Abstract
Objective:
We evaluated whether three neurofunctional domains proposed to be critical in the addiction cycle: Incentive Salience, Negative Emotionality, and Executive Function, could be identified through factor analysis of a deeply phenotyped clinical sample.
Method:
Clinical, behavioral, and self-report measures of addiction, personality, cognition, behavior, and exposure to early life stress were collected as part of a screening and natural history study of alcohol use disorders (AUD) in 454 individuals representing the spectrum of alcohol use and use disorders. The Multiple Indicators Multiple Causes (MIMIC) approach was used to identify significant predictors of the latent factors identified by the analysis.
Results:
We found significant support for both three- and four-factor models to explain biobehavioral variation in this sample of AUD participants and controls, but the three-factor model had the best fit indices. With some nuances, including cross-correlation (lack of independence) between the three factors, the factors corresponded to Incentive Salience, Negative Emotionality, and Executive Function [Executive Control]. The MIMIC model revealed that both exposure to early life stress and sociodemographic variables predicted these factors.
Conclusions:
These findings suggest that three correlated neurofunctional domains are relevant for AUD. More work is required to validate and standardize measures of neurofunctional domains in AUD, to extend these findings to other addictive disorders, and to relate variations in them to predisposition, clinical course, treatment response, neuroimaging data and other psychophysical indicators.
Introduction
Addiction to diverse substances including alcohol, tobacco, and illicit drugs is a leading preventable contributor to global disease burden (1). In 2016, substance use and use disorders (SUD) accounted for two-thirds of the estimated 64,026 drug overdose fatalities in the United States (2), while 88,000 fatalities were associated with alcohol-related adverse effects (3). Although several pharmacologic and behavioral treatments for SUD have shown efficacy in controlled clinical trials, there is a need for more effective treatments. Better knowledge, and measures, of the functional domains underlying SUD could help in assessing severity, modelling heterogeneity, predicting course, targeting treatments, and detecting efficacy and treatment mechanisms.
Based on conceptual frameworks derived from neurobiology, clinical studies, and social psychology (4, 5), we recently proposed a neuroscience-based framework (Addictions Neuroclinical Assessment [ANA]) to better understand the heterogeneity of addiction (6). This framework postulates that three domains are implicated in the development and maintenance of SUD: Incentive Salience, Negative Emotionality, and Executive Function (7). As reviewed in detail elsewhere (6), previous studies have shown group differences between addicted and non-addicted individuals in various assessments, including neuropsychological and neuroimaging, purporting to measure those domains. Additional disruptions in function in those domains have been demonstrated in some individuals at risk for developing addictive disorders, suggesting that compromised function may be a risk factor and/or a consequence of addiction. One constraint of these previous studies is that they typically evaluated a single domain of function, such as cognitive control or emotion regulation, rather than using a comprehensive framework. An exception to this pattern is the National Consortium on Alcohol and Neurodevelopment in Adolescence (NCANDA) study, which evaluated multiple functional domains in adolescents across the spectrum of alcohol use (Sullivan et al., 2016). This study found differences in cognitive, affective, and motor domains related to levels of alcohol consumption. These initial findings suggest that further testing of a comprehensive model in clinical samples is warranted. Thus, the present study attempts to begin such a line of inquiry.
We sought to test the ANA model by applying factor analytic techniques to a deeply phenotyped sample across the spectrum of alcohol use and alcohol use disorders, ranging from healthy individuals up to and including patients with DSM-IV alcohol abuse or dependence, i.e., alcohol use disorder (AUD) in DSM-5 terminology. Based on developmental models of SUD (8–10), we hypothesized that family history, adverse childhood experiences (ACEs), and age at first drink would predict the scores of individuals on factors emergent from this analysis.
Methods
Participants
Participants (n = 454) included individuals seeking treatment for AUD at the NIH Clinical Center and non-treatment-seeking individuals (ranging from healthy volunteers to individuals with AUD not seeking treatment) screened in the NIAAA outpatient clinic for participation in research studies between January 2015 and February 2017. All completed the NIAAA Screening and Natural History Protocol (SNHP), which provides a platform for common assessments and uses minimal inclusion/exclusion criteria, to maximize generalizability. Individuals were only excluded if pregnant, breastfeeding, or under legal confinement. Treatment seeking patients were diagnosed with alcohol dependence or abuse using DSM-IV terminology; we refer to their diagnosis as AUD, using DSM-5 parlance, to include those with dependence and abuse in the same group, as is consistent with the most current nosology. Most patients had multiple relapses despite prior treatment. All study participants provided written informed consent under this NIH IRB-approved protocol.
Assessments and Indicator Variables
Study participants underwent a deep phenotyping with measures relevant for addiction and related constructs, including psychiatric diagnoses, attention, impulsivity, aggression, and personality. Specific instruments appear in the footnotes of Table 2.
Table 2.
Subject characteristics including background measures and indicator measures used in the analysis
| Measure | Total Sample (n = 454) | Alcohol Use Disorder (AUD)a(n = 186) | Non-AUD (n = 252) | P-value | |
|---|---|---|---|---|---|
| Female | 185 (40.8%) | 53 (28.5%) | 124 (49.2%) | <0.0001 | |
| Caucasian | 185 (40.8%) | 74 (39.8%) | 107 (42.5%) | 0.02 | |
| Hispanic | 26 (5.7%) | 4 (2.2%) | 21 (8.3%) | 0.006 | |
| Age | 40.3 (13.5); 18.7–91.4 | 45.2 (11.9); 21.1–71.8 | 36.4 (13.4); 18.7–91.4 | <0.0001 | |
| Years of Education | 14.7 (3.6); 2–41 | 13.7 (3.2); 2–36 | 15.6 (3.6); 2–41 | <0.0001 | |
| Current AUD | 186 (43.3%) | 186 (100%) | 0 (0%) | - | |
| Lifetime AUD | 200 (46.5%) | 186 (100%) | 14 (5.6%) | <0.0001 | |
| Family History of Alcohol Use Problemsb | 0.1 (0.3); 0.0–1.0 | 0.2 (0.3); 0.0–1.0 | 0.1 (0.2); 0.0–1.0 | <0.0001 | |
| Age at First Drink | 16.5 (4.3); 5–45 | 15.2 (5.3); 5–45 | 17.6 (3.1); 10–32 | <0.0001 | |
| Alcohol Dependence Severityc | 8.5 (10.2); 0–42 | 17.6 (9.1); 0–42 | 1.3 (2.3); 0–14 | <0.0001 | |
| Obsessive Drinking Subscaled | 3.2 (4.7); 0–20 | 7 (5.1); 0–20 | 0.3 (1.1); 0–8 | <0.0001 | |
| Compulsive Drinking Subscaled | 5.6 (5.6); 0–20 | 10.6 (4.8); 0–20 | 1.8 (2.3); 0–14 | <0.0001 | |
| Current Substance Use Disorder ( other than alcohol ) | 28 (6.5%) | 28 (15.1%) | 0 (0%) | <0.0001 | |
| Lifetime Substance Use Disorder ( other than alcohol ) | 129 (30.0%) | 111 (59.7%) | 18 (7.1%) | <0.0001 | |
| Current Mood Disorder | 32 (7.4%) | 29 (15.6%) | 3 (1.2%) | <0.0001 | |
| Lifetime Mood Disorder | 80 (18.6%) | 51 (27.4%) | 29 (11.5%) | <0.0001 | |
| Current Anxiety Disorder | 55 (12.8%) | 43 (23.1%) | 12 (4.8%) | <0.0001 | |
| Lifetime Anxiety Disorder | 68 (15.8%) | 56 (30.1%) | 12 (4.8%) | <0.0001 | |
| Current PTSD | 24 (5.6%) | 19 (10.2%) | 5 (2.0%) | 0.0002 | |
| Lifetime PTSD | 34 (7.9%) | 29 (15.6%) | 5 (2.0%) | <0.0001 | |
| Emotional Abusee | 8.3 (4.6); 5–25 | 9.4 (5.2); 5–25 | 7.3 (3.8); 5–24 | <0.0001 | |
| Physical Abusee | 7.3 (3.5); 5–25 | 8.3 (4.4); 5–25 | 6.5 (2.3); 5–20 | <0.0001 | |
| Sexual Abusee | 6.2 (3.9); 5–25 | 6.7 (4.4); 5–25 | 5.7 (2.9); 5–25 | 0.007 | |
| Emotional Neglecte | 9.5 (4.5); 5–25 | 10.2 (4.9); 5–25 | 8.7 (4.0); 5–23 | 0.001 | |
| Physical Neglecte | 6.9 (3.1); 5–25 | 7.5 (3.7); 5–25 | 6.4 (2.3); 5–16 | 0.0002 | |
| Indicator Variables | |||||
| Continuous Measures: | |||||
| Attentional Impulsivityf | 14.1 (4.2); 8–32 | 16.3 (4.6); 8–32 | 12.3 (3.0); 8–23 | <0.0001 | |
| Motor Impulsivityf | 22.3 (4.4); 14–40 | 24.5 (5); 14–40 | 20.6 (3.2); 14–30 | <0.0001 | |
| Non-planning Impulsivityf | 22.6 (6.2); 11–42 | 26.3 (6.1); 13–42 | 19.6 (4.4); 11–35 | <0.0001 | |
| Negative Urgencyg | 2 (0.7); 1.0–3.9 | 2.4 (0.7); 1.0–3.9 | 1.6 (0.5); 1.0–3.7 | <0.0001 | |
| Premeditationg | 1.8 (0.5); 1.0–4.0 | 2 (0.6); 1.0–4.0 | 1.7 (0.4); 1.0–3.5 | <0.0001 | |
| Perseveranceg | 1.8 (0.5); 1.0–3.6 | 2 (0.5); 1.0–3.6 | 1.6 (0.4); 1.0–3.4 | <0.0001 | |
| Positive Urgencyg | 1.7 (0.7); 1.0–4.0 | 2.1 (0.8); 1.0–4.0 | 1.4 (0.5); 1.0–3.9 | <0.0001 | |
| Neuroticismh | 49 (11.6); 23.0–85.0 | 54.9 (11.4); 26.0–84.0 | 45 (10.0); 23.0–85.0 | <0.0001 | |
| Extraversionh | 51 (10.1); 13.0–83.3 | 52 (10.2); 19.3–78.1 | 50.3 (10.0); 13.0–83.3 | 0.11 | |
| Opennessh | 54.1 (10.5); 29.0–83.0 | 51.7 (10); 29.0–77.0 | 55.8 (10.4); 30.0–83.0 | <0.0001 | |
| Agreeablenessh | 48.7 (11); 16.0–78.0 | 46.6 (11.6); 18.0–77.0 | 50.1 (10.3); 16.0–78.0 | 0.002 | |
| Conscientiousnessh | 50.2 (11); 8.0–79.0 | 45.3 (11.4); 8.0–72.0 | 53.7 (9.2); 20.0–79.0 | <0.0001 | |
| Delay Discounting K function | 0.1 (0.2); 0.0–1.0 | 0.1 (0.2); 0.0–1.0 | 0.1 (0.2); 0.0–1.0 | 0.23 | |
| Aggressioni | 84.6 (18.8); 55–147 | 92.4 (19.5); 60–144 | 77.9 (14.8); 55–131 | <0.0001 | |
| MADRS Depression Scorej | 5.9 (8.8); 0–37 | 12.1 (10.1); 0–37 | 1.2 (3); 0–27 | <0.0001 | |
| Spielberger Trait Anxietyk | 35.6 (12.8); 20–75 | 44.6 (13); 20–72 | 28.6 (7.5); 20–75 | <0.0001 | |
| Categorical Measures: |
Response |
||||
| Symptoms Consistent with ADHDl | 0 | 403 (89.2%) | 143 (77.7%) | 245 (97.2%) | <0.0001 |
| 1 | 49 (10.8%) | 41 (22.3%) | 7 (2.8% ) | ||
| ADS Question 18: Do you almost constantly think about drinking alcohol?m | 0 | 351 (77.7%) | 90 (48.9%) | 251 (99.6%) | <0.0001 |
| 1 | 101 (22.3%) | 94 (51.1%) | 1 (0.4% ) | ||
| OCDS Question 1: How much of your time when you’re not drinking is occupied by ideas, thoughts, impulses, or images related to drinking?n | 0 | 238 (52.5%) | 23 (12.4%) | 211 (83.7%) | <0.0001 |
| 1 | 98 (21.6%) | 55 (29.7%) | 37 (14.7% ) | ||
| 2 | 58 (12.8%) | 53 (28.7%) | 3 (1.2% ) | ||
| 3 | 28 (6.2%) | 27 (14.6%) | 0 (0% ) | ||
| 4 | 31 (6.84) | 27 (14.6%) | 1 (0.4% ) | ||
| OCDS Question 11: If you were prevented from drinking alcohol when you desired a drink, how anxious or upset would you become?o | 0 | 263 (58.1%) | 29 (15.7%) | 232 (92.1%) | <0.0001 |
| 1 | 77 (17.0%) | 53 (28.7%) | 18 (7.1% ) | ||
| 2 | 54 (11.9%) | 48 (26.0%) | 2 (0.8% ) | ||
| 3 | 44 (9.7%) | 41 22.2%) | 0 (0% ) | ||
| 4 | 15 (3.3%) | 14 (7.6%) | 0 (0% ) | ||
| OCDS Question 13: How strong is the drive to consume alcoholic beverages?p | 0 | 238 (52.5%) | 23 (12.4%) | 210 (83.3%) | <0.0001 |
| 1 | 89 (19.7%) | 50 (27.0%) | 37 (14.7% ) | ||
| 2 | 42 (9.3%) | 32 (17.3%) | 3 (1.2% ) | ||
| 3 | 55 (12.1%) | 53 (28.7%) | 2 (0.8% ) | ||
| 4 | 29 (6.4%) | 27 (14.6%) | 0 (0% ) | ||
Note: For continuous measures, the values are the mean, standard deviation (in parentheses), the minimum to maximum value range, and the p-value is based on the t-test comparison of AUD vs. non-AUD groups. For categorical measures, the values are N and the percentage (in parentheses), and the p-value is based on the chi-square test comparison of AUD vs. non-AUD groups.
Based on the Structured Clinical Interview for DSM-IV Disorders (SCID-IV); SCID-IV data were missing for 16 subjects
Proportion of parents with a known alcohol use problem
Alcohol Dependence Scale (ADS)
Obsessive-Compulsive Drinking Sale (OCDS)
Childhood Trauma Questionnaire (CTQ)
Barratt Impulsiveness Scale, Version 11
UPPS-P Impulsive Behavior Scale
NEO Five Factor Personality Inventory-Revised
Buss-Perry Aggression Questionnaire
Montgomery-Asberg Depression Rating Scale, derived from the Comprehensive Psychopathology Rating Scale
Spielberger State-Trait Anxiety Inventory
Adult ADHD Self-Report Scale (ASRS-v1.1) Symptom Checklist (binary outcome: 0 = symptoms not consistent with ADHD; 1 = symptoms consistent with ADHD)
ADS Question 18 (binary outcome: 0 = no; 1 = yes)
OCDS Question 1 (categorical outcome: 0 = none; 1 = less than 1 hour a day; 2 = 1–3 hours a day; 3 = 4–8 hours a day; 4 = greater than 8 hours a day)
OCDS Question 11 (categorical outcome: 0 = I would not experience any anxiety or irritation; 1 = I would become only slightly anxious or irritated; 2 = The anxiety or irritation would mount but remain manageable; 3 = I would experience a prominent and very disturbing increase in anxiety or irritation; 4 = I would experience incapacitating anxiety or irritation
OCDS Question 13 (categorical outcome: 0 = no drive; 1 = some pressure to drink; 2 = strong pressure to drink; 3 = very strong drive to drink; 4 = the drive to drink is completely involuntary and overpowering
Statistical Analyses
Exploratory and Confirmatory Factor Analysis
The dataset was randomly split into two halves (each with n = 227); one for discovery and the other for replication. We used exploratory factor analysis (EFA) in the discovery half of the sample to identify latent factors underlying the indicator variables included in the assessments. Analyses were conducted in Mplus version 7.4 (Muthen & Muthen, Copyright © 1998–2015). A robust weighted least squares estimator (WLSMV), which does not assume normally distributed variables (11), was used along with the GEOMIN oblique rotation. The GEOMIN rotation allows correlation between factors and is recommended when indicators are predicted to load onto more than one factor (12). The EFA models were estimated using full information maximum likelihood (FIML). Factor selection was guided by examination of fit indices and overall interpretability. The fit indices examined were the root mean-squared error of approximation (RMSEA), the Comparative Fit Index (CFI), and the Tucker-Lewis Index (TLI). We followed the recommendations of Hu and Bentler (13) who suggest CFI and TLI values above 0.95, and RMSEA values below 0.06 to represent good model fit. Variables with a loading ≥ 0.35 were considered to load onto a particular factor. Confirmatory factor analysis (CFA) was performed in the replication half of the dataset. Variables with loadings ≤ 0.35 in the EFA were fixed at 0, and modification indices (MI) were examined and applied if they improved model fit and were conceptually meaningful.
Multiple Indicators Multiple Causes (MIMIC) Analysis
To assess the influence of several predictor variables on the latent factors and individual indicators, we conducted a Multiple Indicator Multiple Cause (MIMIC) analysis (14) using the final solution of the CFA, with the resulting factors scores as the outcome variables. For this analysis, we recombined the data to include the full sample. Specifically, we evaluated the association of both the latent factors and the indicators with the following: three demographic variables (sex, age, and race/ethnicity), current AUD diagnosis, family history of alcohol problems, age at first drink, and exposure to childhood adversity as assessed by the five subscales of the CTQ. Direct effects (i.e., effects that were not mediated by the latent factors) of the predictors on indicator measures were identified using modification indices with a cutoff of 10 or higher, and a p-value of 0.001 to identify predictors with significant direct effects (15).
Receiver operator characteristic (ROC) curve analysis
To assess the ability of each factor/domain to predict AUD (determined via SCID interview), we plotted ROC curves using the factor scores from each of the three factors, and calculated the area under the curve as a measure of how well the scores distinguished between individuals with and without AUD. This analysis was performed in SAS version 9.4 (SAS Institute, Cary, NC).
Results
Sample
The participant sample included a total of 454 individuals. Approximately 40% of the sample was female and 40% were Caucasian, with most of the rest being African-American. The mean age was just over 40 years. Average educational attainment was 14 yrs. A substantial percentage (43.5%) of the participants had current AUD. Participants had high rates of other lifetime psychiatric disorders, including substance use disorders (30%), mood disorders (18.6%), anxiety disorders (15.8%), and posttraumatic stress disorder (7.9%). Participant demographics and characteristics for the full sample and for AUD and non-AUD participants appear in Table 2.
Factor Structure
In the exploratory factor analysis in the discovery the data set, good model fit was found for both a 3-factor model (RMSEA = 0.04, CFI = 0.97, TLI = 0.96) and a 4-factor model (RMSEA = 0.04, CFI = 0.98, TLI = 0.97). However, the 4-factor model, while yielding roughly the same first 3 factors as the 3-factor solution, included a fourth factor with only a single indicator (Extraversion). Consequently, we chose the 3-factor model as the model for subsequent analyses because of its greater parsimony and better correspondence with the three hypothesized ANA domains. Factor loadings for the 3-factor solution appear in Table 1. Overall, the factors aligned well with the hypothesized ANA domains, with a few nuances. Factor 1 defined a Negative Emotionality domain, with positive loadings for neuroticism, aggression, and trait anxiety, and negative loadings for extraversion and agreeableness; however, positive urgency also loaded onto Factor 1. Factor 2 defined an (impaired) Executive Function (Executive Control) domain, with positive loadings for ADHD, the three impulsivity measures from the BIS, and the four impulsivity measures from the UPPS-P, and negative loading for conscientiousness. Factor 3 defined an Incentive Salience (and mood) domain, with positive loadings for the three items from the OCDS and ADS scales that assess thinking about drinking and drive to consume alcohol; the MADRS depression score and the OCDS item assessing anxiety when not able to drink also loaded onto factor 3. Two variables, Delay Discounting and openness, did not load onto any factor. In addition to Table 1, a correlation matrix of continuous indicators appears in Supplementary Table 1 and the four-factor solution appears in Supplementary Table 2.
Table 1.
Three factor solution from exploratory factor analysis
| Proposed Grouping | Indicator/Measure | Factor 1 | Factor 2 | Factor 3 |
|---|---|---|---|---|
| Executive function | ADHD | 0.166 | 0.443 | 0.193 |
| Executive function | Attentional Impulsivity | 0.259 | 0.675 | −0.024 |
| Executive function | Motor Impulsivity | 0.068 | 0.662 | −0.042 |
| Executive function | Non-planning Impulsivity | 0.093 | 0.73 | 0.074 |
| Executive function | Delay Discounting (K) | 0.174 | −0.184 | 0.027 |
| Executive function | Conscientiousness | 0.036 | −0.723 | −0.014 |
| Executive function | Negative Urgency | 0.329 | 0.536 | 0.143 |
| Executive function | Premeditation | −0.008 | 1.022 | −0.31 |
| Executive function | Perseverance | 0.157 | 0.64 | −0.002 |
| Executive function | Positive Urgency | 0.374 | 0.504 | 0.042 |
| Negative Emotionality | Depression | 0.151 | −0.081 | 0.689 |
| Negative Emotionality | Neuroticism | 0.534 | 0.007 | 0.294 |
| Negative Emotionality | Extraversion | −0.394 | 0.307 | 0.008 |
| Negative Emotionality | Openness | −0.039 | 0.009 | −0.117 |
| Negative Emotionality | Agreeableness | −0.598 | −0.042 | 0.174 |
| Negative Emotionality | Trait Anxiety | 0.35 | 0.112 | 0.524 |
| Negative Emotionality | Aggression | 0.931 | −0.053 | −0.001 |
| Negative Emotionality | OCDS Item 11 | 0.005 | −0.062 | 0.976 |
| Incentive Salience | OCDS Item 1 | −0.123 | 0.032 | 0.903 |
| Incentive Salience | OCDS Item 13 | 0.003 | −0.191 | 0.997 |
| Incentive Salience | ADS Item 18 | −0.119 | 0.017 | 0.986 |
Note: Boldface indicates factor loadings ≥ 0.350. We note that the loading for Premeditation in Factor 2 is larger than 1, however, in applying the oblique GEOMIN rotation, which allows for correlation between factors, the factor loadings are expressed as regression coefficients and not correlations. Thus factor loadings larger than 1 are possible.
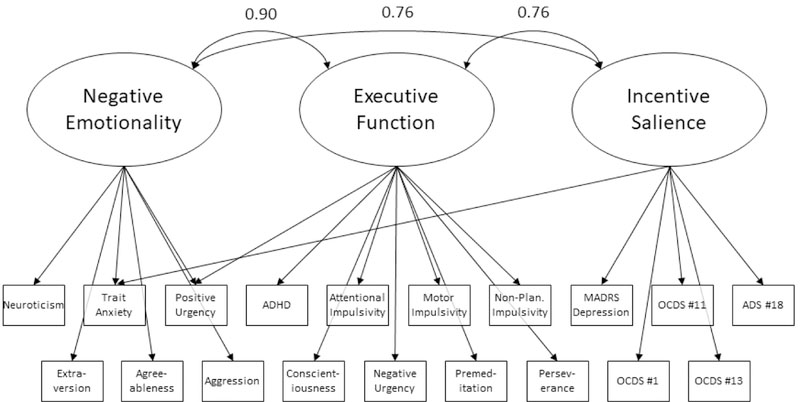
Confirmatory factor analysis of the 3-factor model in the replication half of the dataset also resulted in good model fit (RMSEA = 0.06, CFI = 0.94, TLI = 0.93). We subsequently ran a CFA in the full recombined dataset which also fit well (RMSEA = 0.05, CFI = 0.95, TLI = 0.94). All three factors were correlated with each other (Factor 1/Factor 2 correlation coefficient = 0.90, Factor 1/Factor 3 correlation coefficient = 0.76, Factor 2/Factor 3 correlation coefficient = 0.76). A visual representation of the model is presented in Figure 1.
Figure 1.
Visual representation of the three latent factors and the specific indicators. The numbers indicate the correlations between the three factors.
MIMIC Analysis
The results of the MIMIC analysis are presented in in Table 3 and Figures 2a-2c. Predictors of higher scores for Negative Emotionality included AUD, being female, emotional abuse, sexual abuse, and emotional neglect subscales of the CTQ. Predictors of higher scores for Executive Function included AUD, emotional and sexual abuse, family history, and age at first drink. Non-Caucasian race was associated with a lower score, as was a higher age a first drink (note that lower scores on this factor indicate better executive function). Conversely, emotional abuse, sexual abuse, AUD, and family history were associated with a higher score (and thus poorer executive function). Higher scores in the Incentive Salience factor included AUD, emotional abuse, and family history (although the latter association was marginally significant). Non-Caucasian race was associated with lower scores. There were only four direct effects of predictors on indicator measures: gender on agreeableness and neuroticism, age on agreeableness, and AUD on extraversion.
Table 3.
MIMIC model results
| Effects of Predictors on Latent Factors | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Negative Emotionality |
Executive Function |
Incentive Salience |
|||||||
| Predictor | Estimate | S.E. | p-value | Estimate | S.E. | p-value | Estimate | S.E. | p-value |
| AUD Diagnosisa | 0.641 | 0.105 | <0.0001 | 0.923 | 0.083 | <0.0001 | 1.507 | 0.073 | <0.0001 |
| Genderb | 0.329 | 0.104 | 0.001 | 0.093 | 0.089 | 0.295 | −0.069 | 0.079 | 0.381 |
| Racec | −0.056 | 0.097 | 0.564 | −0.377 | 0.085 | <0.0001 | −0.332 | 0.074 | <0.0001 |
| Age | −0.027 | 0.048 | 0.574 | −0.055 | 0.045 | 0.22 | 0.002 | 0.042 | 0.966 |
| Emotional Abuse | 0.202 | 0.068 | 0.003 | 0.133 | 0.06 | 0.025 | 0.187 | 0.054 | 0.001 |
| Physical Abuse | 0.07 | 0.065 | 0.281 | 0.006 | 0.058 | 0.925 | −0.067 | 0.054 | 0.219 |
| Sexual Abuse | 0.107 | 0.047 | 0.023 | 0.116 | 0.045 | 0.01 | 0.007 | 0.044 | 0.881 |
| Emotional Neglect | 0.19 | 0.072 | 0.008 | 0.014 | 0.063 | 0.821 | −0.031 | 0.053 | 0.56 |
| Physical Neglect | −0.072 | 0.068 | 0.293 | 0.066 | 0.062 | 0.28 | 0.067 | 0.048 | 0.159 |
| Family History | 0.043 | 0.049 | 0.382 | 0.088 | 0.044 | 0.044 | 0.07 | 0.035 | 0.049 |
| Age at First Drink | −0.075 | 0.053 | 0.161 | −0.109 | 0.044 | 0.014 | −0.055 | 0.035 | 0.114 |
| Significant Direct Effects of Predictors on Indicators | |||||||||
| Extraversion | Neuroticism | Agreeableness | |||||||
| Predictor | Estimate | S.E. | p-value | Estimate | S.E. | p-value | Estimate | S.E. | p-value |
| AUD a | 0.447 | 0.134 | 0.001 | -- | -- | -- | -- | -- | -- |
| Genderb | -- | -- | -- | −0.401 | 0.092 | <0.0001 | −0.337 | 0.105 | 0.001 |
| Age | -- | -- | -- | -- | -- | -- | 0.212 | 0.054 | <0.0001 |
Note: Estimates are standardized coefficients. Estimates, S.E., and p-values are bolded for significant predictors of each latent factor.
Current AUD: 0 = no, 1 = yes
Gender: 0 = male, 1 = female
Race: 0 = white/Caucasian, 1 = black/other
Figures 2a – 2c.
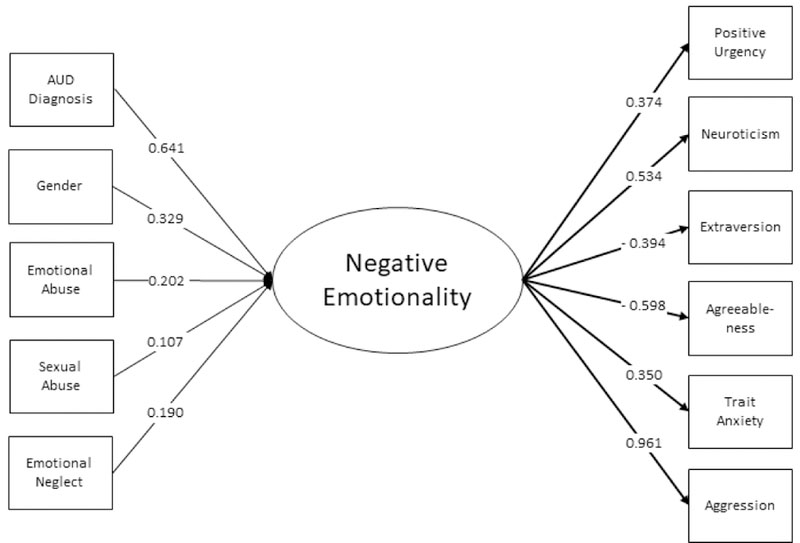
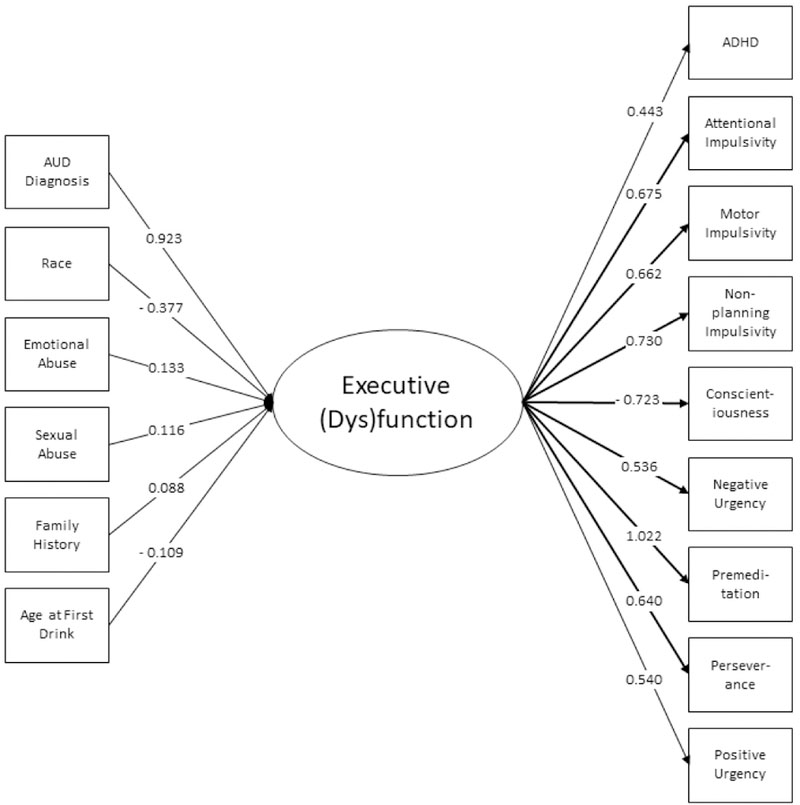
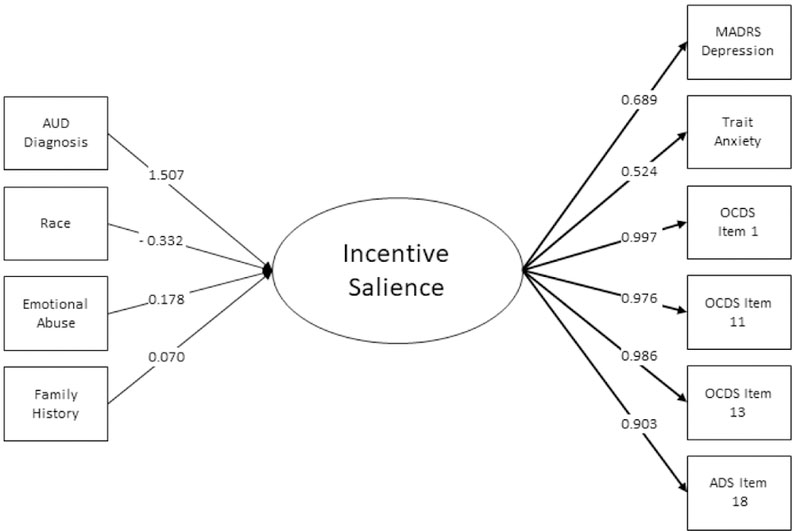
Predictors and indicators of the three latent factors including variable loadings. Only significant predictors are included.
ROC Analysis
All three factors distinguished well between AUD and non-AUD individuals, with Incentive Salience having the highest area under the curve (AUROC) at 0.96. AUROC for Negative Emotionality and Executive Function were 0.86 and 0.85, respectively. The ROC curves based on the scores output for each factor appear in Supplementary Figure 2.
Discussion
In a large and diverse clinical sample enriched for AUD, factors representing three neurofunctional domains, Incentive Salience, Negative Emotionality, and Executive Function underpinned and incorporated results from a broad range of scales and neuropsychological tests. Our findings support the existence of neurofunctional domains relevant to SUD that have been previously theorized (6, 16) and for which prior evidence in model organisms and humans has focused attention to particular aspects of cognition, behavior, emotion, and circuit function (e.g., 7, 17, 18).
Factor analyses of clinical data from SUD are limited by the lack of measures that may directly access the underlying domains, thereby increasing, if not ensuring, the likelihood that such studies return class structures based on severity, onset, exposure and use. Several clinical trials, including Project MATCH for the treatment of alcohol dependence, have targeted treatments to clinical subgroups or patients with particular clinical features. For example, long-term, rewardblunted patients with alcohol dependence may be less responsive to naltrexone, explaining the heterogeneous efficacy of this drug in clinical trials (19, 20).
We hypothesized that non-invasive deep phenotyping of individuals with and without AUD across multiple cognitive, psychologic and behavioral domains might detect latent domains mediating vulnerability and progression of addiction. We asked whether the latent factors discovered from this analysis would align with three neurofunctional domains identified in studies of patients with SUD that have used assessment such as positron emission tomography [PET] and functional Magnetic Resonance Imaging (fMRI) in humans and neuropharmacological, neurocircuit, and genetic manipulations in animal models. Notably, these methods are more invasive and costly and not suitable for clinical practice.
We found that a three-factor model generally demonstrated a good fit with our assessment measures providing strong support for our first hypothesis. Furthermore, the factors aligned closely with the ANA domains of Incentive Salience, Negative Emotionality, and Executive Function. Relatively inexpensive and non-invasive measures recovered a framework predicted by a large body of preclinical data, clinical psychology, and brain imaging studies (4, 5). Preclinical data demonstrates decreases in dopaminergic and serotonergic transmission in the nucleus accumbens during withdrawal, paralleled by upregulation of brain stress systems (e.g., CRF, dynorphin) as addiction progresses (4, 21). This increase in stress during withdrawal is also related to increased vulnerability for relapse and failure to regulate negative affective states in humans. Clinical studies have found that SUD, whether because of innate predisposition or effect of exposure to various environmental stimuli, such as stress or alcohol, is marked by disruptions of function in prefrontal brain regions implicated in executive function (22–24), in striatocortical reward regions implicated in incentive salience (25), and in the extended amygdala, as implicated in negative emotionality (26, 27). Neurobehavioral and cognitive assessment could represent a bridge between clinical assessment of symptoms, use and course, and deeper levels of neurofunctional assessment, recovering indices of the same process.
The three principal factors in our sample were intercorrelated, consistent with models of psychopathology based on clinical, rather than neuroscience-based assessments (28). These intercorrelations are likely explained by shared underlying neural circuitry, within and betweensystem adaptations occurring as part of the addiction process (29, 30), and shared genetic and environmental risk factors. Increasingly, different SUD demonstrate common disruptions and vulnerability processes. An important goal of deep phenotyping is to define how far sharing of vulnerability and consequences extends. Sharing of vulnerability is observed via family studies and several large, epidemiologically sound twin studies demonstrating cross-inheritance of addictions, as well as agent-specific genetic factors (31). Clinical research in general population samples has found that remission of one disorder is associated with remission of other disorders and lower probability of new onset of another disorder (32, 33). Clinical research has also found common outcomes, and for example higher levels of dysphoria and anxiety, in SUD populations, regardless of other subtypology. For example, in one study of SUD patients from different populations, dysphoria and anxiety were elevated regardless of whether patients did or did not have Type II Antisocial Personality Disorder features (34). An important future direction is to examine whether change in any one of the ANA domains is accompanied by change in other domains, in accord with the relatively strong correlation between domains observed in our sample. If that is the case, treatments that target one domain may have positive spillover effects on other domains. Conversely, it may be essential to simultaneously address several deficits to avoid the possibility that unaddressed deficits may interfere with remission or predispose to relapse.
In the ROC analyses, each of the three neurofunctional domains was moderately to highly accurate in predicting AUD, with areas under the curve ranging from 0.84 to 0.96. An important caveat is that a variety of other clinical instruments of different lengths and complexity, but of much greater brevity than the SCID, can also be used to diagnose AUD. In one study, the fourquestion CAGE had a AUROC of 0.81 for alcohol dependence in men and 0.75 in women (35). Our ROC analysis shows that the three neurofunctional domains are germane to AUD but does not demonstrate that measuring these latent factors can improve clinical assessment of AUD or SUD. Nevertheless, the demonstration that the three proposed domains differentiate between AUD and non-AUD participants underscores the relevance of these domains for addiction and is a critical finding of this data analysis.
The replication of the 3-factor structure in the two split samples provides further support for the robustness of the three-factor solution. Because the ANA model is based on a combination of preclinical and clinical data, and had not been previously tested in its entirety, these findings suggest that a development of a battery of assessments designed specifically to measure the ANA domains among of individuals with a broader range of SUD is a critical next step for evaluating the validity and clinical utility of this model.
We hypothesized that sociodemographic characteristics would predict severity of the neurofunctional factors. Our MIMIC model indicated that that the three domains were associated with genetic (e.g., sex, family history), environmental (childhood adversity) and developmental (age at first drink) correlates. These findings, consistent with biopsychosocial models of SUD (8–10), underscore the widespread effects of these risk factors on the neurobiological underpinnings of AUD and offer potential targets for prevention. Gene by environment interactions may play a role in explaining these findings. For example, individuals with impaired executive function due to genetic predisposition may also be early users of alcohol, which itself is an environmental variable shaping AUD onset and progression (36, 37). In this scenario, an individual may have both a genetic predisposition for impaired executive function and exposure early in life to alcohol due to a family environment that may also be influenced by the same genetic liability.
For the negative emotionality domain, it is critical to consider the role of exposure to ACEs. Exposure to ACEs is one of the strongest risk factors for later development of addictive disorders, likely through genetic and environmental mechanisms (38). Childhood trauma has an enduring effect on propensity for negative affect (39) and may lead individuals to consume alcohol and other substances to relieve dysphoria. The MIMIC analysis found that all five subscales of the CTQ predicted the negative emotionality factor, whereas fewer subscales predicted the other two factors. The finding that CTQ subscales predicted all three factors constitutes an example of a single predictor, leading to disruptions in multiple domains of function, i.e., multifinality (Cicchetti & Rogosch, 1996).
Finally, although our sample contained healthy volunteers as well as individuals with other SUD, we note that differences in factor scores may be secondary to AUD, i.e., they occur as a consequence of the disease, while others may be preexisting. Many individuals become dysphoric as a function of the development of AUD or the use of other substances, i.e., the “dark side of addiction” described by Koob and colleagues (40, 41), whereas in others, preexisting dysphoria makes them vulnerable to alcohol use and AUD (42). Individuals with AUD, regardless of subtype, appear to be more anxious and dysphoric (43) and the present study found strong correlation between the Negative Emotionality factor and other variables. Increased sensitivity to incentive salience is another correlate of AUD. It is also possible that some individuals are predisposed to be less motivated for natural rewards, and thus more likely to pursue the exaggerated and immediate reward response to cues and context associated with drugs of abuse. Here, incentive salience is defined as motivation for rewards derived from both one’s physiological state and previously learned associations about a reward cue and is often linked to activation of the mesocorticolimbic dopamine system (44). Indeed, that depression and trait anxiety scores loaded more heavily onto the Incentive Salience factor than the (predicted) Negative Emotionality factor may be considered as evidence of a coupling between negative affect and craving for alcohol as addiction becomes severe, e.g., (7). In this model, craving becomes associated with negative emotions, i.e., shifting from “reward” craving to “relief” craving. Finally, the impact of chronic heavy alcohol consumption on executive function may also be quite profound, while preexisting executive dysfunction is also a known correlate of AUD (45). Thus, across all three domains, chronic alcohol consumption leading to AUD has the potential to induce negative emotional states, exaggerated salience for alcohol and related cues, and executive dysfunction.
These results must be interpreted in the context of the study’s strengths and limitations. Strengths include a well-characterized sample of individuals across the spectrum of alcohol use, including individuals seeking inpatient treatment for AUD, the use of measures with good psychometric properties, and that the factor structure was replicated across both halves of the randomly split sample. Limitations include that measures were not collected prospectively and may be biased by recollection. Furthermore, although study measures allowed us to recover the three factors hypothesized by the ANA, it is possible that additional factors would emerge if other more diverse measures were included. We note that measures hypothesized to represent the ANA Executive Function domain comprise a subset of measures related to cognitive control, rather than the broad capacities included in Executive Function (e.g., working memory, attention, response inhibition, planning, etc.), and delay discounting did not load onto this factor.
In summary, the neuroclinical assessment of addictions can capture important dimensions of neuropsychological functioning in individuals with varying levels of alcohol use and use disorders, and these domains are highly relevant to SUD. Future studies combining brain imaging and standardized measures for ANA will help refine our understanding of the relationship of these measures to neural circuits implicated in executive function, negative emotionality and incentive salience in SUD and other addictive disorders. Future studies will be needed to learn whether assessment of ANA domains can improve prevention or treatment, as already hinted by treatment studies of SUD.
Supplementary Material
Supplementary Figure 1. Three-dimensional plot of the factor scores for Negative Emotionality, Executive Function, and Incentive Salience, indicating the distribution of scores in participants with and without alcohol use disorder (AUD).
Supplementary Figure 2. ROC curves for each of the three latent domains predicting a diagnosis of AUD. AUC = area under the curve.
Acknowledgments
We acknowledge the Division of Intramural Clinical and Biological Research, National Institute on Alcohol Abuse and Alcoholism, including the 1SE Inpatient Behavioral Health Unit and the 1SE Outpatient Clinic. We thank Roshni Janakiraman and Caroline Grant for their assistance with the preparation of this manuscript.
Footnotes
Dr. Blanco owns stock in Sanofi, Eli Lilly, and General Electric. All other authors have no interests to disclose.
References
- 1.Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. The Lancet 2009;373:2223–2233. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad F, Rossen L, Spencer M, Warner M, Sutton P. Provisional Drug Overdose Death Counts. National Center for Health Statistics 2018.
- 3.Prevention CfDCa: Alcohol Related Disease Impact (ARDI) application. Available at www.cdc.gov/ARDI.2013.
- 4.Koob GF, Moal ML. Drug Abuse: Hedonic Homeostatic Dysregulation. Science 1997;278:52–58. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein Rita Z., Volkow Nora D.. Drug Addiction and Its Underlying Neurobiological Basis: Neuroimaging Evidence for the Involvement of the Frontal Cortex. American Journal of Psychiatry 2002;159:1642–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwako LE, Momenan R, Litten RZ, Koob GF, Goldman D. Addictions Neuroclinical Assessment: A Neuroscience-Based Framework for Addictive Disorders. Biological Psychiatry;80:179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology 2010;35:217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanco C, Rafful C, Wall MM, Ridenour TA, Wang S, Kendler KS. Towards a comprehensive developmental model of cannabis use disorders. Addiction (Abingdon, England) 2014;109:284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.García-Rodríguez O, Blanco C, Wall MM, Wang S, Jin CJ, Kendler KS. Toward a comprehensive developmental model of smoking initiation and nicotine dependence. Drug & Alcohol Dependence 2014;144:160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanco C, Hanania J, Petry NM, Wall MM, Wang S, Jin CJ, Kendler KS. Towards a comprehensive developmental model of pathological gambling. Addiction (Abingdon, England) 2015;110:1340–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muthén LK, Muthén BO: Mplus User’s Guide Seventh Edition. Los Angeles, CA, Muthén & Muthén; 1998–2015. [Google Scholar]
- 12.Browne MW. An Overview of Analytic Rotation in Exploratory Factor Analysis. Multivariate Behavioral Research 2001;36:111–150. [Google Scholar]
- 13.Hu LT, Bentler PM, Kano Y. Can test statistics in covariance structure analysis be trusted? Psychol Bull 1992;112:351–362. [DOI] [PubMed] [Google Scholar]
- 14.Jöreskog KG, Goldberger AS. Estimation of a Model with Multiple Indicators and Multiple Causes of a Single Latent Variable. Journal of the American Statistical Association 1975;70:631–639. [Google Scholar]
- 15.Stark S, Chernyshenko OS, Drasgow F. Detecting differential item functioning with confirmatory factor analysis and item response theory: toward a unified strategy. J Appl Psychol 2006;91:1292–1306. [DOI] [PubMed] [Google Scholar]
- 16.Litten RZ, Ryan ML, Falk DE, Reilly M, Fertig JB, Koob GF. Heterogeneity of alcohol use disorder: understanding mechanisms to advance personalized treatment. Alcohol Clin Exp Res 2015;39:579–584. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Serrano MJ, Perez-Garcia M, Schmidt Rio-Valle J, Verdejo-Garcia A. Neuropsychological consequences of alcohol and drug abuse on different components of executive functions. J Psychopharmacol 2010;24:1317–1332. [DOI] [PubMed] [Google Scholar]
- 18.Sinha R, Fox HC, Hong KI, Hansen J, Tuit K, Kreek MJ. Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Arch Gen Psychiatry 2011;68:942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mann K, Vollstädt‐Klein S, Reinhard I, Leménager T, Fauth‐Bühler M, Hermann D, Hoffmann S, Zimmermann US, Kiefer F, Heinz A, Smolka MN. Predicting Naltrexone Response in Alcohol‐Dependent Patients: The Contribution of Functional Magnetic Resonance Imaging. Alcoholism: Clinical and Experimental Research 2014;38:2754–2762. [DOI] [PubMed] [Google Scholar]
- 20.Mann K, Roos CR, Hoffmann S, Nakovics H, Leménager T, Heinz A, Witkiewitz K. Precision Medicine in Alcohol Dependence: A Controlled Trial Testing Pharmacotherapy Response Among Reward and Relief Drinking Phenotypes. Neuropsychopharmacology 2017;43:891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koob GF. A Role for Brain Stress Systems in Addiction. Neuron 2008;59:11–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S, Im S, Lee J, Lee S-G. Disrupted Control Network Connectivity in Abstinent Patients with Alcohol Dependence. Psychiatry Investigation 2017;14:325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Volkow ND, Wang G-J, Fowler JS, Tomasi D, Telang F. Addiction: Beyond dopamine reward circuitry. Proceedings of the National Academy of Sciences 2011;108:15037–15042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldstein RZ, Leskovjan AC, Hoff AL, Hitzemann R, Bashan F, Khalsa SS, Wang G-J, Fowler JS, Volkow ND. Severity of neuropsychological impairment in cocaine and alcohol addiction: association with metabolism in the prefrontal cortex. Neuropsychologia 2004;42:1447–1458. [DOI] [PubMed] [Google Scholar]
- 25.Volkow ND, Wang G-J, Telang F, Fowler JS, Logan J, Jayne M, Ma Y, Pradhan K, Wong C. Profound Decreases in Dopamine Release in Striatum in Detoxified Alcoholics: Possible Orbitofrontal Involvement. The Journal of Neuroscience 2007;27:12700–12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Volkow ND, Wang G, Begleiter H, et al. High levels of dopamine d2 receptors in unaffected members of alcoholic families: Possible protective factors. Archives of General Psychiatry 2006;63:999–1008. [DOI] [PubMed] [Google Scholar]
- 27.Glaser YG, Zubieta J-K, Hsu DT, Villafuerte S, Mickey BJ, Trucco EM, Burmeister M, Zucker RA, Heitzeg MM. Indirect Effect of Corticotropin-Releasing Hormone Receptor 1 Gene Variation on Negative Emotionality and Alcohol Use via Right Ventrolateral Prefrontal Cortex. The Journal of Neuroscience 2014;34:4099–4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blanco C, Krueger RF, Hasin DS, et al. Mapping common psychiatric disorders: Structure and predictive validity in the national epidemiologic survey on alcohol and related conditions. JAMA Psychiatry 2013;70:199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koob G, Bloom F. Cellular and molecular mechanisms of drug dependence. Science 1988;242:715–723. [DOI] [PubMed] [Google Scholar]
- 30.Volkow ND, Fowler JS. Addiction, a Disease of Compulsion and Drive: Involvement of the Orbitofrontal Cortex. Cerebral Cortex 2000;10:318–325. [DOI] [PubMed] [Google Scholar]
- 31.Goldman D, Bergen A. General and specific inheritance of substance abuse and alcoholism. Arch Gen Psychiatry 1998;55:964–965. [DOI] [PubMed] [Google Scholar]
- 32.Blanco C, Okuda M, Wang S, Liu S, Olfson M. testing the drug substitution switchingaddictions hypothesis: A prospective study in a nationally representative sample. JAMA Psychiatry 2014;71:1246–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blanco C, Wall MM, Wang S, Olfson M. Examining heterotypic continuity of psychopathology: a prospective national study. Psychological Medicine 2017;47:2097–2106. [DOI] [PubMed] [Google Scholar]
- 34.Ducci F, Enoch M-A, Funt S, Virkkunen M, Albaugh B, Goldman D. Increased anxiety and other similarities in temperament of alcoholics with and without antisocial personality disorder across three diverse populations. Alcohol 2007;41:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saremi A, Hanson RL, Williams DE, Roumain J, Robin RW, Long JC, Goldman D, Knowler WC. Validity of the CAGE questionnaire in an American Indian population. Journal of Studies on Alcohol 2001;62:294–300. [DOI] [PubMed] [Google Scholar]
- 36.Sher KJ, Grekin ER, Williams NA. The Development of Alcohol Use Disorders. Annual Review of Clinical Psychology 2005;1:493–523. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan EV, Brumback T, Tapert SF, Fama R, Prouty D, Brown SA, Cummins K, Thompson WK, Colrain IM, Baker FC, De Bellis MD, Hooper SR, Clark DB, Chung T, Nagel BJ, Nichols BN, Rohlfing T, Chu W, Pohl KM, Pfefferbaum A. Cognitive, emotion control, and motor performance of adolescents in the NCANDA study: Contributions from alcohol consumption, age, sex, ethnicity, and family history of addiction. Neuropsychology 2016;30:449–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Enoch M-A, Hodgkinson CA, Yuan Q, Shen P-H, Goldman D, Roy A. The Influence of GABRA2, Childhood Trauma, and Their Interaction on Alcohol, Heroin, and Cocaine Dependence. Biological Psychiatry;67:20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, Anda RF. Adverse childhood experiences and the risk of depressive disorders in adulthood. Journal of Affective Disorders;82:217–225. [DOI] [PubMed] [Google Scholar]
- 40.Schulteis G, Koob G. Dark side of drug dependence. Nature 1994;371:108. [DOI] [PubMed] [Google Scholar]
- 41.Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nature Neuroscience 2005;8:1442. [DOI] [PubMed] [Google Scholar]
- 42.Bravo AJ, Pearson MR, Henson JM. Drinking to Cope With Depressive Symptoms and Ruminative Thinking: A Multiple Mediation Model Among College Students. Substance Use & Misuse 2017;52:52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goldman D, Ducci F. Deconstruction of Vulnerability to Complex Diseases: Enhanced Effect Sizes and Power of Intermediate Phenotypes. The Scientific World Journal 2007;7. [DOI] [PMC free article] [PubMed]
- 44.Berridge KC. From prediction error to incentive salience: mesolimbic computation of reward motivation. European Journal of Neuroscience 2012;35:1124–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gierski F, Hubsch B, Stefaniak N, Benzerouk F, Cuervo‐Lombard C, Bera‐Potelle C, Cohen R, Kahn JP, Limosin F. Executive Functions in Adult Offspring of Alcohol‐Dependent Probands: Toward a Cognitive Endophenotype? Alcoholism: Clinical and Experimental Research 2013;37:E356–E363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Three-dimensional plot of the factor scores for Negative Emotionality, Executive Function, and Incentive Salience, indicating the distribution of scores in participants with and without alcohol use disorder (AUD).
Supplementary Figure 2. ROC curves for each of the three latent domains predicting a diagnosis of AUD. AUC = area under the curve.