Abstract
OBJECTIVE
The authors sought to investigate the incidence and predictors of venous thromboembolic events (VTEs) after craniotomy for tumor resection, which are not well established, and the efficacy of and risks associated with VTE chemoprophylaxis, which remains controversial.
METHODS
The authors investigated the incidence of VTEs in a consecutive series of patients presenting to the authors’ institution for resection of an intracranial lesion between 2012 and 2017. Information on patient and tumor characteristics was collected and independent predictors of VTEs were determined using stepwise multivariate logistic regression analysis. Review of the literature was performed by searching MEDLINE using the keywords “venous thromboembolism,” “deep venous thrombosis,” “pulmonary embolism,” “craniotomy,” and “brain neoplasms.”
RESULTS
There were 1622 patients included for analysis. A small majority of patients were female (52.6%) and the mean age of the cohort was 52.9 years (SD 15.8 years). A majority of intracranial lesions were intraaxial (59.3%). The incidence of VTEs was 3.0% and the rates of deep venous thromboses and pulmonary emboli were 2.3% and 0.9%, respectively. On multivariate analysis, increasing patient age (unit OR 1.02, 95% CI 1.00–1.05; p = 0.018), history of VTE (OR 7.26, 95% CI 3.24–16.27; p < 0.001), presence of motor deficit (OR 2.64, 95% CI 1.43–4.88; p = 0.002), postoperative intracranial hemorrhage (OR 4.35, 95% CI 1.51–12.55; p < 0.001), and prolonged intubation or reintubation (OR 3.27, 95% CI 1.28–8.32; p < 0.001) were independently associated with increased odds of a VTE. There were 192 patients who received VTE chemoprophylaxis (11.8%); the mean postoperative day of chemoprophylaxis initiation was 4.6 (SD 3.8). The incidence of VTEs was higher in patients receiving chemoprophylaxis than in patients not receiving chemoprophylaxis (8.3% vs 2.2%; p < 0.001). There were 30 instances of clinically significant postoperative hemorrhage (1.9%), with only 1 hemorrhage occurring after initiation of VTE chemoprophylaxis (0.1%).
CONCLUSIONS
The study results show the incidence and predictors of VTEs after craniotomy for tumor resection in this patient population. The incidence of VTE within this cohort appears low and comparable to that observed in other institutional series, despite the lack of routine prophylactic anticoagulation in the postoperative setting.
Keywords: brain neoplasms, chemoprevention, deep venous thrombosis, neurosurgery, pulmonary embolism, venous thromboembolism, vascular disorders
VENOUS thromboembolic events (VTEs) are a known complication of intracranial tumor surgery, though the reported incidence of VTEs in recent literature is variable.1,10,21,39,49 Given the morbidity and potential mortality associated with VTEs,37 as well as the need for subsequent therapeutic anticoagulation, there is significant clinical interest in identifying patient risk factors for VTEs, as well as in developing optimal VTE prevention strategies.
The results of recent systematic reviews have suggested that while prophylactic anticoagulation administered in the postoperative setting may be protective against VTEs,3,5,24,44 the cost-effectiveness of chemoprophylaxis in addition to mechanical prophylaxis for a general neurosurgical population is less certain,3 in part because of a potentially increased risk of significant postoperative bleeding.24,44 At our institution, patients undergoing craniotomy for tumor resection do not routinely receive prophylactic anticoagulation after surgery. Given that this practice is at odds with that of other institutions as reported in recent large retrospective series,1,10,26,41 we assessed the relative efficacy of our VTE prevention strategy by reviewing the clinical course of patients undergoing intracranial tumor surgery over a 5-year period to determine the incidence and predictors of VTEs at our institution. We also collected information on the use of prophylactic anticoagulation, when administered, to gain insight into the safety and efficacy of this intervention. Finally, we performed a review of available literature to place our practice habits and results into a broader context.
Methods
Patient Selection
After receiving approval from our institutional review board (IRB no. 15–001684), we retrospectively reviewed the outcomes of patients undergoing intracranial tumor surgery at our institution from January 2012 to June 2017. Patients undergoing stereotactic or open biopsy and transsphenoidal procedures for pituitary or other sellar tumors were excluded as these patients were typically discharged on postoperative day 1, which was in contrast to the more prolonged inpatient course of most patients undergoing intracranial tumor surgery. Given the tendency for very early mobilization and discharge in this excluded subgroup, it was felt that the inclusion of a large number of these patients would bias our study toward lower VTE rates. In addition, patients undergoing anatomical resection for epilepsy were also excluded given the frequent need for invasive intracranial monitoring requiring bed rest prior to lesional resection.
Institutional Protocol for VTE Prevention
In general, patients undergoing intracranial tumor surgery at our institution receive mechanical VTE prophylaxis in the form of sequential pneumatic compression devices during and after surgery unless contraindicated. Aggressive mobilization is pursued starting on postoperative day 1. Patients do not routinely receive prophylactic anticoagulation, which is administered at the discretion of the consultant neurosurgeon when deemed clinically indicated. In patients receiving prophylactic anticoagulation, specific regimens included subcutaneous heparin (low-molecular-weight heparin [LMWH]) 5000 units either 2 or 3 times daily or LMWH (enoxaparin) 30 mg twice daily or 40 mg daily. Dosages of oral anticoagulants were determined by the clinical pharmacist based on previous regimens and clinical circumstances. Screening ultrasounds were not ordered routinely and instead obtained when there was clinical suspicion of thrombosis. The number of patients who received screening ultrasounds of the upper or lower extremities during their inpatient admission was recorded.
Outcomes and Variables of Interest
The primary outcome of interest was a VTE in the form of an acute deep venous thrombosis (DVT) or pulmonary embolism (PE) within 30 days of surgery. DVTs and PEs were diagnosed on upper- or lower-extremity ultrasound or chest CT angiography studies, respectively. The postoperative day of VTE diagnosis and whether the VTE was discovered in an inpatient versus outpatient setting were also noted. A secondary outcome of interest was clinically significant intracranial hemorrhage (ICH) in the postoperative period. Clinically significant hemorrhage was defined as a hemorrhage requiring surgical evacuation or one resulting in worsening of neurological status.
Information collected on patient and tumor characteristics included patient age, sex, BMI, prior history of VTE, Karnofsky Performance Scale (KPS) score, presence of pre- or postoperative motor deficit, chronic corticosteroid use, preoperative use of antiplatelet or anticoagulation medication, elective or nonelective admission, history of prior craniotomy, supratentorial versus infratentorial and intraaxial versus extraaxial tumor location, tumor pathology and grade, administration and type of prophylactic anticoagulation agent and day of initiation, intubation for longer than 24 hours or reintubation after extubation in the postoperative period, inpatient length of stay, and discharge location. KPS score was determined by review of the neurological and neurosurgical preoperative evaluation; patient scores were dichotomized as greater than or equal to or less than 80. Pre- or postoperative motor deficit was defined as weakness of 4 out of 5 or below measured on the manual muscle testing scale in at least one upper or lower extremity. Preoperative use of either antiplatelet or anticoagulation medication was noted; in general, these medications were stopped at least 1 week prior to surgery and resumed postoperatively at the discretion of the treating neurosurgeon. Tumor pathology was categorized as belonging to one of the following groups: glioma, schwannoma or meningioma, metastatic lesion, or other. Discharge location was defined as home, inpatient rehabilitation facility, skilled nursing facility, or death during admission.
Literature Review
We searched MEDLINE using the medical subject headings “venous thromboembolism,” “deep venous thrombosis,” or “pulmonary embolism,” AND “craniotomy” or “brain neoplasms.” This search yielded a total of 1049 articles, which were screened by title and abstract for publications investigating the incidence of postoperative VTEs in patients undergoing elective craniotomy. Articles passed the initial screen if the population of interest included patients undergoing elective craniotomy for intracranial lesions and data were available on the incidence of VTE, use of prophylactic anticoagulation, and whether patients were screened for DVTs during admission. Articles examining the incidence of VTEs in patients undergoing nonelective craniotomy for trauma, minor intracranial procedures (e.g., ventriculostomy, ventricular shunting, stereotactic biopsy, or transsphenoidal surgery), or spinal neurosurgery were included only if the incidence of VTEs and frequency of prophylactic anticoagulation administration for patients undergoing elective craniotomy were detailed separately. An exception was made to allow the inclusion of 2 randomized clinical trials that did not differentiate VTE rates between cranial and spinal patients,2,37 because these studies presented a high level of evidence and spinal patients represented a small minority of the overall population. Articles that were unavailable, had incomplete data, or were in a foreign language were excluded, as were reviews, case reports, and case series with fewer than 10 patients. The initial screen identified 86 potential articles of interest. These articles were closely reviewed for exclusion criteria, ultimately yielding 33 articles of interest. Information collected from these articles included year of publication, study design, population of interest, sample size, incidence of VTEs and whether patients were screened for DVTs during admission, rate of prophylactic anticoagulation administration, regimen of prophylactic anticoagulation and timing of postoperative initiation, whether the study reported efficacy of prophylactic anticoagulation in preventing VTEs, incidence of ICH, and observed risk factors for VTEs. Recorded sample size for each study included only patients undergoing elective craniotomy. Efficacy of prophylactic anticoagulation was based on the study authors’ interpretation of results. Risk factors for VTEs were noted only if they were found to be independently associated with VTEs on multivariate analysis.
Statistical Analysis
Descriptive statistics were reported as a frequency and percentage for categorical variables and mean, standard deviation, and range for continuous variables. Comparative statistics were performed using the Student t-test or Pearson’s chi-square test, when appropriate, or one-way ANOVA. Predictors of VTE were identified using logistic regression analysis. Variables associated with VTEs on univariate analysis with a p value of ≤ 0.100 were included in a stepwise multivariate logistic regression model. Using a backward elimination method, variables were removed in a stepwise fashion according to strength of association as determined by p value until only variables significantly associated with VTEs remained. Administration of prophylactic anticoagulation was not included as a variable in univariate or multivariate logistic regression given the clear selection bias affecting which patients received this treatment, i.e., in our cohort patients at high risk for VTEs were far more likely to be treated with anticoagulation than were patients perceived to be at lower risk (see Results). For articles included in the literature review, the relationship between year of publication and rate of VTEs was assessed using linear regression analysis, with results reported as a correlation coefficient (r2) and p value. VTE rates were weighted according to study sample size in linear regression models. The alpha level for statistical significance was set at 0.05. Statistical analyses were performed using commercially available software (JMP 10.0.0, 2012 SAS Institute Inc.).
A power analysis was performed to determine necessary sample size in a theoretical randomized clinical trial aimed at determining the efficacy of prophylactic anticoagulation in preventing VTEs. For this analysis, desired power was set at 90.0%. The alpha level for statistical significance using a one-sided test was set at 0.05. All calculations were performed using the software EAST 6.4, 2018 (Cytel).
Results
Patient Characteristics and Outcomes
There were 1622 patients who met criteria for inclusion. The mean patient age was 52.9 years (SD 15.8), and a majority of patients were female (52.9%). There were 50 patients with a previous history of a VTE (3.1%), 25 of whom (50.0%) were on anticoagulation medication prior to surgery. In total, there were 64 patients who were taking anticoagulation medication prior to surgery. A majority of tumors were supratentorial (84.0%) and intraaxial (59.4%). The most common tumor pathology was a glial-based tumor (48.3%), followed by schwannoma or meningioma (35.1%). The most common pathological grade was 1 (35.5%). There were 328 patients (20.2%) with a preoperative or new postoperative motor deficit.
There were 137 patients (8.4%) who received an ultra-sound due to clinical suspicion of VTE. A VTE occurred in 48 patients (3.0%), with 38 and 14 patients suffering a DVT and PE (0.9%), respectively. There were 4 patients who suffered both a DVT and PE (0.2%). The mean postoperative day of VTE diagnosis was 13.1 (SD 9.5); the mean postoperative days of diagnosis for VTEs discovered in the inpatient and outpatient settings were 5.4 (SD 4.3) and 18.6 (SD 8.3), respectively. Prophylactic anticoagulation was administered in 192 patients (11.8%), with a majority of these patients receiving LMWH (57.3%). The mean day of anticoagulation initiation was 4.6 (SD 3.8). A postoperative hemorrhage occurred in 30 patients (1.9%); there was only 1 hemorrhage that occurred after initiation of anticoagulation. In this patient, the hemorrhage occurred 8 days after the initiation of warfarin for a history of VTE prior to surgery. The mean length of stay was 3.8 days (SD 4.5 days), and a majority of patients were discharged to home (80.0%). A complete list of patient characteristics and outcomes is presented in Table 1.
TABLE 1.
Patient characteristics and outcomes
| Variable | Value |
|---|---|
| No. of patients | 1622 |
| Age in yrs, mean (SD) | 52.9 (15.8) |
| Range | (18–89) |
| Sex | |
| Male | 769 (47.4) |
| Female | 853 (52.6) |
| BMI in kg/m2 | 28.9 (6.2) |
| Range | (14.6–59.8) |
| History of VTE | 50 (3.1) |
| KPS score <80 | 281 (17.3) |
| Preop motor deficit | 212 (13.1) |
| Chronic steroid use | 544 (33.5) |
| Preop antiplatelet use | 336 (20.7) |
| Preop anticoagulation | 64 (3.9) |
| Admission type | |
| Elective | 1502 (92.6) |
| Nonelective | 120 (7.4) |
| Revision craniotomy | 317 (19.5) |
| Location | |
| Supratentorial | 1362 (84.0) |
| Infratentorial | 260 (16.0) |
| Intraaxial | 963 (59.4) |
| Extraaxial | 659 (40.6) |
| Pathology | |
| Glial-based tumor | 784 (48.3) |
| Schwannoma/meningioma | 570 (35.1) |
| Metastatic lesion | 108 (6.7) |
| Other | 160 (9.9) |
| Pathological grade* | |
| 1 | 568 (35.5) |
| 2 | 270 (16.9) |
| 3 | 247 (15.4) |
| 4 | 516 (32.2) |
| New postop motor deficit | 165 (10.1) |
| Inpatient extremity ultrasound | 137 (8.4) |
| VTE | 48 (3.0) |
| Inpatient VTE | 20 (1.2) |
| Outpatient VTE | 28 (1.7) |
| DVT | 38 (2.3) |
| PE | 14 (0.9) |
| DVT & PE | 4 (0.2) |
| Day of VTE diagnosis | 13.1 (9.5) |
| Inpatient VTE | 5.4 (4.3) |
| Range | (0–18) |
| Outpatient VTE | 18.6 (8.3) |
| Range | (6–30) |
| Prophylactic anticoagulation | 192 (11.8) |
| Subcutaneous heparin | 77 (40.1)† |
| Subcutaneous LMWH | 110 (57.3)† |
| Oral anticoagulant | 5 (2.6)† |
| Day of chemoprophylaxis initiation | 4.6 (3.8) |
| Range | (0–22) |
| Postop ICH | 30 (1.9) |
| ICH after DVT chemoprophylaxis | 1 (0.5)† |
| Intubated >24 hr/reintubated | 58 (3.6) |
| Mean LOS in days | 3.8 (4.5) |
| Range | (1–119) |
| Discharge location | |
| Home | 1298 (80.0) |
| Inpatient rehabilitation | 240 (14.8) |
| Skilled nursing facility | 82 (5.1) |
| Inpatient mortality | 2 (0.1) |
LOS = length of stay.
Values are presented as number of patients (%) unless otherwise indicated.
There were 21 lesions that were not assigned a pathological grade.
Denominator for percentage was number of patients receiving VTE chemoprophylaxis.
Patient Characteristics Associated With Incidence of VTE
Patient and tumor characteristics of patients who did and those who did not suffer a VTE were compared. Patients with a VTE were older than patients without a VTE (60.1 vs 52.7 years; p = 0.001). Patients with a VTE were more likely to have a history of a VTE than patients without a VTE (20.2% vs 2.5%; p < 0.001). Patients with a VTE were more likely to have a low KPS score (31.2% vs 16.9%; p = 0.001) and to have a motor deficit (45.8% vs 19.4%; p < 0.001). Patients with a VTE were also more likely to be chronic steroid users (50.0% vs 33.0%; p = 0.014) and were more likely to be taking antiplatelet (33.3% vs 20.3%; p = 0.029) and anticoagulation medication (16.7% vs 3.6%; p < 0.001) prior to surgery. VTEs were more common in patients who suffered a postoperative ICH (12.5% vs 1.5%; p < 0.001) and in patients who were treated with prophylactic anticoagulation (33.3% vs 11.2%; p < 0.001). VTEs were also more common in patients with prolonged intubation or reintubation after extubation (14.6% vs 3.2%; p < 0.001). The mean length of stay of patients with a VTE was longer than that of patients without a VTE (7.0 vs 3.7 days; p < 0.001), and patients with a VTE were less likely to discharge to home (56.3% vs 80.8%; p < 0.001). The complete results of comparisons between patients with and without a VTE are visible in Table 2.
TABLE 2.
Comparison of patients with and those without VTE
| Variable | No VTE (n = 1574) | VTE (n = 48) | p Value |
|---|---|---|---|
| Age in yrs, mean (SD) | 52.7 (15.9) | 60.1 (11.9) | 0.001 |
| Range | (18–89) | (33–79) | |
| Sex | |||
| Male | 743 (47.2) | 22 (45.8) | |
| Female | 831 (52.8) | 26 (54.2) | 0.341 |
| BMI in kg/m2 | 28.8 (6.2) | 29.7 (6.5) | 0.353 |
| Range | (14.6–59.8) | (20.7–47.8) | |
| History of VTE | |||
| No | 1534 (97.5) | 38 (79.2) | |
| Yes | 40 (2.5) | 10 (20.2) | <0.001 |
| KPS score <80 | |||
| No | 1308 (83.1) | 33 (68.8) | |
| Yes | 266 (16.9) | 15 (31.2) | 0.001 |
| Pre- or postop motor deficit | |||
| No | 1268 (80.6) | 26 (54.2) | |
| Yes | 306 (19.4) | 22 (45.8) | <0.001 |
| Chronic steroid use | |||
| No | 1054 (67.0) | 24 (50.0) | |
| Yes | 520 (33.0) | 24 (50.0) | 0.014 |
| Preop antiplatelet use | |||
| No | 1254 (79.7) | 32 (66.7) | |
| Yes | 320 (20.3) | 16 (33.3) | 0.029 |
| Preop anticoagulation | |||
| No | 1518 (96.4) | 40 (83.3) | |
| Yes | 56 (3.6) | 8 (16.7) | <0.001 |
| Admission type | |||
| Elective | 1461 (92.8) | 41 (85.4) | |
| Nonelective | 113 (7.2) | 7 (14.6) | 0.054 |
| Revision craniotomy | |||
| No | 1267 (80.5) | 38 (79.2) | |
| Yes | 307 (19.5) | 10 (20.8) | 0.819 |
| Location | |||
| Supratentorial | 1325 (84.2) | 37 (77.1) | |
| Infratentorial | 249 (15.8) | 11 (22.9) | 0.189 |
| Intraaxial | 936 (59.5) | 27 (56.3) | |
| Extraaxial | 638 (40.5) | 21 (43.7) | 0.655 |
| Pathology | |||
| Glial-based tumor | 765 (48.6) | 19 (39.6) | |
| Schwannoma/meningioma | 554 (34.2) | 16 (33.3) | |
| Metastatic lesion | 102 (6.5) | 6 (12.5) | |
| Other | 153 (9.7) | 7 (14.6) | 0.214 |
| Pathological grade* | |||
| 1 | 554 (35.7) | 14 (29.2) | |
| 2 | 262 (16.9) | 8 (16.7) | |
| 3 | 240 (15.5) | 7 (14.6) | |
| 4 | 497 (32.0) | 19 (39.6) | 0.703 |
| Postop ICH | |||
| No | 1550 (98.5) | 42 (87.5) | |
| Yes | 24 (1.5) | 6 (12.5) | <0.001 |
| Prophylactic anticoagulation | |||
| No | 1398 (88.8) | 32 (66.7) | |
| Yes | 176 (11.2) | 16 (33.3) | <0.001 |
| Intubated >24 hrs/reintubated | |||
| No | 1523 (96.8) | 41 (85.4) | |
| Yes | 51 (3.2) | 7 (14.6) | <0.001 |
| LOS in days | 3.7 (4.3) | 7.0 (7.9) | <0.001 |
| Range | (1–119) | (1–46) | |
| Discharge location† | |||
| Home | 1271 (80.8) | 27 (56.3) | |
| Inpatient rehabilitation | 223 (14.2) | 17 (35.4) | |
| Skilled nursing facility | 78 (5.0) | 4 (8.3) | <0.001 |
Values are presented as number of patients (%) unless otherwise indicated.
Boldface type indicates statistical significance.
There were 21 lesions that were not assigned a pathological grade.
The 2 patients who died during their hospital admission were excluded from this analysis.
Characteristics of Patients Receiving Prophylactic Anticoagulation
Given the strong association of prophylactic anticoagulation administration with incidence of VTEs (OR 3.97, 95% CI 2.14–7.38; p < 0.001), we performed a comparison between patients who did and did not receive anticoagulation. There were a number of differences in baseline patient characteristics between patients who did and did not receive prophylactic anticoagulation, including older age (56.3 vs 52.4 years; p = 0.002), higher frequency of VTE history (40.0% vs 2.1%; p < 0.001) and preoperative anticoagulation use (10.4% vs 3.1%; p < 0.001), poor KPS score (35.4% vs 14.9%; p < 0.001), and motor deficit (48.4% vs 16.4%; p < 0.001), which occurred more frequently in patients receiving prophylactic anticoagulation. Patients receiving prophylactic anticoagulation were also more likely to have suffered a postoperative ICH (4.7% vs 1.5%; p = 0.002) and to be intubated for more than 24 hours or be reintubated (9.9% vs 2.7%; p < 0.001). Patients receiving prophylactic anticoagulation had longer lengths of stay (8.1 vs 3.2 days; p < 0.001) and were much less likely to be discharged home (30.4% vs 86.8%; p < 0.001). These findings are summarized in Table 3.
TABLE 3.
Comparison of patients who did and those who did not receive prophylactic anticoagulation
| Variable | No Prophylaxis (n = 1430) | Prophylaxis (n = 192) | p Value |
|---|---|---|---|
| Age in yrs, mean (SD) | 52.4 (15.9) | 56.3 (14.6) | 0.002 |
| Range | (18–89) | (18–84) | |
| Sex | |||
| Male | 681 (47.6) | 88 (45.8) | |
| Female | 749 (52.4) | 104 (54.2) | 0.641 |
| BMI in kg/m2 | 28.8 (6.1) | 29.5 (7.0) | 0.124 |
| Range | (14.6–59.8) | (16.3–57.5) | |
| History of VTE | |||
| No | 1400 (97.9) | 30 (60.0) | |
| Yes | 30 (2.1) | 20 (40.0) | <0.001 |
| KPS score <80 | |||
| No | 1217 (85.1) | 124 (64.6) | |
| Yes | 213 (14.9) | 68 (35.4) | <0.001 |
| Pre- or postop motor deficit | |||
| No | 1195 (83.6) | 99 (51.6) | |
| Yes | 235 (16.4) | 93 (48.4) | <0.001 |
| Chronic steroid use | |||
| No | 956 (66.9) | 122 (63.5) | |
| Yes | 474 (33.1) | 70 (36.5) | 0.362 |
| Preop antiplatelet use | |||
| No | 1139 (79.7) | 147 (76.6) | |
| Yes | 291 (20.3) | 45 (23.4) | 0.322 |
| Preop anticoagulant use | |||
| No | 1386 (96.9) | 172 (89.6) | |
| Yes | 44 (3.1) | 20 (10.4) | <0.001 |
| Admission type | |||
| Elective | 1330 (93.0) | 172 (89.6) | |
| Nonelective | 100 (7.0) | 20 (10.4) | 0.088 |
| Revision craniotomy | |||
| No | 1159 (81.1) | 146 (76.0) | |
| Yes | 271 (18.9) | 46 (24.0) | 0.100 |
| VTE | |||
| No | 1398 (97.8) | 176 (91.7) | |
| Yes | 32 (2.2) | 16 (8.3) | <0.001 |
| Postop ICH | |||
| No | 1409 (98.5) | 183 (95.3) | |
| Yes | 21 (1.5) | 9 (4.7) | 0.002 |
| Intubated >24 hrs/reintubated | |||
| No | 1391 (97.3) | 173 (90.1) | |
| Yes | 39 (2.7) | 19 (9.9) | <0.001 |
| LOS in days | 3.2 (2.2) | 8.1 (10.8) | <0.001 |
| Discharge location* | |||
| Home | 1240 (86.8) | 58 (30.4) | |
| Inpatient rehabilitation | 131 (9.2) | 109 (57.1) | |
| Skilled nursing facility | 58 (4.1) | 24 (12.6) | <0.001 |
Values are presented as number of patients (%) unless otherwise indicated.
Boldface type indicates statistical significance.
The 2 patients who died during their hospital admission were excluded from this analysis.
Logistic Regression Analysis Identifying Predictors of VTE
On univariate analysis, increasing patient age (unit OR 1.03, 95% CI 1.01–1.05; p = 0.002), history of VTE (OR 10.09, 95% CI 4.70–21.67; p < 0.001), poor KPS score (OR 2.24, 95% CI 1.20–4.17; p = 0.012), preoperative use of antiplatelets (OR 1.96, 95% CI 1.06–3.62; p = 0.031) or anticoagulation (OR 5.42, 95% CI 2.42–12.12; p < 0.001), presence of motor deficit (OR 3.51, 95% CI 1.96–6.27; p < 0.001), chronic steroid use (OR 2.03, 95% CI 1.14–3.60; p = 0.016), postoperative ICH (OR 9.23, 95% CI 3.58–23.75; p < 0.001), and prolonged intubation or reintubation (OR 5.10, 95% CI 2.18–11.91; p < 0.001) were associated with increased odds of a VTE (Table 4).
TABLE 4.
Results of univariate logistic regression analysis indicating predictors of VTE
| Variable | OR (95% CI) | p Value |
|---|---|---|
| Age | 1.03 (1.01–1.05)* | 0.002 |
| Female | 0.76 (0.43–1.35) | 0.343 |
| BMI | 1.02 (0.98–1.07)* | 0.352 |
| History of VTE | 10.09 (4.70–21.67) | <0.001 |
| KPS score <80 | 2.24 (1.20–4.17) | 0.012 |
| Pre- or postop motor deficit | 3.51 (1.96–6.27) | <0.001 |
| Chronic steroid use | 2.03 (1.14–3.60) | 0.016 |
| Preop antiplatelet use | 1.96 (1.06–3.62) | 0.031 |
| Preop anticoagulation | 5.42 (2.42–12.12) | <0.001 |
| Nonelective admission | 2.21 (0.97–5.03) | 0.060 |
| Revision craniotomy | 1.09 (0.54–2.20) | 0.819 |
| Supratentorial location | 0.63 (0.32–1.26) | 0.190 |
| Intraaxial location | 0.88 (0.49–1.56) | 0.655 |
| Pathology | ||
| Glial-based tumor | 0.86 (0.44–1.69) | 0.661 |
| Schwannoma/meningioma | Reference | — |
| Metastatic lesion | 2.04 (0.78–5.33) | 0.147 |
| Other | 1.58 (0.64–3.91) | 0.320 |
| Grade | ||
| 1 | Reference | — |
| 2 | 1.21 (0.50–2.92) | 0.674 |
| 3 | 1.15 (0.46–2.90) | 0.760 |
| 4 | 1.51 (0.75–3.05) | 0.247 |
| Postop ICH | 9.23 (3.58–23.75) | <0.001 |
| Intubated >24 hr/reintubated | 5.10 (2.18–11.91) | <0.001 |
Boldface type indicates statistical significance.
Unit OR, denoting increase in odds for every 1 unit increase in variable.
On multivariate analysis, increasing patient age (unit OR 1.02, 95% CI 1.00–1.05; p = 0.018), history of VTE (OR 7.26, 95% CI 3.24–16.27; p < 0.001), presence of motor deficit (OR 2.64, 95% CI 1.43–4.88; p = 0.002), postoperative ICH (OR 4.35, 95% CI 1.51–12.55; p < 0.001), and prolonged intubation or reintubation (OR 3.27, 95% CI 1.28–8.32; p < 0.001) were independently associated with increased odds of a VTE (Table 5).
TABLE 5.
Multivariate stepwise logistic regression analysis indicating independent predictors of VTE
| Variable | OR (95% CI) | p Value | C Statistic |
|---|---|---|---|
| Age | 1.02 (1.00–1.05)* | 0.018 | |
| History of VTE | 7.26 (3.24–16.27) | <0.001 | |
| Pre- or postop motor deficit | 2.64 (1.43–4.88) | 0.002 | |
| Postop ICH | 4.35 (1.51–12.55) | 0.007 | |
| Intubated >24 hrs/reintubated | 3.27 (1.28–8.32) | 0.013 | 0.769 |
Boldface type indicates statistical significance.
Unit OR, denoting increase in odds for every 1 unit increase in variable.
Literature Review
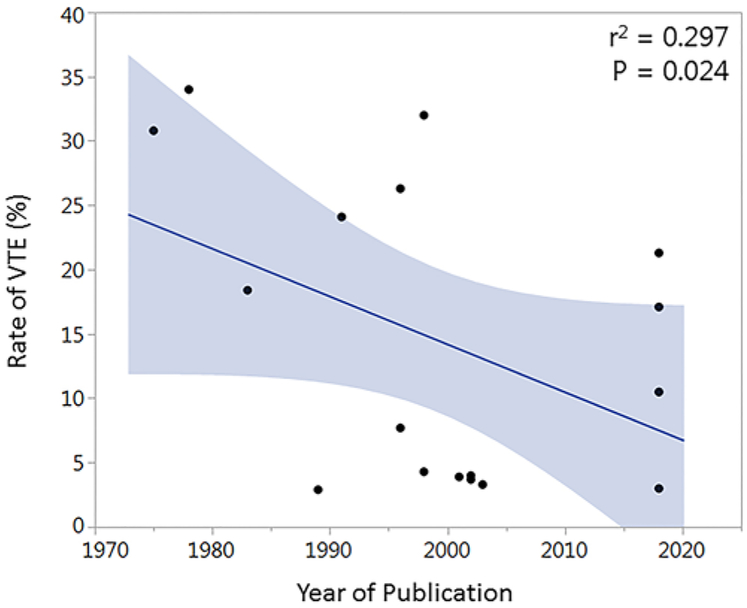
There were 33 articles of interest identified through a search of the literature. Data from included articles are presented in Table 6. Articles were published between the years 1975 and 2018 and included 10 randomized clinical trials, 7 prospective studies, and 16 retrospective studies. Mean study sample size was 469.5 patients (SD 788.9). The mean rate of postoperative VTEs was 11.1% (SD 9.8%); in 19 of 33 (57.6%) studies patients were routinely screened for DVTs. The reported rate of VTEs was significantly higher in studies that screened for DVTs compared to studies in which diagnostic testing was performed only in the setting of clinically suspected VTE (14.8% vs 6.2%; p = 0.010). The mean rate of prophylactic anticoagulation administration was 49.4% (SD 42.5%), which did not correlate with rate of VTEs on linear regression analysis (r2 = 0.017; p = 0.475). Of the articles reporting on the efficacy of prophylactic anticoagulation in preventing VTEs, 6 of 11 (54.5%) reported a benefit. The mean rate of postoperative hemorrhage among 18 reporting studies was 3.1% (SD 3.0%). Study design did not appear to affect VTE rates (randomized controlled trial [RCT] vs prospective vs retrospective study: 12.9% vs 12.3% vs 9.6%; p = 0.680). When all studies and all patients were included, year of publication (r2 = 0.022; p = 0.587) did not correlate with VTE rates. Given the heterogenous rate of prophylactic anticoagulation administration in studies included for analysis, to better assess trends in VTE incidence over time the rate of VTEs among patients who did not receive prophylactic anticoagulation as a function of publication date was also assessed. VTE rates among patients in control arms of RCTs2,7,9,12,16,34,37 and those reported in prospective or retrospective studies in which no patients received anticoagulation6,17,23,27,30,35,36,43,50 were found to be inversely correlated to year of publication (r2 = 0.297; p = 0.024; Fig. 1). Data from the present study were also included in this analysis given the small minority of patients receiving prophylactic anticoagulation. Independent risk factors for VTEs included high-grade glioma,10,49 hypertension,10 history of VTE,48 increasing age,10,20 increasing ICU length of stay,48,49 increasing weight,26 both male20 and female sex,49 motor deficit,10 nonambulatory status,20,26 non-Caucasian ethnicity,49 poor functional status,10 postoperative infection,35 and postoperative seizure.48
TABLE 6.
Summary of previous literature investigating the incidence of VTEs in adult patients undergoing elective craniotomy
| Authors & Year |
Study Design |
Population | Sample Size |
VTE (%) | DVT Screening |
PA (%) | Regimen | Day of PA Initiation |
Efficacy of PA* |
Hemorrhage (%) | VTE Risk Factors† |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Present study | Retro | Brain tumor | 1622 | 3.0 | No | 11.8 | Heparin 5000 U BID or TID, LMWH 30–40 mg QD, or oral AC | POD 4‡ | NA | 1.9 | ICH, motor deficit, older age, prior VTE, prolonged intubation |
| Joffe, 1975 | Retro | Craniotomy | 13 | 30.8 | Yes | 0.0 | — | — | — | NA | NA |
| Cerratoetal., 1978 | RCT | Craniotomy | 100 | 20.0 | Yes | 50.0 | Heparin 5000 U BID | PODO | Yes | 3.0 | NA |
| Ruff & Posner, 1983 | Retro | Glioma | 381 | 18.4 | No | 0.0 | — | — | — | 2.1 | NA |
| Bucci etal., 1989 | RCT | Craniotomy | 70 | 2.9 | Yes | 0.0 | — | — | — | NA | NA |
| Melon etal., 1991 | RCT | Craniotomy | 130 | 19.8 | Yes | 51.5 | LMWH 20 mg QD | POD 1 | No | 0.0 | NA |
| Frim et al., 1992 | Retro | Brain tumor | 611 | 2.5 | No | 22.6 | Heparin 5000 U BID | POD 1 | Yes | 0.0 | NA |
| Nurmohamed etal., 1996 | RCT | Craniotomy§ | 485 | 16.1 | Yes | 49.7 | LMWH¶ | PODO | Yes | 1.6 | NA |
| Flinn etal., 1996 | Prospect | Craniotomy | 1439 | 7.7 | Yes | 0.0 | — | — | — | NA | NA |
| Agnelli et al., 1998 | RCT | Craniotomy** | 259 | 24.6 | Yes | 50.2 | LMWH 40 mg QD | POD 1 | Yes | 3.0 | NA |
| Dickinson etal., 1998 | RCT | Brain tumor | 68 | 11.8 | Yes | 67.6 | LMWH 30 mg BID | Preop | No | 7.4 | NA |
| Constantini etal., 2001 | RCT | Brain tumor | 103 | 3.9 | No | 53.4 | Heparin 5000 U BID | Preop | NA | 2.9 | NA |
| Raabe etal., 2001 | Retro | Brain tumor | 690 | 1.2 | No | 100.0 | Heparin 5000 U TID | PODO | — | 2.5 | NA |
| Goldhaber et al., 2002 | RCT | Brain tumor | 150 | 9.3 | Yes | 100.0 | LMWH 40 mg QD | POD 1 | — | 0.7 | NA |
| Kumar et al., 2002 | Prospect | Brain tumor | 54 | 3.7 | Yes | 0.0 | — | — | — | NA | NA |
| Ting etal., 2002 | Prospect | Brain tumor | 100 | 4.0 | Yes | 0.0 | — | — | — | 1.0 | NA |
| Auguste et al., 2003 | Retro | Glioma | 180 | 3.3§ | No | 0.0 | — | — | — | NA | NA |
| Gerlach et al., 2003 | Prospect | Craniotomy | 1319 | 0.5 | No†† | 100.0 | LMWH 2850 U QD | Preop | — | 3.2 | NA |
| Macdonald et al., 2003 | RCT | Craniotomy | 100 | 2.0 | Yes | 100.0 | LMWH 2500 U BID or heparin 5000 UBID | PODO | No | 3.0 | NA |
| Gerber et al., 2007 | Retro | Meningioma | 224 | 4.9 | No | 90.0 | Heparin 5000 U BID | POD 1 | NA | 2.7 | Male, nonambulatory, increasing age |
| Cage etal., 2009 | Retro | Meningioma | 86 | 3.5 | No | 27.9 | LMWH 30–40 mg QD | POD 1–2 | No | 12.8 | NA |
| Dermody et al., 2011 | Retro | Cranioto-my‡‡ | 123 | 12.2 | Yes | 83.3 | Heparin 5000 U BID/TID or LMWH 40 mg QD | NA | Yes | NA | NA |
| Chaichana et al., 2013 | Retro | Brain tumor | 4293 | 3.0 | No | 100.0 | Heparin 5000 U BID or TID | POD 1 | NA | HGG, HTN, motor deficit, increasing age, poor functional status | |
| Prell etal., 2013 | Prospect | Craniotomy | 101 | 42.6 | Yes | 100.0 | LMWH 3000 IU QD | POD 1 | — | 2.0 | NA |
| Hoefnagel etal., 2014 | Retro | Brain tumor | 581 | 7.2 | No | 100.0 | LMWH 2850 or 5700 IU QD | POD 1 | — | 2.9 | Increasing weight, nonambulatory |
| Smith etal., 2014 | Retro | HGG | 336 | 15.7 | No | 7.4 | Heparin or LMWH¶ | POD 3‡ | Yes | 1.3 | ICU LOS, prior VTE, seizure |
| Frisius etal., 2015 | Retro | Brain tumor | 200 | 7.0 | Yes | 100.0 | LMWH¶ | POD 1–2 | NA | NA | NA |
| Smith et al., 2015 | Retro | Brain tumor | 1148 | 17.1 | No | 10.5 | Heparin or LMWH¶ | POD 5‡ | NA | 2.2 | Female, ICU LOS, HGG, metastasis, non-Caucasian, prior VTE |
| Sjavik et al., 2016 | Retro | Meningioma | 979 | 3.6 | No | 65.0 | LMWH 40 mg/5000 IU QD | Preop | No | 8.1 | NA |
| Agarwal etal., 2018 | Retro | Brain tumor | 800 | 1.6 | No | 100.0 | Heparin 5000 U TID | POD 2 | — | NA | NA |
| Guo et al., 2018 | Prospect | Brain tumor | 133 | 10.5 | Yes | 0.0 | — | — | — | NA | NA |
| Nakano et al., 2018 | Retro | Brain tumor | 61 | 21.3 | Yes | 0.0 | — | — | — | NA | Postop infection |
| Natsumeda et al., 2018 | Prospect | Craniotomy | 82 | 17.1 | Yes | 0.0 | — | — | — | NA | NA |
| Prell etal., 2018 | RCT | Craniotomy | 94 | 18.1 | Yes | 100.0 | LMWH 3000 U QD | POD 1 | — | NA | NA |
| 1975–2018§§ | — | — | 13–4293 (469.5) | 0.5–42.6(11.1) | Yes: 19/33 (57.6%) | 0.0–100.0(49.4) | — | — | Yes: 6/11 (54.5%) | 0.0–12.8(3.1) | — |
AC = anticoagulation; BID = twice daily; HGG = high-grade glioma; HTN = hypertension; ICU = intensive care unit; NA = not available; PA = prophylactic anticoagulation; POD = postoperative day; prospect = prospective; QD = daily; retro = retrospective; TID = 3 times daily; — = variable was not applicable to the study in question (e.g., regimen of PA in study in which no PA was administered).
Based on the study authors’ interpretation of results.
Risk factors noted only if found to be independently associated with VTEs on multivariate analysis.
Represents the mean postoperative day of prophylactic anticoagulation.
1.4% of patients in this study underwent spinal surgery.
Information on dosage and frequency of administration was not available.
15.0% of patients in this study had spine surgery.
100 patients were randomly screened for VTE in this study.
Diagnosis of postoperative VTE was assessed in first 6 weeks after surgery, as opposed to 30 days.
Summary of data for all studies.
FIG. 1.
Relationship of publication date to VTE rate. Plot of VTE rate reported in studies included for analysis as a function of year of publication. The r2 and p values obtained through linear regression analysis are weighted by study sample size. Shaded areas denote 95% CI. Figure is available in color online only.
Power Analysis
A power analysis was performed to determine the necessary sample size of a potential RCT investigating the effect of routine prophylactic anticoagulation administration on VTE rates. Assuming a VTE incidence of 3.0% among patients not receiving prophylactic anticoagulation, to detect a 1.0% reduction in VTE rate (3.0% vs 2.0%), a total of 8440 patients, with 4220 patients per treatment arm, would have to be enrolled.
Discussion
In the present study, we investigated the incidence and predictors of VTEs in a consecutive series of patients undergoing intracranial tumor surgery at our institution. We also determined the frequency of and complications associated with usage of prophylactic anticoagulation.
In our patient cohort, we observed a VTE rate of 3.0%, with respective rates of DVT and PE of 2.3% and 0.9%. In comparison, the rate of VTEs reported in the literature was highly variable, ranging from 0.5% to 42.6% (mean 11.1%, SD 9.8%; Table 6). As expected, the rate of VTEs was significantly higher in studies that screened asymptomatic patients for lower-extremity DVTs (14.8% vs 6.2%; p = 0.010). The utility of screening asymptomatic patients is not well established, particularly in a neurosurgical setting. A majority of postoperative DVTs detected by screening are isolated to deep veins of the calf, which do not pose a risk for a PE unless there is subsequent proximal venous extension, and thus do not require treatment.28 Moreover, asymptomatic distal DVTs typically resolve spontaneously, with progression observed in roughly one-sixth of cases.28 In contrast, though most if not all DVTs originate in the calf, in previous studies 90% of symptomatic patients were found to have thrombi in the proximal vasculature,11 suggesting that limiting diagnostic testing only to symptomatic patients may be more likely to yield actionable results. On the other hand, in a study of patients with a confirmed PE, fewer than half of patients with a confirmed concomitant proximal DVT had symptoms attributable to lower-extremity thrombosis,25 suggesting that clinical suspicion alone is not particularly sensitive for high-risk thrombi. While proximal extension of distal DVTs is effectively halted by anticoagulation treatment,31 in neurosurgical patients the benefit of this intervention must be weighed against the risk of hemorrhage. Though recent studies have suggested that the risk of therapeutic anticoagulation in the postcraniotomy setting is acceptable,45 given the relatively low rate of PE observed in our study (0.9%), the morbidity of routine screening and treatment of asymptomatic DVTs may outweigh any benefit. Further investigation is warranted to determine whether the use of screening protocols for high-risk patients is beneficial. Our rate of VTEs was comparable to those observed in other large single-center studies in which patients were not routinely screened for DVTs,10 as well as studies employing the use of national databases.4,13,29,42,46 Interestingly, patients within our cohort did not routinely receive prophylactic anticoagulation, which was administered at the discretion of the consultant neurosurgeon in cases assessed to be high risk (Table 3). By comparison, Chaichana and colleagues reported that patients treated at their institution were routinely administered prophylactic anticoagulation in the form of subcutaneous heparin starting 24 hours after surgery. Despite these stark differences in practice, we report a rate of VTE that is essentially identical to that reported by Chaichana et al. (3.0% vs 3.0%).10 Given the clear selection bias affecting which patients received prophylactic anticoagulation in our series (Table 3), we are unable to draw conclusions on the efficacy of prophylactic anticoagulation in preventing postoperative VTEs. Moreover, recent meta-analyses have found a benefit to prophylactic anticoagulation in preventing VTEs,3,5,24 though the added benefit in patients already receiving mechanical prophylaxis is less certain.3 In addition, VTE rates may be lower in the current era relative to when much of the available randomized data were collected (Fig. 1), possibly due to routine implementation of nonpharmacological modes of VTE prophylaxis.19,40 Regardless, our findings indicate that the eschewing of routine prophylactic anticoagulation in postoperative patients does not necessarily result in high rates of symptomatic VTEs when combined with routine mechanical prophylaxis and early mobilization. Further randomized trials may be necessary to obtain dispositive evidence on the benefit of prophylactic anticoagulation, though the sample sizes necessary to perform a sufficiently powered study may be prohibitive (see Power Analysis).
Of primary concern with the initiation of anticoagulation in the postoperative setting is the increased risk of ICH. We observed an overall postoperative hemorrhage rate of 1.9%, with only 1 patient experiencing an ICH after initiation of prophylactic anticoagulation (0.5%). There were no observed hemorrhages after initiation of sub-cutaneous heparin or LMWH. Similar to previous studies,4,8,10,21,33,41 our findings suggest that the risk of hemorrhage associated with prophylactic anticoagulation is not prohibitive and should not deter the use of anticoagulation medication in patients deemed to be at high risk for VTEs. It is important to note, however, that the time to prophylactic anticoagulation initiation was frequently delayed in our study (mean time to initiation: 4.6 days; Table 1). Thus our results are not generalizable to prophylactic anticoagulation routinely started within 24 to 48 hours after surgery and should be interpreted with caution, particularly given the association of LMWH with hemorrhage in previous studies.14,16,24,44 Further work is needed to characterize the effect of time from surgery on the risk profile of prophylactic anticoagulation.
To improve the cost-effectiveness of prophylactic anticoagulation, as well as to mitigate potential risks, identification of patients at high risk for VTEs and thus most likely to benefit from prophylactic anticoagulation is of paramount importance. In our study, we identified several factors independently associated with an increased risk of a VTE (Table 5). Among these variables, older age,4,10,13,20,29,46 history of VTE,49 motor deficit,10,20,26 and prolonged postoperative intubation or reintubation4,29 have been previously identified as risk factors for VTEs. Given the ability to identify prior history of a VTE preoperatively, patients should be thoroughly screened for even remote VTEs, and for patients who screen positively strong consideration should be given to early initiation of postoperative prophylactic anticoagulation. Regarding the remainder of the independent risk factors, apart from a motor deficit present preoperatively, they appear to apply almost exclusively to patients suffering postoperative complications (Table 5). Indeed, the mean postoperative day of VTE diagnosis was 13.1 and patients suffering a VTE in our cohort had significantly longer lengths of stay and more frequent discharge to either an inpatient rehabilitation or skilled nursing facility (Tables 1 and 2). While a VTE can certainly contribute to patient morbidity potentially affecting both length of stay and discharge location, these results suggest that postoperative complications may be the primary contributor to VTE risk in many cases. A corollary to this conclusion may be that routine use of prophylactic anticoagulation in patients undergoing uncomplicated tumor resection may not be cost-effective.3
Limitations
Our study is limited by the single-center, retrospective design. In addition, clear baseline differences between patients who did and those who did not receive prophylactic anticoagulation, as well as nonstandardized criteria for initiation and timing of prophylactic anticoagulation, preclude the possibility of drawing definitive conclusions on the efficacy of this intervention.
Conclusions
Herein, we report the incidence and predictors of VTEs in a consecutive series of patients undergoing surgery for resection of an intracranial tumor. Given the absence of dispositive randomized evidence on the efficacy of prophylactic anticoagulation, considering our institutional policy of administering prophylactic anticoagulation on a limited and discretionary basis, our hope is that our results will provide a reference point, along with other large institutional series, to aid neurosurgical providers in the development of protocols for VTE prophylaxis in the postoperative setting.
ABBREVIATIONS
- DVT
deep venous thrombosis
- ICH
intracranial hemorrhage
- KPS
Karnofsky Performance Scale
- LMWH
low-molecular-weight heparin
- PE
pulmonary embolism
- RCT
randomized controlled trial
- VTE
venous thromboembolic event
Footnotes
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1.Agarwal N, Zenonos GA, Agarwal P, Walch FJ, Roach E, Stokes SJ, et al. : Risk-to-benefit ratio of venous thromboembolism prophylaxis for neurosurgical procedures at a quaternary referral center. Neurosurgery [epub ahead of print], 2018 [DOI] [PubMed] [Google Scholar]
- 2.Agnelli G, Piovella F, Buoncristiani P, Severi P, Pini M, D’Angelo A, et al. : Enoxaparin plus compression stockings compared with compression stockings alone in the prevention of venous thromboembolism after elective neurosurgery. N Engl J Med 339:80–85, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Algattas H, Damania D, DeAndrea-Lazarus I, Kimmell KT, Marko NF, Walter KA, et al. : Systematic review of safety and cost-effectiveness of venous thromboembolism prophylaxis strategies in patients undergoing craniotomy for brain tumor. Neurosurgery 82:142–154, 2018 [DOI] [PubMed] [Google Scholar]
- 4.Algattas H, Kimmell KT, Vates GE, Jahromi BS: Analysis of venous thromboembolism risk in patients undergoing craniotomy. World Neurosurg 84:1372–1379, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Alshehri N, Cote DJ, Hulou MM, Alghamdi A, Alshahrani A, Mekary RA, et al. : Venous thromboembolism prophylaxis in brain tumor patients undergoing craniotomy: a meta-analysis. J Neurooncol 130:561–570, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Auguste KI, Quinones-Hinojosa A, Gadkary C, Zada G, Lamborn KR, Berger MS: Incidence of venous thromboembolism in patients undergoing craniotomy and motor mapping for glioma without intraoperative mechanical prophylaxis to the contralateral leg. J Neurosurg 99:680–684, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Bucci MN, Papadopoulos SM, Chen JC, Campbell JA, Hoff JT: Mechanical prophylaxis of venous thrombosis in patients undergoing craniotomy: a randomized trial. Surg Neurol 32:285–288, 1989 [DOI] [PubMed] [Google Scholar]
- 8.Cage TA, Lamborn KR, Ware ML, Frankfurt A, Chakalian L, Berger MS, et al. : Adjuvant enoxaparin therapy may decrease the incidence of postoperative thrombotic events though does not increase the incidence of postoperative intracranial hemorrhage in patients with meningiomas. J Neurooncol 93:151–156, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Cerrato D, Ariano C, Fiacchino F: Deep vein thrombosis and low-dose heparin prophylaxis in neurosurgical patients. J Neurosurg 49:378–381, 1978 [DOI] [PubMed] [Google Scholar]
- 10.Chaichana KL, Pendleton C, Jackson C, Martinez-Gutierrez JC, Diaz-Stransky A, Aguayo J, et al. : Deep venous thrombosis and pulmonary embolisms in adult patients undergoing craniotomy for brain tumors. Neurol Res 35:206–211, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cogo A, Lensing AWA, Prandoni P, Hirsh J: Distribution of thrombosis in patients with symptomatic deep vein thrombosis. Implications for simplifying the diagnostic process with compression ultrasound. Arch Intern Med 153:2777–2780, 1993 [PubMed] [Google Scholar]
- 12.Constantini S, Kanner A, Friedman A, Shoshan Y, Israel Z, Ashkenazi E, et al. : Safety of perioperative minidose heparin in patients undergoing brain tumor surgery: a prospective, randomized, double-blind study. J Neurosurg 94:918–921, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Cote DJ, Dubois HM, Karhade AV, Smith TR: Venous thromboembolism in patients undergoing craniotomy for brain tumors: a U.S. nationwide analysis. Semin Thromb Hemost 42:870–876, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Danish SF, Burnett MG, Ong JG, Sonnad SS, Maloney-Wilensky E, Stein SC: Prophylaxis for deep venous thrombosis in craniotomy patients: a decision analysis. Neurosurgery 56:1286–1294, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Dermody M, Alessi-Chinetti J, Iafrati MD, Estes JM: The utility of screening for deep venous thrombosis in asymptomatic, non-ambulatory neurosurgical patients. J Vasc Surg 53:1309–1315, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Dickinson LD, Miller LD, Patel CP, Gupta SK: Enoxaparin increases the incidence of postoperative intracranial hemorrhage when initiated preoperatively for deep venous thrombosis prophylaxis in patients with brain tumors. Neurosurgery 43:1074–1081, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Flinn WR, Sandager GP, Silva MB Jr, Benjamin ME, Cerullo LJ, Taylor M: Prospective surveillance for perioperative venous thrombosis. Experience in 2643 patients. Arch Surg 131:472–480, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Frim DM, Barker FG II, Poletti CE, Hamilton AJ: Postoperative low-dose heparin decreases thromboembolic complications in neurosurgical patients. Neurosurgery 30:830–833, 1992 [PubMed] [Google Scholar]
- 19.Frisius J, Ebeling M, Karst M, Fahlbusch R, Schedel I, Gerganov V, et al. : Prevention of venous thromboembolic complications with and without intermittent pneumatic compression in neurosurgical cranial procedures using intraoperative magnetic resonance imaging. A retrospective analysis. Clin Neurol Neurosurg 133:46–54, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Gerber DE, Segal JB, Salhotra A, Olivi A, Grossman SA, Streiff MB: Venous thromboembolism occurs infrequently in meningioma patients receiving combined modality prophylaxis. Cancer 109:300–305, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Gerlach R, Scheuer T, Beck J, Woszczyk A, Seifert V, Raabe A: Risk of postoperative hemorrhage after intracranial surgery after early nadroparin administration: results of a prospective study. Neurosurgery 53:1028–1035, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Goldhaber SZ, Dunn K, Gerhard-Herman M, Park JK, Black PM: Low rate of venous thromboembolism after craniotomy for brain tumor using multimodality prophylaxis. Chest 122:1933–1937, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Guo X, Zhang F, Wu Y, Gao L, Wang Q, Wang Z, et al. : Coagulation alteration and deep vein thrombosis in brain tumor patients during the perioperative period. World Neurosurg 114:e982–e991, 2018 [DOI] [PubMed] [Google Scholar]
- 24.Hamilton MG, Yee WH, Hull RD, Ghali WA: Venous thromboembolism prophylaxis in patients undergoing cranial neurosurgery: a systematic review and meta-analysis. Neurosurgery 68:571–581, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Hirmerova J, Seidlerova J, Chudacek Z: The prevalence of concomitant deep vein thrombosis, symptomatic or asymptomatic, proximal or distal, in patients with symptomatic pulmonary embolism. Clin Appl Thromb Hemost 24:1352–1357, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoefnagel D, Kwee LE, van Putten EH, Kros JM, Dirven CM, Dammers R: The incidence of postoperative thromboembolic complications following surgical resection of intracranial meningioma. A retrospective study of a large single center patient cohort. Clin Neurol Neurosurg 123:150–154, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Joffe SN: Incidence of postoperative deep vein thrombosis in neurosurgical patients. J Neurosurg 42:201–203, 1975 [DOI] [PubMed] [Google Scholar]
- 28.Kearon C: Natural history of venous thromboembolism. Circulation 107 (23 Suppl 1):I22–I30, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Kimmell KT, Jahromi BS: Clinical factors associated with venous thromboembolism risk in patients undergoing craniotomy. J Neurosurg 122:1004–1011, 2015 [DOI] [PubMed] [Google Scholar]
- 30.Kumar K, Tang KK, Thomas J, Chumpon C: Is postoperative deep vein thrombosis a problem in neurosurgical patients with brain tumours in Singapore? Singapore Med J 43:345–349, 2002 [PubMed] [Google Scholar]
- 31.Lagerstedt CI, Olsson CG, Fagher BO, Oqvist BW, Albrechtsson U: Need for long-term anticoagulant treatment in symptomatic calf-vein thrombosis. Lancet 2:515–518, 1985 [DOI] [PubMed] [Google Scholar]
- 32.Macdonald RL, Amidei C, Baron J, Weir B, Brown F, Erickson RK, et al. : Randomized, pilot study of intermittent pneumatic compression devices plus dalteparin versus intermittent pneumatic compression devices plus heparin for prevention of venous thromboembolism in patients undergoing craniotomy. Surg Neurol 59:363–374, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Macdonald RL, Amidei C, Lin G, Munshi I, Baron J, Weir BK, et al. : Safety of perioperative subcutaneous heparin for prophylaxis of venous thromboembolism in patients undergoing craniotomy. Neurosurgery 45:245–252, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Melon E, Keravel Y, Gaston A, Huet Y, Combes S, the NEURO-NOX Group: Deep venous thrombosis prophylaxis by low molecular weight heparin in neurosurgical patients. Anesthesiology 75:A214, 1991. (Abstract) [Google Scholar]
- 35.Nakano F, Matsubara T, Ishigaki T, Hatazaki S, Mouri G, Nakatsuka Y, et al. : Incidence and risk factor of deep venous thrombosis in patients undergoing craniotomy for brain tumors: A Japanese single-center, retrospective study. Thromb Res 165:95–100, 2018 [DOI] [PubMed] [Google Scholar]
- 36.Natsumeda M, Uzuka T, Watanabe J, Fukuda M, Akaiwa Y, Hanzawa K, et al. : High incidence of deep vein thrombosis in the perioperative period of neurosurgical patients. World Neurosurg 112:e103–e112, 2018 [DOI] [PubMed] [Google Scholar]
- 37.Nuño M, Carico C, Mukherjee D, Ly D, Ortega A, Black KL, et al. : Association between in-hospital adverse events and mortality for patients with brain tumors. J Neurosurg 123:1247–1255, 2015 [DOI] [PubMed] [Google Scholar]
- 38.Nurmohamed MT, van Riel AM, Henkens CM, Koopman MM, Que GT, d’Azemar P, et al. : Low molecular weight heparin and compression stockings in the prevention of venous thromboembolism in neurosurgery. Thromb Haemost 75:233–238, 1996 [PubMed] [Google Scholar]
- 39.Prell J, Rachinger J, Smaczny R, Taute BM, Rampp S, Illert J, et al. : D-dimer plasma level: a reliable marker for venous thromboembolism after elective craniotomy. J Neurosurg 119:1340–1346, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Prell J, Schenk G, Taute BM, Scheller C, Marquart C, Strauss C, et al. : Reduced risk of venous thromboembolism with the use of intermittent pneumatic compression after craniotomy: a randomized controlled prospective study. J Neurosurg [epub ahead of print March 30, 2018. DOI: 10.3171/2017.9.JNS17533] [DOI] [PubMed] [Google Scholar]
- 41.Raabe A, Gerlach R, Zimmermann M, Seifert V: The risk of haemorrhage associated with early postoperative heparin administration after intracranial surgery. Acta Neurochir (Wien) 143:1–7, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Rolston JD, Han SJ, Bloch O, Parsa AT: What clinical factors predict the incidence of deep venous thrombosis and pulmonary embolism in neurosurgical patients? J Neurosurg 121:908–918, 2014 [DOI] [PubMed] [Google Scholar]
- 43.Ruff RL, Posner JB: Incidence and treatment of peripheral venous thrombosis in patients with glioma. Ann Neurol 13:334–336, 1983 [DOI] [PubMed] [Google Scholar]
- 44.Salmaggi A, Simonetti G, Trevisan E, Beecher D, Carapella CM, DiMeco F, et al. : Perioperative thromboprophylaxis in patients with craniotomy for brain tumours: a systematic review. J Neurooncol 113:293–303, 2013 [DOI] [PubMed] [Google Scholar]
- 45.Scheller C, Rachinger J, Strauss C, Alfieri A, Prell J, Koman G: Therapeutic anticoagulation after craniotomies: is the risk for secondary hemorrhage overestimated? J Neurol Surg A Cent Eur Neurosurg 75:2–6, 2014 [DOI] [PubMed] [Google Scholar]
- 46.Senders JT, Goldhaber NH, Cote DJ, Muskens IS, Dawood HY, De Vos FYFL, et al. : Venous thromboembolism and intracranial hemorrhage after craniotomy for primary malignant brain tumors: a National Surgical Quality Improvement Program analysis. J Neurooncol 136:135–145, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sjåvik K, Bartek J Jr, Solheim O, Ingebrigtsen T, Gulati S, Sagberg LM, et al. : Venous thromboembolism prophylaxis in meningioma surgery: a population-based comparative effectiveness study of routine mechanical prophylaxis with or without preoperative low-molecular-weight heparin. World Neurosurg 88:320–326, 2016 [DOI] [PubMed] [Google Scholar]
- 48.Smith TR, Lall RR, Graham RB, Mcclendon J Jr, Lall RR, Nanney AD, et al. : Venous thromboembolism in high grade glioma among surgical patients: results from a single center over a 10 year period. J Neurooncol 120:347–352, 2014 [DOI] [PubMed] [Google Scholar]
- 49.Smith TR, Nanney AD III, Lall RR, Graham RB, McClendon J Jr, Lall RR, et al. : Development of venous thromboembolism (VTE) in patients undergoing surgery for brain tumors: results from a single center over a 10 year period. J Clin Neurosci 22:519–525, 2015 [DOI] [PubMed] [Google Scholar]
- 50.Ting AC, Cheng SW, Cheung GC, Wu LL, Hung KN, Fan YW: Perioperative deep vein thrombosis in Chinese patients undergoing craniotomy. Surg Neurol 58:274–279, 2002 [DOI] [PubMed] [Google Scholar]