Abstract
Background and Purpose
Semantic verbal fluency test is a neuropsychological assessment that can sensitively detect neuropathological changes. Considering its multifactorial features tapping various cognitive domains such as semantic memory, executive function, and working memory, it is necessary to examine verbal fluency performance in association with underlying cognitive functions. The objective of the current study was to investigate semantic fluency patterns of people with mild cognitive impairment (MCI) based on clustering and switching and their relationship with working memory.
Methods
Twenty-six individuals with MCI and 23 normal elderly adults participated in this study. A semantic verbal fluency test (animal version) was administered and the performance was analyzed using the following measures: number of correct words, cluster size, and number of switches. Scores of digit forward (DF) and backward span tasks were employed as working memory measures.
Results
Analyses of variance revealed significant group differences in the numbers of correct words and switches. Multivariate logistic regression and receiver-operating characteristic analyses showed that the number of switches more sensitively distinguished MCI existence than the number of correct words. Stepwise linear regression analysis showed that DF task and age significantly predicted the number of correct words while only the DF task significantly predicted the number of switches.
Conclusions
Decrement in semantic verbal fluency in MCI seems to be associated with impaired switching abilities. Working memory capacity might serve as the underlying cognitive factor related to decreased verbal fluency in MCI.
Keywords: Mild Cognitive Impairment, Semantic Fluency, Clustering, Switching, Working Memory
INTRODUCTION
Mild cognitive impairment (MCI) is a relatively widespread clinical condition. It is at an intermediate stage from normal aging to clinical stages of dementia. Patients with MCI might have independent daily life. However, memory impairment is the main symptom of MCI.1,2 The risk of MCI to progress to dementia has been reported to be 10 to 15% per year or 50 to 80% per year, which is much higher than the risk of 1%–2% in normal elderly people.3,4,5,6 Over a period of 10 years or more before patients are diagnosed with dementia, patients with MCI undergo a slight cognitive change and gradually progress to dementia.7,8
MCI includes various cognitive dysfunctions ranging from mild memory impairment to early dementia. Because memory impairment is its main symptom, it is possible that not only high-risk group of dementia, but also various patient groups such as memory disorders due to depression are included in MCI. It is important to understand cognitive mechanisms that influence the progression of dementia due to the heterogeneous nature of these MCI groups. Episodic memory impairment is known to be an important indicator of pre-dementia including MCI.9,10 However, recent studies have reported impairment in various areas (such as general cognitive ability, speaking ability, orientation, attention, executive function, abstract thinking ability, and complex time and space related functions) and episodic memory deterioration in MCI patients.11,12,13,14 Particularly, memory impairment and impairment of executive function have been reported as cognitive functions associated with progression to dementia.15,16
Semantic verbal fluency test evaluates the executive function and the semantic memory. It is a highly sensitive test that can distinguish MCI group from normal elderly people.17,18,19 In addition, it can distinguish normal group from the high-risk group of dementia20,21,22. It has been reported that it can distinguish between dementia progressive group and non-progressive group in MCI.12,23,24,25,26 Therefore, analyzing the performance of semantic verbal fluency task of the MCI group may help us understand not only cognitive characteristics of MCI, but also cognitive mechanisms that influence the progression to dementia.
Semantic verbal fluency task is a task to produce as many words belonging to a specific category as possible within a given time. To accomplish this task, it is necessary to access semantic network and retrieve information. While confrontation naming task that gives a stimulus corresponding to a certain word is used to evaluate the degree of damage to the semantic memory, the semantic verbal fluency task taker must find and retrieve a word belonging to a specific category. Therefore, it is a multi-domain task that could evaluate semantic memory and executive function at the same time.27 In order to efficiently retrieve a specific category of words, semantic knowledge must be preserved while cognitive mechanism capable of productively searching and retrieving relevant information from the semantic knowledge must also operate functionally.28 This self-initiated retrieval is known to be influenced by various cognitive factors such as cognitive flexibility, search strategy, executive function, working memory, processing speed, and verbal ability.29,30,31
In particular, working memory has been reported to be associated with performance of fluency tasks in several previous studies.32,33,34,35,36,37 Working memory is a cognitive mechanism that temporarily stores and processes needed information while performing complex cognitive tasks.38 According to the model proposed by Baddeley and Hitch,38 working memory consists of two short-term storage systems: a visuo-spatial sketchpad which temporarily maintain and manipulate visuo-spatial information, and a phonological loop which stores and rehearses phonological information. In addition, there is a central executive, an attention system of limited capacity to control and process such information. This central executive is an attentional-controlling system that can intervene in the conduct of complex cognitive tasks, particularly a cognitive system vulnerable to brain damage due to Alzheimer's dementia (AD).39
Many studies have suggested that working memory has a significant impact on exploration and retrieval of information from long-term memory.36,40,41 In particular, Rosen and Engle36 have analyzed differences in verbal fluency task performance among participants with different levels of working memory capacity. For participants with high working memory, a larger number of category exemplars were calculated. The number of words decreased with increasing difficulty of the task. Based on these results, they proposed a component model of retrieval related to fluency task performance, suggesting that working memory is an important factor in information retrieval. The component model of retrieval comprises four retrieval components: activation that automatically spreads from clues, self-monitoring of output to avoid repetition and error, suppression of previously retrieved reactions, and creation of clues to access new names. The first stage of these steps is automatic diffusion of semantic memory stored in the long-term memory when categorical clue is given in the task. It happens without active control. However, the remaining steps are processes requiring attention capacity, meaning that working memory capacity is involved.31,36
Studies to elucidate various cognitive functions involved in performing a fluency task have emphasized the importance of analyzing qualitative aspects of the process beyond the number of appropriately retrieved words or categorical error responses.28,42 Troyer et al.28 have argued that qualitative analysis of components such as clustering and switching is needed to figure out the characteristic of cognitive processing underlying the fluency task. Clustering is to produce words belonging to a particular subcategory relying on semantic memory and word knowledge. Switching is a process that moves efficiently into a new subcategory depending on the executive function that is responsible for the strategic search and retrieval process. In the fluency task, there is a time constraint. Therefore, a strategy to efficiently retrieve information within a limited time is needed. In order to perform the task optimally, words related to a specific subcategory are clustered. If a subcategory is exhausted, one has to shift to a new subcategory and retrieve.43
Clustering and switching have been discussed in relation to cognitive functions related to specific areas of the brain. Clustering depends on temporal processing known as verbal memory and word storage as a process of accessing and retrieving words. Switching depends on frontal processing such as strategic search processes and cognitive flexibility, attention switching, and executive function.28,43 These distinctive mechanisms of clustering and switching have become more persuasive through studies on patients with localized brain injury or functional brain imaging studies. Related studies have reported switching defects in patients with frontal lobe damages and clustering defects in patients with temporal lobe damages. In the case of patients with generalized brain injury or dementia, it has been reported that semantic fluency tasks are defective in both clustering and switching as well as in the number of correct words.43,44,45,46 In addition, brain imaging studies have shown that activation of temporal and frontal lobes is increased during performing semantic fluency task, indicating that it is a task involving both frontal and temporal lobes.47,48,49,50
Studies on fluency tasks for dementia patients have reported performance deficits primarily based on the number of correct words. In AD patients, decreased performance has been consistently reported.21,45,51,52,53,54 Significant decreases in performance were also observed in patients with Lewy body dementia and vascular dementia,55,56,57 although such decreases were not as consistent as those in AD patients. Retrieved number of words is the most commonly used index of fluency task performance. However, it is less sensitive for distinguishing subgroups of dementia. In addition, it has the disadvantage of not providing information about underlying cognitive mechanisms.58,59 Considering these drawbacks, some studies have analyzed the performance of AD patients based on clustering and switching and reported relatively consistent results, showing deficiencies in both clustering and switching as well as number of correct words. Furthermore, qualitative analysis such as clustering and switching has been reported to be a sensitive measure to distinguish even a mild AD from a control group.22,54,60 There are also attempts to classify dementia subgroups using clustering and switching. Troyer et al.53 have reported that delicate measurements to distinguish AD group and Parkinson group with dementia are clustering and switching rather than the number of correct words. Park et al.61 have also reported the number of correct words is more sensitive measurement for distinguishing vascular dementia and Alzheimer's disease patients.
Analysis of fluency task performance has indicated that the number of words produced in the MCI group is significantly lower than that of the control group.14,20,21,22,62,63,64,65 However, studies on performance of the MCI group through qualitative analysis of the fluency task have reported opposite findings. Although the semantic memory of the MCI group is impaired, both stances that the executive function is preserved20,22 and the executive function is impaired with semantic memory65 are reported. These various results may reflect the heterogeneity of the MCI group. However, in a study that reported no significant difference in cluster size or number of switches between MCI group and control group, clustering and switching reduction patterns observed in the MCI group followed the tendency of the AD group, suggesting that qualitative analysis of semantic fluency task might be a good screening test for early diagnosis of dementia.22
Domestic studies reporting the fluency task performance in the MCI group mainly provided results based on the number of words.14,51,63,64 Few studies have analyzed qualitative analysis using clustering and switching. Therefore, the objective of this study was to examine cluster size and the number of switches in addition to the number of correct words in MCI group and determine which measures sensitively distinguish MCI group from the normal elderly, taking the multidimensional characteristic of the semantic fluency task into account. We also attempted to verify the degree to which each measure to distinguish MCI existence. Based on a previous study showing that working memory could affect fluency task performance, we also examined the effect of working memory on semantic fluency task performance by analyzing whether digit forward (DF) and digit backward (DB) scores used as indicators of working memory might be related to measures of fluency task.
METHODS
Subjects
This study was performed on 26 patients with MCI and 23 normal elderly adults in Seoul and Gyeonggi province, Korea. The MCI group was diagnosed as a MCI by a neurologist. Scores of the Short version of Geriatric Depression Scale66 of subjects were less than 8 points which belonged to normal range. Those with Korean-Instrumental Activities of Daily Living67 score of less than 0.43, no difficulty in everyday life, and a Clinical Dementia Rating68 of 0.5 were enrolled. In addition, subjects were recruited based on the criteria for the diagnosis of MCI presented by Petersen2: 1) subjective memory impairment appealed by patients and caregivers, 2) cognitive impairment in memory areas in neuropsychological tests, 3) overall cognitive function was normal except memory area, 4) complete daily activities ability, and 5) not diagnosed as dementia. Normal elderly was those aged over 60 years with normal range of Korean-Mini Mental State Examination (K-MMSE),69,70 16%ile or more, and normal range of the Seoul Verbal Learning Test-Elderly's version (SVLT-E).71 Based on results of questionnaire on health screening,72 subjects without neuropsychiatric history were selected. They were matched in age, gender, and education level according to the MCI group. There were no statistically significant differences in age, education years, or gender between the normal elderly group and the MCI group. Demographic information for groups, descriptive statistics for K-MMSE, DF, DB, and SVLT, and inference statistics for verifying differences between the two groups are presented in Table 1.
Table 1. Demographic characteristics and descriptive data from neuropsychological tests.
| Characteristics | Gender | Age (yr) | Education (yr) | K-MMSE | DF score | DB score | SVLT (immediate) | SVLT (delayed) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Man | Woman | Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| MCI (n=26) | 6 | 20 | 72.85 (5.64) | 61–83 | 6.40 (5.36) | 0.5–18 | 24.54 (3.04) | 4.23 (2.02) | 3.58 (1.77) | 17.35 (6.24) | 4.04 (3.36) |
| Normal (n=23) | 6 | 17 | 70.07 (5.93) | 63–88 | 9.19 (4.46) | 0.5–16 | 27.61 (1.87) | 7.30 (2.94) | 4.87 (1.42) | 19.57 (5.53) | 6.48 (2.62) |
| Statistical value | 0.060 | 1.666 | −1.965 | −3.865* | −4.297* | −2.792* | −1.309 | −2.803* | |||
For statistical analysis, t-test and χ2 test were performed for continuous variables and categorical variable, respectively.
SD: standard deviation, DF: digit forward, DB: digit backward, SVLT: Seoul Verbal Learning Test.
*p<0.01.
Analysis of tasks and data
The test for evaluating semantic fluency was an animal naming test included in Seoul Neuropsychological Screening Battery 2nd Edition.71 Participants were instructed to speak names of animals as quickly as possible for one minute. In order to measure their working memory, DF and DB tests included in the Korean version of the Wechsler Adult Intelligence Scale IV73 were administered. Total score of correct response was used as a measurement of working memory capacity.
Based on the analysis method of Troyer,46 we calculated the number of correct words, the mean cluster size, and the number of switches from the word list produced by participants for qualitative analysis of fluency task performance. Repeated responses and intrusion error responses not in the category were excluded from the number of correct words, but included in the mean cluster size and the number of switches.28,46 The mean cluster size was calculated by dividing the total sum of cluster size by the number of clusters. Cluster size was calculated by counting from the second word in the cluster of words belonging to a specific subcategory. The number of switches was calculated as the number of movements between clusters. Analysis of data was performed by two doctoral students majoring in speech-language pathology. Their agreement was 100%.
In statistical analysis, t-test was used to find out difference between the MCI group and the control group in three types of fluency task scores: number of correct words, mean cluster size, and number of switches. Multivariate logistic regression analysis and receiver-operating characteristics (ROCs) analysis were performed to find if each score correlated with MCI presence. In addition, stepwise multiple linear regression analysis was performed to investigate whether demographic variables and DF and DB scores as indicators of working memory were significantly associated with fluency task scores. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and statistical significance was tested at significance level of 0.05.
RESULTS
Comparison of intergroup performance by semantic fluency task type
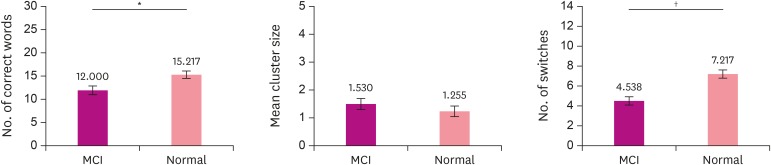
In order to figure out difference between the normal elderly group and the MCI group for the number of correct words, the mean cluster size, and the number of switches, t-test was performed for each measure. Intergroup difference in the number of correct words was significant (t(47)=−2.62; p<0.05). The mean number of correct words (M=12.000) in the MCI group was significantly lower than that (M=15.217) in the normal elderly group. For mean cluster size, the intergroup difference was not significant (t(47)=1.06; p>0.05). Regarding the number of switches, the intergroup difference was significant (t(47)=−4.42; p<0.000). The mean number of switches in the MCI group (M=4.538) was significantly lower than that in the normal elderly group (M=7.217) (Fig. 1).
Fig. 1. Number of correct words, mean cluster size, and number of switches in MCI and normal elderly groups.
MCI: mild cognitive impairment.
*p<0.05; †p<0.01.
Results of multivariate logistic regression analysis
As the number of correct words and the number of switches were significantly different between the MCI group and the normal elderly group, multivariate logistic regression analysis was performed to determine whether the number of correct words and number of switches were correlated with MCI after controlling for age, gender, and years of education. Between MCI presence and the number of correct words, the number of correct words was found to be a significant risk factor for discriminating the MCI group. When the number of correct words was increased, the risk of MCI was about 20% lower (odds ratio [OR], 0.818; 95% confidence interval [CI], 0.671–0.996). In addition, between MCI presence and the number of switches, the number of switches was found to be a significant variable for effectively discriminating the MCI group. When the number of switches was increased, the risk of MCI was about 50% lower (OR, 0.511; 95% CI, 0.326–0.802). Logistic regression analysis results for each model are shown in Table 2.
Table 2. Adjusted ORs for MCI by the number of correct words and the number of switches.
| Risk factors | OR | 95% CI | p-value |
|---|---|---|---|
| No. of correct words | 0.818 | 0.671–0.996 | 0.046 |
| No. of switches | 0.511 | 0.326–0.802 | 0.004 |
OR: odds ratio, MCI: mild cognitive impairment, CI: confidence interval.
Sensitivity and specificity analysis by ROC analysis
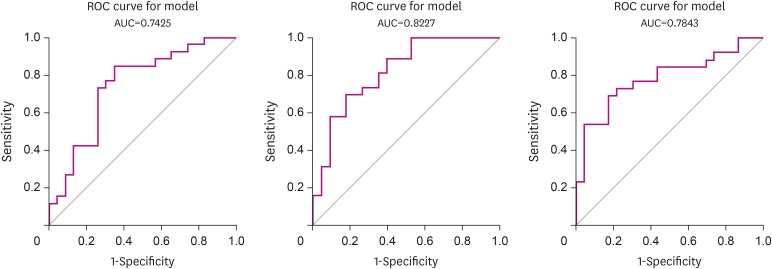
To determine whether the number of correct words and the number of switches were sensitive to the presence of MCI, ROC analysis was conducted on models after controlling for age, years of education, and gender. The area under the curve (AUC) for the number of correct words was 0.742 and the AUC for the number of switches was 0.823. In addition, the AUC for Mini Mental State Examination (MMSE) was 0.784 when ROC analysis for MMSE was performed to see whether these two measures of fluency test were more sensitive to MCI than MMSE, a measure of overall cognitive function. The ROC curve for each measurement is shown in Fig. 2.
Fig. 2. ROC curves of number of correct words, number of switches, and K-MMSE for MCI.
ROC: receiver-operating characteristic, AUC: area under the curve, K-MMSE: Korean-Mini Mental State Examination, MCI: mild cognitive impairment.
Results of correlation analysis and stepwise multiple linear regression analysis
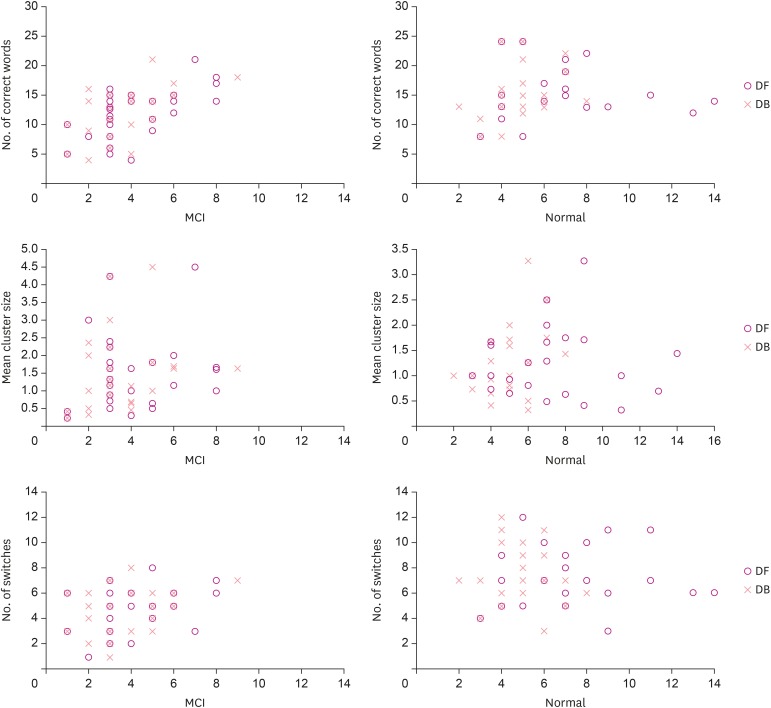
Correlation analysis was performed to determine whether number of correct words, mean cluster size, and number of switches in the MCI group were significantly related to age, years of education, K-MMSE, DF score, and DB score. Results showed that number of correct words significantly correlated with age (r=−0.446; p<0.05), DF score (r=0.606; p<0.01), and DB score (r=0.534; p<0.01). In particular, DF and DB scores showed strong positive correlations with number of correct words. Mean cluster size did not correlate with any variables (all p>0.05). In the case of number of switches, it showed a significant correlation with DF score (r=0.411; p<0.05). To investigate correlation between fluency task scores, we analyzed the correlations among number of correct words, mean cluster size, and number of switches. As a result, correlations of number of correct words with cluster size (r=0.498; p<0.01), and number of switches (r=0.541; p<0.01) were significant. Regarding the normal elderly group, there were no significant correlations of age, years of education, K-MMSE, DF, and DB (all p>0.05) with any score of the fluency test. For fluency task scores, correlations of number of switches with number of correct words (r=0.431; p<0.05) and cluster size (r=−0.595; p<0.01) were significant. Correlation coefficients between variables are shown in Table 3. DF and DB scatterplots of the fluency task are shown in Fig. 3.
Table 3. Pearson correlation coefficients among fluency measures, demographic information, DF score, and DB score.
| Characteristics | Age (yr) | Education (yr) | K-MMSE | DF score | DB score | No. of correct words | Mean cluster size | No. of switches | |
|---|---|---|---|---|---|---|---|---|---|
| No. of correct words | |||||||||
| MCI | −0.446* | 0.206 | 0.361 | 0.606† | 0.534† | 1.000 | |||
| Normal | −0.026 | 0.177 | −0.028 | −0.070 | 0.337 | 1.000 | |||
| Mean cluster size | |||||||||
| MCI | −0.088 | −0.001 | 0.021 | 0.150 | 0.222 | 0.498* | 1.000 | ||
| Normal | −0.162 | 0.341 | 0.089 | −0.007 | 0.368 | 0.305 | 1.000 | ||
| No. of switches | |||||||||
| MCI | −0.315 | 0.186 | 0.251 | 0.411* | 0.323 | 0.541† | −0.373 | 1.000 | |
| Normal | 0.059 | −0.245 | −0.121 | 0.035 | −0.044 | 0.431* | −0.595† | 1.000 | |
MCI: mild cognitive impairment, K-MMSE: Korean-Mini Mental State Examination69, DF: digit forward, DB: digit backward.
*p<0.05; †p<0.01.
Fig. 3. Scatterplot for both groups (MCI and normal elderly) in number of correct words, mean cluster size, and number of switches.
MCI: mild cognitive impairment, DF: digit forward, DB: digit backward.
To investigate whether age, years of education, K-MMSE, DF, and DB scores were related to measures of the fluency task, a stepwise multiple linear regression analysis was conducted for each group. As a result, in the MCI group, DF and age were significantly associated with number of correct words, explaining 51.9% of total variance of number of correct words (F(2, 23)=12.422; p<0.001; R2=0.519). For standardized coefficients showing relative contribution of independent variables, DF (β=0.568) had the greatest effect on number of correct words, followed by age (β=−0.392). In addition, DF (β=0.411) showed a significant correlation with the number of switches (F(1, 24)=4.866; p<0.05; R2=0.169). The model predicting cluster size in the MCI group and the models predicting all measures of semantic fluency in the normal elderly group were not significant (all p>0.05).
DISCUSSION
In this study, we explored the performance of the MCI group in semantic fluency task using number of correct words, cluster size, and number of switches to determine which measurements were sensitive to distinguish the MCI group from the normal elderly group. We also tried to investigate the extent to which each measure could distinguish MCI existence. Furthermore, we examined whether the working memory measured by the DF/DB task showed a significant correlation with fluency measures in order to determine effects of working memory on semantic fluency task performance.
As a result of analyzing the significant difference between the MCI group and the normal elderly group for each measure of the semantic fluency task, it was found that the number of correct words and the number of switches in the semantic fluency task could distinguish the MCI group from the normal elderly group. Results of previous studies have reported that the number of correct words and the number of switches are sensitive measures that can distinguish the MCI group from the control group.65,74 The number of correct words in the MCI group was significantly lower than that in the normal elderly group. This phenomenon can be seen in the relationship between cluster size and number of switches. The decrease in the number of correct words might be the result of a decrease in the number of switches in that the cluster size of the MCI group did not show a significant difference from the normal elderly group. In other words, the absence of group differences in cluster size suggests that the ability to retrieve words in a particular category of the MCI group is not different from that of the normal elderly group. On the other hand, results of a significant decrease in the number of switches in the MCI group suggest that their abilities to search for various subcategories under the category of animals on the semantic network or to switch between subcategories are impaired. That is, the decrease in total number of correct words in the MCI group can be interpreted as a result of stopping generating words without switching to other subcategories when completing word production in a certain subcategory. In this study, we showed that the number of switches (pη2=0.294) is a more sensitive measure of the MCI group than the number of correct words (pη2=0.127). This is in agreement with results of Nutter-Upham et al.65 showing that switching is the most reliable measure of group classification. The result that the number of switches was a more useful indicator to distinguish between the two groups was supported by results of multiple logistic regression analyses that revealed the correlation of MCI presence. As the number of correct words was increased, the risk of becoming MCI was 20% lower, but the risk was 50% lower when the number of switches was increased. Also, through ROC analysis, the AUC of the number of switches was 0.823, indicating that it was superior to the number of correct words or MMSE in discriminating MCI. The decrease in the number of switches suggests the possibility of defects in the executive function of the MCI group. This result was supported by results showing that DF and DB scores of the MCI group were significantly lower than those in the normal elderly group and that the DF score in multiple linear regression analysis was significantly associated with the number of switches in the MCI group. Therefore, performance degradation of the fluency task of the MCI group is a phenomenon that occurs due to damage of other cognitive domains besides the semantic memory.54 Specifically, it suggests that damage to the executive function involved in category switching such as category discovery and access might be the cause. In addition, switching deficits have been reported as a factor in MCI for distinguishing dementia progressing group from non-dementia group.50,75 In that respect, switching may be an important factor in understanding the cognitive mechanisms that influence progression to dementia.76
The absence of difference between groups in cluster size has also been reported in previous studies.22,74 These results suggest that cluster size is not a useful measure in distinguishing between MCI and normal elderly groups. However, in studies of AD patients, there was a significant difference in cluster size as well as the number of correct words and the number of switches in the AD group compared to the control group.22,53 Therefore, as the process proceeds to AD, it may be difficult to access the semantic network and retrieve semantic knowledge. Understanding the change in cluster size can also help us understand the progress of this disease.
This study showed that the reduction in the number of correct words and the number of switches in the MCI group could be explained by a decrease in the working memory capacity of the MCI group. This supports results of previous studies that suggest working memory may be an important variable affecting semantic fluency task performance.31,36 When investigating whether working memory is associated with measurements of the semantic fluency task by using scores of DF and DB tasks as an indicator of working memory, DF scores and age were associated with the number of correct words in the MCI group while the number of switches was related to DF score. In particular, the association of DF scores with the number of correct words was found to be higher than the association of age with the number of correct words. These results suggest that impaired executive function in the MCI group is sensitively associated with the number of correct words of semantic fluency tasks and the number of switches.65,74,75 The fact that working memory is significantly more related to the performance of semantic fluency task than the K-MMSE score indicating the overall cognitive function or the number of years of education suggests that category switching deficiencies driven by working memory deficits can explain the difficulty of semantic fluency task performance. Therefore, deterioration of the executive attention related to manipulating information in short term memory may be an important cognitive mechanism to explain the deterioration of semantic fluency task performance. These associations did not appear in normal elderly adults, consistent with results of Rosen and Engle36 reporting that people with higher working memory did not have problems with semantic fluency task performance while those with lower working memory had difficulty to perform fluency task due to limited working memory capacity in memory retrieval stage requiring attentional control. In addition, these findings suggest that delicate executive function impairment may occur from the MCI stage, unlike the claim that executive difficulties appear prominent in later stages of neurodegenerative disease.65 Age also appeared to be a variable associated with the number of correct words in the MCI group, suggesting that cognitive aging might have a greater impact on semantic fluency task performance in the MCI group than that in the normal elderly group. Age is known to be an important risk factor causing cognitive impairment.77 Impaired semantic memory or executive function of the MCI group is more vulnerable due to overall cognitive impairment caused by aging. Therefore, it may be more apparent in MCI group that the number of words generated decreases as age increases.
DB score as another measure of working memory failed to approach significance in the stepwise multiple linear regression analysis. This seems to be attributed to the effects of range restriction of DB scores in the MCI group.78 In the case of DF, the score is distributed relatively evenly between 1 and 8. However, in the case of DB, the majority of scores are narrowly distributed around lower scores. The range restriction of scores for tasks with a high degree of difficulty in the clinical population has been reported in several studies. In order to accurately identify cognitive characteristics of the clinical population, it is important to select a task that can reflect their individual differences.79 Also, a high correlation between DF and DB scores (r=0.642; p<0.000) might be the reason for excluding DB score in the stepwise multiple linear regression analysis model where the number of correct words was a dependent variable. The number of correct words and DB score showed a strong positive correlation (r=0.523; p<0.01). However, in a stepwise multiple regression analysis, the most relevant variable is firstly put into the regression equation After that, when the next relevant variable is put into the model, the covariance of the first variable and the second variable is excluded. Therefore, in the current data, DB variable which shared a large portion of variance with DF variable could not be put into the model predicting the number of correct words.
Analyzing correlations between measurements of the semantic fluency task provides additional information on the task performance aspect of the MCI group. The participants who performed switching many times also generated many correct words in both the MCI and normal elderly groups. In other words, the number of correct words of people who actively moved between categories through active search of sub-categories was higher than those who did not. In the MCI group, the larger the cluster size, the more the number of correct words was increased. This reflects that in MCI group, cluster size has a large influence on the number of correct words due to the lack of switching ability. These results suggest that both ability to search subcategories and retrieval ability of words within the category can be important variables affecting the number of correct words in the MCI group. The larger the cluster size, the fewer switching occurred in normal elderly. The negative relationship between cluster size and the number of switches can be interpreted as a natural phenomenon due to time constraints. This phenomenon did not appear in the MCI group, indicating that the MCI group might have less time constraints on task performance than the normal elderly.
This study analyzed the semantic fluency task performance of the MCI group using the analysis method of clustering and switching. Measurements that could distinguish the MCI group from normal elderly were found to be the number of correct words and number of switches, with the number of switches being a more sensitive measure. DF score was significantly associated with the number of correct words and the number of switches in the MCI group, indicating that semantic fluency task performance in MCI group was associated with working memory. In clinical practice, fluency task is used to evaluate frontal and temporal functions in a relatively short period of time. However, the evaluation is mainly based on the number of correct words. This means that information revealing various cognitive characteristics is not being fully utilized. This study shows that analysis of qualitative characteristics such as clustering and switching needs to be used to understand cognitive characteristics of MCI group.
This study has some limitations. First, it did not use various tasks to measure working memory or executive function. In this study, we used a standardized DF/DB task as a measure of working memory for Korean speakers. However, in order to further strengthen the result that working memory has an important effect on semantic fluency task performance, it is necessary to look at the impact of working memory measured through various tasks. In addition, semantic fluency task is a multidisciplinary task involving various cognitive factors. Thus, further study needs to analyze factors contributing to task performance by examining the relationship with various cognitive functions such as language ability and verbal/nonverbal memory. Finally, this study focused on semantic fluency test to examine patterns of clustering and switching. Analyzing features of phonemic fluency test in subsequent studies is needed to identify cognitive impairment patterns of the MCI group on their fluency task.
Footnotes
Funding: This work was supported by the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (NRF-2017R1A2B4006604).
Conflicts of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Oh SJ, Sung JE.
- Data curation: Oh SJ, Choi SJ.
- Formal analysis: Oh SJ, Sung JE, Choi SJ.
- Funding acquisition: Sung JE.
- Methodology: Oh SJ, Sung JE.
- Project administration: Oh SJ, Sung JE, Jeong JH.
- Resources: Choi SJ, Jeong JH.
- Supervision: Sung JE.
- Validation: Oh SJ, Sung JE.
- Visualization: Oh SJ.
- Writing - original draft: Oh SJ, Choi SJ.
- Writing - review & editing: Oh SJ, Sung JE.
References
- 1.Morris JC. Mild cognitive impairment is early-stage Alzheimer disease: time to revise diagnostic criteria. Arch Neurol. 2006;63:15–16. doi: 10.1001/archneur.63.1.15. [DOI] [PubMed] [Google Scholar]
- 2.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 3.Chertkow H. Mild cognitive impairment. Curr Opin Neurol. 2002;15:401–407. doi: 10.1097/00019052-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Flicker C, Ferris SH, Reisberg B. Mild cognitive impairment in the elderly: predictors of dementia. Neurology. 1991;41:1006–1009. doi: 10.1212/wnl.41.7.1006. [DOI] [PubMed] [Google Scholar]
- 5.Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 6.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 7.Aguirre-Acevedo DC, Lopera F, Henao E, Tirado V, Muñoz C, Giraldo M, et al. Cognitive decline in a Colombian kindred with autosomal dominant Alzheimer disease: a retrospective cohort study. JAMA Neurol. 2016;73:431–438. doi: 10.1001/jamaneurol.2015.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajan KB, Wilson RS, Weuve J, Barnes LL, Evans DA. Cognitive impairment 18 years before clinical diagnosis of Alzheimer disease dementia. Neurology. 2015;85:898–904. doi: 10.1212/WNL.0000000000001774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Jager CA, Budge MM. Stability and predictability of the classification of mild cognitive impairment as assessed by episodic memory test performance over time. Neurocase. 2005;11:72–79. doi: 10.1080/13554790490896820. [DOI] [PubMed] [Google Scholar]
- 10.Ganguli M, Dodge HH, Shen C, DeKosky ST. Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology. 2004;63:115–121. doi: 10.1212/01.wnl.0000132523.27540.81. [DOI] [PubMed] [Google Scholar]
- 11.Bäckman L, Jones S, Berger AK, Laukka EJ, Small BJ. Cognitive impairment in preclinical Alzheimer's disease: a meta-analysis. Neuropsychology. 2005;19:520–531. doi: 10.1037/0894-4105.19.4.520. [DOI] [PubMed] [Google Scholar]
- 12.Han NE, Chin JH, Lee BH, Seo SW, Na DY. Neuropsychological characteristics and rates of conversion to dementia in early-and late-onset mild cognitive impairment. Korean J Clin Psychol. 2012;31:373–390. [Google Scholar]
- 13.Pérès K, Chrysostome V, Fabrigoule C, Orgogozo JM, Dartigues JF, Barberger-Gateau P. Restriction in complex activities of daily living in MCI: impact on outcome. Neurology. 2006;67:461–466. doi: 10.1212/01.wnl.0000228228.70065.f1. [DOI] [PubMed] [Google Scholar]
- 14.Yoon JH, Ahn IS, Kim DK, Kim JH. Neuropsychological evaluation of mild cognitive impairment: comparison of no cognitive impairment and early Alzheimer's disease. Korean J Clin Psychol Annu Conf. 2005;6:408–409. [Google Scholar]
- 15.Belleville S, Chertkow H, Gauthier S. Working memory and control of attention in persons with Alzheimer's disease and mild cognitive impairment. Neuropsychology. 2007;21:458–469. doi: 10.1037/0894-4105.21.4.458. [DOI] [PubMed] [Google Scholar]
- 16.Taler V, Voronchikhina A, Gorfine G, Lukasik M. Knowledge of semantic features in mild cognitive impairment. J Neurolinguist. 2016;38:56–70. [Google Scholar]
- 17.Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 18.Cunje A, Molloy DW, Standish TI, Lewis DL. Alternate forms of logical memory and verbal fluency tasks for repeated testing in early cognitive changes. Int Psychogeriatr. 2007;19:65–75. doi: 10.1017/S1041610206003425. [DOI] [PubMed] [Google Scholar]
- 19.Ribeiro F, de Mendonça A, Guerreiro M. Mild cognitive impairment: deficits in cognitive domains other than memory. Dement Geriatr Cogn Disord. 2006;21:284–290. doi: 10.1159/000091435. [DOI] [PubMed] [Google Scholar]
- 20.Adlam AL, Bozeat S, Arnold R, Watson P, Hodges JR. Semantic knowledge in mild cognitive impairment and mild Alzheimer's disease. Cortex. 2006;42:675–684. doi: 10.1016/s0010-9452(08)70404-0. [DOI] [PubMed] [Google Scholar]
- 21.Choi H. A comparison of the performances of confrontation naming test and verbal fluency task in patients with prodromal Alzheimer's disease and mild Alzheimer's disease. Speech Sci. 2008;15:111–118. [Google Scholar]
- 22.Murphy KJ, Rich JB, Troyer AK. Verbal fluency patterns in amnestic mild cognitive impairment are characteristic of Alzheimer's type dementia. J Int Neuropsychol Soc. 2006;12:570–574. doi: 10.1017/s1355617706060590. [DOI] [PubMed] [Google Scholar]
- 23.Artero S, Tierney MC, Touchon J, Ritchie K. Prediction of transition from cognitive impairment to senile dementia: a prospective, longitudinal study. Acta Psychiatr Scand. 2003;107:390–393. doi: 10.1034/j.1600-0447.2003.00081.x. [DOI] [PubMed] [Google Scholar]
- 24.Fuld PA, Masur DM, Blau AD, Crystal H, Aronson MK. Object-memory evaluation for prospective detection of dementia in normal functioning elderly: predictive and normative data. J Clin Exp Neuropsychol. 1990;12:520–528. doi: 10.1080/01688639008400998. [DOI] [PubMed] [Google Scholar]
- 25.Saxton J, Lopez OL, Ratcliff G, Dulberg C, Fried LP, Carlson MC, et al. Preclinical Alzheimer disease: neuropsychological test performance 1.5 to 8 years prior to onset. Neurology. 2004;63:2341–2347. doi: 10.1212/01.wnl.0000147470.58328.50. [DOI] [PubMed] [Google Scholar]
- 26.Tabert MH, Manly JJ, Liu X, Pelton GH, Rosenblum S, Jacobs M, et al. Neuropsychological prediction of conversion to Alzheimer disease in patients with mild cognitive impairment. Arch Gen Psychiatry. 2006;63:916–924. doi: 10.1001/archpsyc.63.8.916. [DOI] [PubMed] [Google Scholar]
- 27.Nebes RD. Semantic memory in Alzheimer's disease. Psychol Bull. 1989;106:377–394. doi: 10.1037/0033-2909.106.3.377. [DOI] [PubMed] [Google Scholar]
- 28.Troyer AK, Moscovitch M, Winocur G. Clustering and switching as two components of verbal fluency: evidence from younger and older healthy adults. Neuropsychology. 1997;11:138–146. doi: 10.1037//0894-4105.11.1.138. [DOI] [PubMed] [Google Scholar]
- 29.Henry JD, Crawford JR, Phillips LH. Verbal fluency performance in dementia of the Alzheimer's type: a meta-analysis. Neuropsychologia. 2004;42:1212–1222. doi: 10.1016/j.neuropsychologia.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Kessler J, Bley M, Mielke R, Kalbe E. Strategies and structures in verbal fluency tasks in patients with Alzheimer's disease. Behav Neurol. 1997;10:133–135. doi: 10.3233/BEN-1997-10406. [DOI] [PubMed] [Google Scholar]
- 31.Shiffrin RM, Schneider W. Controlled and automatic human information processing: II. Perceptual learning, automatic attending and a general theory. Psychol Rev. 1977;84:127–190. [Google Scholar]
- 32.Azuma T. Working memory and perseveration in verbal fluency. Neuropsychology. 2004;18:69–77. doi: 10.1037/0894-4105.18.1.69. [DOI] [PubMed] [Google Scholar]
- 33.Fischer-Baum S, Miozzo M, Laiacona M, Capitani E. Perseveration during verbal fluency in traumatic brain injury reflects impairments in working memory. Neuropsychology. 2016;30:791–799. doi: 10.1037/neu0000286. [DOI] [PubMed] [Google Scholar]
- 34.Kraan C, Stolwyk RJ, Testa R. The abilities associated with verbal fluency performance in a young, healthy population are multifactorial and differ across fluency variants. Appl Neuropsychol Adult. 2013;20:159–168. doi: 10.1080/09084282.2012.670157. [DOI] [PubMed] [Google Scholar]
- 35.Pakhomov SV, Eberly LE, Knopman DS. Recurrent perseverations on semantic verbal fluency tasks as an early marker of cognitive impairment. J Clin Exp Neuropsychol. 2018;40:832–840. doi: 10.1080/13803395.2018.1438372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosen VM, Engle RW. Forward and backward serial recall. Intelligence. 1997;25:37–47. [Google Scholar]
- 37.Unsworth N, Spillers GJ, Brewer GA. Working memory capacity and retrieval limitations from long-term memory: an examination of differences in accessibility. Q J Exp Psychol (Hove) 2012;65:2397–2410. doi: 10.1080/17470218.2012.690438. [DOI] [PubMed] [Google Scholar]
- 38.Baddeley AD, Hitch GJ. Working memory. In: Bower GA, editor. Recent Advances in Learning and Motivation. Vol 8. New York: Academic Press; 1974. pp. 47–89. [Google Scholar]
- 39.Baddeley A, Logie R, Bressi S, Della Sala S, Spinnler H. Dementia and working memory. Q J Exp Psychol A. 1986;38:603–618. doi: 10.1080/14640748608401616. [DOI] [PubMed] [Google Scholar]
- 40.Cowan N, Towse JN, Hamilton Z, Saults JS, Elliott EM, Lacey JF, et al. Children's working-memory processes: a response-timing analysis. J Exp Psychol Gen. 2003;132:113–132. doi: 10.1037/0096-3445.132.1.113. [DOI] [PubMed] [Google Scholar]
- 41.Healey MK, Miyake A. The role of attention during retrieval in working-memory span: a dual-task study. Q J Exp Psychol (Hove) 2009;62:733–745. doi: 10.1080/17470210802229005. [DOI] [PubMed] [Google Scholar]
- 42.Estes WK. Learning theory and intelligence. Am Psychol. 1974;29:740–749. [Google Scholar]
- 43.Troyer AK, Moscovitch M, Winocur G, Alexander MP, Stuss D. Clustering and switching on verbal fluency: the effects of focal frontal- and temporal-lobe lesions. Neuropsychologia. 1998;36:499–504. doi: 10.1016/s0028-3932(97)00152-8. [DOI] [PubMed] [Google Scholar]
- 44.Butters N, Granholm E, Salmon DP, Grant I, Wolfe J. Episodic and semantic memory: a comparison of amnesic and demented patients. J Clin Exp Neuropsychol. 1987;9:479–497. doi: 10.1080/01688638708410764. [DOI] [PubMed] [Google Scholar]
- 45.Ober BA, Dronkers NF, Koss E, Delis DC, Friedland RP. Retrieval from semantic memory in Alzheimer-type dementia. J Clin Exp Neuropsychol. 1986;8:75–92. doi: 10.1080/01688638608401298. [DOI] [PubMed] [Google Scholar]
- 46.Troyer AK. Normative data for clustering and switching on verbal fluency tasks. J Clin Exp Neuropsychol. 2000;22:370–378. doi: 10.1076/1380-3395(200006)22:3;1-V;FT370. [DOI] [PubMed] [Google Scholar]
- 47.Birn RM, Kenworthy L, Case L, Caravella R, Jones TB, Bandettini PA, et al. Neural systems supporting lexical search guided by letter and semantic category cues: a self-paced overt response fMRI study of verbal fluency. Neuroimage. 2010;49:1099–1107. doi: 10.1016/j.neuroimage.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Henry JD, Crawford JR. A meta-analytic review of verbal fluency performance following focal cortical lesions. Neuropsychology. 2004;18:284–295. doi: 10.1037/0894-4105.18.2.284. [DOI] [PubMed] [Google Scholar]
- 49.Iudicello JE, Woods SP, Weber E, Dawson MS, Scott JC, Carey CL, et al. Cognitive mechanisms of switching in HIV-associated category fluency deficits. J Clin Exp Neuropsychol. 2008;30:797–804. doi: 10.1080/13803390701779578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taler V, Phillips NA. Language performance in Alzheimer's disease and mild cognitive impairment: a comparative review. J Clin Exp Neuropsychol. 2008;30:501–556. doi: 10.1080/13803390701550128. [DOI] [PubMed] [Google Scholar]
- 51.Choi H. Category-specific impairment of patients with dementia of Alzheimer's type in category fluency tasks. Korean J Commun Disord. 2010;15:572–580. [Google Scholar]
- 52.Tröster AI, Salmon DP, McCullough D, Butters N. A comparison of the category fluency deficits associated with Alzheimer's and Huntington's disease. Brain Lang. 1989;37:500–513. doi: 10.1016/0093-934x(89)90032-1. [DOI] [PubMed] [Google Scholar]
- 53.Troyer AK, Moscovitch M, Winocur G, Leach L, Freedman M. Clustering and switching on verbal fluency tests in Alzheimer's and Parkinson's disease. J Int Neuropsychol Soc. 1998;4:137–143. doi: 10.1017/s1355617798001374. [DOI] [PubMed] [Google Scholar]
- 54.Weakley A, Schmitter-Edgecombe M. Analysis of verbal fluency ability in Alzheimer's disease: the role of clustering, switching and semantic proximities. Arch Clin Neuropsychol. 2014;29:256–268. doi: 10.1093/arclin/acu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lambon Ralph MA, Powell J, Howard D, Whitworth AB, Garrard P, Hodges JR. Semantic memory is impaired in both dementia with Lewy bodies and dementia of Alzheimer's type: a comparative neuropsychological study and literature review. J Neurol Neurosurg Psychiatry. 2001;70:149–156. doi: 10.1136/jnnp.70.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Laukka EJ, Jones S, Small BJ, Fratiglioni L, Bäckman L. Similar patterns of cognitive deficits in the preclinical phases of vascular dementia and Alzheimer's disease. J Int Neuropsychol Soc. 2004;10:382–391. doi: 10.1017/S1355617704103068. [DOI] [PubMed] [Google Scholar]
- 57.De Jager CA, Hogervorst E, Combrinck M, Budge MM. Sensitivity and specificity of neuropsychological tests for mild cognitive impairment, vascular cognitive impairment and Alzheimer's disease. Psychol Med. 2003;33:1039–1050. doi: 10.1017/s0033291703008031. [DOI] [PubMed] [Google Scholar]
- 58.Bayles KA, Trosset MW, Tomoeda CK, Montgomery EB, Jr, Wilson J. Generative naming in Parkinson disease patients. J Clin Exp Neuropsychol. 1993;15:547–562. doi: 10.1080/01688639308402578. [DOI] [PubMed] [Google Scholar]
- 59.Cummings JL, Darkins A, Mendez M, Hill MA, Benson DF. Alzheimer's disease and Parkinson's disease: comparison of speech and language alterations. Neurology. 1988;38:680–684. doi: 10.1212/wnl.38.5.680. [DOI] [PubMed] [Google Scholar]
- 60.Gomez RG, White DA. Using verbal fluency to detect very mild dementia of the Alzheimer type. Arch Clin Neuropsychol. 2006;21:771–775. doi: 10.1016/j.acn.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 61.Park J, Kang Y, Chang EJ, Oh E, Yu KH, Lee BC. Clustering and switching on verbal fluency in vascular dementia and dementia of the Alzheimer's type. Korean J Commun Disord. 2006;11:99–112. [Google Scholar]
- 62.Choi H, Kim JH, Lee CM, Kim JI. Features of semantic language impairment in patients with amnestic mild cognitive impairment. Dement Neurocogn Disord. 2013;12:33–40. [Google Scholar]
- 63.Kim H, Kang Y, Yu KH, Lee BC. A comparison of the deterioration characteristics in verbal fluency between amnestic mild cognitive impairment and vascular mild cognitive impairment. Commun Sci Disord. 2015;20:587–595. [Google Scholar]
- 64.Kim C, Kim S, Kang Y. A comparison of the Controlled Oral Word Association Test performances between amnestic multi-domain MCI and MCI in Parkinson's disease. Korean J Psychol Annu Conf. 2013:234–235. [Google Scholar]
- 65.Nutter-Upham KE, Saykin AJ, Rabin LA, Roth RM, Wishart HA, Pare N, et al. Verbal fluency performance in amnestic MCI and older adults with cognitive complaints. Arch Clin Neuropsychol. 2008;23:229–241. doi: 10.1016/j.acn.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jung IK, Kwak DI, Joe SH, Lee HS. A study of standardization of Korean form of Geriatric Depression Scale(KGDS) J Korean Geriatr Psychiatry. 1997;1:61–72. [Google Scholar]
- 67.Won CW, Yang KY, Rho YG, Kim SY, Lee EJ, Yoon JL, et al. The development of Korean activities of daily living(K-ADL) and Korean instrumental activities of daily living(K-IADL) scale. J Korean Geriatr Soc. 2002;6:107–120. [Google Scholar]
- 68.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 69.Kang Y, Na DL, Hahn S. A validity study on the Korean Mini-Mental State Examination (K-MMSE) in dementia patients. J Korean Neurol Assoc. 1997;15:300–308. [Google Scholar]
- 70.Kang YW. A normative study of the Korean-Mini Mental State Examination (K-MMSE) in the elderly. Korean J Psychol Gen. 2006;25:1–12. [Google Scholar]
- 71.Kang Y, Jang S, Na D. Seoul Neuropsychological Screening Battery II. Seoul: Human Brain Research & Consulting Co.; 2012. [Google Scholar]
- 72.Christensen KJ, Multhaup KS, Nordstrom S, Voss K. A cognitive battery for dementia: development and measurement characteristics. J Consult Clin Psychol. 1991;3:168–174. [Google Scholar]
- 73.Hwang S, Kim J, Park G, Choi J, Hong S. Korean Wechsler Adult Intelligence Scale IV. Daegu: Korea Psychology Co. Ltd; 2012. [Google Scholar]
- 74.Weakley A, Schmitter-Edgecombe M, Anderson J. Analysis of verbal fluency ability in amnestic and non-amnestic mild cognitive impairment. Arch Clin Neuropsychol. 2013;28:721–731. doi: 10.1093/arclin/act058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Raoux N, Amieva H, Le Goff M, Auriacombe S, Carcaillon L, Letenneur L, et al. Clustering and switching processes in semantic verbal fluency in the course of Alzheimer's disease subjects: results from the PAQUID longitudinal study. Cortex. 2008;44:1188–1196. doi: 10.1016/j.cortex.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 76.Alexopoulos P, Grimmer T, Perneczky R, Domes G, Kurz A. Progression to dementia in clinical subtypes of mild cognitive impairment. Dement Geriatr Cogn Disord. 2006;22:27–34. doi: 10.1159/000093101. [DOI] [PubMed] [Google Scholar]
- 77.Kawas C, Gray S, Brookmeyer R, Fozard J, Zonderman A. Age-specific incidence rates of Alzheimer's disease: the Baltimore Longitudinal Study of Aging. Neurology. 2000;54:2072–2077. doi: 10.1212/wnl.54.11.2072. [DOI] [PubMed] [Google Scholar]
- 78.Sackett PR, Lievens F, Berry CM, Landers RN. A cautionary note on the effects of range restriction on predictor intercorrelations. J Appl Psychol. 2007;92:538–544. doi: 10.1037/0021-9010.92.2.538. [DOI] [PubMed] [Google Scholar]
- 79.Sung JE. Performances on short-term and working memory tasks and their relationships to aphasia severity and auditory comprehension in normal elderly adults and people with aphasia. Korean J Commun Disord. 2010;15:285–297. [Google Scholar]