Abstract
Background & Aims:
Noninvasive methods are needed to determine disease stage in patients with nonalcoholic fatty liver disease (NAFLD). We evaluated the diagnostic performance of several widely available fibrosis models for the assessment of hepatic fibrosis in patients with NAFLD.
Methods:
We performed a retrospective analysis of data from individuals enrolled in the NIDDK NASH Clinical Research Network, from 2004 through 2018. Using biopsy as the reference standard, we determined the diagnostic performance of the aspartate aminotransferase (AST):platelet ratio (APRI), FIB-4, ratio of AST:alanine aminotransferase (ALT) and the NAFLD fibrosis score (NFS) in a cross-sectional study of 1904 subjects. The ability of these models to detect changes in fibrosis stage was assessed in a longitudinal data set of 292 subjects with 2 biopsies and accompanying laboratory data. Outcomes were detection of fibrosis of any stage (stages 0 to 4), detection of moderate fibrosis (stages 0–1 vs 2–4), and detection of advanced fibrosis (stages 0–2 vs 3–4). Diagnostic performance was evaluated using the C-statistic, sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) analyses.
Results:
In the cross-sectional study, FIB-4 and NFS outperformed other non-invasive models for detecting advanced fibrosis; the C-statistics were 0.80 for FIB-4 and 0.78 for NFS. In the longitudinal study, 216 patients had non-advanced fibrosis at baseline and 35 patients progressed to advanced fibrosis after median follow up of 2.6 years. After we adjusted for fibrosis stage and model score at initial biopsy, change in APRI, FIB-4, and NFS were significantly associated with change in fibrosis. A unit change in APRI, FIB-4, or NFS was associated with changes in fibrosis stage of 0.33 (95% CI, 0.20-0.45; P<.001), 0.26 (95% CI, 0.15–0.37; P<.001), and 0.19 (95% CI, 0.07–0.31; P=.002), respectively. The cross-validated C-statistic for detecting progression to advanced fibrosis for APRI was 0.82 (95% CI, 0.74–0.89), for FIB-4 was 0.81 (95% CI, 0.73–0.81), and for NFS was 0.80 (95% CI, 0.71–0.88).
Conclusion:
In a combined analysis of data from 2 large studies, we found that FIB-4, APRI, and NFS can detect advanced fibrosis and fibrosis progression in patients with NAFLD.
Keywords: nonalcoholic steatohepatitis, diagnostic, prognostic, scoring system comparison
INTRODUCTION:
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease in the United States1. Histologically NAFLD can be classified into either nonalcoholic fatty liver (NAFL) or nonalcoholic steatohepatitis (NASH)2. The mortality risk is linked to the fibrosis stage in patients with NAFLD3. The assessment of hepatic fibrosis stage is therefore a cornerstone of current diagnostic and prognostic assessment of NAFLD. The current reference standard for this purpose is a liver biopsy with histological assessment of fibrosis stage using the NIDDK NASH CRN histological scoring system2. Unfortunately, liver biopsies are invasive, uncomfortable and occasionally associated with severe morbidity and even mortality. Histological assessment is further limited by both sampling and inter- as well as intra-observed variability. There is therefore an urgent unmet need to develop noninvasive methods for the assessment of fibrosis stage in those with NAFLD.
A multitude of noninvasive tools to detect hepatic fibrosis including imaging, circulating and dynamic markers of fibrogenesis are undergoing evaluation4. These are however either early in development or involve additional costs, time and resources. While major international consortia are currently engaged in efforts to qualify non-invasive measures of disease assessment in NAFLD, they are still years away from fruition. There is therefore an unmet need to further refine existing clinical tools to assess disease stage and its progression.
The AST:platelet ratio index (APRI), FIB4, AST/ALT ratio and NAFLD fibrosis score (NFS) are simple clinical aids that are related to the fibrosis stage in NAFLD5–8. Several studies have established the general relationship of these aids with fibrosis stage and even clinical outcomes9,10. However, their diagnostic performance across the full spectrum of individuals with NAFLD including those of varying age, BMI, race, type 2 diabetes etc. are not established. Their ability to detect changes in fibrosis stage over time is also unknown. The objective of this study was therefore to establish the diagnostic utility of such clinical laboratory aids in a large cross-sectional study that captured the full clinical and histological spectrum of NAFLD and determine their ability to detect changes in fibrosis stage over time in this population.
METHODS:
This study is a retrospective analysis of data from individuals enrolled in the NIDDK NASH Clinical Research Network (CRN). This network conducted multiple studies and the current analysis included individuals enrolled in several studies if they met enrollment criteria. These included the NASH CRN NAFLD database 1 study (2004-2009) and database 2 study (2009-present) and PIVENS and FLINT clinical trials11,12. Subjects enrolled in these studies, who also met inclusion criteria for this trial were included for this analysis. The NASH CRN data-coordinating center performed the analysis reported below in alignment with TRIPOD standards for reporting biomarker studies. The authors take full responsibility for meeting NIH standards for rigor and transparency in reporting and all aspects of this study.
Context of Use statement:
This study provides data on the performance of selected clinical-laboratory aids in two specific contexts of use in individuals with full spectrum of NAFLD seen in a tertiary care setting. The first context was to evaluate the diagnostic performance of specific diagnostic aids in the static assessment of fibrosis stage. The second context of use was to evaluate the performance of these aids as dynamic measures to evaluate longitudinal changes in fibrosis.
Study Populations:
The study population included subjects with histological findings consistent with NAFLD after negative serological evaluation for common causes of chronic liver diseases (i.e. hepatitis B or C, etc.) and less than mild alcohol consumption13. The inclusion criteria for the analysis related to the static assessment of fibrosis included: (1) the presence of NAFLD confirmed by central histological assessment of a liver biopsy by the NASH CRN pathology committee, (2) the availability of clinical and laboratory data within 90 days of the liver biopsy to allow construction of non-invasive fibrosis models. For the dynamic assessment of fibrosis change, subjects with at least two evaluable liver biopsies. Pairs of the first and last eligible samples were used if more than 2 eligible samples were available per patient. For individuals enrolled in the PIVENS and FLINT trials, only the baseline biopsies prior to therapeutic intervention and follow-up biopsies of the placebo group were included. The exclusion criterion was the lack of a full data set available for analysis.
Procurement of data, chain of custody and data integrity:
Both prospective and retrospectively collected data were included in the two database studies, which served as the source for this analysis. In some instances in database 1, individuals were included if they had an evaluable liver biopsy from the distant past i.e. one that was available for assessment by the NASH CRN pathology committee as long as they had verifiable laboratory data within 90 days of the biopsy. On the other hand, all individuals in database 2 had a liver biopsy within 90 days of entry in to the database along with laboratory assessment. The baseline data for those entering the clinical trials were either obtained from biopsies performed within the time-frame specified by the trials or prospectively during the screening process.
Unstained paraffin-embedded 4 μm thick liver tissue sections were sent to the data coordinating center and stained in a core laboratory using identical methods and internal quality standards as previously described13. The NASH CRN pathology committee performed a masked assessment of the stained sections. The clinical centers entered the clinical and laboratory data in to the CRN database using secure data transfer systems. All data were stored, monitored for integrity and analyzed at the Data Coordinating Center at the John Hopkins Bloomberg School of Public Health. The Institutional Review Boards at participating centers approved the study (NCT01030484) and all participants provided written informed consent prior to enrollment.
Histological Assessment:
The presence of NAFLD and the fibrosis stage was scored using the NASH CRN scoring system2. The fibrosis stage was scored as follows: 0= no fibrosis, 1= perisinusoidal fibrosis, 2= perisinusoidal and portal fibrosis, 3= bridging fibrosis and 4= cirrhosis. For purposes of this analysis, stage 2 or higher (moderate fibrosis) represented clinically significant fibrosis. The case definition of advanced fibrosis was stage 3 or 4 fibrosis.
Non-Invasive Fibrosis Models:
A literature search was performed for non-proprietary fibrosis models that could be constructed on routinely available clinical data. Models that had previously been specifically validated in NAFLD were used10,14,15. The models chosen include AST to platelet ratio index (APRI)5, FIB-47, AST to ALT ratio16, and NAFLD fibrosis score (NFS)8. Although BARD and BAAT scores have been evaluated in NAFLD, they were not considered in this study since these provide discrete score and it is not feasible to determine optimal cutoffs at fixed specificity or at fixed sensitivity10,14. These models are also inferior to the APRI, FIB4 and NFS for the static assessment of fibrosis14. The specific formula used to calculate these fibrosis models are listed in supplemental table 1. The normal ALT and AST values was set as <19IU/L in women and <31 IU/L in men17.
Statistical Analysis
The diagnostic performance of the selected fibrosis model for three fibrosis outcomes was evaluated. The first outcome was the presence of any fibrosis i.e. stages 0 vs 1-4, the second was the presence of moderate fibrosis (stages 0-1 vs. 2-4) and the third was advanced fibrosis (stages 0-2 vs 3-4). The diagnostic performance was evaluated using the C-statistic; sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) at 90% sensitivity, 90% specificity, and the maximum Youen’s index. PPV and NPV curves were constructed at 90% specificity.
In the longitudinal assessment of dynamic changes in fibrosis, the association between change in diagnostic model score and change in fibrosis stage was assessed. First, scatter plots were used to visualize changes in model scores vs changes in fibrosis stage. The validity of the association accounting for fibrosis stage and model score at the first biopsy was determined by multiple linear regression. Next, a logistic regression model was constructed for each diagnostic model with any progression in fibrosis stage or the progression to advanced fibrosis as an outcome, and initial fibrosis stage, initial model score, and change in model score as covariates. A leave-one-out cross-validated C-statistic (area under Receiver operating characteristic (ROC) curve), and diagnostic statistics at 90% specificity, 90% specificity and the maximum Youden’s index were calculated.
In order to check appropriateness in mixing paired samples with varying time intervals between two biopsies, we did sensitivity analyses by: comparing prediction models with and without time term; and splitting samples by the time interval. To check potential confounding by indication between non-trial and trial patients, we split samples by type of studies in which patients were initially enrolled (cohort study or clinical trial). Mean (± S.D.) values of continuous variables and proportions for categorical variables are reported for both sets of analyses. Analysis of variance for across group comparisons of continuous variables and chi square for categorical variables was used. Statistical significance was set at a p-value of 0.05 or less.
RESULTS
A: Cross-sectional static assessment of fibrosis stage
A total of 1904 patients met criteria and were included in the cross-sectional analysis (Table 1 and Supplemental Figure 1). The mean age and BMI of the cohort was 48.9±11.7 years and 34.4±6.4 kg/m2, respectively. The distribution of diabetes mellitus, dyslipidemia, and hypertension was 39%, 62%, and 58%, respectively. Definite NASH was present in 58% of the cohort. The number of subjects with varying fibrosis stage were 463 (stage 0, 24%), 532 (stage 1, 28%), 372 (stage 2, 20%), 379 (stage 3, 20%) and 158 (stage 4, 8%).
TABLE 1:
Characteristics of the study population, cross-sectional study (n = 1,904)
| Patient characteristics | Mean (± SD) or N (%) |
|---|---|
| Demographic characteristics | |
| Age (year) | 50.3 (±12.2) |
| Gender (% female) | 1201 (63%) |
| Race (%) | |
| White, non-Hispanic | 1382 (73%) |
| Black, non-Hispanic | 70 (4%) |
| Hispanic | 240 (13%) |
| Other | 212 (11%) |
| Clinical characteristics | |
| BMI (kg/m2) | 34.4 (±6.4) |
| Diabetes† (%) | 747 (39%) |
| Hyperlipidemia† (%) | 1173 (62%) |
| Hypertension† (%) | 1102 (58%) |
| Laboratory measures (mean) | |
| Platelet (109/L) | 236.5 (±69.8) |
| Albumin (g/dL) | 4.3 (±0.4) |
| ALT (U/L) | 69.8 (±50.6) |
| AST (U/L) | 51.2 (±36.7) |
| Glucose (mg/dL) | 110.6 (±38.4) |
| Insulin‡(μU/mL) | 25.8 (±26.9) |
| Histological characteristics | |
| Biopsy length (mm) | 20 (±9) |
| Steatosis grade (%) | |
| < 5% | 41 (2%) |
| 5-33% | 742 (39%) |
| 34-66% | 669 (35%) |
| >66% | 452 (24%) |
| Fibrosis stage (%) | |
| None (stage 0) | 463 (24%) |
| Mild (stage 1) | 532 (28%) |
| Moderate (stage 2) | 372 (20%) |
| Bridging (stage 3) | 379 (20%) |
| Cirrhosis (stage 4) | 158 (8%) |
| NASH stage (%) | |
| NAFLD | 437 (23%) |
| Borderline NASH | 368 (19%) |
| Definite NASH | 1099 (58%) |
Defined as those ever diagnosed as having the disease.
Based on sample size of 1,864 due to missing values.
Diagnostic Performance of Noninvasive Fibrosis Models
The diagnostic performance of non-invasive fibrosis models to detect presence of varying degrees of fibrosis is shown in supplemental figure 2. With specificity fixed at 90%, all the non-invasive fibrosis models had high PPV for detecting presence of any fibrosis (Table 2). APRI and FIB-4 had higher diagnostic accuracy for detecting presence of any fibrosis (Table 2). APRI had a C-statistic of 0.75 (95% confidence interval = 0.72, 0.77); and at 90% specificity, a cutoff of 1.10 had PPV of 92% and NPV of 32% (Supplemental Figure 2). FIB-4 had a C-statistic of 0.72 (0.70, 0.75); and at 90% specificity, a cutoff value of 1.69 had a PPV of 92% and NPV of 31% (Supplemental Figure 2). The C-statistic for detecting presence of moderate fibrosis was 0.76 (0.74, 0.78) for FIB-4, 0.73 (0.71, 0.76) for APRI, and 0.73 (0.71, 0.75) for NFS (Table 2). FIB-4, APRI and NFS were also accurate for detecting presence of advanced fibrosis (Table 2). FIB-4 had a C-statistic of 0.80 (0.78, 0.82). The PPV was 65% and the NPV was 81% at specificity of 90% for a FIB-4 cutoff of 1.95. NFS had a C-statistic of 0.78 (0.76, 0.80) with PPV and NPV of 63% and 80%. APRI had a C-statistic of 0.76 (0.74, 0.79) with PPV and NPV of 61% and 79%, respectively, at specificity of 90% for an APRI cutoff of 1.53.
Table 2:
Diagnostic performance of non-invasive fibrosis models for detection of fibrosis (n = 1,904)
| Specificity Fixed at 90%† |
Sensitivity Fixed at 90%‡ | At maximum Youden’s index | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fibrosis Model | C-statistic (95% CI) |
Model score cutoff |
Sens. (%) |
PPV (%) |
NPV (%) |
Model score cutoff |
Sens. (%) |
PPV (%) |
NPV (%) |
Model score cutoff |
Sens. (%) |
Spec. (%) |
PPV (%) |
NPV (%) |
| Any Fibrosis§ (stage 1-4 vs. stage 0) | ||||||||||||||
| APRI | 0.75 (0.72, 0.77) | 1.10 | 39 | 92 | 32 | 0.42 | 31 | 80 | 50 | 0.74 | 63 | 76 | 89 | 40 |
| FIB-4 | 0.72 (0.70, 0.75) | 1.69 | 35 | 92 | 31 | 0.64 | 25 | 79 | 45 | 1.04 | 70 | 68 | 87 | 42 |
| NAFLD Fibrosis Score | 0.69 (0.66, 0.72) | −0.22 | 31 | 91 | 30 | −2.95 | 24 | 79 | 44 | −1.51 | 64 | 66 | 85 | 37 |
| AST/ALT ratio | 0.59 (0.56, 0.62) | 1.06 | 18 | 85 | 26 | 0.50 | 14 | 77 | 33 | 0.85 | 38 | 76 | 83 | 28 |
| Moderate Fibrosis§ (stage 2-4 vs. stage 1-2) | ||||||||||||||
| FIB-4 | 0.76 (0.74, 0.78) | 1.81 | 40 | 79 | 62 | 0.81 | 38 | 57 | 81 | 1.31 | 66 | 74 | 70 | 71 |
| APRI | 0.73 (0.71,0.76) | 1.34 | 39 | 78 | 62 | 0.48 | 33 | 55 | 78 | 0.84 | 65 | 71 | 67 | 69 |
| NAFLD Fibrosis Score | 0.73 (0.71, 0.75) | 0.03 | 35 | 76 | 60 | −2.45 | 32 | 55 | 78 | −0.81 | 57 | 77 | 70 | 67 |
| AST/ALT ratio | 0.65 (0.63, 0.68) | 1.06 | 22 | 67 | 56 | 0.56 | 26 | 53 | 74 | 0.81 | 54 | 68 | 61 | 62 |
| Advanced Fibrosis§ (stage 3-4 vs. stage 0-2) | ||||||||||||||
| FIB-4 | 0.80 (0.78, 0.82) | 1.95 | 47 | 65 | 81 | 1.02 | 49 | 41 | 93 | 1.37 | 75 | 71 | 51 | 88 |
| NAFLD Fibrosis Score | 0.78 (0.76, 0.80) | 0.19 | 43 | 63 | 80 | −1.98 | 41 | 37 | 91 | −0.76 | 70 | 74 | 51 | 86 |
| APRI | 0.76 (0.74, 0.79) | 1.53 | 40 | 61 | 79 | 0.57 | 39 | 37 | 91 | 0.84 | 75 | 65 | 46 | 87 |
| AST/ALT ratio | 0.68 (0.66, 0.71) | 1.09 | 26 | 51 | 76 | 0.59 | 29 | 33 | 88 | 0.85 | 54 | 73 | 44 | 80 |
CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value; sens., sensitivity; spec. specificity.
Fixed at specificity closest to 90% but ≥ 90%.
Fixed at sensitivity closest to 90% but ≥ 90%.
Prevalence of any fibrosis was 76%; prevalence of mild or advanced fibrosis was 48%; prevalence of advanced fibrosis was 28%.
The diagnostic performance of AST/ALT Ratio was lower across all fibrosis stages when compared to FIB-4, APRI or NFS (Table 2). At 90% sensitivity, the NPV for detecting advanced fibrosis was 93%, 91%, 91%, and 88% for FIB-4, APRI, NAFLD Fibrosis Score and AST/ALT ratio, respectively.
Longitudinal Assessment of dynamic changes in fibrosis over time
A total of 292 patients had repeat liver biopsy with median time interval of 2.6 years between biopsies (minimum 0.5, first quartile 1.6, median 2.6, third quartile 4.8, maximum 11.0 years; Table 3). The mean age and BMI of this cohort at the time of initial liver biopsy was 50±12 years and 34.7±6.3 kg/m2, respectively. Definite NASH was present in 201 (69%) and advanced fibrosis in 76 (26%) of the cohort on their initial biopsy (Table 3). The distribution of fibrosis stage 0, 1, 2, 3, and 4 was 62 (21%), 85 (29%), 69 (24%), 66 (23%), and 10 (3%) on the first liver biopsy (Table 3). Of the 292 patients with two liver biopsies, 74 (25%) had at least 1 stage fibrosis regression, 126 (43%) remained stable, and 92 (32%) had fibrosis progression.
Table 3:
Characteristics of the study population at first biopsy in the longitudinal study (n = 292)
| Mean ± S.D. or N (%) |
Change in fibrosis stage† | p-value‡ |
||||
|---|---|---|---|---|---|---|
| Regression (n = 74) |
No change (n = 126) |
Progression (n = 92) |
Regres- sion vs. no change |
Progres- sion vs. no change |
||
| DEMOGRAPHICS | ||||||
| Age (year) | 48.9 (±11.7) | 48.3 (±11.5) | 47.9 (±11.6) | 50.7 (±11.9) | 0.40 | 0.03 |
| Gender (% female) | 186 (64%) | 45 (61%) | 79 (63%) | 62 (67%) | 0.44 | 0.34 |
| Race (%) | 0.43 | 0.11 | ||||
| White, non-Hispanic | 224 (77%) | 51 (69%) | 94 (75%) | 79 (86%) | ||
| Black | 4 (1%) | 2 (3%) | 2 (2%) | 0 (0%) | ||
| Hispanic | 37 (13%) | 12 (16%) | 17 (13%) | 8 (9%) | ||
| Other | 27 (9%) | 9 (12%) | 13 (10%) | 5 (5%) | ||
| CLINICAL | ||||||
| BMI (kg/m2) | 34.7 (±6.3) | 34.0 (±4.6) | 35.2 (±7.2) | 34.5 (±6.0) | 0.04 | 0.42 |
| Diabetes§ (%) | 113 (39%) | 29 (39%) | 45 (36%) | 39 (42%) | 0.59 | 0.26 |
| Hyperlipidemia§ (%) | 173 (59%) | 42 (57%) | 76 (60%) | 55 (60%) | 0.25 | 0.76 |
| Hypertension§ (%) | 160 (55%) | 35 (47%) | 74 (59%) | 51 (55%) | 0.001 | 0.75 |
| LABORATORY | ||||||
| Platelet (109/L) | 248.0 (±64.8) | 251.8 (±67.7) | 251.8 (±62.9) | 239.8 (±65.1) | 0.57 | 0.08 |
| Albumin (g/dL) | 4.3 (±0.4) | 4.3 (±0.4) | 4.3 (±0.4) | 4.2 (±0.4) | 0.48 | 0.07 |
| ALT (U/L) | 75.8 (±50.5) | 80.9 (±55.8) | 71.0 (±41.7) | 78.3 (±56.8) | 0.48 | 0.32 |
| AST (U/L) | 53.9 (±36.8) | 54.0 (±36.8) | 52.2 (±30.7) | 56.0 (±44.1) | 0.62 | 0.45 |
| Glucose (mg/dL) | 110.3 (±41.9) | 110.1 (±37.0) | 109.2 (±42.2) | 111.9 (±45.5) | 0.47 | 0.57 |
| Insulin¶ (μU/mL) | 25.2 (±28.9) | 20.1 (±10.9) | 28.4 (±38.3) | 25.0 (±22.6) | 0.02 | 0.47 |
| HISTOLOGY | ||||||
| Biopsy length (mm) | 19 (±9) | 21 (±9) | 19 (±9) | 19 (±8) | 0.22 | 0.95 |
| Time between biopsyǁ (yrs) | 3.3 (±2.1) | 3.5 (±1.9) | 3.1 (±2.1) | 3.5 (±2.2) | 0.04 | 0.12 |
| Steatosis grade (%) | 0.60 | 0.24 | ||||
| < 5% | 2 (1%) | 1 (1%) | 1 (1%) | 0 | ||
| 5-33% | 92 (32%) | 19 (26%) | 46 (37%) | 27 (29%) | ||
| 34-66% | 105 (36%) | 33 (45%) | 45 (36%) | 27 (29%) | ||
| >66% | 93 (32%) | 21 (28%) | 34 (27%) | 38 (41%) | ||
| Fibrosis stage | 0.14 | 0.10 | ||||
| None (stage 0) | 62 (21%) | 0 (0%) | 36 (29%) | 26 (28%) | ||
| Mild (stage 1) | 85 (29%) | 26 (35%) | 32 (25%) | 27 (29%) | ||
| Moderate (stage 2) | 69 (24%) | 25 (34%) | 19 (15%) | 25 (27%) | ||
| Bridging (stage 3) | 66 (23%) | 21 (28%) | 31 (25%) | 14 (15%) | ||
| Cirrhosis (stage 4) | 10 (3%) | 2 (3%) | 8 (6%) | 0 | ||
| NASH stage (%) | 0.18 | 0.32 | ||||
| NAFLD, not NASH | 48 (16%) | 7 (9%) | 26 (21%) | 15 (16%) | ||
| Borderline NASH | 43 (15%) | 12 (16%) | 20 (16%) | 11 (12%) | ||
| Definite NASH | 201 (69%) | 55 (74%) | 80 (63%) | 66 (72%) | ||
Regression = any decline in fibrosis stage; no change = no change in fibrosis stage; progression = any increase in fibrosis stage.
ANOVA for continuous variables and chi-squared test for categorical variables comparing: the regression group with the no change group among patients with fibrosis stage 1-4 (n = 164); and the progression group with the no change group among patients with fibrosis stage 0-3 (n = 210).
Defined as those ever diagnosed as having the disease.
Based on sample size of 281 due to missing values.
Minimum = 0.5, first quartile = 1.6, median = 2.6, third quartile = 4.8, maximum = 11.0 years.
Diagnostic Performance of Models to detect change in fibrosis stage
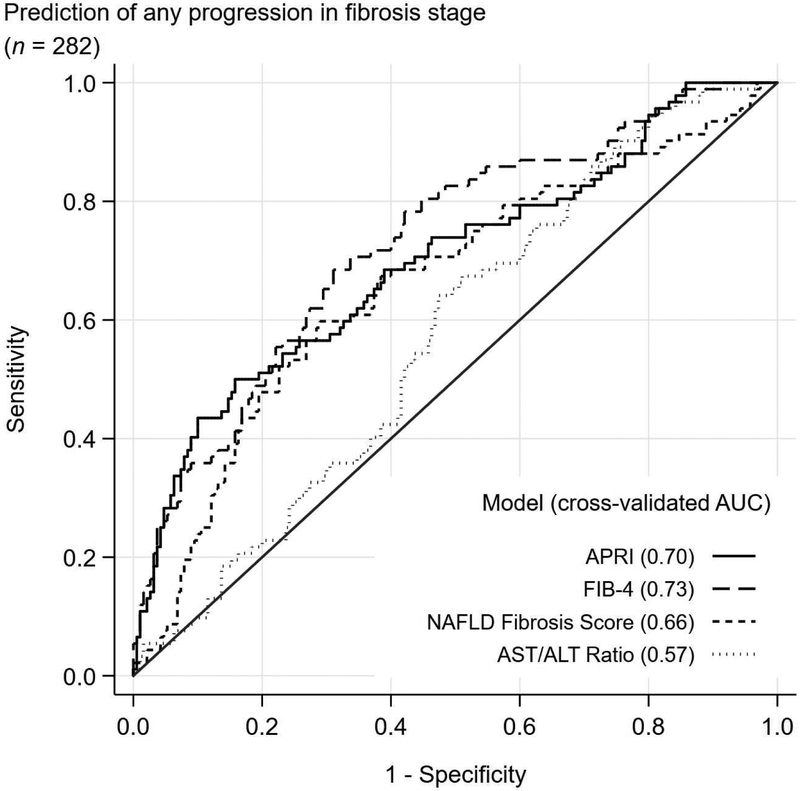
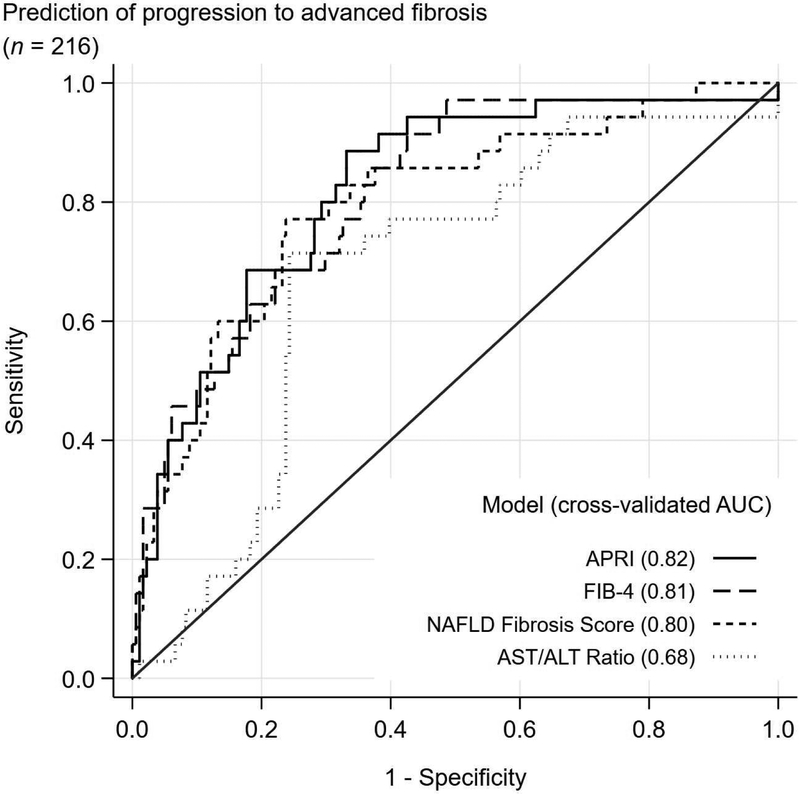
The C-statistic to detect any fibrosis progression ranged from 0.57 for AST/ALT ratio to 0.73 for FIB4 (Figure 1). The C-statistic to detect progression to advanced fibrosis was higher for all models with C-statistic of 0.82, 0.81, 0.80, and 0.68 for APRI, FIB4, NFS and AST/ALT ratio, respectively. Similar results were observed when the time interval between two biopsies was included in the prediction model and no differences in c-statistics were observed when the samples were split by the time interval and by type of studies patients participated in initially.
Figure 1:
Cross-validated receiver operating characteristics (ROC) curves for detecting any progression in fibrosis stage and progression to advanced fibrosis
A significant increase in all the diagnostic model scores comparing patients who progressed with those who did not change their fibrosis stage was noted (Supplemental Table 2). However, only ALT/AST ratio was able to detect any decrease in fibrosis stage from first to second biopsy. (Supplemental Table 3). The mean change in APRI scores in patients with no change, fibrosis regression, and fibrosis progression was −0.2±0.7, −0.3±0.8 (P=0.34 for regression vs. no change), and 0.2±1.3 (P=0.006 for progression vs. no change), respectively (Supplemental Table 2). Similarly, the mean change in FIB-4 in patients with no change in fibrosis, fibrosis regression and fibrosis progression was 0.1±0.7, 0.0±0.5 (P=0.61 for regression vs. no change), and 0.5±1.3 (P<0.001 for fibrosis progression vs. no change), respectively.
In unadjusted models with change in fibrosis as the outcome variable, one-unit change in model score was significantly associated with change in fibrosis by 0.19 (0.07, 0.31; P = 0.002) for APRI and by 0.22 (0.10, 0.34, P < 0.001) for FIB-4 (Table 4). While adjusting for initial fibrosis stage, fibrosis model score at initial biopsy and time interval between two biopsies both APRI and FIB-4 remained significantly associated with change in fibrosis (Table 4). In adjusted analysis, one unit change in model score was associated with change in fibrosis by 0.33 (0.20, 0.45; P < 0.001) for APRI, 0.26 (0.15, 0.37; P < 0.001) for FIB-4, and 0.19 (0.07, 0.31; P = 0.002). The AST/ALT ratio was not linearly associated with change in fibrosis.
Table 4:
Estimated change in fibrosis score associated with a unit change in model score (n = 292)
| Model index | Unadjusted model† |
Adjusted model‡ |
||||
|---|---|---|---|---|---|---|
| Coefficient (95% CI) | Standardized coefficient (95% CI) | p-value | Coefficient (95% CI) | Standardized coefficient (95% CI) | p-value | |
| APRI | 0.19 (0.07, 0.31) |
0.18 (0.07, 0.29) |
0.002 | 0.33 (0.20, 0.45) |
0.32 (0.20, 0.44) |
<0.001 |
| FIB-4 | 0.22 (0.10, 0.34) |
0.21 (0.10, 0.32) |
<0.001 | 0.26 (0.15, 0.37) |
0.24 (0.14, 0.35) |
<0.001 |
| AST/ALT ratio | 0.28 (−0.21,0.76) |
0.07 (−0.05, 0.18) |
0.26 | 0.42 (−0.08, 0.91) |
0.10 (−0.02, 0.22) |
0.10 |
| NAFLD fibrosis score | 0.09 (−0.04, 0.21) |
0.08 (−0.03, 0.20) |
0.16 | 0.19 (0.07, 0.31) |
0.17 (0.06, 0.29) |
0.002 |
Results of simple linear regression. The outcome variable is the change in fibrosis score. The predictor is the change in model score.
Results of multiple linear regression. To the unadjusted model, initial fibrosis stage (categorical) and model score at the 1st biopsy (continuous) were added. Interaction between the change in model score and the initial fibrosis stage were assessed using another model but not significant (data not shown).
Diagnostic Performance of Non-Invasive Fibrosis Models in Predicting Progression to Advanced Fibrosis
Of the 216 patients with non-advanced fibrosis at baseline, 35 patients progressed to advanced fibrosis (16%) after a median follow up of 2.5 years (IQR = 3.2). Patients with baseline fibrosis stage of 2 were more likely to progress to advanced fibrosis when compared to those with less fibrosis stages at baseline (Supplemental Table 3). APRI, FIB-4 and NFS had better capability for detecting progression to advanced fibrosis with cross-validated C-statistic of 0.82 (0.74, 0.89), 0.81 (0.73, 0.89) and 0.80 (0.71, 0.88), respectively (Figure 1). With specificity fixed at 90%, the PPV of predicting progression to advance fibrosis was 47%, 49%, 18% and 44% for APRI, FIB- 4, AST/ALT Ratio, and NAFLD Fibrosis Score, respectively (Table 5). At fixed sensitivity of 90%, the NPV for detecting progression to advanced fibrosis was 97%, 97%, 96% and 93% for APRI, FIB-4, AST/ALT ratio and NFS, respectively (Supplemental Figure 3).
Table 5:
Diagnostic performance of change in model score for any progression in fibrosis stage and progression to advanced fibrosis
| Mode score | Cross-validated C-statistic† (95 CI%) |
Cutoff criteria | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Specificity fixed at 90%‡ |
Sensitivity fixed at 90%§ |
At maximum Youden’s index | |||||||||
| Sens. (%) |
PPV (%) |
NPV (%) |
Spec. (%) |
PPV (%) |
NPV (%) |
Sens. (%) |
Spec. (%) |
PPV (%) |
NPV (%) |
||
| Any progression in fibrosis stage (n = 282)ǁ | |||||||||||
| APRI | 0.70 (0.63, 0.77) |
43 | 68 | 77 | 21 | 36 | 82 | 50 | 84 | 61 | 78 |
| FIB-4 | 0.73 (0.67, 0.79) |
36 | 63 | 74 | 26 | 37 | 85 | 68 | 69 | 52 | 82 |
| AST/ALT ratio | 0.57 (0.50, 0.64) |
10 | 32 | 67 | 24 | 37 | 84 | 64 | 53 | 40 | 75 |
| NAFLD fibrosis score | 0.66 (0.59, 0.73) |
24 | 54 | 71 | 17 | 35 | 79 | 60 | 71 | 50 | 78 |
| Progression to advanced fibrosis (n = 216)¶ | |||||||||||
| APRI | 0.82 (0.74, 0.89) |
46 | 47 | 90 | 62 | 32 | 97 | 89 | 67 | 34 | 97 |
| FIB-4 | 0.81 (0.73, 0.89) |
49 | 49 | 90 | 57 | 29 | 97 | 91 | 57 | 29 | 97 |
| AST/ALT ratio | 0.68 (0.59, 0.77) |
11 | 18 | 84 | 35 | 21 | 96 | 71 | 76 | 36 | 93 |
| NAFLD fibrosis score | 0.80 (0.71,0.88) |
40 | 44 | 89 | 43 | 24 | 96 | 77 | 76 | 39 | 95 |
CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value; sens., sensitivity; spec. specificity.
Based on leave-one-out cross-validation.
Fixed at specificity larger than and closest to 90%.
Fixed at sensitivity larger than and closest to 90%.
Among 216 patients with initial fibrosis stage 0-2, 35 (16%) progressed to advanced fibrosis.
Among 282 patients with initial fibrosis stage 0-3, 92 (33%) progressed in fibrosis stage.
DISCUSSION
The present study evaluated the accuracy of panel of non-invasive fibrosis models to detect hepatic fibrosis and change in fibrosis in patients with histologically proven NAFLD. FIB-4, NFS and APRI outperformed other models in detecting the presence of any, moderate and advanced fibrosis. These data are in line with published literature showing similar overall performance of these models and higher performance of FIB-4, NFS and APRI compared to other non-invasive models of hepatic fibrosis10,14 (Supplemental Table 4). The diagnostic accuracy of these models increased marginally to be able to detect presence of advanced fibrosis. Our findings confirm published literature showing limited sensitivity of these models. However, the NPV for these models is high and thus may be used clinically to exclude the presence of moderate and advanced fibrosis to identify patients who can be safely spared confirmatory yet invasive testing. While the accuracy of these non-invasive fibrosis models is inferior to elastography, both MRE and VCTE, these models do no incur additional expense since they are constructed with readily available clinical information and can therefore be implemented widely with relative ease.
NAFLD is a dynamic process, in which patients transition between histological states over time18. The ability to predict histological changes non-invasively is vital in managing patients with NAFLD. Currently there are no point of care test that can be widely deployed for routine use in clinical practice, and these non-invasive models may fill this potential void. While elastography has becoming increasing common in clinical management of NAFLD, the ability of VCTE to detect change in fibrosis stage is unknown. Although MRE can detect longitudinal change in fibrosis, it cannot be deployed as a point of care test. These non-invasive models use routinely collected data to construct fibrosis models and therefore do not incur any additional cost. Hepatic fibrosis, particularly advanced fibrosis is a strong surrogate for disease progression and associated mortality3. Changes in APRI, FIB-4, NFS and AST/ALT ratio were significantly associated with disease progression, however, only AST/ALT ratio was associated reduction in fibrosis stage. The relationship between APRI, NFS and FIB-4 and change in fibrosis persisted while controlling for baseline biopsy stage, time between biopsy and model score at baseline biopsy. Finally, FIB-4 and APRI had the greatest accuracy in predicting progression to advanced fibrosis and while the PPV for all non-invasive fibrosis models were suboptimal, the NPV for these models were high.
The current study used prospectively collected data in a large, multicenter cohort of patients with histologically well-characterized patients, thus represents the typical NAFLD patient population that is seen in hepatology clinics. The bio-clinical data used to construct fibrosis models were collected within close proximity to liver biopsy, thus avoiding the impact of time on these models since changes in hepatic fibrosis are slow.
There are several notable limitations to the current study. First, the current study limited the analysis to fibrosis models with output that was continuous thereby excluding BARD and BAAT. However, these models have been shown to be less effective than the models that were evaluated in the current study10,15. The time interval between two biopsies was variable. The current study was performed in a population with high prevalence of advanced fibrosis, reflective of a typical tertiary care hepatology practice. Therefore, the PPV and NPV obtained cannot be readily applied to other clinical practice where the prevalence of the disease is lower. We reported cross-validated C-statistic to assess the predictive performance of change in model’s cores. However, it would necessary to use split-sample and external sample for further validation of their performance. Finally, we excluded all patients who were on medical therapy for NASH, thus another study would be necessary to assess how well these models would perform to evaluate response to therapy.
In summary, change in FIB-4, APRI and NFS are able to predict fibrosis progression and development of advanced fibrosis in patients with NAFLD. These non-invasive fibrosis models can be used to identify individuals at risk individuals in whom additional histological evaluation maybe warranted both cross-sectionally and longitudinally.
Supplementary Material
WHAT YOU NEED TO KNOW.
Background: We evaluated the diagnostic performance of tools for assessment of hepatic fibrosis in patients with NAFLD.
Findings: In a cross-sectional study, FIB-4 and NFS outperformed other non-invasive models for detecting advanced fibrosis. In a longitudinal study, after we adjusted for fibrosis stage and model score at initial biopsy, change in APRI, FIB-4, and NFS were significantly associated with change in fibrosis.
Limitations: These were retrospective studies.
Implications for patient care: FIB-4, APRI, and NFS can detect advanced fibrosis and fibrosis progression in patients with NAFLD.
NAFLD was defined as presence of hepatic steatosis in the absence of cytological ballooning or lobular inflammation. Definite NASH was defined histologically by a pattern of injury composed of steatosis, lobular inflammation, and ballooning degeneration by expert histopathologist. Borderline NASH was defined as hepatic steatosis that is accompanied by mild lobular inflammation and none to rare cytological ballooning not typical of definite NASH.
Acknowledgments
Source of funding (adult and pediatric)
The Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (grants U01DK061718, U01DK061728, U01DK061731, U01DK061732, U01DK061734, U01DK061737, U01DK061738, U01DK061730, U01DK061713).
Abbreviations:
- NAFLD:
Nonalcoholic Fatty Liver Disease
- NASH:
Nonalcoholic Steatohepatitis
- NASH CRN:
NASH Clinical Research Network
- VCTE:
Vibration Controlled Transient Elastography
- LSM:
Stiffness Measurement
- CAP:
Controlled Attenuation Parameter
Footnotes
Conflicts of Interests: None that pertains to the current manuscript and are disclosed in conflict of interest form
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
M. Shadab Siddiqui, Email: mosiddiq@gmail.com.
Goro Yamada, Email: gyamada1@jhu.edu.
Raj Vuppalanchi, Email: rvuppala@iu.edu.
Mark Van Natta, Email: mvnatta@jhu.edu.
Rohit Loomba, Email: roloomba@ucsd.edu.
Cynthia Guy, Email: cynthia.guy@duke.edu.
Danielle Brandman, Email: Danielle.Brandman@ucsf.edu.
James Tonanscia, Email: james.tonascia@jhu.edu.
Naga Chalasani, Email: nchalasa@iupui.edu.
Brent Neuschander-Tetri, Email: brent.tetri@health.slu.edu.
Arun J Sanyal, Email: arun.sanyal@vcuhealth.org.
REFERENCES:
- 1.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- 2.Kleiner DE, Brunt EM, Natta M Van, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313–1321. [DOI] [PubMed] [Google Scholar]
- 3.Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2015;149:389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shadab Siddiqui M, Harrison SA, Abdelmalek MF, et al. Case definitions for inclusion and analysis of endpoints in clinical trials for NASH through the lens of regulatory science. Hepatology 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kruger FC, Daniels CR, Kidd M, et al. APRI: a simple bedside marker for advanced fibrosis that can avoid liver biopsy in patients with NAFLD/NASH. S Afr Med J 2011;101:477–480. [PubMed] [Google Scholar]
- 6.Sheth SG, Flamm SL, Gordon FD, et al. AST/ALT ratio predicts cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol 1998;93:44–48. [DOI] [PubMed] [Google Scholar]
- 7.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317–1325. [DOI] [PubMed] [Google Scholar]
- 8.Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007;45:846–854. [DOI] [PubMed] [Google Scholar]
- 9.Siddiqui MS, Patidar KR, Boyett S, et al. Performance of non-invasive models of fibrosis in predicting mild to moderate fibrosis in patients with non-alcoholic fatty liver disease. Liver Int 2016;36:572–579. [DOI] [PubMed] [Google Scholar]
- 10.Shah AG, Lydecker A, Murray K, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009;7:1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanyal AJ, Chalasani N, Kowdley K V, et al. Pioglitazone, Vitamin E, or Placebo for Nonalcoholic Steatohepatitis. N Engl J Med 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 2015;385:956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neuschwander-Tetri BA, Clark JM, Bass NM, et al. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology 2010;52:913–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siddiqui MS, Patidar KR, Boyett S, et al. Performance of Non-invasive Models of Fibrosis in Predicting Mild to Moderate Fibrosis in Patients with Nonalcoholic Fatty Liver Disease (NAFLD). Liver Int 2015. [DOI] [PubMed] [Google Scholar]
- 15.Imajo K, Kessoku T, Honda Y, et al. Magnetic Resonance Imaging More Accurately Classifies Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease Than Transient Elastography. Gastroenterology 2016;150:626–637.e7. [DOI] [PubMed] [Google Scholar]
- 16.Sheth SG, Flamm SL, Gordon FD, et al. AST/ALT ratio predicts cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol 1998;93:44–48. [DOI] [PubMed] [Google Scholar]
- 17.Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med 2002;137:1–10. [DOI] [PubMed] [Google Scholar]
- 18.McPherson S, Hardy T, Henderson E, et al. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol 2015;62:1148–1155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.