears to mediate the stabilization of relevant task-set representations supporting “top-down” regulation. Increases in cholinergic neuromodulation can be seen in the SAT as increases in extracellular acetylcholine (ACh) levels in rPFC as rodents move from a no-task baseline to the task itself, and further increases in the face of attentional challenge [21,22]. These longer-term increases in rPFC ACh occur despite reductions in hits, and thus cholinergic transients, during attentional challenges, indicating an independent, though interacting (see below) pathway. Human fMRI studies show parallel increases in rPFC activation from no-task baseline, to the SAT, to the challenge condition [23–25]. Notably, the challenge-related rPFC increase is not seen in individuals expressing a low-capacity variant of the choline high-affinity transporter thought to limit the ability to sustain cholinergic activity [25].
These increases in rPFC ACh and/or activation are linked with top-down “attentional effort” and maintenance, stabilization, or recovery of the relevant task set rather than successful performance per se. They occur even when the task is only expected based on past experience, not actually introduced [26], interact closely with dopaminergically-mediated motivational systems [27], and persist or even increase when other factors make performance declines inevitable [22]. In humans, rPFC activation increases during the SAT are correlated with larger, not smaller, performance declines in response to the attentional challenge condition, and individuals expressing the low-CHT variant are able to maintain successful performance.
While initially counterintuitive, functional connectivity analyses clarified the potential role of rPFC activation: Increased connectivity with the anterior cingulate, associated with error signaling, was associated with greater vulnerability to the attentional challenge, whereas increased connectivity to superior parietal regions, associated with the control of spatial attention, correlated with preserved performance [25]. In other words, rPFC activation/cholinergic neuromodulation appears to interact with conflict or error detection and motivation systems to support the recruitment of the task representations necessary for preserving performance in the face of challenge, but actual preserved performance may depend more heavily on the degree to which those representations can be successfully implemented.
More specifically, the top-down neuromodulatory component appears to play a critical role in stabilizing the relevant task set, presumably by maintaining the synchrony of the representative cortical ensembles. It is thus more directly involved in preserving performance when challenge comes from “competitive distractors” – stimuli with strong bottom-up salience that are associated with a competing response or task set. This is illustrated by comparing the performance of low-cholinergic populations across different task parameters. As a general rule, they show stimulus-driven, rather than goal-driven behavior.
For example, “sign trackers” (STs) are defined by their propensity to direct attention and behavior towards Pavlovian cues, even though these cues only predict reward delivery and do not require any response. Rodent STs exhibit an attenuated capacity for sustained elevated cholinergic neuromodulation, due to a dysregulation in the CHT that limits ACh synthesis [28]. This is associated with poor attentional control and a bias towards bottom-up, stimulus-directed processing, with the sign-tracking behavior itself (orienting towards a salient but irrelevant cue) as one example. In the SAT, where the target cue has a great deal of bottom-up salience but top-down control is required to maintain the appropriate cue-response set associations, STs exhibit above-chance but highly unstable performance [29]. The top-down attentional control vulnerabilities of STs were also revealed by the demonstration that their ability to perform complex movements is relatively poor when such movement require attentional control, such as during the traversal of rotating straight or zigzag rods [30].
Humans expressing a relatively low-capacity CHT variant (I89V variant), and thus presumably with a reduced capacity to sustain cortical cholinergic modulation, show a similar pattern of parameter-dependent preserved versus impaired performance. As noted above, these humans fail to activate rPFC regions associated with attentional effort in response to changing background illumination in the SAT, but demonstrate good performance by relying on the bottom-up salience of the cue. In contrast, they report high levels of distractibility in everyday life, and show marked distractor vulnerability in a task where the target has very low bottom-up salience (differentiated from nontargets only by its duration), but the distractor (videos playing on a nearby laptop) has strong bottom-up salience [31]. Notably, a different human CHT variant suspected to yield a relatively superior capacity of the CHT is associated with a strikingly near-perfect resistance to this distractor [32]. Providing converging evidence, in patients with Parkinson’s disease, cortical cholinergic denervation does not predict SAT performance, but does predict vulnerability to the video distractor [33].
Interactive cognitive control functions of cholinergic signaling
The evidence from PD patients also points to the ways that different components of the cholinergic system interact with each other and other systems to support cognitive control. Although cortical cholinergic denervation did not predict hits to the visually salient cue in SAT, thalamic denervation did, perhaps reflecting early-stage “orienting” [34]. The importance of thalamic cue processing was also revealed by the finding that cue-evoked glutamate bursts from thalamo-cortical neurons - which express α4β2* nicotinic acetylcholine receptors (nAChRs) - are necessary (though not sufficient) for the generation of cholinergic transients in cortex [35,36]. In simplified, functional terms, thalamic inputs “insert” information about the cue into the cortex. Elevated levels of cholinergic neuromodulation facilitate the reliability and efficacy of cue representation in cortical circuitry [35–38]. However, a cholinergic transient is then required for cue to be detected, in part via synchronizing multiple neuronal populations across larger brain regions to activate cueassociated task and response set representations [39,40]. The results from studies using pharmacological means to elevate cholinergic activity in humans are consistent with the view that detection processes and associated attentional biases are under cholinergic control [41,42].
We have focused on the specific roles of transient and neuromodulatory cholinergic signaling in detecting relevant cues and avoiding disruption from irrelevant stimuli as these operations are fundamental to successful behavior in many situations. Moreover, divided attention, cross-modal cue processing, and working memory capacity all depend on cholinergic signaling [43–46]. Because of the multilevel nature of cholinergic contributions, cholinergic deficiencies affect all forms of attention and attention-dependent learning [47–50]. In addition, cholinergically-mediated top-down control and response to movement cues becomes especially important when motor function is impaired, and thus poor attentional control causes complex movement deficits and falls in older adults and patients with Parkinson’s disease [51–53]. The dual role of cholinergic contributions in maintaining stable representations and detecting change may be especially important for timing in both movement and working memory [26,30,54,55].
In conclusion, converging evidence from animal models, human genetic populations, and patients with dysregulated or degenerating cholinergic systems continues to illuminate the multidimensional role that cholinergic activity plays in attention and cognitive control. Given the fundamental limitations of acetylcholinesterase inhibitors [discussed in 56], further development of this more detailed and mechanistic perspective will be essential for improving the cognition and daily life of patients with diverse neurological and psychiatric disorders [57,58].
Acknowledgements:
The authors’ research was supported by PHS grants PO1 DA031656 and P50NS091856 (Morris K. Udall Center for Excellence in Parkinson’s Disease Research, University of Michigan).
Footnotes
Competing Financial Interests: The authors declare no competing financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Posner MI, Snyder CR, Davidson BJ: Attention and the detection of signals. J Exp Psychol (1980) 109(2):160–174. [PubMed] [Google Scholar]
- 2.Posner MI, Snyder CRR: Attention and cognitive control In: Information processing and cognition: The Loyola symposium. Solso R (Ed) Erlbaum, Hillsdale, NJ: (1975):55–85. [Google Scholar]
- 3.Demeter E, Sarter M, Lustig C: Rats and humans paying attention: Cross-species task development for translational research. Neuropsychology (2008) 22(6):787–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang YV: Habitual versus goal-driven attention. Cortex (2018) 102(107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGaughy J, Kaiser T, Sarter M: Behavioral vigilance following infusions of 192 iggsaporin into the basal forebrain: Selectivity of the behavioral impairment and relation to cortical ache-positive fiber density. Behav Neurosci (1996) 110(2):247–265. [DOI] [PubMed] [Google Scholar]
- 6.Apparsundaram S, Martinez V, Parikh V, Kozak R, Sarter M: Increased capacity and density of choline transporters situated in synaptic membranes of the right medial prefrontal cortex of attentional task-performing rats. J Neurosci (2005) 25(15):3851–3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez V, Sarter M: Lateralized attentional functions of cortical cholinergic inputs. Behavioral Neuroscience (2004) 118(984–991. [DOI] [PubMed] [Google Scholar]
- 8.Sarter M, Turchi J: Age- and dementia-associated impairments in divided attention: Psychological constructs, animal models, and underlying neuronal mechanisms. Dement Geriatr Cogn Disord (2002) 13(1):46–58. [DOI] [PubMed] [Google Scholar]
- 9.Bergert S: How do our brain hemispheres cooperate to avoid false memories? Cortex (2013) 49(2):572–581. [DOI] [PubMed] [Google Scholar]
- 10.Parkin AJ, Bindschaedler C, Harsent L, Metzler C: Pathological false alarm rates following damage to the left frontal cortex. Brain Cogn (1996) 32(1):14–27. [DOI] [PubMed] [Google Scholar]
- 11.Parikh V, Pomerleau F, Huettl P, Gerhardt GA, Sarter M, Bruno JP: Rapid assessment of in vivo cholinergic transmission by amperometric detection of changes in extracellular choline levels. Eur J Neurosci (2004) 20(6):1545–1554. [DOI] [PubMed] [Google Scholar]
- 12.Parikh V, Kozak R, Martinez V, Sarter M: Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron (2007) 56(1):141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howe WM, Berry AS, Francois J, Gilmour G, Carp JM, Tricklebank M, Lustig C, Sarter M: Prefrontal cholinergic mechanisms instigating shifts from monitoring for cues to cue-guided performance: Converging electrochemical and fmri evidence from rats and humans. J Neurosci (2013) 33(20):8742–8752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gritton HJ, Howe WM, Mallory CS, Hetrick VL, Berke JD, Sarter M: Cortical cholinergic signaling controls the detection of cues. Proc Natl Acad Sci U S A (2016) 113(8):E1089–1097.Using optogenetic methods to generate or suppress cholinergic transients in mice performing a sustained attention task, this study shows the necessity and sufficiency of such transients for the detection of hits.
- 15.Pinto L, Goard MJ, Estandian D, Xu M, Kwan AC, Lee S-H, Harrison TC, Feng G, Dan Y: Fast modulation of visual perception by basal forebrain cholinergic neurons. Nature Neurosci (2013) 16(1857–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hangya B, Ranade SP, Lorenc M, Kepecs A: Central cholinergic neurons are rapidly recruited by reinforcement feedback. Cell (2015) 162(5):1155–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howe WM, Gritton HJ, Lusk NA, Roberts EA, Hetrick VL, Berke JD, Sarter M: Acetylcholine release in prefrontal cortex promotes gamma oscillations and thetagamma coupling during cue detection. J Neurosci (2017) 37(12):3215–3230.By combining electrochemical with neurophysiological recordings, this study demonstrated that cholinergic transients generate high frequency oscillations in prefrontal cortex and that cue detection was disrupted if such oscillations were prevented by blocking muscarinic acetylcholine M1 receptors in this region.
- 18.Chen N, Sugihara H, Sur M: An acetylcholine-activated microcircuit drives temporal dynamics of cortical activity. Nat Neurosci (2015) 18(6):892–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goard M, Dan Y: Basal forebrain activation enhances cortical coding of natural scenes. Nat Neurosci (2009) 12(11):1444–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruff DA, Cohen MR: A normalization model suggests that attention changes the weighting of inputs between visual areas. P Natl Acad Sci USA (2017) 114(20):E4085–E4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.St Peters M, Demeter E, Lustig C, Bruno JP, Sarter M: Enhanced control of attention by stimulating mesolimbic-corticopetal cholinergic circuitry. J Neurosci (2011) 31(26):9760–9771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozak R, Bruno JP, Sarter M: Augmented prefrontal acetylcholine release during challenged attentional performance. Cereb Cortex (2006) 16(1):9–17. [DOI] [PubMed] [Google Scholar]
- 23.Demeter E, Hernandez-Garcia L, Sarter M, Lustig C: Challenges to attention: A continuous arterial spin labeling (asl) study of the effects of distraction on sustained attention. Neuroimage (2011) 54(2):1518–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berry AS, Blakely RD, Sarter M, Lustig C: Cholinergic capacity mediates prefrontal engagement during challenges to attention: Evidence from imaging genetics. Neuroimage (2015) 108(386–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berry AS, Sarter M, Lustig C: Distinct frontoparietal networks underlying attentional effort and cognitive control. J Cogn Neurosci (2017) 29(7):1212–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paolone G, Lee TM, Sarter M: Time to pay attention: Attentional performance timestamped prefrontal cholinergic activation, diurnality, and performance. J Neurosci (2012) 32(35):12115–12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.St Peters M, Demeter E, Lustig C, Bruno JP, Sarter M: Enhanced control of attention by stimulating mesolimbic-corticopetal cholinergic circuitry. J Neurosci (2011) 31(26):9760–9771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koshy Cherian A, Kucinski A, Pitchers KK, Yegla B, Parikh V, Kim Y, Valuskova P, Gurnarni S, Lindsley CW, Blakely RD, Sarter M: Unresponsive choline transporter as a trait neuromarker and a causal mediator of bottom-up attentional biases. J Neurosci (2017) 37(2947–2959.This study revealed that disrupted regulation of the neuronal choline transporter represents the cellular mechanism responsible for a “dampened” cholinergic neuromodulator system in rats which, therefore, exhibit poor attentional control as a behavioral trait.
- 29.Paolone G, Angelakos CC, Meyer PJ, Robinson TE, Sarter M: Cholinergic control over attention in rats prone to attribute incentive salience to reward cues. J Neurosci (2013) 33(19):8321–8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kucinski A, Lustig C, Sarter M: Addiction vulnerability trait impacts complex movement control: Evidence from sign-trackers. Behav Brain Res (2018) 350(139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berry AS, Demeter E, Sabhapathy S, English BA, Blakely RD, Sarter M, Lustig C: Disposed to distraction: Genetic variation in the cholinergic system influences distractibility but not time-on-task effects. J Cogn Neurosci (2014) 26(9):1981–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarter M, Lustig C, Blakely RD, Koshy Cherian A: Cholinergic genetics of visual attention: Human and mouse choline transporter capacity variants influence distractibility. J Physiol Paris (2016) 110(1–2):10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim K, Muller M, Bohnen NI, Sarter M, Lustig C: The cortical cholinergic system contributes to the top-down control of distraction: Evidence from patients with parkinson’s disease. Neuroimage (2017) in press.Examining the impact of cholinergic losses on attentional performance in patients with Parkinson’s disease, the authors concluded that the capacity for distractor resistance is related to the integrity of the cortical projections of basal forebrain cholinergic neurons, as opposed to the cholinergic inputs to thalamus.
- 34.Kim K, Muller M, Bohnen NI, Sarter M, Lustig C: Thalamic cholinergic innervation makes a specific bottom-up contribution to signal detection: Evidence from parkinson’s disease patients with defined cholinergic losses. Neuroimage (2017) 149(295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parikh V, Man K, Decker MW, Sarter M: Glutamatergic contributions to nicotinic acetylcholine receptor agonist-evoked cholinergic transients in the prefrontal cortex. J Neurosci (2008) 28(14):3769–3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parikh V, Ji J, Decker MW, Sarter M: Prefrontal beta2 subunit-containing and alpha7 nicotinic acetylcholine receptors differentially control glutamatergic and cholinergic signaling. J Neurosci (2010) 30(9):3518–3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howe WM, Ji J, Parikh V, Williams S, Mocaër E, Trocmé-Thibierge C, Sarter M: Enhancement of attentional performance by selective stimulation of alpha4beta2* nachrs: Underlying cholinergic mechanisms. Neuropsychopharmacology (2010) 35(6):1391–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lambe EK, Picciotto MR, Aghajanian GK: Nicotine induces glutamate release from thalamocortical terminals in prefrontal cortex. Neuropsychopharmacology (2003) 28(2):216–225. [DOI] [PubMed] [Google Scholar]
- 39.Hasselmo ME, Sarter M: Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology (2011) 36(1):52–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarter M, Lustig C, Berry AS, Gritton HJ, Howe WM, Parikh V: What do phasic cholinergic signals do? Neurobiology of Learning and Memory (2016) 130(135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gratton C, Yousef S, Aarts E, Wallace DL, D’Esposito M, Silver MA: Cholinergic, but not dopaminergic or noradrenergic, enhancement sharpens visual spatial perception in humans. J Neurosci (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rokem A, Landau AN, Garg D, Prinzmetal W, Silver MA: Cholinergic enhancement increases the effects of voluntary attention but does not affect involuntary attention. Neuropsychopharmacology (2010) 35(13):2538–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Botly LCP, De Rosa E: Cholinergic deafferentation of the neocortex using 192 iggsaporin impairs feature binding in rats. J Neurosci (2009) 29(13):4120–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ljubojevic V, Luu P, De Rosa E: Cholinergic contributions to supramodal attentional processes in rats. J Neurosci (2014) 34(6):2264–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turchi J, Sarter M: Cortical acetylcholine and processing capacity: Effects of cortical cholinergic deafferentation on crossmodal divided attention in rats. Cogn Brain Res (1997) 6(2):147–158. [DOI] [PubMed] [Google Scholar]
- 46.Turchi J, Sarter M: Cortical cholinergic inputs mediate processing capacity: Effects of 192 igg-saporin-induced lesions on olfactory span performance. Eur J Neurosci (2000) 12(12):4505–4514. [PubMed] [Google Scholar]
- 47.Mesulam M: The cholinergic lesion of alzheimer’s disease: Pivotal factor or side show? Learn Mem (2004) 11(1):43–49. [DOI] [PubMed] [Google Scholar]
- 48.Bohnen NI, Muller ML, Kotagal V, Koeppe RA, Kilbourn MR, Gilman S, Albin RL, Frey KA: Heterogeneity of cholinergic denervation in parkinson’s disease without dementia. J Cereb Blood Flow Metab (2012) 32(8):1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sarter M, Bruno JP, Givens B: Attentional functions of cortical cholinergic inputs: What does it mean for learning and memory? Neurobiology of Learning and Memory (2003) 80(3):245–256. [DOI] [PubMed] [Google Scholar]
- 50.Lustig C, Sarter M: Attention and the cholinergic system: Relevance to schizophrenia. Curr Top Behav Neurosci (2016) 28(327–362. [DOI] [PubMed] [Google Scholar]
- 51.Bohnen NI, Müller MLTM, Koeppe RA, Studenski SA, Kilbourn MA, Frey KA, Albin RL: History of falls in parkinson disease is associated with reduced cholinergic activity. Neurology (2009) 73(20):1670–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kucinski A, Paolone G, Bradshaw M, Albin R, Sarter M: Modeling fall propensity in parkinson’s disease: Deficits in the attentional control of complex movements in rats with cortical-cholinergic and striatal-dopaminergic deafferentation. J Neurosci (2013) 33(42):16522–16539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sarter M, Albin RL, Kucinski A, Lustig C: Where attention falls: Increased risk of falls from the converging impact of cortical cholinergic and midbrain dopamine loss on striatal function. Exp Neurol (2014) 257(120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matell MS, Meck WH, Lustig C: Not “just” a coincidence: Frontal-striatal interactions in working memory and interval timing. Memory (2007) 13(3–4):441–448. [DOI] [PubMed] [Google Scholar]
- 55.Meck WH: Neuropharmacology of timing and time perception. Cogn Brain Res (1996) 3(3–4):227–242. [DOI] [PubMed] [Google Scholar]
- 56.Sarter M: Behavioral-cognitive targets for cholinergic enhancement. Curr Opin Behav Sci (2015) 4(22–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bain EE, Robieson W, Pritchett Y, Garimella T, Abi-Saab W, Apostol G, McGough JJ, Saltarelli MD: A randomized, double-blind, placebo-controlled phase 2 study of alpha4beta2 agonist abt-894 in adults with adhd. Neuropsychopharmacology (2013) 38(3):405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moran SP, Dickerson JW, Cho HP, Xiang Z, Maksymetz J, Remke DH, Lv X, Doyle CA, Rajan DH, Niswender CM, Engers DW et al. : M1-positive allosteric modulators lacking agonist activity provide the optimal profile for enhancing cognition. Neuropsychopharmacology (2018) 43(8):1763–1771.Consistent with the role of cholinergic neuromodulation proposed herein, these authors demonstrate the potential usefulness of postsynaptic positive modulators of muscarinic receptors for treating a wide range of cognitive disorders.
- 59.St Peters M, Cherian AK, Bradshaw M, Sarter M: Sustained attention in mice: Expanding the translational utility of the sat by incorporating the michigan controlled access response port (micarp). Behav Brain Res (2011) 225(2):574–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McGaughy J, Sarter M: Behavioral vigilance in rats: Task validation and effects of age, amphetamine, and benzodiazepine receptor ligands. Psychopharmacology (Berl) (1995) 117(3):340–357. [DOI] [PubMed] [Google Scholar]