Abstract
OBJECTIVE
In this multicenter study, the authors reviewed the results obtained in patients who underwent Gamma Knife radiosurgery (GKRS) for dural arteriovenous fistulas (dAVFs) and determined predictors of outcome.
METHODS
Data from a cohort of 114 patients who underwent GKRS for cerebral dAVFs were compiled from the International Gamma Knife Research Foundation. Favorable outcome was defined as dAVF obliteration and no posttreatment hemorrhage or permanent symptomatic radiation-induced complications. Patient and dAVF characteristics were assessed to determine predictors of outcome in a multivariate logistic regression analysis; dAVF-free obliteration was calculated in a competing-risk survival analysis; and Youden indices were used to determine optimal radiosurgical dose.
RESULTS
A mean margin dose of 21.8 Gy was delivered. The mean follow-up duration was 4 years (range 0.5–18 years). The overall obliteration rate was 68.4%. The postradiosurgery actuarial rates of obliteration at 3, 5, 7, and 10 years were 41.3%, 61.1%, 70.1%, and 82.0%, respectively. Post-GRKS hemorrhage occurred in 4 patients (annual risk of 0.9%). Radiation-induced imaging changes occurred in 10.4% of patients; 5.2% were symptomatic, and 3.5% had permanent deficits. Favorable outcome was achieved in 63.2% of patients. Patients with middle fossa and tentorial dAVFs (OR 2.4, p = 0.048) and those receiving a margin dose greater than 23 Gy (OR 2.6, p = 0.030) were less likely to achieve a favorable outcome. Commonly used grading scales (e.g., Borden and Cognard) were not predictive of outcome. Female sex (OR 1.7, p = 0.03), absent venous ectasia (OR 3.4, p < 0.001), and cavernous carotid location (OR 2.1, p = 0.019) were predictors of GKRS-induced dAVF obliteration.
CONCLUSIONS
GKRS for cerebral dAVFs achieved obliteration and avoided permanent complications in the majority of patients. Those with cavernous carotid location and no venous ectasia were more likely to have fistula obliteration following radiosurgery. Commonly used grading scales were not reliable predictors of outcome following radiosurgery.
Keywords: Gamma Knife, arteriovenous, fistula, dural, stereotactic radiosurgery, outcome, vascular disorders
DURAL arteriovenous fistulas (dAVFs) represent approximately 15% of all cerebral vascular malformations.4,10,12 These lesions are commonly treated with endovascular embolization, microsurgery, or radiosurgery.10 Optimal therapeutic strategies based on patient and lesion characteristics remain unknown, and there remains significant variation in treatments among highvolume centers. Additionally, patients with dAVFs often require multiple treatment sessions or modalities. With regard to the treatment of dAVF, the literature is primarily composed of small single-center experiences.6,15,16,26,27 Specifically for radiosurgery, these reports often comprise a small number of patients that precludes analysis of independent predictors of dAVF obliteration.5 In this multicenter study, we review the results following Gamma Knife radiosurgery (GKRS) of cerebral dAVFs in a multicenter, international cohort to determine predictors of outcome.
Methods
Patient Population
Nine medical centers participating in the International Gamma Knife Research Foundation obtained individual institutional review board approvals to participate in this study. A total of 133 patients were identified with cerebral dAVFs treated with GKRS from 1988 to 2016. At each center, retrospective clinical outcome analysis of patients was performed. The following centers contributed data for this study: University of Pittsburgh (43 patients), University of Pennsylvania (9 patients), University of Sherbrooke (2 patients), University of Manitoba (1 patient), West Virginia University (2 patients), University of Puerto Rico (1 patient), Beaumont Health System (1 patient), Na Homolce Hospital (14 patient), and the University of Virginia (60 patients).
The records of dAVF patients who underwent Gamma Knife (Elekta AB) treatment between 1988 and 2016 were evaluated by clinicians at each center for study inclusion. A database with selected variables of patient and dAVF characteristics was created and sent to all participating centers. Documented dAVF characteristics were determined by physicians at the treating facility and included lesion size, location, and popular classification grades. Participants at each center reviewed the medical records of their patients, entered the data in the spreadsheet, and removed all patient identifiers from the database. Pooled and de-identified data were screened by an independent third party for errors. Any uncertainties or ambiguities in the data were addressed at the contributing center. Afterward, data were transmitted to the first and senior authors who, along with their coauthors, developed this report.
Patients were included in the study if they had a cerebral dAVF treated with GKRS and had a minimum of 6 months of neuroimaging and clinical follow-up, though patients with a complication within 6 months of treatment were also included. Additionally, patients with volume-staged radiosurgery were excluded. As such, 19 patients were excluded.
Radiosurgical Technique
The Gamma Knife models U, B, C, 4C, and Perfexion were used depending on the technology available at the time of GKRS for each participating center. The radiosurgery procedure began with the application of the Leksell model G stereotactic frame (Elekta AB) using local anesthetic supplemented by additional sedation as needed. All radiosurgery procedures were performed in a single fraction. After stereotactic frame placement, high-resolution stereotactic MRI was performed. In cases in which MRI was not feasible or when MRI distortion was a concern, a stereotactic CT scan was obtained. Thin-slice axial and/or coronal images were obtained after intravenous contrast administration. Stereotactic cerebral angiography was performed, and the images were incorporated in treatment planning for nidus definition and dose planning. Radiosurgery dose planning was then performed by the neurosurgeon in conjunction with a radiation oncologist and medical physicist.
Clinical and Neuroimaging Follow-Up
Clinical and neuroimaging evaluations were generally performed at follow-up intervals of 6 months for the first 2 years following radiosurgery and then yearly thereafter. When there was no dAVF visible on MRl and/or CT, the patient underwent angiography to confirm the obliteration of the nidus. All images were analyzed by both a neuro-surgeon and a neuroradiologist. Patients were instructed to continue MRI every 1–5 years to monitor for long-term complications, even after their angiogram demonstrated complete dAVF obliteration. For those patients for whom MRI was contraindicated (e.g., when a cardiac pacemaker was present), CT was performed instead. Whenever feasible, patients underwent follow-up neurological examination and neuroimaging at the respective treating center. However, since participating institutions represent tertiary referral centers, some patients underwent follow-up evaluations by their local physicians. For such patients, clinical notes and actual neuroimaging studies (i.e., not just the radiological reports) were received and reviewed by the treating clinicians who performed the GKRS procedure. The follow-up images were compared with those obtained at the time of GKRS. dAVF dimensions were assessed in the axial, sagittal, and coronal planes in relation to comparable measurements on the GKRS neuroimaging studies.
Statistical Analysis
Much of our methods have been published in our previous work(s).8,9,19,21 Data are presented as the median or mean and range for continuous variables and as frequency and percentage for categorical variables. Calculations of normality were assessed graphically and statistically. Statistical analyses of categorical variables were carried out using chi-square and Fisher’s exact tests of associations as appropriate. Statistics of means were carried out using unpaired Student t-tests, both with and without equal variance (Levene’s test), as necessary, and Wilcoxon rank sum tests when variables were not normally distributed. Favorable outcome was defined as dAVF obliteration and no posttreatment hemorrhage or permanent symptomatic complications following treatment. Patient, dAVF, and treatment characteristics were assessed in a univariate analysis to test for covariates predictive of outcome. Common dAVF classification scale scores were analyzed as continuous variables in a univariate analysis and precluded from multivariate analysis. Clinically significant variables and interaction expansion covariates were further assessed in multivariable analyses as deemed relevant. Factors predictive in univariate analysis (p < 0.15) were entered into multivariate logistic regression analysis models, both with and without treatment characteristics.1 Additionally, competing risk survival analysis of dAVF-free obliteration was calculated using the modified Kaplan-Meier method and Gray’s method.13 After confirmation of the proportional hazards assumption, factors predictive of obliteration (p < 0.15) were entered into modified multivariate Cox regression analysis to assess hazard ratios in the presence of competing mortality risk.11 Multivariate regression models and commonly used grading scale scores were assessed using area under the receiver operating characteristic curve. Youden indices were calculated to determine cutoffs for the dichotomized continuous variable margin dose (Gy) that yielded the optimal discrimination of outcome. p ≤ 0.05 was considered statistically significant. Statistical analysis was carried out with Stata 14.0 (StataCorp LLC) and SAS 9.4 (SAS Institute Inc.).
Results
Demographics and Clinical Presentation
From a total of 133 patients with dAVF treated with GKRS from 1988 to 2016 in the 9 contributing centers, 114 patients met study inclusion criteria. Patient baseline and presenting characteristics are shown in Table 1. Our cohort had 48 females (42%) and a mean age of 55 years. Headache was reported in 60 patients (52.6%), tinnitus in 38 (33.3%), visual changes in 27 (23.7%), neurological deficits in 28 (25%), and seizures in 8 (7%). About half (51.8%) of the patients had received prior treatment: endovascular treatment in 54 patients (47.4%), craniotomy in 6 (5.3%), and stereotactic radiosurgery in 3 (2.6%). Of note, multiple prior endovascular procedures failed in 22 patients (19.3%), 2 patients (1.8%) had prior craniotomy and endovascular treatments, and 2 patients (1.8%) underwent prior stereotactic radiosurgery and endovascular treatments.
TABLE 1.
Summary of patient and dAVF characteristics
| Characteristic | Value (%) |
|---|---|
| Age (mean ± SD), yrs | 55 ± 14 |
| Female | 48 (42) |
| Prior treatment for dAVF | 59 (51.8) |
| Any endovascular | 54 (47.4) |
| Endovascular treatment ≥2 times | 22 (19.3) |
| Craniotomy | 6 (5.3) |
| Stereotactic radiosurgery | 3 (2.6) |
| Presenting patient characteristic | |
| Headache | 60 (52.6) |
| Tinnitus | 38 (33.3) |
| Visual changes | 27 (23.7) |
| Neurological deficit | 28 (25) |
| Seizures | 8 (7.0) |
| Asymptomatic | 8 (7.0) |
| ICH | 27 (23.7) |
| Spinal drainage | 24 (21.1) |
| Associated aneurysms | 2 (1.8) |
| Associated edema | 10 (8.8) |
| Multihole dAVF | 48 (42.1) |
| Cortical venous reflux | 59 (51.7) |
| Venous ectasia | 23 (20.2) |
| Maximum dAVF diameter* | |
| Small (≤10 mm) | 19 (27.1) |
| Medium (11–20 mm) | 28 (40.0) |
| Large (>20 mm) | 23 (32.9) |
| Location of dAVF | |
| Transverse/sigmoid | 35 (30.7) |
| Tentorial | 30 (26.3) |
| Carotid cavernous | 19 (16.7) |
| Torcular | 10 (8.8) |
| Anterior fossa | 8 (7.0) |
| Convexity | 8 (7.0) |
| Middle fossa | 6 (5.3) |
| Sagittal | 6 (5.3) |
| Borden grade | |
| I | 44 (38.6) |
| II | 26 (23.8) |
| III | 44 (38.6) |
| Cognard classification† | |
| I | 38 (33.3) |
| IIa | 9 (7.9) |
| IIb | 6 (5.3) |
| IIab | 10 (8.8) |
| III | 6 (5.3) |
| IV | 20 (17.5) |
| V | 24 (21.1) |
Values are presented as the number (%) of patients unless otherwise specified.
Maximum diameter size available for 70 patients.
Cognard classifications available for 113 patients.
Pretreatment Radiographic Evaluation and GKRS Treatment
Preradiosurgery CT or MRI studies were available for all patients. dAVF details are shown in Table 1. Twenty-seven patients (23.7%) had intracerebral hemorrhage (ICH), 5 (18.5%) of whom had concurrent intraventricular hemorrhage. Fourteen patients (12.3%) had subarachnoid hemorrhage (SAH), with or without concurrent ICH. Forty-four patients had Borden grade III dAVFs and 26 had Borden grade II dAVFs; 18 of the 44 Borden grade Ill patients (40.9%) and 8 of 26 Borden grade II patients (30.8%) presented with either ICH or SAH. There were 59 dAVFs (51.7%) demonstrating cortical venous reflux, 23 (20.2%) demonstrating venous ectasia, 24 (21.1%) with spinal drainage, 10 (8.8%) with associated cerebral edema, and 2 (1.8%) with associated aneurysms. The most common dAVF location was the transverse or sigmoid sinus. More females presented with cavernous dAVF (C-dAVF) (14 in females vs 5 in males, p = 0.002).
Table 2 details the GKRS parameters. The mean margin dose was 21.8 Gy, and the mean maximum dose was 40.7 Gy. A total of 51 patients (44.7%) had margin radiation doses exceeding 23 Gy, with 29 patients (25.4%) receiving margin doses exceeding 25 Gy.
TABLE 2.
Summary of radiosurgery treatment characteristics
| Maximum dose, Gy | 40.7 ± 7.3 |
| Marginal dose, Gy | 21.9 ± 3.1 |
| Isodose line, % | 54.1 ± 11.2 |
| Number of isocenters | 3.1 ± 2.6 |
| Follow-up duration, mos | 46.7 ± 40.1 |
Values are presented as the mean ± SD.
dAVF Obliteration
Post-GKRS follow-up angiographic studies were obtained for 76 patients (66.6%); follow-up MRI studies were available for 36 patients (31.5%). dAVF obliteration was confirmed in 78 patients (68.4%), including 60 (76.9%) verified angiographically and 16 (20.5%) verified by MRI alone. Post-GKRS actuarial rates of obliteration at 3, 5, 7, and 10 years were 41.3%, 61.1%, 70.1%, and 82.0%, respectively (Fig. 1). The median time to obliteration was 39 months (95% CI 36–58 months). The rate of C-dAVF obliteration was 84.2% (16 of 19), whereas the rate of noncavernous obliteration was 65.3% (62 of 95). Table 3 displays the results of the univariate and multivariate regression analyses for predictors of GKRS-induced dAVF obliteration. Multivariate regression revealed female sex (p = 0.03), absent venous ectasia (p < 0.001), and cavernous carotid location (p = 0.019) as independent predictors of post-GKRS dAVF obliteration (Fig. 2). Of the patients whose dAVF failed to achieve obliteration following GKRS, 13 received further endovascular treatment, 2 had repeat radiosurgery, and 1 had a craniotomy.
FIG. 1.
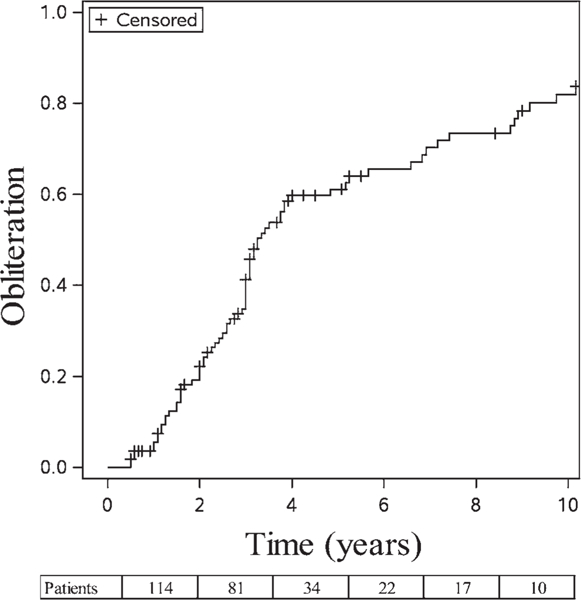
Kaplan-Meier plot of overall dAVF obliteration over time. The postradiosurgery actuarial rates of obliteration at 3, 5, 7, and 10 years were 41.3%, 61.1%, 70.1%, and 82.0%, respectively. Values at the bottom of the figure correspond to the number of patients available for each follow-up interval (114 for 2 years, 81 for 4 years, and so on).
TABLE 3.
Univariate and multivariate analyses for predictors of dAVF obliteration after GKRS
| Univariate Analysis |
Multivariate Analysis |
|||||
|---|---|---|---|---|---|---|
| Factor | Hazard Ratio | 95% CI | p Value | Hazard Ratio | 95% CI | p Value |
| Female sex | 1.52 | 0.97–2.37 | 0.066 | 1.71 | 1.05–2.78 | 0.030 |
| Presenting tinnitus | 1.51 | 0.95–2.41 | 0.083 | NS | NS | NS |
| Presenting vision changes | 1.49 | 0.91–2.45 | 0.117 | NS | NS | NS |
| Nonhemorrhagic presentation | 1.61 | 0.97–2.68 | 0.065 | NS | NS | NS |
| Presenting w/o ICH | 1.51 | 0.88–2.60 | 0.138 | NS | NS | NS |
| Presenting w/o IVH | 3.06 | 0.95–9.78 | 0.060 | NS | NS | NS |
| Presenting w/o SAH | 2.26 | 1.12–4.58 | 0.023* | NS | NS | NS |
| Lower Borden grade† | 1.44 | 1.12–1.85 | 0.004* | NA | NA | NA |
| Lower Cognard classification† | 1.16 | 1.06–1.27 | 0.001* | NA | NA | NA |
| Absent venous ectasia | 2.73 | 1.43–5.21 | 0.002* | 3.39 | 1.74–6.61 | <0.001 |
| Absent cortical venous reflux | 1.73 | 1.09–2.72 | 0.019* | NS | NS | NS |
| Absent spinal drainage | 2.55 | 1.37–4.75 | 0.003* | NS | NS | NS |
| Decreasing size of the dAVF | 1.71 | 1.15–2.54 | 0.009* | NS | NS | NS |
| Carotid cavernous location | 2.29 | 1.29–4.08 | 0.005* | 2.08 | 1.13–3.84 | 0.019 |
IVH = intraventricular hemorrhage; NA = not applicable; NS = not significant in the multivariate analysis.
Univariate factors with significance at p < 0.15 are listed.
Statistically significant in the univariate analysis.
Grading scales excluded from multivariate analysis.
FIG. 2.
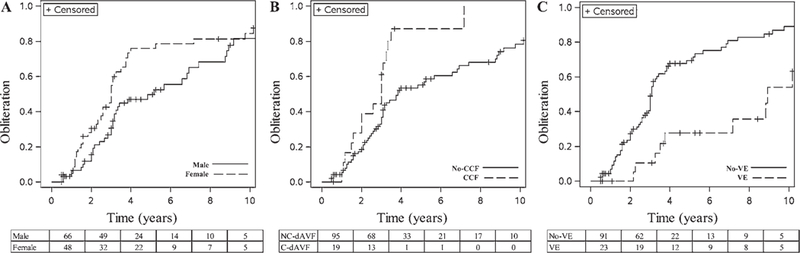
Kaplan-Meier plot of dAVF obliterations over time stratified by predictive factor. The postradiosurgery Kaplan-Meier plots of obliteration stratified by sex (A), cavernous carotid fistula location (CCF) and noncavernous location (No-CCF) (B), and the presence of venous ectasia (VE) and the absence of venous ectasia (No-VE) (C). Values at the bottom of each panel correspond to the number of male and female patients (A), the number of patients with noncavernous dAVFs (NC-dAVFs) and C-dAVFs (B), and the number of patients without venous ectasia and with venous ectasia (C) available for each follow-up interval (66 males and 48 females at 2 months [A], 95 NC-dAVFs and 19 C-dAVFs at 2 months [B], and 91 patients without venous ectasia and 23 patients with venous ectasia at 2 months [C], and so on).
Post-GKRS Hemorrhage, Complications, and Clinical Outcomes
Over 443 person-years of follow-up, 4 separate dAVFs hemorrhaged (3.5%) following GKRS treatment, yielding an annual hemorrhage risk of 0.9%. Time from GKRS to hemorrhage for each patient was 9, 10, 11, and 35 months. Of the dAVFs that hemorrhaged, 3 were Borden grade II, and one was Borden grade III. All the post-GKRS dAVFs that hemorrhaged had failed to respond to alternative treatment prior to radiosurgery (3 endovascular, 1 resection). In dAVFs without cortical venous drainage (CVD), no hemorrhagic complications were observed. Radiation-induced changes were evident in 11 patients (9.7%). Two patients experienced transient symptomatic radiation-induced complications (RICs) and 4 patients (3.5%) experienced permanent RICs. Three patients required shunt placement.
On follow-up, 76 patients (77.5%) reported improvement of original symptoms, with 22 patients (22.5%) reporting persistent symptoms.
A total of 42 patients (36.8%) had an unfavorable outcome, defined as failure of dAVF obliteration, permanent symptomatic RICs, or post-GKRS hemorrhage. Failure to achieve dAVF obliteration was the most common reason for an unfavorable outcome (85.7%). Table 4 displays the results of the univariate and multivariate regression analyses for predictors of unfavorable outcome following dAVF GKRS. A tentorial or middle fossa location (p = 0.048) and a mean peripheral dose greater than 23 Gy (p = 0.030) were independent predictors of an unfavorable outcome following GKRS for dAVF. Venous ectasia did not reach statistical significance in the multivariate analysis (p = 0.085). The Borden grade, modified Borden grade, and Cognard classification were not predictive of post-GKRS outcome.29 Additionally, clinical presentation subgroups, including aggressive presentation, hemorrhagic presentation, and nonhemorrhagic neural deficits, were not predictive of a post-GKRS favorable outcome.
TABLE 4.
Univariate and multivariate analyses for predictors of unfavorable outcome after dAVF GKRS
| Univariate Analysis |
Multivariate Analysis |
|||||
|---|---|---|---|---|---|---|
| Factor | Odds Ratio | 95% CI | p Value | Odds Ratio | 95% CI | p Value |
| Male sex | 1.79 | 0.81–1.90 | 0.149 | NS | NS | NS |
| Presenting seizure | 3.12 | 0.70–13.7 | 0.135 | NS | NS | NS |
| Venous ectasia | 2.22 | 0.88–5.61 | 0.092 | NS | NS | NS |
| Cortical venous reflux | 1.92 | 0.88–4.17 | 0.099 | NS | NS | NS |
| Spinal drainage | 2.00 | 0.80–4.98 | 0.136 | NS | NS | NS |
| Associated edema | 2.83 | 0.75–10.7 | 0.124 | NS | NS | NS |
| Tentorial or middle fossa dAVF | 2.11 | 0.90–4.94 | 0.085 | 2.39 | 1.01–5.68 | 0.048 |
| Prior resection | 3.68 | 0.64–21.0 | 0.142 | NS | NS | NS |
| Larger peripheral dose | 1.11 | 097–1.27 | 0.126 | NS | NS | NS |
| Peripheral dose >23 Gy | 1.87 | 0.86–4.09 | 0.116 | 2.59 | 1.10–6.15 | 0.030 |
NS = not significant in the multivariate analysis.
Univariate factors with p < 0.15 are listed.
Discussion
In this retrospective study, we analyzed data obtained in 114 patients, from 9 medical centers, with dAVFs treated with GKRS. Our study demonstrated an effective GKRS dAVF obliteration rate of 68.4%, which is similar to rates reported in the literature (55%−68%).5,6,15,16,20 We identified female sex, absent venous ectasia, and carotid cavernous sinus location as predictors of post-GKRS dAVF obliteration. Additionally, a middle fossa or tentorial location and mean peripheral radiation dose greater than 23 Gy were predictors of unfavorable outcome.
Dural Fistula GKRS Obliteration
In our cohort, venous ectasia was the greatest independent predictor of failure of dAVF obliteration following GKRS. The reasons behind this are unclear. In AVF literature, larger nidus is also independently associated with GKRS obliteration failure.28 In dAVF patients with venous ectasia, the nidus may be obscured on neuroimaging studies, and planning may be more challenging, which could explain the lower obliteration rates in these patients. Alternatively, high venous strain indicated by the formation of venous ectasia might counteract and prevent the radiation-induced endothelial luminal closure. Similarly, patients with complex dAVFs and cortical venous drainage were less likely to achieve obliteration in the univariate analysis, but this was not predictive in the multivariate analysis. We also found carotid cavernous location to be a predictive factor for GKRS obliteration. Although Yang et al. also found cavernous sinus location to be predictive of GKRS obliteration, we failed to observe a significant difference in our 2015 systematic review of dAVFs (OR 1.72, 95% CI 0.66–4.46; p = 0.27).5,27 In their single-institution study, Wu et al. also failed to observe a difference in obliteration rates between C-dAVFs and noncavernous dAVFs. Compared with their study, our cohort had a lower proportion of C-dAVFs (16.7% our study vs 64.3% in theirs).26
Consistent with the literature, more females presented with a C-dAVF.23,26 Regardless of dAVF location, we found that female sex was an independent predictor of GKRS dAVF obliteration, contradicting the findings of some prior studies.6,15,26 Suh et al. described 3 distinct venous drainage patterns for C-dAVFs: proliferative type, restrictive type, and late restrictive type.23 The proliferative type consisted of two subtypes: diffuse proliferative, involving the entire cavernous sinus with many arterial feeders, and posterior proliferative, involving the posterior cavernous sinus with fewer arterial feeders. Since the commonly proposed mechanisms of dAVF development include neighboring venous stasis and overexpression of angiogenic factors, it would be interesting to investigate whether these C-dAVF subgroups respond differently to GKRS treatment.6 Furthermore, Suh et al. found that the diffuse proliferative subtype only appeared in females.23 If these lesions respond differently to GKRS, it would be a potential confounder, possibly explaining why we found female sex and cavernous sinus location to be predictive of obliteration. This proliferative type variable was not available for assessment by all centers.
Dural Fistula GKRS Outcomes
To our knowledge, this is the first multicenter study to assess for predictors of outcome following GKRS of dAVF. We found that commonly utilized dAVF grading scales (Borden,2 modified Borden,29 and Cognard7), were not predictive of post-GKRS outcomes. Furthermore, factors used in these grading scales, CVD, spinal drainage, and venous ectasia were also not predictive of outcome. While it has been shown that venous ectasia is an independent risk factor for dAVF hemorrhage, it failed to reach statistical significance in our multivariate analysis as a predictor of unfavorable outcome following GKRS (p = 0.085).3 There has been an increasing number of reports in the literature that stratify dAVFs by clinical presentation, nonaggressive versus aggressive status, and hemorrhagic versus nonhemorrhagic neurological deficits.14,22,29 None of these clinical presentation groups were predictive of favorable outcomes following GKRS. Instead, a mean peripheral dose greater than 23 Gy and middle fossa or tentorial location were predictive of unfavorable outcomes. The nidus in patients with tentorial dAVFs may be more difficult to target, or these patients may be more likely to experience symptomatic GKRS-related complications. Patients treated with a dose greater than 23 Gy were more likely to experience complications without the benefit of increased chance of dAVF obliteration. Of the patients who received a mean peripheral dose greater than 23 Gy, 3 (6%) experienced permanent RICs and 2 (4%) had post-GKRS hemorrhage.
We observed a 3.5% rate of post-GKRS hemorrhage, similar to rates reported in the literature (0.4%–5%5,6,16,20,26). Of the 4 dAVFs that hemorrhaged posttreatment, all demonstrated significant CVD, a known risk factor for hemorrhage.2,7 Our overall post-GKRS annual hemorrhage risk was 0.9%, similar to reported rates.24 In their recent paper, Tonetti et al. observed a 0% postradiosurgery hemorrhage risk in 279 patient-years of follow-up in cases involving dAVFs with CVD presenting in a nonaggressive manner (no neurological deficits or hemorrhage).24 They proposed that radiosurgery should be considered for these dAVFs with CVD that present in a nonaggressive fashion. In our study, 0 of the 24 nonaggressive-presenting dAVFs with CVD treated with GKRS hemorrhaged posttreatment during 95 person-years of follow-up; this yields a 0% post-GKRS hemorrhage risk for these lesions. This finding agrees with the reported 0% post-GKRS risk reported by Tonetti et al.24 The post-GKRS annual hemorrhage risk for aggressively presenting dAVFs with CVD was 1.8% over 224 years of follow-up, substantially lower than the reported 7.4%–19% natural hemorrhage risk for these lesions.14,18,22,25 Our study further validated the concept that radiosurgery is likely safe for these nonaggressive dAVFs with CVD. None of the dAVFs that hemorrhaged post-treatment demonstrated venous ectasia.
Our study has a few of the limitations inherent in retrospective analyses and multicenter collaborations, including selection and follow-up biases. Twenty-four patients (21%) were missing angiographic follow-up, and as such the actual obliteration rates may be slightly overestimated. Al-though several patients had long-term follow-up (18 years), the treatment period can also influence the outcome.17
Conclusions
GKRS is an effective treatment option for cerebral dAVF and achieves obliteration while avoiding permanent complications in most patients. Patients without angiographic evidence of venous ectasia were most likely to achieve GKRS-induced dAVF obliteration, whereas middle fossa or tentorial location and a mean peripheral radiation dose greater than 23 Gy were predictors of an unfavorable outcome.
Acknowledgments
Disclosures
Dr. Vargo reports receiving a speaking honorarium from Brain-LAB. Dr. Hideyuki Kano reports receiving an Elekta AB research grant. Dr. Grills reports stock ownership in Greater Michigan Gamma Knife and is on the executive board of directors for Greater Michigan Gamma Knife. Dr. Lunsford reports direct stock ownership in Elekta AB; he is a consultant for Insightec.
ABBREVIATIONS
- C-dAVF
cavernous dAVF
- CVD
cortical venous drainage
- dAVF
dural arteriovenous fistula
- GKRS
Gamma Knife radiosurgery
- ICH
intracerebral hemorrhage
- RIC
radiation-induced complication
- SAH
subarachnoid hemorrhage
References
- 1.Altman DG: Practical Statistics for Medical Research. Boca Raton, FL: CRC Press, 1990 [Google Scholar]
- 2.Borden JA, Wu JK, Shucart WA: A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg 82:166–179, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Bulters DO, Mathad N, Culliford D, Millar J, Sparrow OC: The natural history of cranial dural arteriovenous fistulae with cortical venous reflux—the significance of venous ectasia. Neurosurgery 70:312–319, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Chaichana KL, Coon AL, Tamargo RJ, Huang J: Dural arteriovenous fistulas: epidemiology and clinical presentation. Neurosurg Clin N Am 23:7–13, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Chen CJ, Lee CC, Ding D, Starke RM, Chivukula S, Yen CP, et al. : Stereotactic radiosurgery for intracranial dural arteriovenous fistulas: a systematic review. J Neurosurg 122:353–362, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Cifarelli CP, Kaptain G, Yen CP, Schlesinger D, Sheehan JP: Gamma knife radiosurgery for dural arteriovenous fistulas. Neurosurgery 67:1230–1235, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Cognard C, Gobin YP, Pierot L, Bailly AL, Houdart E, Casasco A, et al. : Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology 194:671–680, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Cohen-Inbar O, Starke RM, Kano H, Bowden G, Huang P, Rodriguez-Mercado R, et al. : Stereotactic radiosurgery for cerebellar arteriovenous malformations: an international multicenter study. J Neurosurg 127:512–521, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Cohen-Inbar O, Starke RM, Paisan G, Kano H, Huang PP, Rodriguez-Mercado R, et al. : Early versus late arteriovenous malformation responders after stereotactic radiosurgery: an international multicenter study. J Neurosurg 127:503–511, 2017 [DOI] [PubMed] [Google Scholar]
- 10.Elhammady MS, Ambekar S, Heros RC: Epidemiology, clinical presentation, diagnostic evaluation, and prognosis of cerebral dural arteriovenous fistulas. Handb Clin Neurol 143:99–105, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Fine JP, Gray RJ: A proportional hazards model for the sub-distribution of a competing risk. J Am Stat Assoc 94:496–509, 1999 [Google Scholar]
- 12.Gomez J, Amin AG, Gregg L, Gailloud P: Classification schemes of cranial dural arteriovenous fistulas. Neurosurg Clin N Am 23:55–62, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Gray RJ: A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 16:1141–1154, 1988 [Google Scholar]
- 14.Gross BA, Du R: The natural history of cerebral dural arteriovenous fistulae. Neurosurgery 71:594–603, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Hanakita S, Koga T, Shin M, Shojima M, Igaki H, Saito N: Role of Gamma Knife surgery in the treatment of intracranial dural arteriovenous fistulas. J Neurosurg 117 Suppl: 158–163, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Park KS, Kang DH, Park SH, Kim YS: The efficacy of gamma knife radiosurgery alone as a primary treatment for intracranial dural arteriovenous fistulas. Acta Neurochir (Wien) 158:821–828, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Patibandla MR, Ding D, Kano H, Starke RM, Lee JYK, Mathieu D, et al. : Effect of treatment period on outcomes after stereotactic radiosurgery for brain arteriovenous malformations: an international multicenter study. J Neurosurg [epub ahead of print February 2, 2018. DOI: 10.3171/2017.8.JNS171336] [DOI] [PubMed] [Google Scholar]
- 18.Reynolds MR, Lanzino G, Zipfel GJ: Intracranial dural arteriovenous fistulae. Stroke 48:1424–1431, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheehan JP, Starke RM, Kano H, Barnett GH, Mathieu D, Chiang V, et al. : Gamma Knife radiosurgery for posterior fossa meningiomas: a multicenter study. J Neurosurg 122:1479–1489, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Söderman M, Edner G, Ericson K, Karlsson B, Rähn T, Ulfarsson E, et al. : Gamma knife surgery for dural arteriovenous shunts: 25 years of experience. J Neurosurg 104:867–875, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Starke RM, Kano H, Ding D, Lee JY, Mathieu D, Whitesell J, et al. : Stereotactic radiosurgery for cerebral arteriovenous malformations: evaluation of long-term outcomes in a multi-center cohort. J Neurosurg 126:36–44, 2017 [DOI] [PubMed] [Google Scholar]
- 22.Strom RG, Botros JA, Refai D, Moran CJ, Cross DT III, Chicoine MR, et al. : Cranial dural arteriovenous fistulae: asymptomatic cortical venous drainage portends less aggressive clinical course. Neurosurgery 64:241–248, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Suh DC, Lee JH, Kim SJ, Chung SJ, Choi CG, Kim HJ, et al. : New concept in cavernous sinus dural arteriovenous fistula: correlation with presenting symptom and venous drainage patterns. Stroke 36:11–1139, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Tonetti DA, Gross BA, Jankowitz BT, Kano H, Monaco EA III, Niranjan A, et al. : Reconsidering an important subclass of high-risk dural arteriovenous fistulas for stereotactic radio-surgery. J Neurosurg [epub ahead of print March 16, 2018. DOI: 10.3171/2017.10.JNS171802] [DOI] [PubMed] [Google Scholar]
- 25.van Dijk JM, terBrugge KG, Willinsky RA, Wallace MC: Clinical course of cranial dural arteriovenous fistulas with long-term persistent cortical venous reflux. Stroke 33:1233–1236, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Wu HM, Pan DHC, Chung WY, Guo WY, Liu KD, Shiau CY, et al. : Gamma Knife surgery for the management of intracranial dural arteriovenous fistulas. J Neurosurg 105 Suppl:43–51, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Yang HC, Kano H, Kondziolka D, Niranjan A, Flickinger JC, Horowitz MB, et al. : Stereotactic radiosurgery with or without embolization for intracranial dural arteriovenous fistulas. Neurosurgery 67:1276–1285, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Yen CP, Ding D, Cheng CH, Starke RM, Shaffrey M, Sheehan J: Gamma Knife surgery for incidental cerebral arteriovenous malformations. J Neurosurg 121:1015–1021, 2014 [DOI] [PubMed] [Google Scholar]
- 29.Zipfel GJ, Shah MN, Refai D, Dacey RG Jr, Derdeyn CP: Cranial dural arteriovenous fistulas: modification of angiographic classification scales based on new natural history data. Neurosurg Focus 26(5): E14, 2009 [DOI] [PubMed] [Google Scholar]


