Abstract
Background:
This study aims to investigate the dynamic interactions among three neural systems that are implicated in substance and behavioral addictions, in response to food cues in young adults. These include an impulsive system involving the striatum, a reflective system involving the prefrontal cortex, and a homeostasis sensing system involving the insular cortex.
Methods:
College students (N = 45) with various levels of body mass index (BMI) were recruited. fMRI data were acquired while participants perform food-related Go/Nogo tasks, with low- and high-calorie food cues. Participants were scanned under both food satiety and deprivation conditions. Dynamic causal modeling (DCM) was applied to the data to examine the causal architecture of coupled or distributed dynamics among the abovementioned systems.
Results:
Participants showed difficulty inhibiting responses to high calorie foods as suggested by higher false alarm rate and C for low-calorie food Go task. This difficulty was enhanced during food deprivation condition. Deprivation increased neural activity of (1) both the insula and the striatum, bilaterally, in response to high calorie foods during Go trials, and (2) the anterior cingulate cortex (ACC) and dorsolateral prefrontal cortex (DLPFC) activity during Nogo trials. DCM analysis revealed that (1) food deprivation modulates the communications between the insula, striatum and DLPFC, and (2) the modulations were positively associated with BMI.
Conclusions:
The results support tripartite views of decision making. The deprivation states such as hunger triggers insular activity, which in turn modulates the balance between the impulsive and reflective systems when facing tempting food cues.
Keywords: Food, Dynamic Causal Modeling, fMRI, Insula, Deprivation, Striatum, Dorsolateral Prefrontal Cortex
INTRODUCTION
Food is very important for human survival. Nevertheless, overconsumption of food can lead to overweight and obesity, which have become a key global public health concern (1–3). The reason is that they are associated with increased risk for cardiovascular/metabolic diseases, as well as several types of cancer in adults (4). Research on excess weight gain has been dominated by a focus on underlying metabolic mechanisms (5). The question of why people decide to eat “unhealthy” food that drive excess weight gain in the first place; that is, viewing excess weight gain as partially associated with food choice decisions, is relatively newer and has been relatively understudied. Taking this perspective, several studies have suggested that the difficulty to resist “unhealthy” foods is subserved by alterations in neural systems implicated in impaired decision making and behavioral addictions (6–11). They suggest that an individual who persistently chooses to eat high-calorie foods, despite the risk of potential long-term negative consequences of excess weight, may be similar to someone who is addicted to other substances or behaviors in terms of underlying brain mechanisms; they are simply drawn to immediate food rewards , while ignoring long term adverse consequences (6).
Adapting this behavioral addiction view, we contend that the loss of willpower to resist the temptation of high-calorie food, and the development of eating habits that lead to excess weight, may be explained in part in terms of abnormal activity in any one, or a combination of three key neural systems that underlie addiction development and maintenance (12). These include: (1) an impulsive system involving the striatum that drives reward seeking, (2) a reflective system involving the prefrontal cortex that exerts self-control, and (3) an interoceptive awareness / homeostasis sensing system involving the insular cortex (i.e., “the insula”) that responds to deprivation states (e.g., hunger) and consequently alters the dynamics of the impulsive and reflective systems. It does so by intensifying impulsive system activity and reducing reflective system activity. Specifically, individuals with addictive disorders often have a hyper-active impulsive system that promotes automatic, habitual reward seeking behaviors (3, 13–17) and show higher level of impulsivity (18–21). They also can have a hypo-active reflective system, which hinders their impulse control capacities and the ability to resist stimuli that are rewarding in the short-term, but lead to negative consequences in the long term (3, 13–15). They show loss of cognitive control over addiction cues and this may be modulated by reduced striatal dopamine D2 receptor (22, 23). The insular cortex system is very sensitive to the sense of body (18, 24–27). In addictive disorders, it translates homeostatic and interoceptive signals triggered by states of deprivation, or by exposure to reward cues (e.g., high calorie food cues), into craving and what may become subjectively experienced as an “urge” to generate the reward via the behavior that produces it (e.g., eat the food) (28). This model was based on the Dual Process Theory of addiction proposed by B consistent with the dual model of food addiction proposed by Consistent with this perspective, a rising number of studies suggest that these same brain regions that have been implicated in substance and behavioral addiction research, have altered responses to food cues in excess weight individuals (6–11).
The present study aims to investigate the neural dynamics among these three neural systems in response to food cues in young adults. To do so, participants were scanned twice: (1) under a food satiation condition, and (2) under a food deprivation condition. We used a food-related Go/Nogo task with low- and high-calorie food cues, and we then applied a dynamic causal modeling (DCM) analysis to the data (29), in order to examine the causal architecture of coupled or distributed dynamics of central brain regions of the impulsive, reflective, and interoceptive brain systems. Consistent with theoretical dynamics portrayed in neural models of addictive behaviors (14) and the view that excess weight may be associated in part with deficits in systems that govern decision making (30), we tested the hypotheses that (1) food deprivation states engage the insula, which in turn (2) exacerbates the activity of the impulsive system, and (3) weakens activity of the control function of the reflective system. Consistent with the view of excess weight as stemming in part from decision making deficits (30), we also expected that these effects will be positively associated with body mass index (BMI).
METHODS AND MATERIALS
Participants
Forty-five volunteers (28 females, mean age = 20 ± 1.1 years, 18 to 22 years old) were recruited from the University of Southern California. Participants with neurological or psychiatric disorders were excluded (details see Supplementary Materials). All participants gave informed consent to the study procedures, which were approved by the University of Southern California Institutional Review Board.
Procedures
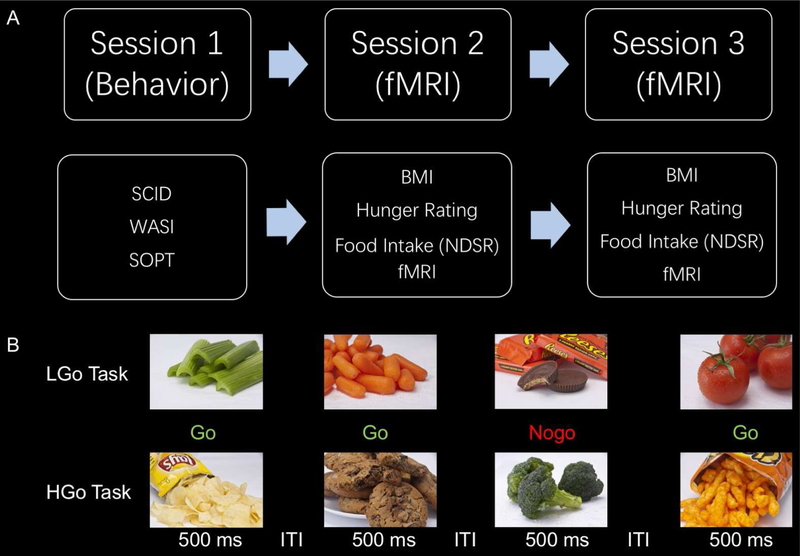
Participants were asked to come to the lab for three sessions (Figure 1A). During the first visit, participants were asked to complete and sign the consent form, and complete the screening interview and behavioral tasks. Participants were then scheduled to return for two functional Magnetic Resonance Imaging (fMRI) scans on two different dates: one in a food satiated (full) condition and another in a food deprived (hunger) condition. Right before each fMRI session, their height and weight were measured, and a 24-hour dietary recall was conducted with the Nutrition Data Systems for Research (NDSR). During each session, they completed two versions of the food-specific Go/Nogo task (8 min each) and one structural scan for registration purpose; these lasted about 35 min.
Figure 1:
Design of the study. A) The schematic of the procedure. Participants were asked to visit the lab for three sessions: one behavioral session, and two fMRI sessions (one under satiated/full condition, the other under deprived/hunger condition). The order of the two fMRI sessions was counterbalanced across participants. Participants who were scanned under the satiated/full condition were asked to eat normally and to have their usual meal before they arrived for the fMRI session. For the food deprived/hunger condition, participants were asked refrain from eating from 8 PM the day before the scan. B) The illustration of the event-related food-specific Go/Nogo tasks 1) low-calorie food Go/high-calorie food Nogo task (LGo task), and 2) high-calorie food Go/low-calorie food Nogo task (HGo task). Participants were asked to press a button as soon as possible to the Go trials (vegetable pictures in LGo task and snack pictures in HGo task) and withhold the response to the Nogo trials (snack pictures in LGo task and vegetable pictures in HGo task). The order of tasks was counterbalanced across subjects. SCID: structured clinical interview for DSM-IV; WASI: Wechsler abbreviated scale of intelligence; SOPT: self-ordered pointing task; BMI: body mass index; NDSR: nutrition data system for research; fMRI: functional magnetic resonance imaging; ITI: interatrial interval.
Under the satiation condition, participants were asked to eat normally and to have their usual meal right before they arrived for the fMRI session. They were asked to eat a variety of snacks (such as crackers, and chocolate) provided to them until they feel full at the lab. For the food deprivation condition, participants were asked to refrain from eating starting at 8 pm on the day before the scan. All scans were scheduled in the morning between 10 AM and 11 AM to avoid variation of scanning time and potential development of hunger. For manipulation-check purposes, participants were asked to rate their hunger levels on a 1 (not hungry at all) to 10 (very hungry) scale right before the scan. The satiation and deprivation sessions were counterbalanced across participants, and they were separated by more than 30 days (on average 41.2 ± 7.3 days, from 33 to 52 days) to avoid practice effect. The time between the scans did not correlate with either session or BMI (both ps > 0.05)
Measures
Neuropsychological Assessment.
Participants were asked to complete two behavioral tasks (see Table 1). The Wechsler Abbreviated Scale of Intelligence (WASI, 31) was used as an estimate for IQ, and the Self-Ordered Pointing Task (SOPT, 32) was used as an index of working memory and executive function.
Table 1.
Descriptive statistics in the two sessions (M ± SD)
| Satiation Session (Range) | Hunger Session (Range) | Statistics | |
|---|---|---|---|
| BMI | 22.9 ± 3.5 (19.1–33.7) | 22.8 ± 3.0 (18.5–31.3) | t(44) = 0.46, p = 0.65 |
| Hunger Rating | 2.5 ± 2.0 (1–5) | 6.6 ± 1.9 (6–10) | t(44) = −2.86, p < 0.01 |
| NDSR Low-Calorie Foods | 2.7 ± 1.7 (0.3–7.5) | 2.8 ± 1.5 (0.3–5.4) | t(44) = −0.30, p = 0.77 |
| NDSR High-Calorie Foods | 1.9 ± 1.5 (0.0–5.7) | 1.8 ± 1.5 (0.0–6.0) | t(44) = 0.82, p = 0.42 |
Dietary Intake.
A single, in-person 24-hour dietary recall was conducted by trained research staff using a multi-pass method facilitated by the NDSR (33, 34). Details of NDSR procedure could be found in the Supplementary Materials. The software calculated the low and high-calorie food consumption (servings/day), which served as the dietary intake index in the present study.
Food-specific Go/Nogo tasks
Although using different cohort of participants from He, Xiao (15), participants performed two event-related food-specific Go/Nogo tasks adapted from He, Xiao (15) in each scan: 1) a low-calorie food Go and high-calorie food Nogo task (LGo task), and 2) high-calorie food Go and low-calorie food Nogo task (HGo task). The only difference from He, Xiao (15), is that in the current study, participants were scanned twice, several weeks apart. Detail description of these tasks could be found in Supplementary Materials. Figure 1B portrays a sample of food pictures.
The hit rate, false alarm rate, sensitivity index d’ and decision bias C were calculated for each task following prior research with the same paradigm (15, 35). The mean reaction time for Go trials and Nogo trials (false alarm trials only) for each task was also calculated. The reaction time for Go trials served as an index for automatic responding to the stimuli, with longer reaction times indicating less habitual responses; decision bias C served as an index of response inhibition, with higher values indicating better inhibitory control.
fMRI Data Analysis
Participants finished two runs of Go/Nogo tasks in each scanning session. Details of fMRI data acquisition and preprocessing could be found in Supplementary Materials. Univariate fMRI data were modeled using a general linear model by convolving the onsets of each condition with the canonical hemodynamic response function. There were eight conditions in the first level model, representing each cell in the 2 Condition (Satiated vs Deprived) × 2 Task (Go vs Nogo) × 2 Stimuli (Low-calorie vs High-calorie food cues) within-subject factor design. Statistical significance was defined as cluster forming threshold at p < 0.001, and family wise error (FWE) corrected cluster threshold p < 0.05. Behavior and brain data correlation were calculated using robust correlation and Bonferroni corrections were applied to adjust for family-wise error.
Stochastic Dynamic Causal Modeling
Dynamic Causal Modeling with DCM12 (revision 6906) was used for connectivity analysis. DCM is described in detail elsewhere (29). In a nutshell, it develops a biophysical model of the underlying neuronal connectivity and how the neuronal connectivity generates the observed BOLD signal. The DCM analysis included three regressors: 1) habitual response manifested in onsets of all Go trials; 2) reflective response manifested in onsets of all Nogo trials; and 3) deprivation response onsets of all trials under deprivation condition.
Regions of interest (ROI) were regions that met all of the following criteria: (1) showed significant activation in the univariate second-level analysis; (2) showed activation in previous fMRI studies using Go/NoGo tasks (36, 37), and (3) had a theoretical reason to be activated as per the tripartite model of addictive behaviors (12, 28, 38). Eight nodes (Figure S1) met these criteria and were consequently used in the DCM analyses: (1) left insula; (2) right insula; (3) ventromedial prefrontal cortex (VMPFC); (4) anterior cingulate cortex (ACC); (5) left striatum; (6) right striatum; (7) left dorsolateral prefrontal cortex (DLPFC); and (8) right DLPFC.
Volumes of interest (VOIs) were obtained by significant activation clusters, which were determined by the second-level random effects univariate analysis. Cluster maxima locations were used as VOI centers around which 6 mm sphere were extracted as the VOI region. The standard SPM procedure was followed by using the principal eigenvariate of each VOI as a summary of the functional activity time-series in that VOI (39), and each principal eigenvariate time series was adjusted for the F-contrast of effects of interest (40). The same VOIs were used across subjects.
DCM structure inference was conducted using DCM Network Discovery (DND) (41). Before the DND analysis was conducted, a single “full” model was specified for each subject. Group-level post-hoc optimization was conducted by selecting all inverted “full” models (one per subject). The optimal sparse model was found at the group-level by using Bayesian parameter averaging. The correlation of behavioral and DCM data were calculated using the robust correlation.
RESULTS
Behavioral Results
The average IQ of our sample was normal (Mean = 118.4, SD = 9.8, ranged from 103–136), and the executive function performance was also normal according to SOPT (Mean = 65.1, SD = 3.8, ranged from 56 to 70). Descriptive statistics regarding BMI, hunger rating, and food intake are given in Table 1. Only hunger ratings significantly differed between the two sessions; in support of intervention validity, Hunger Deprived > Hunger Satiated.
Food Go/Nogo task behavior measures were analyzed with 2 Condition (satiated VS deprived) × 2 Task (HGo VS LGo) within-subject ANOVA (Table S1). After Bonferroni correction, only two main effects of task were significant; on: (1) false alarm: F(1, 42) = 11.46, p = 0.002, partial η2 = 0.42, and on (2) C: F(1, 42) = 9.89, p = 0.003, partial η2 = 0.45), suggesting that in the HGo task, participants had smaller false alarm rate and higher inhibition control (C) than in the LGo task. We also found a significant main effect of task on RT in Go trials (F(1, 42) = 4.89, p = 0.03, partial η2 = 0.23), and a significant effect of condition on RT in Nogo trials (F(1, 42) = 5.24, p = 0.03, partial η2 = 0.32). However, these effects were not sustained after multiple comparison correction.
fMRI Univariate Analysis
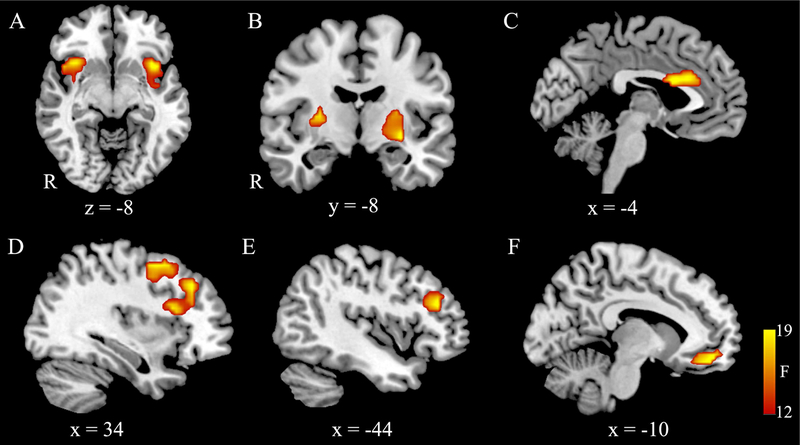
fMRI second-level random effects one-sample t-test analyses revealed several statistically significant clusters for main effects and interactions (Table 2 and Figure 2). They suggested that (1) the left and right insula were significantly activated in deprivation vs satiation conditions, (2) the ACC, left and right DLPFC showed more activation in Nogo trials compared to Go trials, (3) the left and right striatum showed significant activation in interaction between task and stimuli vs baseline, and (4) the VMPFC showed significant activation in the three-way interaction vs baseline.
Table 2:
Summary of Univariate fMRI results
| L/R | Brain region | Voxels | MNI x | MNI y | MNI z | F | P |
|---|---|---|---|---|---|---|---|
| Main Effect of Condition (Hungry > Satiated) | |||||||
| R | Insula | 132 | 33 | 20 | −7 | 22.11 | < .001 |
| L | Insula | 124 | −39 | 14 | −7 | 19.45 | < .001 |
| Main Effect of Task (Nogo > Go) | |||||||
| R | DLPFC | 503 | 30 | 20 | 20 | 20.46 | < .001 |
| L/R | ACC | 254 | 6 | 20 | 23 | 18.79 | < .001 |
| L | DLPFC | 100 | −45 | 35 | 23 | 18.22 | < .001 |
| Interaction between Task and Stimuli | |||||||
| L | Putamen/Striatum | 107 | −30 | −13 | −7 | 19.02 | < .001 |
| R | Putamen/Striatum | 103 | 33 | 2 | −1 | 18.58 | < .001 |
| Three-way Interaction (Task x Stimuli x Condition) | |||||||
| L/R | VMPFC | 159 | −9 | 47 | −16 | 19.11 | < .001 |
L: Left; R: Right; DLPFC: Dorsolateral Prefrontal Cortex; ACC: Anterior Cingulate Cortex; VMPFC: Ventromedial Prefrontal Cortex.
Note: The voxel size in fMRI analysis is 2 mm × 2 mm × 2 mm = 8 mm3. The x, y, and z coordinates and the F, and p values were reported on the peak voxel.
Figure 2:
Univariate 2-way fMRI ANOVA results. (A) Left and right insula showed significant activation in main effect of condition. (B) Left and right striatum showed significant activation in interaction between task and stimuli. The main effect of task showed activation in the ACC (C), left DLPFC (D), and right DLPFC (E). (F) The VMPFC showed significant activation in the three-way interaction. R indicated the right hemisphere.
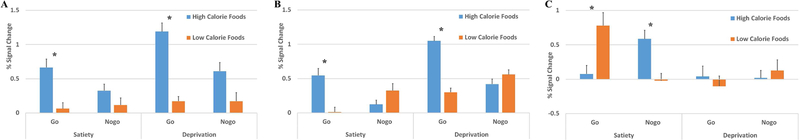
The percent signal change of the significant clusters showing interaction effects were extracted and plotted for different groups (Figure 3). Further analysis showed that left (Figure 3A) and right striatum (Figure 3B) were more highly activated in Go trials of high calorie food than in low calorie food trials (all ts > 3.23, ps < 0.001), but this difference was not present in the Nogo trials (ps > 0.05). The VMPFC (Figure 3C) showed higher activity in low-calorie food Go trials than in high-calorie food trials. It also showed higher activity in high-calorie food Nogo trials than in low-calorie food trials. This difference was present during the satiation condition (both ts > 3.58, ps < 0.001) but not in the deprivation condition (ps > 0.05).
Figure 3:
The brain activation pattern illustrated in bar graphs for A) left striatum, B) right striatum, and C) VMPFC. The percent signal change for each region was extracted using MarsBaR region of interest toolbox for SPM (http://marsbar.sourceforge.net/). Star denotes the significant activation difference between high calorie foods and low calorie foods in that condition. Error bars represent standard error.
The extracted percent signal changes were also correlated with BMI and NDSR scores. To reduce the number of multiple comparisons, the left and right striatum, the ACC and right DLPFC, and the left and right insula were linearly paired, respectively. The two sessions of NDSR and BMI scores were also combined. Results suggest that striatal activity when a person sees high-calorie foods in the Go trials, under either satiation and deprivation condition, correlated positively with NDSR High-Calorie Foods (Satiation: r(45) = 0.528, p < 0.001; Deprivation: r(45) = 0.623, p < 0.001), BMI (Satiation : r(45) = 0.453, p < 0.002; Deprivation: r(45) = 0.578, p < 0.001). The ACC/DLPFC activity for high calorie foods in the Nogo trials under either satiation or deprivation condition negatively correlated with NDSR High-Calorie Foods (Satiety: r(45) = −0.531, p < 0.001; Deprivation: r(45) = −0.572, p < 0.001), BMI (Satiety: r(45) = −0.488, p < 0.001; Deprivation: r(45) = −0.612, p < 0.001). The insular activity difference between deprivation and satiation conditions was positively correlated with NDSR High-Calorie Foods (Satiety: r(45) = 0.723, p < 0.001; Deprivation: r(45) = 0.651, p < 0.001), BMI (Satiety: r(45) = 0.568, p < 0.002; Deprivation: r(45) = 0.645, p < 0.001). These results suggest that people who consume more high-calorie foods or have higher BMI present stronger activation of the striatum in response to high-calorie foods, have more difficulty to activate the ACC and DLPFC to inhibit automatic responses to high- calorie food, and show higher insular activity when food-deprived.
DCM Results
Starting from a full model, post-hoc optimization revealed a sparse model structure at the group level. The sparse structure regarding driving inputs is given in Table 3, which shows the posterior mean strength and posterior probability for each driving input and each location. Consistent with our hypothesis, the table shows that the left and right striatum were driving input locations for Go trials; left and right DLPFC and ACC were driving input locations for Nogo trials; and left and right insular activity was driven by deprivation.
Table 3:
Sparse Structure Matrix - Driving Input Effects
| Location | Go Trials |
Nogo Trials |
Deprivation |
|||
|---|---|---|---|---|---|---|
| Strength (Hz) | Posterior probability | Strength (Hz) | Posterior probability | Strength (Hz) | Posterior probability | |
| L Striatum | 0.0352 | 0.9999 | 0 | 0 | 0 | 0 |
| R Striatum | 0.0323 | 0.9999 | 0 | 0 | 0 | 0 |
| ACC | 0 | 0 | 0.0528 | 0.9999 | 0 | 0 |
| L DLPFC | 0 | 0 | 0.0144 | 0.9999 | 0 | 0 |
| R DLPFC | 0 | 0 | 0.0156 | 0.9999 | 0 | 0 |
| L Insula | 0 | 0 | 0 | 0 | 0.0022 | 0.9999 |
| R Insula | 0 | 0 | 0 | 0 | 0.0034 | 0.9999 |
| VMPFC | 0 | 0 | 0 | 0 | 0 | 0 |
Posterior mean strength (Hz) and posterior probability (Seghier and Friston, 2013) of each driving input and each driving input location are demonstrated.
The group-level sparse structure regarding modulation effects is given in Table 4 and Figure 4, which shows the posterior mean strength and posterior probability for each modulatory input and each connection. Given the large number of connections (56), only connections that had posterior probability of modulation effect greater than 0.999 are given in Table 4. Results demonstrated that five connections were modulated by deprivation, including left insula to left DLPFC, right insula to right DLPFC, left insula to left striatum; right insula to right striatum, and right insula to ACC.
Table 4:
Sparse matrix structure - modulation effect
| Location | Deprivation |
Correlated with BMI | Correlated with NDSR-High Calorie Foods | |
|---|---|---|---|---|
| Strength (Hz) | Posterior probability | |||
| L Insula → L DLPFC | −0.5647 | 0.9999 | r = −0.468, p = 0.001 | r = −0.711, p < 0.001 |
| R Insula → R DLPFC | −0.5273 | 0.9999 | r = −0.678, p < 0.001 | r = −0.672, p < 0.001 |
| L Insula → L Striatum | 0.7132 | 0.9999 | r = 0.682, p < 0.001 | r = 0.543, p < 0.001 |
| R Insula → R Striatum | 0.6950 | 0.9999 | r = 0.526, p < 0.001 | r = 0.669, p < 0.001 |
| R Insula → ACC | −0.3165 | 0.9999 | r = −0.317, p = 0.034a | r = −0.308, p = 0.040a |
Among the 56 connections, only those that were identified (posterior probability > 0.999) to be modulated by one of the three conditions are listed. Posterior mean strength (Hz) and posterior probability of each identified (posterior probability > 0.999) modulation effect are demonstrated.
not significant anymore after Bonferroni multiple comparison correction.
Figure 4:
Schematic diagram representing effective connectivity modulated by food deprivation. For clarity, not all nodes are shown in this figure. L = left. R = right. The numbers represent the posterior mean strength (Hz) of the modulation. The positive modulations were presented in orange, and negative modulations were presented in blue. The diagram was visualized with the BrainNet Viewer (Xia et al., 2013). It was illustrated in a custom view with Azimuch = 20 and Elevation = 60 to show all brain regions and connections.
Xia M, Wang J, He Y (2013) BrainNet Viewer: A Network Visualization Tool for Human Brain Connectomics. PLoS ONE 8: e68910.
The group-level sparse structure regarding the endogenous connections is given in Table 5, which shows the posterior mean strength and posterior probability of each endogenous connection. It shows that nineteen connections became non-significant after the post-hoc optimization. These include left insula to right DLPFC, left insula to right striatum, left DLPFC to right striatum, left DLPFC to right insula, left DLPFC to ACC, ACC to left DLPFC, ACC to right DLPFC, right insula to left DLPFC, right insula to left striatum, right DLPFC to left DLPFC, right DLPFC to left striatum, right DLPFC to ACC, right DLPFC to left insula, left striatum to ACC, left striatum to right DLPFC, left striatum to right insula, right striatum to ACC, right striatum to left DLPFC, and right striatum to left insula.
Table 5:
Sparse matrix structure - endogenous connection
| Connection | Strength (Hz) | Posterior probability | Connection | Strength (Hz) | Posterior probability |
|---|---|---|---|---|---|
| L Striatum → R Striatum | 0.0176 | 0.9999 | ACC → L Striatum | 0.0238 | 0.9999 |
| L Striatum → L DLPFC | 0.0159 | 0.9999 | ACC → R Striatum | 0.0349 | 0.9999 |
| L Striatum → R DLPFC | 0.0358 | 0 | ACC → L DLPFC | 0.0309 | 0 |
| L Striatum → ACC | 0.0354 | 0 | ACC → R DLPFC | 0.0163 | 0 |
| L Striatum → L Insula | 0.0277 | 0.9999 | ACC → L Insula | 0.0214 | 0.9999 |
| L Striatum → R Insula | 0.0320 | 0 | ACC → R Insula | 0.0143 | 0.9999 |
| L Striatum → VMPFC | 0.0341 | 0.9999 | ACC → VMPFC | 0.0115 | 0.9999 |
| R Striatum → L Striatum | 0.0425 | 0.9999 | L Insula → L Striatum | 0.0075 | 0.9999 |
| R Striatum → L DLPFC | 0.0173 | 0 | L Insula → R Striatum | 0.0136 | 0 |
| R Striatum → R DLPFC | 0.0098 | 0.9999 | L Insula → L DLPFC | 0.0301 | 0.9999 |
| R Striatum → ACC | 0.0342 | 0 | L Insula →R DLPFC | 0.0346 | 0 |
| R Striatum → L Insula | 0.0144 | 0 | L Insula → ACC | 0.0277 | 0.9999 |
| R Striatum → R Insula | 0.0371 | 0.9999 | L Insula → R Insula | 0.0156 | 0.9999 |
| R Striatum → VMPFC | 0.0126 | 0.9999 | L Insula → VMPFC | 0.0231 | 0.9999 |
| L DLPFC → L Striatum | 0.0172 | 0.9999 | R Insula → L Striatum | 0.0104 | 0 |
| L DLPFC → R Striatum | 0.0400 | 0 | R Insula → R Striatum | 0.0135 | 0.9999 |
| L DLPFC → R DLPFC | 0.0080 | 0.9999 | R Insula → L DLPFC | 0.0256 | 0 |
| L DLPFC → ACC | 0.0132 | 0 | R Insula → R DLPFC | 0.0118 | 0.9999 |
| L DLPFC → L Insula | 0.0336 | 0.9999 | R Insula → ACC | 0.0360 | 0.9999 |
| L DLPFC → R Insula | 0.0193 | 0 | R Insula → L Insula | 0.0334 | 0.9999 |
| L DLPFC → VMPFC | 0.0108 | 0.9999 | R Insula → VMPFC | 0.0427 | 0.9999 |
| R DLPFC → L Striatum | 0.0119 | 0 | VMPFC → L Striatum | 0.0162 | 0.9999 |
| R DLPFC → R Striatum | 0.0147 | 0.9999 | VMPFC → R Striatum | 0.0260 | 0.9999 |
| R DLPFC → L DLPFC | 0.0173 | 0 | VMPFC → L DLPFC | 0.0122 | 0.9999 |
| R DLPFC → ACC | 0.0141 | 0 | VMPFC → R DLPFC | 0.0082 | 0.9999 |
| R DLPFC → L Insula | 0.0169 | 0 | VMPFC → ACC | 0.0045 | 0.9999 |
| R DLPFC → R Insula | 0.0258 | 0.9999 | VMPFC → L Insula | 0.0153 | 0.9999 |
| R DLPFC → VMPFC | 0.0190 | 0.9999 | VMPFC → R Insula | 0.0336 | 0.9999 |
Posterior mean strength (Hz) and posterior probability of each endogenous connection are outlined.
The extracted posterior strength of all modulation effects were correlated with the BMI and NDSR total scores (combined across the two sessions). The results in Table 4 suggest that all modulation effects were correlated with both BMI and NDSR-High Calorie Foods, except for the modulation of right insula to ACC path, which was not significant after Bonferroni correction.
DISCUSSION
The present study aimed to investigate the network dynamics of three neural systems involved in making food-related choices. Using fMRI and DCM analyses, the results revealed that participants have difficulty inhibiting high-calorie foods when performing a food-specific Go/Nogo task. This behavioral inhibition difficulty is linked to imbalanced among activations of three neural systems, with the insula responding to the deprivation state, bilaterally, the striatum responding to high-calorie foods, bilaterally, and the ACC and DLPFC responding to Nogo trials (i.e., behavioral inhibition attempts). This inhibition difficulty was stronger for higher BMI participants, and for those who consume higher calorie foods. DCM analysis showed that food deprivation modulated the connections between the insula, the striatum, and DLPFC. These modulations were larger for higher BMI participants and for those who consume higher calorie foods. Altogether, these results provide new insights on the dynamic nature among three neural systems involved in making food-related choices; they also reinforce and extend models that portray the neural mechanism of choices pertaining to other rewarding substances and behaviors (6–8, 10, 11, 14, 40, 42).
Understanding the underlying neural mechanisms that give rise to food choices that are tempting and rewarding in the short term, but can lead to health consequences in the long term, can help inform the development of new therapeutic strategies that potentially improve, not only food related choices, but perhaps other short-term rewarding choices that lead to addictive behaviors. More specifically, the amygdala-striatal (dopamine dependent) neural system (i.e., impulsive system) is critical for the incentive motivational effects of a variety of non-natural rewards (e.g., psychoactive drugs), and natural rewards (e.g., food) (43). The present study showed that regardless of one’s state (satiation or hunger) higher impulsive/habitual system activity is generated in response to high-calorie foods. This is consistent with the idea that the mesolimbic dopamine-striatal system mediates habitual responses to primary reward food cues (44–46). In further support of this notion, functional neuroimaging studies showed that, compared to non-food pictures, food pictures activate the amygdala (47) and the ventral striatum (48) in healthy individuals. It would be noted that the striatum activated in the present study was mostly located to the dorsolateral part in the mid (right) and posterior (left) putamen. Although there were studies showing different roles of ventral and dorsal striatum in response to reward, there were mounting evidence suggested that activation of dorsal striatum is common for food-related (13, 15, 49, 50) and other substance-related (51, 52) studies. Anyway, we extend such findings and show that habitual response in the striatum to high-calorie foods is positively correlated with BMI and NDSR High-Calorie food intake. These results were in line with studies showing that food may induce greater incentive values in excess weight individuals compared to normal controls (53), and in those who generally eat more high-calorie foods (13, 15) despite other factors that might influence the neural responses to food in excess weight individuals (e.g. emotional, other psychiatric comorbidities etc.).
While the impulsive system and its related mesolimbic dopamine may explain the drive and seeking of food-related rewards, humans possess decision-making and self-control neural systems that enable them to resist these reward temptations, especially when they lead to long term negative consequences (14). This decision-making and self-control system has been called the “reflective” system, and it depends primarily on the functions of the prefrontal cortex, which is necessary for controlling these more basic motivational drives toward reward, and which allows for more flexible pursuit of long-term goals (14). The present study found that the reflective system (and specifically the ACC and DLPFC) was engaged when attempts to control food-related responses were made. We also found that the VMPFC was activated when engaging in inhibitory control, when participants were satiated. This supports extant views regarding the central role of the VMPFC in decision making (54–56) and specifically inhibitory control (57, 58). Adequate food related decision-making reflects an integration of both cognitive and affective assessments, and the ability to more optimally weigh short-term gains against long-term losses and choose between conflicting alternatives (e.g., consume food now or later). Accordingly, our findings reinforce the role of VMPFC, ACC and DLPFC in decision making (14), and extend prior models to the food choice domain.
We also found that the activity of the reflective system was inversely correlated with BMI and NDSR-high calorie food consumption. This finding was in line with previous studies suggesting weaker inhibitory control in excess weight individuals (59, 60), and those who consume more high-calorie foods (3, 13, 15). It should be noted that we could not conclude that DLPFC plays the same role in obese individuals than normal weight participants, as in the present study we only tested normal weight participants. In fMRI studies, it has also been shown that excess weight people have less activation in the left DLPFC in response to a meal than do their lean counterparts (61). Considering the roles of the impulsive and reflective systems we observe here, our findings lend further support to the idea that excess weight can be explained in part via the lens of decision making deficits.
The current results also show that the insular cortex is significantly more active in deprivation relative to satiation conditions. Indeed, it has been argued that the insular cortex plays a key role in translating interoceptive signals into what one subjectively experiences as a feeling of desire, anticipation, or urge (14, 28). Evidence shows that the insular cortex is also implicated in food craving (49). This is consistent across substances; strokes that damage the insular cortex tend to eliminate the urge to smoke in individuals previously addicted to cigarette smoking (62, 63). One proposed mechanism for how this may take place is that activation of interoceptive representations through the insula can, on the one hand, sensitize the impulsive system and on the other, tax the prefrontal cortex, so that it cannot subvert attention, reasoning, planning, and decision-making processes as related to food consumption. Put differently, these interoceptive representations have the capacity to “hijack” the cognitive resources necessary for exerting inhibitory control to resist calorie-rich food items (14).
Consistent with this idea, the present study demonstrated that the insula triggers changes that alter the dynamic neural balance between the impulsive and reflective systems. This insula induced modulation of the neural dynamics was larger in higher BMI participants and in those who eat more high-calorie foods. These results extend several neuroimaging studies showing that food versus non-food pictures activate the insular cortex in healthy individuals (64, 65). The increased activity induced by food presentation in the insular cortex significantly correlated with self-reports of hunger and desire for food in normal-weight subjects (49, 66). One recent fMRI study (67) reports that compared to lean adolescent girls, obese girls show greater activation in the gustatory cortex (anterior and mid insular, frontal operculum) and in somatosensory regions (parietal operculum and Rolandic operculum) in response to the anticipated intake of chocolate milkshake (versus a tasteless solution) and to actual consumption of milkshake (versus a tasteless solution). Another recent study examined resting state functional connectivity difference of the dorsal mid-insula both pre- and post-meal; findings suggested obesity-related alterations in dorsal mid-insula functional connectivity patterns (68). Overall, our results provide further evidence regarding the centrality of the insular system in decision making, and extend this perspective to show how the insula dynamically modulates neural processes that govern food-related behaviors.
Several limitations of this study are noteworthy. First, the sample included presumably healthy individuals with normal weights. Although our study suggested that the individual difference of DCM networks were correlated with their BMI, and in line with other previous report suggesting insular functional connectivity difference between obese and normal weight participants (68), we could not extend our inferences to clinically obese people or people with higher levels of BMI. Second, the generalization of conclusion to females may be influenced by the phase of menstrual cycle because there are studies suggesting females’ reward processing (69, 70) including food-cues processing (71, 72) are influenced by the phase of menstrual cycle, and the intake of contraceptives (73). Third, we did not find a significant interaction between condition and task. This might be an artefact of the sample (healthy young individuals) and/or the task condition (unfamiliar fMRI environment). Nevertheless, such non-significant differences might also suggest that groups were matched on task difficulty, making fMRI difference more prominent. Fourth, the main conclusion were drawn from the DCM results. Nevertheless, it is debated if fMRI signals could be used for casual inference because of the time lag that limits the accuracy of causal modeling with fMRI data (74, 75). Lastly, we could not fully enforce deprivation and satiation. We did not measure or actually control how much participants consumed before the scans. While hunger was indeed higher in the deprivation condition, future studies may replicate ours in a controlled environment where food intake (or lack of in deprivation condition) can be fully controlled by the researchers.
Ultimately, our results portray a tripartite model of key neural systems that govern food-related choices. This is consistent with the recent literature that supports the idea of an overlap between the neural systems underlying drug seeking behaviors and eating high-calorie food (6, 11, 76). The findings advance our understanding of excess weight, possibly extended to obesity, especially of food choices in adolescents who have under-developed prefrontal cortex (77), and in environments such as school or University campuses where unhealthy food options are prominent.
Supplementary Material
Acknowledgments
QH was supported by research grants from the National Natural Science Foundation of China (31400959), Entrepreneurship and Innovation Program for Chongqing Overseas Returned Scholars (cx2017049), and Fundamental Research Funds for Central Universities (SWU1809003 and SWU1709106). AB was supported by research grants from National Institute on Drug Abuse (NIDA) R01DA023051, National Cancer Institute (NCI) R01CA152062, the National Heart, Lung, and Blood Institute and the National Institute of Child Health and Human Development (U01HL097839).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
QH, OT, and AB designed the study, QH collected the data, QH, XH, SZ, OT, and LM analyzed the data, QH, XH, SZ, OT, LM, and AB wrote the manuscript, all authors approved the final version of the manuscript. All authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES:
- 1.Stein CJ, Colditz GA (2004): The epidemic of obesity. Journal of Clinical Endocrinology & Metabolism 89:2522–2525. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Mi J, Shan XY, Wang QJ, Ge KY (2007): Is China facing an obesity epidemic and the consequences? The trends in obesity and chronic disease in China. Int J Obes (Lond) 31:177–188. [DOI] [PubMed] [Google Scholar]
- 3.He Q, Chen C, Dong Q, Xue G, Chen C, Lu Z, et al. (2015): Gray and White Matter Structures in the Midcingulate Cortex Region Contribute to Body Mass Index in Chinese Young Adults. Brain Struct Funct 220:319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M (2008): Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. The Lancet 371:569–578. [DOI] [PubMed] [Google Scholar]
- 5.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. (2004): Obesity and the metabolic syndrome in children and adolescents. New England Journal of Medicine 350:2362–2374. [DOI] [PubMed] [Google Scholar]
- 6.Volkow ND, Baler RD (2015): NOW vs LATER brain circuits: implications for obesity and addiction. Trends in neurosciences 38:345–352. [DOI] [PubMed] [Google Scholar]
- 7.Volkow ND, Wang GJ, Fowler JS, Telang F (2008): Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philosophical Transactions of the Royal Society B: Biological Sciences 363:3191–3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelley AE, Berridge KC (2002): The neuroscience of natural rewards: relevance to addictive drugs. The Journal of Neuroscience 22:3306–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rolls E (2007): Understanding the mechanisms of food intake and obesity. Obesity Reviews 8:67–72. [DOI] [PubMed] [Google Scholar]
- 10.Trinko R, Sears RM, Guarnieri DJ, DiLeone RJ (2007): Neural mechanisms underlying obesity and drug addiction. Physiology & behavior 91:499–505. [DOI] [PubMed] [Google Scholar]
- 11.Volkow ND, Wise RA (2005): How can drug addiction help us understand obesity? Nature neuroscience 8:555–560. [DOI] [PubMed] [Google Scholar]
- 12.Noel X, Brevers D, Bechara A (2013): A neurocognitive approach to understanding the neurobiology of addiction. Curr Opin Neurobiol 23:632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He Q, Xiao L, Xue G, wong s, Ames SL, Bechara A (2014): Altered Dynamics Between Neural Systems Sub-serving Decisions for Unhealthy Food. Frontiers in neuroscience 8:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noël X, Brevers D, Bechara A (2013): A neurocognitive approach to understanding the neurobiology of addiction. Current opinion in neurobiology 23:632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He Q, Xiao L, Xue G, Wong S, Ames SL, Schembre SM, et al. (2014): Poor ability to resist tempting calorie rich food is linked to altered balance between neural systems involved in urge and self-control. Nutrition Journal 13:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bechara A (2005): Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci 8:1458–1463. [DOI] [PubMed] [Google Scholar]
- 17.Bechara A, Van Der Linden M (2005): Decision-making and impulse control after frontal lobe injuries. Curr Opin Neurol 18:734–739. [DOI] [PubMed] [Google Scholar]
- 18.Crews FT, Boettiger CA (2009): Impulsivity, frontal lobes and risk for addiction. Pharmacology Biochemistry Behavior 93:237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao F, Su L, Liu T, Gao X (2007): The relationship between impulsivity and Internet addiction in a sample of Chinese adolescents. European Psychiatry 22:466–471. [DOI] [PubMed] [Google Scholar]
- 20.Kim SM, Huh HJ, Cho H, Kwon M, Choi JH, Ahn HJ, et al. (2014): The effect of depression, impulsivity, and resilience on smartphone addiction in university students. Journal of Korean Neuropsychiatric Association 53:214–220. [Google Scholar]
- 21.Kreek MJ, Nielsen DA, Butelman ER, LaForge KS (2005): Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nature neuroscience 8:1450. [DOI] [PubMed] [Google Scholar]
- 22.Ghahremani DG, Lee B, Robertson CL, Tabibnia G, Morgan AT, De Shetler N, et al. (2012): Striatal dopamine D2/D3 receptors mediate response inhibition and related activity in frontostriatal neural circuitry in humans. Journal of Neuroscience 32:7316–7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, et al. (2009): Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. Journal of Neuroscience 29:14734–14740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Craig AD (2009): How do you feel--now? The anterior insula and human awareness. Nature reviews neuroscience 10. [DOI] [PubMed] [Google Scholar]
- 25.Craig A (2011): Significance of the insula for the evolution of human awareness of feelings from the body. Annals of the New York Academy of Sciences 1225:72–82. [DOI] [PubMed] [Google Scholar]
- 26.Craig A (2009): Emotional moments across time: a possible neural basis for time perception in the anterior insula. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences 364:1933–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Craig A (2003): Interoception: the sense of the physiological condition of the body. Current opinion in neurobiology 13:500–505. [DOI] [PubMed] [Google Scholar]
- 28.Droutman V, Read SJ, Bechara A (2015): Revisiting the role of the insula in addiction. Trends in Cognitive Sciences 19:414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friston KJ, Harrison L, Penny W (2003): Dynamic causal modelling. Neuroimage 19:1273–1302. [DOI] [PubMed] [Google Scholar]
- 30.He Q, Chen C, Dong Q, Xue G, Chen C, Lu Z-L, et al. (2013): Gray and white matter structures in the midcingulate cortex region contribute to body mass index in Chinese young adults. Brain Struct Funct 1–11. [DOI] [PMC free article] [PubMed]
- 31.Wechsler D (1999): Wechsler Abbreviated Scale of Intelligence New York, NY: The Psychological Corporation: Harcourt Brace & Company. [Google Scholar]
- 32.Cragg L, Nation K (2007): Self-ordered pointing as a test of working memory in typically developing children. Memory 15:526–535. [DOI] [PubMed] [Google Scholar]
- 33.Feskanich D, Sielaff BH, Chong K, Buzzard IM (1989): Computerized collection and analysis of dietary intake information. Computer methods and programs in biomedicine 30:47–57. [DOI] [PubMed] [Google Scholar]
- 34.Johnson RK, Driscoll P, Goran MI (1996): Comparison of multiple-pass 24-hour recall estimates of energy intake with total energy expenditure determined by the doubly labeled water method in young children. Journal of the American Dietetic Association 96:1140. [DOI] [PubMed] [Google Scholar]
- 35.Turel O, He Q, Xue G, Xiao L, Bechara A (2014): Examination of neural systems sub-serving Facebook “addiction”. Psychol Rep 115:675–695. [DOI] [PubMed] [Google Scholar]
- 36.Simmonds DJ, Pekar JJ, Mostofsky SH (2008): Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia 46:224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swick D, Ashley V, Turken U (2011): Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage 56:1655–1665. [DOI] [PubMed] [Google Scholar]
- 38.Turel O, Bechara A (2016): A triadic reflective-impulsive-interoceptive awareness model of general and impulsive information system use: Behavioral tests of neuro-cognitive theory. Frontiers in Psychology 7:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma L, Steinberg JL, Cunningham KA, Lane SD, Kramer LA, Narayana PA, et al. (2015): Inhibitory behavioral control: a stochastic dynamic causal modeling study using network discovery analysis. Brain connectivity 5:177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stephan KE, Penny WD, Moran RJ, den Ouden HE, Daunizeau J, Friston KJ (2010): Ten simple rules for dynamic causal modeling. Neuroimage 49:3099–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friston KJ, Li B, Daunizeau J, Stephan KE (2011): Network discovery with DCM. Neuroimage 56:1202–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW (2003): Mapping cortical change across the human life span. Nature neuroscience 6:309–315. [DOI] [PubMed] [Google Scholar]
- 43.Wise RA (2002): Brain reward circuitry: insights from unsensed incentives. Neuron 36:229–240. [DOI] [PubMed] [Google Scholar]
- 44.Siep N, Roefs A, Roebroeck A, Havermans R, Bonte ML, Jansen A (2009): Hunger is the best spice: an fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behavioural brain research 198:149–158. [DOI] [PubMed] [Google Scholar]
- 45.Ng J, Stice E, Yokum S, Bohon C (2011): An fMRI study of obesity, food reward, and perceived caloric density. Does a low-fat label make food less appealing? Appetite 57:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grill HJ, Skibicka KP, Hayes MR (2007): Imaging obesity: fMRI, food reward, and feeding. Cell Metabolism 6:423–425. [DOI] [PubMed] [Google Scholar]
- 47.Gottfried JA, O’Doherty J, Dolan RJ (2003): Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science 301:1104–1107. [DOI] [PubMed] [Google Scholar]
- 48.Morris J, Dolan R (2001): Involvement of human amygdala and orbitofrontal cortex in hunger-enhanced memory for food stimuli. Journal of Neuroscience 21:5304–5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD (2004): Images of desire: food-craving activation during fMRI. Neuroimage 23:1486–1493. [DOI] [PubMed] [Google Scholar]
- 50.Rothemund Y, Preuschhof C, Bohner G, Bauknecht H-C, Klingebiel R, Flor H, et al. (2007): Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage 37:410–421. [DOI] [PubMed] [Google Scholar]
- 51.Volkow ND, Wang G-J, Telang F, Fowler JS, Logan J, Childress A-R, et al. (2006): Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. Journal of Neuroscience 26:6583–6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McClernon FJ, Kozink RV, Lutz AM, Rose JE (2009): 24-h smoking abstinence potentiates fMRI-BOLD activation to smoking cues in cerebral cortex and dorsal striatum. Psychopharmacology 204:25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Temple JL, Legierski CM, Giacomelli AM, Salvy S-J, Epstein LH (2008): Overweight children find food more reinforcing and consume more energy than do nonoverweight children. The American journal of clinical nutrition 87:1121–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bechara A, Tranel D, Damasio H, Damasio AR (1996): Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cereb Cortex 6:215–225. [DOI] [PubMed] [Google Scholar]
- 55.Bechara A, Damasio H, Tranel D, Damasio AR (1997): Deciding advantageously before knowing the advantageous strategy. Science 275:1293–1295. [DOI] [PubMed] [Google Scholar]
- 56.Bechara A, Damasio H, Tranel D, Anderson SW (1998): Dissociation Of working memory from decision making within the human prefrontal cortex. J Neurosci 18:428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW (2003): Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature neuroscience 6:115–116. [DOI] [PubMed] [Google Scholar]
- 58.Aron A, Robbins T, Poldrack R (2004): Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences 8:170–177. [DOI] [PubMed] [Google Scholar]
- 59.Nederkoorn C, Braet C, Van Eijs Y, Tanghe A, Jansen A (2006): Why obese children cannot resist food: the role of impulsivity. Eating behaviors 7:315–322. [DOI] [PubMed] [Google Scholar]
- 60.Loeber S, Grosshans M, Korucuoglu O, Vollmert C, Vollstädt-Klein S, Schneider S, et al. (2012): Impairment of inhibitory control in response to food-associated cues and attentional bias of obese participants and normal-weight controls. International journal of obesity 36:1334–1339. [DOI] [PubMed] [Google Scholar]
- 61.Le DSN, Pannacciulli N, Chen K, Del Parigi A, Salbe AD, Reiman EM, et al. (2006): Less activation of the left dorsolateral prefrontal cortex in response to a meal: a feature of obesity. The American journal of clinical nutrition 84:725–731. [DOI] [PubMed] [Google Scholar]
- 62.Naqvi NH, Rudrauf D, Damasio H, Bechara A (2007): Damage to the insula disrupts addiction to cigarette smoking. Science 315:531–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Naqvi NH, Bechara A (2009): The hidden island of addiction: the insula. Trends Neurosci 32:56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Frank S, Laharnar N, Kullmann S, Veit R, Canova C, Hegner YL, et al. (2010): Processing of food pictures: influence of hunger, gender and calorie content. Brain research 1350:159–166. [DOI] [PubMed] [Google Scholar]
- 65.Simmons WK, Martin A, Barsalou LW (2005): Pictures of appetizing foods activate gustatory cortices for taste and reward. Cerebral Cortex 15:1602–1608. [DOI] [PubMed] [Google Scholar]
- 66.Schienle A, Schäfer A, Hermann A, Vaitl D (2009): Binge-eating disorder: reward sensitivity and brain activation to images of food. Biological psychiatry 65:654–661. [DOI] [PubMed] [Google Scholar]
- 67.Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M (2001): Changes in brain activity related to eating chocolate. Brain 124:1720–1733. [DOI] [PubMed] [Google Scholar]
- 68.Avery JA, Powell JN, Breslin FJ, Lepping RJ, Martin LE, Patrician TM, et al. (2017): Obesity is associated with altered mid-insula functional connectivity to limbic regions underlying appetitive responses to foods. J Psychopharmacol 31:1475–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berns GS, McClure SM, Pagnoni G, Montague PR (2001): Predictability modulates human brain response to reward. Journal of neuroscience 21:2793–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dreher J-C, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF (2007): Menstrual cycle phase modulates reward-related neural function in women. Proceedings of the National Academy of Sciences 104:2465–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Van Vugt DA (2009): Brain imaging studies of appetite in the context of obesity and the menstrual cycle. Human reproduction update 16:276–292. [DOI] [PubMed] [Google Scholar]
- 72.Frank TC, Kim GL, Krzemien A, Van Vugt DA (2010): Effect of menstrual cycle phase on corticolimbic brain activation by visual food cues. Brain research 1363:81–92. [DOI] [PubMed] [Google Scholar]
- 73.De Silva A, Salem V, Long CJ, Makwana A, Newbould RD, Rabiner EA, et al. (2011): The gut hormones PYY3–36 and GLP-17–36 amide reduce food intake and modulate brain activity in appetite centers in humans. Cell metabolism 14:700–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ramsey JD, Hanson SJ, Hanson C, Halchenko YO, Poldrack RA, Glymour C (2010): Six problems for causal inference from fMRI. neuroimage 49:1545–1558. [DOI] [PubMed] [Google Scholar]
- 75.Smith SM (2012): The future of FMRI connectivity. Neuroimage 62:1257–1266. [DOI] [PubMed] [Google Scholar]
- 76.Wang G-J, Volkow ND, Telang F, Jayne M, Ma J, Rao M, et al. (2004): Exposure to appetitive food stimuli markedly activates the human brain. Neuroimage 21:1790–1797. [DOI] [PubMed] [Google Scholar]
- 77.Giedd J, Blumenthal J, Jeffries N, Castellanos F, Liu H, Zijdenbos A, et al. (1999): Brain development during childhood and adolescence: a longitudinal MRI study. Nature neuroscience 2:861–862. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.