Abstract
Background and Purpose
Sacubitril/valsartan (Sac/val) is more effective than valsartan in lowering BP and mortality in patients with heart failure. Here, we proposed that Sac/val treatment would be more effective in preventing pathological vascular remodelling in 129X1/SvJ (129X1), than in C57BL/6J (B6) inbred mice.
Experimental Approach
Sac/val (60 mg·kg−1·day−1) and valsartan (27 mg·kg−1·day−1) were given as prophylactic or therapeutic treatments, to 129X1 or B6 mice with carotid artery ligation for 14 days. Blood flow was measured by ultrasound. Ex vivo, carotid tissue was analysed with histological and morphometric techniques, together with RNA sequencing and gene ontology.
Key Results
Sac/val was more effective than valsartan in lowering BP in 129X1 compared with B6 mice. Liver expression of CYP2C9 and plasma cGMP levels were similar across treatments. A reduction in carotid thickening after prophylactic treatment with valsartan or Sac/val also resulted in significant arterial shrinkage in B6 mice. In 129X1 mice, Sac/val and prophylactic treatment with valsartan had no effect on carotid thickening but preserved carotid size. BP lowering significantly correlated with a decline in carotid stiffness (R 2 = .37, P = .0096) in 129X1 but not in B6 mice. The gene expression signature associated with hyalurononglucosaminidase activity was down‐regulated in injured arteries after both regimens of Sac/val only in 129X1 mice. Administration of Sac/val but not valsartan significantly reduced deposition of hyaluronic acid and carotid fibrosis in 129X1 mice.
Conclusion and Implications
These results underscore the importance of the genetic background in the efficacy of the Sac/val on vascular fibrosis.
Abbreviations
- 129X1
129X1/SvJ inbred mouse strain
- ACEI
ACE inhibitor
- ARB
angiotensin II receptor blocker
- ARNI
angiotensin receptor and neprilysin inhibitor
- B6
C57BL/6J inbred mouse strain
- CYP2C9
cytochrome P450 2C9
- GO
gene ontology
- HF
heart failure
- HR
heart rate
- LCA
left carotid artery
- ‐P
Prophylactic regimen
- PARADIGM‐HF
Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure
- PARAMETER
Prospective Comparison of Angiotensin Receptor Neprilysin Inhibitor With Angiotensin Receptor Blocker Measuring Arterial Stiffness in the Elderly
- PC
principal component
- RCA
right carotid artery
- RF
radio frequency echo data
- Sac/val
sacubitril/valsartan; Entresto
- ‐T
therapeutic regimen
- Val
valsartan
- Veh
vehicle or normal saline
- US
ultrasound method
What is already known
Sacubitril/valsartan is more effective than enalapril in reducing mortality in heart failure.
What this study adds
Sacubitril/valsartan was more effective than valsartan in reducing BP in 129X1/SvJ, than in C57BL/6J mice.
Genetic signatures associated with decreased carotid fibrosis after sacubitril/valsartan in 129X1/SvJ but not C57BL/6J mice.
What is the clinical significance
Superiority of sacubitril/valsartan over valsartan due to inhibition of vascular fibrosis in some patients.
1. INTRODUCTION
Sacubitril/valsartan (Sac/val) or Entresto, a first‐in‐class angiotensin receptor and neprilysin inhibitor (ARNI), showed favourable effects compared to valsartan (Val), an angiotensin II receptor blocker (ARB), in patients with heart failure (HF) with preserved ejection fraction (Solomon et al., 2012). The Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM‐HF) study indicated that patients with chronic HF with reduced ejection fraction who received Entresto lived longer without being hospitalized for HF than those who received standard care with enalapril, an ACE inhibitor (ACEI; McMurray et al., 2014). A recent report from PARADIGM‐HF showed that Entresto was effective regardless of baseline left ventricular ejection fraction (Solomon et al., 2016). However, we have limited knowledge of the therapeutic mechanisms underlying the effects of Sac/val in HF, which could be also driven by reversing pathological changes in arteries. Treatments with ARNI and ACEI improved arterial function in hypertensive rats (Seki et al., 2017). Our group has previously reported that dual inhibition of neutral endopeptidase and ACE with omapatrilat had greater protective effects than ACEI (fosinopril) on arterial remodelling in hypertensive rats (Korshunov, Massett, Carey, & Berk, 2004). We and others have also shown that endothelial dysfunction and vascular remodelling varied across inbred mouse strains (Korshunov & Berk, 2004; Ryan, Didion, Davis, Faraci, & Sigmund, 2002). Specifically, endothelium‐dependent dysfunction was evident in 129X1/SvJ (129X1) mice (Ryan et al., 2002). Among thousands of differentially expressed genes in the aortas of more than 100 inbred mouse strains, 129X1 mice exhibited a higher basal expression of neprilysin (Mme) than C57BL/6J (B6) mice (Bennett et al., 2015). Given the pharmacological activity of Sac/val, we postulated that treating 129X1 mice with Sac/val would provide greater protection against pathological remodelling than treatment with Val alone.
2. METHODS
2.1. Animals
All animal care and experimental procedures complied with the Guide for the Care and Use of Laboratory Animals (Animals, 2011) and were approved by the University of Rochester Animal Care Committee. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, & Altman, 2010) and with the recommendations made by the British Journal of Pharmacology. Experiments were carried out using male B6 (Stock: 000664) and 129X1 (Stock: 000691) mice, 8–10 weeks old. Animals were purchased from the Jackson Laboratory (Bar Harbor, ME). The mice were individually housed under 12‐hr light/12‐hr dark cycle (lights on from 6a.m. to 6p.m.) with free access to water and chow.
2.2. Administration of agents
To deliver a final amount of 60 mg·kg−1 day‐1 in two doses, Sac/val was dissolved in water to a concentration of 15 mg ml‐1 which was administered to mice at 0.02 ml per 10 g. Valsartan was initially dissolved in 1‐N NaOH, titrated to pH~8–9 with 1‐N HCl and diluted to a working concentration of 6.75 mg·ml−1. Two doses (0.02 ml per 10 g) of this solution of Val provided the final amount of 27 mg·kg−1·day−1. Both working solutions were stored at +4°C for up to 2 weeks. Normal saline (Veh) was used as the vehicle treatment. Each treatment (Veh, Sac/val or Val) was given by gavage in the morning and evening. The administration schedules were based on equal pharmacokinetic profiles for Val and Sac/val in mice, as provided by Novartis Pharmaceuticals (data not shown).
2.3. Experimental design
The overall experimental design of the study that lasted 23 days is shown in Figure S1. To compare protective effects of Sac/val and Val, we induced vascular remodelling in these mice by carotid ligation and treated 129X1 or B6 mice with two protocols: (a) a prophylactic regimen (P‐regimen) began 2 days before ligation and (b) a therapeutic regimen (T‐regimen) started 7 days after ligation. Mice were randomly divided into 12 experimental groups: (a) B6, Veh‐P, n = 11; (b) 129X1, Veh‐P, n = 11; (c) B6, Val‐P, n = 10; (d) 129X1, Val‐P, n = 11; (e) B6, Sac/val‐P, n = 11; (f) 129X1, Sac/val‐P, n = 11; (g) B6, Veh‐T, n = 11; (h) 129X1, Veh‐T, n = 10; (i) B6, Val‐T, n = 11; (j) 129X1, Val‐T, n = 10; 11) B6, Sac/val‐T, n = 11; and (k) 129X1, Sac/val‐T, n = 10.
Mice were exposed to the procedures for indirect measurement of haemodynamic parameters using tail‐cuff plethysmography, for 7 days before interventions. We measured BP for 3 days at the end of Weeks 1 and 2 (BP‐1 and BP‐2, Figure S1). Blood flow in carotid arteries was evaluated by non‐invasive ultrasound (US) at the end of Weeks 1 and 2 (US‐1 and US‐2, Figure S1). Peripheral blood, fresh and fixed tissues were collected 14 days after the carotid ligation.
2.4. Tail‐cuff plethysmography
We used a 6‐channel BP‐2000 system (Visitech System) to measure systolic BP, diastolic BP, mean arterial pressure and heart rate (HR), as described (Batchu, Hughson, Gerloff, Fowell, & Korshunov, 2013; Korshunov & Berk, 2003; Smolock et al., 2012). Each mouse spent ~40 min on BP platform for seven consecutive days before the experiment to minimize the stress. Measurements were taken approximately 1 hr after the morning dose to reduce any acute effects on BP.
2.5. Carotid artery ligation
The flow‐induced carotid remodelling was studied in all mice over 14 days after carotid ligation, as described (Korshunov & Berk, 2003). Briefly, animals were anaesthetized with a mixture of ketamine and xylazine (130 mg·kg−1 and 9 mg·kg−1, respectively, i.p.). The neck area was open by a midline incision and the bifurcation of the left carotid artery (LCA) was isolated. The internal and external LCAs were ligated with 6–0 silk, leaving the occipital artery intact. The neck opening was closed with 6–0 coated vicryl suture. An analgesic (flunixin meglumine, 120 mg·kg−1, i.p.) was given immediately after closure and once a day for 3 days after the surgery. A plastic house and additional bedding material were introduced to individually housed mice for an enhanced environment after the surgery. About 80–100% of B6 mice survived after the surgery (Figure S2A,C). In contrast, 60–70% of 129X1 mice were alive after P‐regimen (Figure S2B). The worst survival rates (40–60%) were observed in 129X1 mice after T‐regimen (Figure S2D). Both strains of mice tended to lose weight in 2 weeks but only those treated with the Sac/val‐T reached statistical significance (Figure S2E–F).
2.6. Vascular ultrasound in experimental mice
Echo imaging was done with a Vevo2100 machine (FUJIFILM VisualSonics; Toronto, Canada) as described (Korshunov et al., 2017). Mice were anaesthetized with isoflurane and HR maintained above 400 beats per min during measurements with a 128‐element transducer operating at 40 MHz (MS550D). The first set of ultrasound imaging, US‐1, confirmed successful surgical procedure (Figure S1). We identified only two mice (Veh‐treated B6 and 129X1) with unsuccessful surgery, shown as a significantly higher blood flow velocities (300–400 mm·s−1) in the LCA and were excluded from the study. The US‐2 imaging set collected radio frequency (RF) echo data to calculate Young's modulus, a measure of the LCA stiffness based on systolic BP values at the 2‐week time point (Figure S1). The carotid artery blood flow velocities and RF data were analysed as before (Korshunov et al., 2017).
2.7. Plasma cGMP levels in treated mice
Peripheral blood was collected in EDTA‐coated tubes after cardiac puncture of anaesthetized animals at the end of experiment. Plasma was immediately separated and stored at −80°C until use as described (Batchu, Smolock, Dyachenko, Murashev, & Korshunov, 2015). The levels of cGMP in the plasma were quantified using an ELISA kit according to the manufacturer's instructions (Enzo Life Sciences). Plasma samples were diluted 1:1 in a diluent, and 100 μl was used per reaction. Plates were read at 440 nm using Fluostar Optima version 2.20R2 (BMG LabTech), and sample concentrations were calculated based on a standard curve.
2.8. Protein expression in livers from treated mice
The antibody‐based procedures used in this study comply with the recommendations made by the British Journal of Pharmacology. Livers were frozen in liquid nitrogen and stored in −80°C and protein was isolated as before (Batchu, Xia, et al., 2015). A portion of liver (about 100 mg) from each mouse was removed and homogenized in 1‐ml 10× lysis buffer (Cell Signaling) with 1× protease inhibitor cocktail (Sigma) with the aid of a hand‐held homogenizer. Protein concentrations were determined by the Bradford assay (BioRad) and SDS samples were made by adding 30‐μg protein to 5× SDS sample buffer. Samples were run on a 10% SDS running gel with a 4% SDS stacking gel, then transferred to a PVDF membrane. Membranes were incubated in 4% milk in PBS‐T for 1 hr at room temperature, then incubated overnight with primary antibodies rabbit anti‐cytochrome P450 2C9 (CYP2C9; 1:500; Biorbyt Cat# orb25530, RRID:AB_10935294; https://scicrunch.org/resources/Any/search?q=AB_10935294&l=AB_10935294) or mouse anti‐GAPDH (1:5,000; Cell Signaling) at 4°C. Membranes were washed with PBS‐T then incubated in HRP‐conjugated goat anti‐rabbit secondary antibody (1:10,000; Cell Signaling) at room temperature for 1 hr. Membranes were washed with PBS‐T and incubated in Peirce ECL Western blotting substrate (Thermo Scientific, 0.125 ml·cm−2) for 5 min. Membranes were exposed in the Bio‐Rad gel doc for 30 s at the highest sensitivity. Band intensities were measured with the rectangular volume tool on ImageLab software (Bio‐Rad, version 5.2).
2.9. Histology and morphometry
At the end of each experiment, mice were anaesthetized with a mixture of ketamine and xylazine (130 mg·kg−1 and 9 mg·kg−1, respectively, i.p.). They were then perfusion fixed via the left ventricle, tissues processed and stained with haematoxylin and eosin (DAKO). Morphometric analysis was carried out with MCID image software. These procedures are detailed in Korshunov & Berk (2003). Specifically, a series of cross sections (5 μm) were made from the carotid bifurcation every 200 μm through a 2‐mm length (10 area divisions) of the artery. Compartmental volumes were computed based on area measurements for each mouse and 3‐dimentional (3D) images were generated as before (Korshunov et al., 2017). In our pilot studies, we found that both inbred mouse strains exhibited no difference in thickness of the treated contralateral right carotid artery (RCA), compared with the sham‐operated LCA (not shown). Similarly, the external elastic lamina of the treated contralateral RCA, compared with the sham‐operated LCA volumes were unchanged in 129X1 but increased in B6 mice (not shown). Unstained LCA sections located ~1 mm proximal to the bifurcation were used for staining with Alcian Blue kit (ScienCell Research Laboratories; Carlsbad, CA) or PicroSirius Red kit (Abcam; Cambridge, MA). LCA staining was analysed in 5–6 mice per group (2–3 sections per mouse) using Image‐Pro Plus (RRID:SCR_016879; https://scicrunch.org/resources/Any/search?q=SCR_016879&l=SCR_016879) as we reported (Gerloff & Korshunov, 2012). A relative content of positive staining (red over yellow for PicroSirius Red; blue over background for Alcian Blue) was quantitated by ImagePro analyser (v.6.2) in a blind manner and expressed as % of positive‐coloured area within the LCA intima‐media or adventitia compartments.
2.10. RNA sequencing of carotids from treated mice
mRNA was isolated from snap‐frozen single LCAs (n = 3 per group) by the University of Rochester Genomic Research Center, as previously reported (Smolock et al., 2014). Multiple quality controls showed a reliable RNA sequencing (RNAseq) of mRNAs from the LCA. Specifically, there was less than 2.6% drop in reads that ranged between 26.5 and 37.7 million (M) reads (Table S1). A very good alignment and assignment (mapping) to a mouse genome (82.4–91.0%) were found in this RNAseq study (not shown). Raw RNAseq data were deposited in the NCBI gene expression and hybridization array data repository (GEO, http://www.ncbi.nlm.nih.gov/geo/) and allocated the GEO Series accession number GSE129689 (Edgar, Domrachev, & Lash, 2002). A differential gene expression analysis was done by DESeq2‐1.16.1 within R‐3.4.1 for data normalization and differential expression analysis with an adjusted P value threshold of .05 on each set of raw expression measures. Heatmaps showed hierarchically clustered Euclidean distances between strains from the regularized log transformation of the normalized count data (Figure S3). A principal component analysis (PCA) of prophylactic and therapeutic regimens is shown in the 2D‐plane spanned by their first two principal components, which are the two components explaining most of the variance (Figure S4). The plots suggested differences in variance for principal component 1 (PC1) and PC2 after T‐ and P‐regimens (Figure S4). Because of the large numbers of differentially expressed genes between B6 and 129X1 mice (Tables S2–S13), we utilized an online Gene List Venn Diagram tool. For up‐regulated and down‐regulated gene lists between 129X1 and B6, we plotted results after P‐ or T‐regimens and the treatment: (a) Veh, (b) Val, and (c) Sac/val.
2.11. Gene ontology analyses
To further understand molecular mechanisms in arterial responses to Sac/val and Val between 129X1 and B6 mice, we ran gene ontology (GO) analyses by using the online Enrichr tool (Kuleshov et al., 2016). This type of analysis allowed us to uncover molecular activities of gene products from the identified lists. Specifically, the up‐regulated or down‐regulated genes after P‐ or T‐regimens were investigated based on three grouping factors: common gene differences between 129X1 and B6 after Veh + Val + Sac/val (Tables S14–S15); unique gene differences between 129X1 and B6 attributed to Val + Sac/val (Tables S16–S17); and unique gene differences between 129X1 and B6 after Sac/val (Tables S18–S19).
2.12. Data and statistical analysis
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology. Data are presented as mean ± SEM. We used JMP13.0.0 software (SAS) for evaluation of statistical significance. Differences between three or more groups were determined by two‐way ANOVA between treatment groups and various parameters (mouse strain, carotid, or time) with post hoc comparisons of all means by the Tukey–Kramer Honest Significant Difference test. Pairwise correlations were determined by multivariate analyses between systolic BP and Young's modulus after P‐regimen for each mouse strain. The level of P < .05 was regarded as significant.
2.13. Materials
Sac/val and valsartan were supplied by Novartis Pharmaceuticals.
2.14. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Christopoulos et al., 2017; Alexander, Fabbro et al., 2017).
3. RESULTS
3.1. BP‐lowering effects of drugs in inbred strains of mice
The diastolic BP and mean arterial pressure were higher in 129X1 mice than in B6 mice, while systolic BP was similar between the strains (Figures 1a–c and S5A–C). Treatment with the P‐regimen of Sac/val significantly reduced systolic BP and mean arterial pressure compared to baseline or Veh in both strains, but diastolic BP was only reduced in 129X1 mice (Figure 1a–c). The T‐regimen of Sac/val lowered systolic BP in both strains compared with the corresponding Vehicles but, in 129X1 mice, diastolic BP and mean arterial pressure were decreased (Figure S5A–C). In B6 mice, after Val‐P there was only a trend towards reduction of systolic BP and mean arterial pressure that became significant after Val‐T (Figures 1a,c and S5A,C). We found that interventions reduced HR in B6 with little or no effect in 129X1 mice (Figures 1d and S5D). Thus, Sac/val was more effective than Val in the 129X1 mice.
Figure 1.
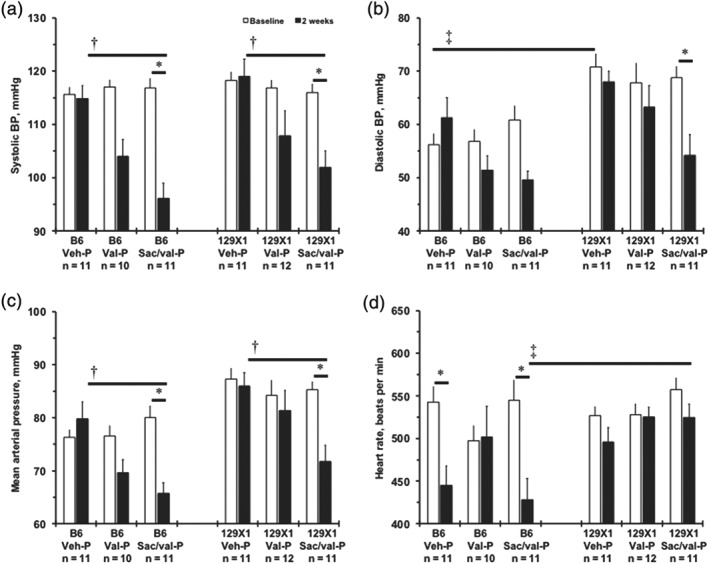
Haemodynamic changes after prophylactic (P‐) treatments in two inbred mouse strains. (a) Systolic BP (mmHg) of mice after prophylactic treatment with Veh, Val or Sac/val). (b) Diastolic BP, mmHg. (c) Mean arterial pressure, mmHg. (d) Heart rate, beats per min. Measurements were made at baseline and then at after 2 weeks of treatment. Values are mean ± SEM; *P < .05, significantly different from baseline; † P < .05, significantly different from Veh‐P, 2 weeks; ‡ P < .05, significantly different from B6 mice. n, number of animals per group
3.2. No differences in the primary metabolic enzyme across the experimental groups
To rule out the possibility of genetic variation in hepatic P450 metabolism of the compounds (Ayalasomayajula, Langenickel, Pal, Boggarapu, & Sunkara, 2017; de Vries et al., 2016), we measured levels of CYP2C9, a major enzyme that metabolizes Val and Sac/val in mice and humans (Figure S6). Despite significant changes in BP, we found no effect of the strain or regimen on liver expression of CYP2C9 (Figure S6A). Correspondingly, we found that plasma cGMP levels were similar between 129X1 and B6 mice, regardless of the compound or the regimen (Figure S6B). Our data suggest that the major metabolic enzyme and systemic levels of the secondary messenger cGMP were the same after Val or Sac/val in B6 and 129X1 mice.
3.3. Blood flow changes in carotid arteries across the treated mice
A non‐invasive ultrasound imaging confirmed successful surgical ligation procedure with lower values of blood flow velocities in the LCA (73–176 mm·s−1) than in the RCA (254–476 mm·s−1) in experimental mice (Tables S20–S21). Mean blood flow velocity in LCA was lower in 129X1 compared to B6 mice (Table S20). These differences were associated with a lower end diastolic, peak systolic velocities, velocity time integral, and mean gradient in 129X1 mice compared with those in B6 mice (Table S20). Pulsatility and resistive indexes were similar between the mouse strains. A mean blood flow velocity in RCA was also lower in 129X1 mice (~300 mm·s−1), compared to B6 (>400 mm·s−1) mice (Table S21). These differences were associated with low end diastolic, peak systolic velocities, velocity time integral, and mean gradient in 129X1 mice, compared with those in B6 mice (Table S21). In contrast, pulsatility and resistive indexes for Veh‐P and Val‐T were higher in 129X1 mice than in B6 mice (Table S21). There was a significant increase in resistive indexes after Sac/val‐P, compared with Veh‐P in B6 mice. These data suggest an impairment of responses in 129X1 mice, to a physiological adaptation to increased blood flow.
3.4. BP‐lowering effects on carotid artery stiffness in mouse strains
We recently introduced a method of assessing LCA stiffness in mice, using ultrasound to measure Young's modulus for the artery (Korshunov et al., 2017). Here, we observed slightly higher values of Young's modulus, in Veh‐treated 129X1 mice, compared with those in B6 mice (Figure 2a). There was a significant reduction of the Young's modulus in the LCA after Sac/val‐P in 129X1 mice, but not after the Val‐P regimen (Figure 2a). In order to investigate a relationship of decreased BP to stiffness in ligated arteries, we plotted values of the systolic BP against the LCA Young's modulus in B6 and 129X1 mice, after the P‐regimen (Figure 2b). There was essentially no correlation between systolic BP and LCA stiffness in B6 mice (Figure 2b). However, a significant correlation (R 2 = .37) was found between systolic BP and LCA stiffness in 129X1 mice (Figure 2b). These results suggest functional improvements in elasticity or structure of the injured carotid artery were provided by treatment with Sac/val, only in 129X1 mice.
Figure 2.
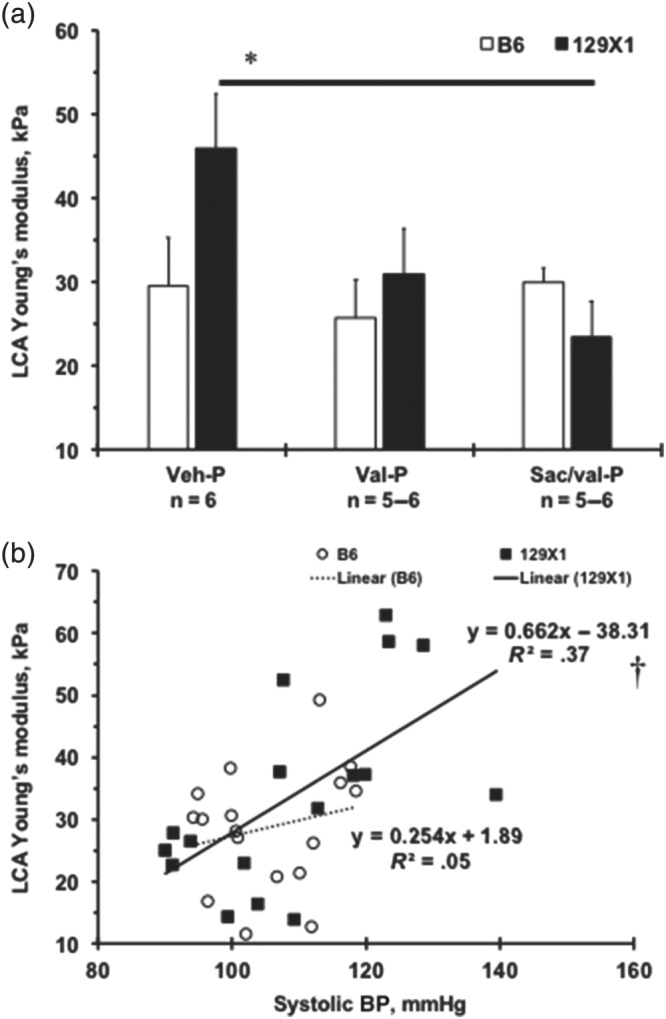
Relationships between BP‐lowering effects of the compounds and carotid artery stiffness in two mouse strains. (a) Young's modulus (kPa) of the ligated left carotid artery (LCA) after prophylactic treatment (P) with the compounds. Values are mean ± SEM; *P < .05, significantly different from Veh‐P. n, number of animals per group. (b) LCA Young's modulus (kPa) values on X‐axis are plotted against systolic BP (mmHg) on Y‐axis for each experimental mouse after prophylactic treatment. There was a significant correlation (R 2 = .37, † P = .0096) between systolic BP and LCA stiffness in 129X1 mice
3.5. Histological analyses of carotid remodelling in treated mice
The 3D models of the carotid histology displayed larger RCA lumen volumes in 129X1 mice, compared with B6 mice, after Veh‐P or Veh‐T (Figures 3 and S7). P‐regimens of Val and Sac/val significantly reduced LCA lumen volumes in B6 mice, compared with those in 129X1 mice (Figure 3b–d). Val‐P and Sac/val‐P reduced LCA intima/media ratio in B6 mice compared with Veh‐P, while the intima/media ratio was similar across the groups in 129X1 mice (Figure 3d). Although the LCA intima/media ratio after Sac/val‐P was not statistically significant compared to RCA in 129X1 mice (Figure 3d), there was no detectable intima compartment in RCA in both strains (Figures 3 and S7). We also found that the T‐regimen had no effect on intima/media ratio in both strains (Figure S7D). The LCA adventitia significantly increased compared to RCA in B6 mice (with only a trend in 129X1 mice) and only Sac/val‐P reduced LCA adventitia in B6 mice (Figure 3d). We noted a smaller LCA adventitia and intima/media ratio after T‐than after P‐regimens in 129X1 mice (Figures 3d and S7D). Ligation increased the external elastic lamina in LCA compared with the RCA, after Veh‐P in B6 mice (Figure 3d). Similar to LCA lumen volume, Sac/val‐P significantly decreased the external elastic lamina in the LCA of B6 mice, compared to Veh‐P or Sac/val‐P 129X1 mice (Figure 3d). These data suggest a dose‐dependent effect of Sac/val and Val against LCA intimal thickening in B6 mice. However, the beneficial effects of the P‐regimens with Sac/val or Val also resulted in a significant shrinkage of the LCA in B6 mice. In 129X1 mice, we found that treatment with Sac/val or Val were ineffective in decreasing carotid thickening but prevented a decrease in carotid size, after the injury.
Figure 3.
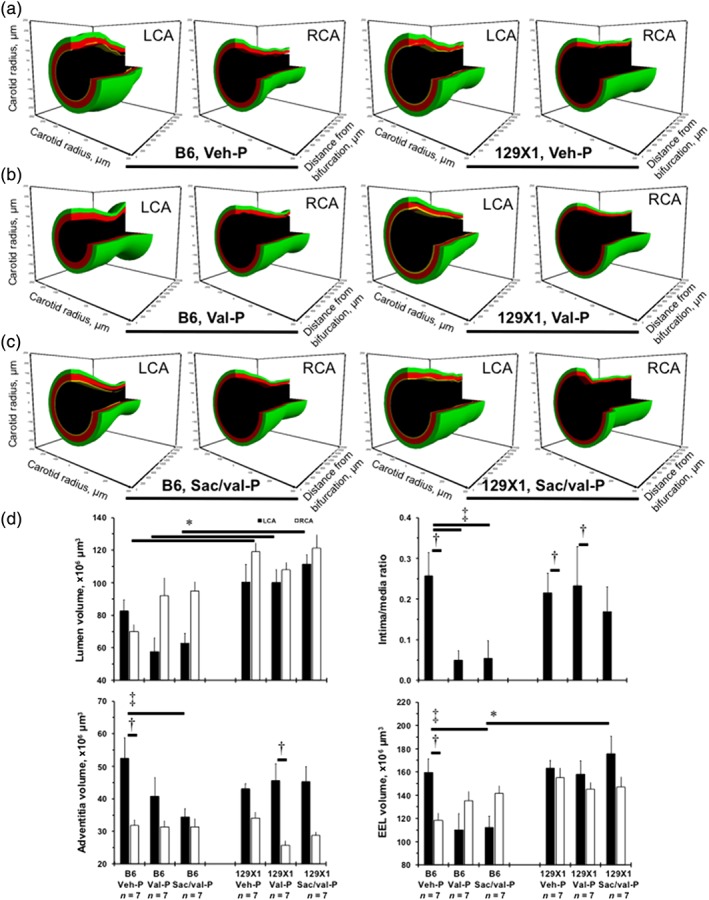
Carotid artery remodelling after prophylactic (P‐) regimen of treatments in two inbred mouse strains. (a) Representative 3‐dimensional (3D) reconstructions of the 2‐mm length from the bifurcation of the ligated left carotid artery (LCA) and right carotid artery (RCA) after Veh‐P in B6 and 129X1 mice. (b) Representative 3D carotids after Val‐P in B6 and 129X1 mice. (c) Representative 3D carotids after Sac/val‐P in B6 and 129X1 mice. Black colour shows lumen, yellow—intima, red—media, and green—adventitia volume. (d) Quantifications of the lumen volumes (top left), ratio of intima to media (top right), adventitia volume (bottom left), and external elastic lamina (EEL) volume (bottom right). Values are mean ± SEM; *P < .05, significantly different from B6; † P < 0.05, significantly different from RCA; ‡ P < 0.05, significantly different from Veh‐P. n, number of animals per group
3.6. Comparative gene expression analyses of the carotids from treated mice
To obtain insight into strain‐dependent differences in vascular remodelling, we analysed gene expression profiles of the LCA after interventions in B6 and 129X1 mice (Tables S2–S13). Pairwise comparisons of LCA gene expressions for each regimen were computed and revealed differences between B6 and 129X1 mice after Veh (Figure S3). We confirmed earlier strain‐dependent differences in aorta (Bennett et al., 2015), carotid expression of Mme was higher (1.185‐fold) after Veh‐T, while that for the angiotensin II AT1 receptor associated protein (Agtrap) was significantly lower (0.525‐fold) in 129X1 mice, compared with expression in B6 mice (Tables S4–S5). In addition, treatment with Val‐P did not reverse strain‐dependent differences in Mme or Agtrap expression (Tables S6–S7). The up‐regulated genes in LCA in 129X1 and B6 mice were common after three interventions (Veh , Val, and Sac/val; in the centre of the Venn diagram) for P‐ (n = 213) and T‐regimens (n = 227; Figures 4a and S8). Also, commonly down‐regulated genes in three treatment comparisons were common after P‐ (n = 209) and T‐regimens (n = 203; Figures 4A and S8). Mouse‐strain differences of these genes are likely to be independent of any intervention. There was a smaller number of the shared genes in the overlapping area between light grey and dark grey circles: Val + Sac/val up‐regulated genes in the carotid artery for P‐ (n = 10) and T‐regimen (n = 29) were less than down‐regulated for P‐ (n = 25) and T‐regimen (n = 56; Figures 4A and S8). The latter suggest a transcriptional similarity in responses to both compounds in 129X1 and B6 mice. Finally, we identified unique genes that differed between Val‐ and Sac/val‐treated mice (far right vs. low number on each Venn diagram), except the up‐regulated genes after T‐regimens (n = 87 vs. n = 148; Figure S8). Thus, the smallest number of genes are controlled by Sac/val in response to carotid injury between 129X1 and B6 mice.
Figure 4.

Gene expression analyses of the effects of treatment with Sac/val or Val in injured carotid artery, in two mouse strains. (a) Venn diagrams of up‐regulated and down‐regulated gene numbers in 129X1 and B6 mice after prophylactic (P‐) regimen. Open circles represent differentially expressed gene numbers Veh‐treated mice. Light grey circles—Val‐treated mice. Dark grey circles—Sac/val‐treated mice. The numbers in overlay areas show shared genes between the treatments. (b) Commonly up‐regulated gene ontology (GO) molecular functions in Veh‐, Val‐, and Sac/val‐treated 129X1and B6 mice after therapeutic (T‐) and P‐regimens. (c) Down‐regulated GO molecular functions shared between Sac/val‐ and Val‐treated 129X1 and B6 mice after T‐ and P‐regimens. (d) A down‐regulated hyalurononglucosaminidase activity (GO:0004415) was only observed in Sac/val‐treated 129X1 mice and not in B6 mice, after T‐ and P‐regimens. (e) Representative images of Alcian Blue‐stained ligated carotid arteries after P‐regimen to visualize connective tissues rich in hyaluronic acid. Scale bar is 50 μm. Black brackets indicate the intima‐media area. Quantification of the positive (blue) staining in intima‐media area, as %. Values are mean ± SEM; *P < .05, significantly different from B6; † P < .05, significantly different from Sac/val. n, number of animals per group
To discover molecular mechanisms that explain the beneficial effects of Sac/val on pathological remodelling in 129X1 mice, compared with those in B6 mice, we cross‐referenced GO molecular functions found between P‐ and T‐regimens (Tables S14–S19). First, three GO molecular functions were up‐regulated in 129X1 mice, which are common to Veh, Val and Sac/val treatments (Figure 4b). Second, Sac/val and Val reduced gene expression in LCA and down‐regulated three GO molecular functions in 129X1 carotid arteries (Figure 4c). Only one GO molecular function was consistently down‐regulated in 129X1 mice after Sac/val: a hyalurononglucosaminidase activity GO:0004415 (Figure 4d). We validated our bioinformatics findings, using Alcian Blue staining of the LCAs after P‐regimen to visualize connective tissues rich in hyaluronic acid (Figure 4e). As predicted, there was a higher intensity of the positive (blue) staining in the LCA intima‐media in 129X1 mice, compared with that in B6 mice, after Veh‐P (Figure 4e). Sac/val‐P but not Val‐P significantly reduced the Alcian Blue staining in 129X1 mice (Figure 4e). Taken together, our data suggest that a strain‐specific beneficial effect of Sac/val over Val on pathological vascular remodelling could be due to a down‐regulation of the hyalurononglucosaminidase activity.
3.7. Differences between treatments in protection against carotid fibrosis
Hyaluronic acid is important for pericellular matrix remodelling and fibrosis, especially in early vascular disease (Fischer, 2018; Sadowitz, Seymour, Gahtan, & Maier, 2012). Representative images are shown after P‐regimens in B6 and 129X1 mice (Figure 5a). There was twice as much fibrosis in the LCA intima‐media compartment in 129X1 mice, compared with that in B6 mice (black brackets), while adventitial (white box) fibrosis exhibited a trend to be higher in 129X1 mice than in B6 mice, after Veh‐P (Figure 5b–c). Both regimens of Val were ineffective in reducing LCA fibrosis despite significant reduction in LCA thickness after P‐regimen in B6 mice (Figures 5 and S9). Sac/val significantly reduced intima‐media fibrosis in 129X1 mice (Figures 5b and S9A). Only Sac/val‐P reduced LCA adventitial fibrosis in 129X1 mice (Figure 5c). Thus, anti‐fibrosis mechanisms of Sac/val might explain its efficacy in preventing carotid artery size decline and decreasing stiffness in 129X1 mice, compared with those in B6 mice.
Figure 5.
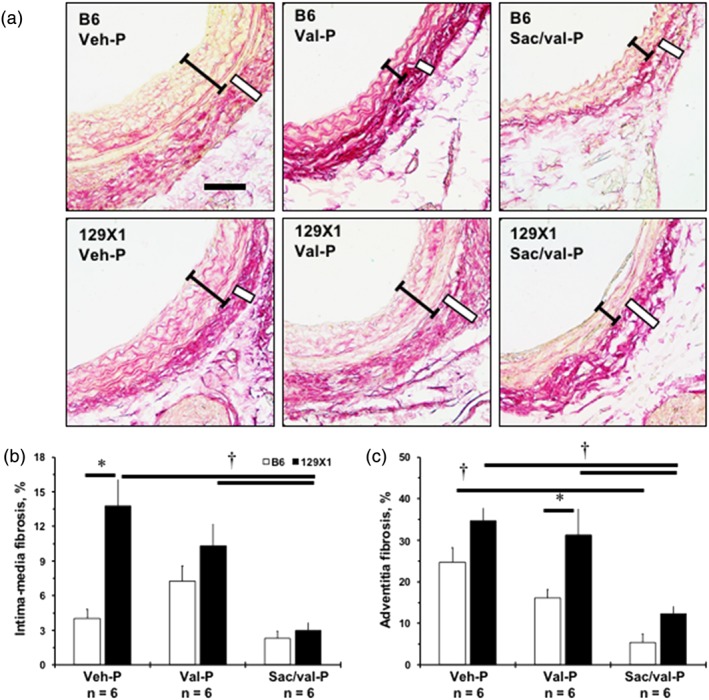
Strain‐dependent differences in efficacy of Sac/val compared with Val in inhibiting fibrosis in the injured carotid artery. (a) Representative images of PicroSirius Red‐stained ligated left carotid artery (LCA) after prophylactic (P‐) regimen. Scale bar is 50 μm. Black brackets indicate intima‐media area, white box—adventitia. (b) Quantification of fibrosis (red colour) in intima‐media area of the LCA, as %. (c) Quantification of fibrosis in adventitia of the LCA, as %. Values are mean ± SEM; *P < .05, significantly different from B6; † P < .05, significantly different from Sac/val. n, Number of animals per group
4. DISCUSSION
In this study, we found that treatment with Sac/val was more effective than Val in decreasing of BP in 129X1 mouse strain compared to B6 mice. This could be in part due to higher levels of mean arterial pressure and diastolic BP at the baseline in 129X1 mice, compared with those in B6 mice. Mouse strain‐dependent differences in metabolism of Sac/val and Val are unlikely as CYP2C9 or plasma cGMP were similar across experimental mice. In B6 mice, we observed significant protection against carotid intimal thickening after P‐ but not T‐regimens of Val or Sac/val. However, the reduction in carotid thickening after Val‐P or Sac/val‐P also resulted in a significant shrinkage of the LCA in B6 mice. In 129X1 mice, we found that Sac/val or Val did not change carotid thickening but preserved LCA size. Correspondingly, BP lowering was correlated with reduction of LCA stiffness in 129X1, but not in B6, mice. We discovered a unique list of hundreds of genes that were significantly down‐regulated in ligated LCAs after Sac/val in 129X1 and B6 mice. Our bioinformatics approaches revealed that a hyalurononglucosaminidase activity was the only GO molecular function that was consistently down‐regulated after Sac/val in 129X1 mice. Correspondingly, Sac/val but not Val significantly reduced intensity of the Alcian Blue staining suggesting a decline in deposition of the hyaluronic acid within the ligated LCA. A higher efficacy of Sac/val compared with Val was implied by the greater inhibition of vascular fibrosis in 129X1 mice. Our data suggest that Sac/val decreases vascular fibrosis and vascular stiffness due to its effects on extracellular matrix remodelling.
A genetic variation in metabolic enzymes and altered metabolism of these compounds is another possibility to explain the greater efficacy of Sac/val over Val (Ayalasomayajula et al., 2017; de Vries et al., 2016). Our results showing similar expression of CYP2C9 in livers and plasma cGMP levels imply no effect of the mouse genetic background on Sac/val and Val metabolism. Genetic testing for neutral endopeptidase polymorphisms has been suggested to monitor long‐term side effects (cognitive dysfunction or Alzheimer's disease) in ARNI‐treated HF patients so far (Krittanawong & Kitai, 2017). Comparisons of the efficacy of Entresto between cohorts (e.g., German IMS® Disease Analyzer database and PARADIGM‐HF) have been the main focus on demographic and clinical characteristics of patients (Wachter et al., 2018). Entresto is likely to modify vascular structure without direct correlation with systemic biomarkers, despite strong beneficial data in patients with HF (Zile et al., 2016). A discovery of a decreased hyalurononglucosaminidase activity after Sac/val treatment in genetically predisposed “fibrotic” 129X1 mice could explain a greater efficacy of Sac/val over Val in pathological vascular remodelling. We previously proposed multiple genetic and biochemical mechanisms that govern vascular remodelling including proliferation, inflammation, oxidative stress, and extracellular matrix remodelling (Korshunov, Schwartz, & Berk, 2007). In the current study, we found that 129X1 mice had a greater fibrotic response in the ligated LCAs than B6 mice. These findings were further supported by the Sac/val‐dependent inhibition of the unique gene expression signature and molecular functions related to LCA fibrosis after ligation in 129X1 mice. Similarly, Sac/val administration reduced cardiac fibrosis rather than cardiac hypertrophy in its protection against pathological cardiac dysfunction (Miyoshi et al., 2018; von Lueder et al., 2015). In a recent study on rats with chronic kidney disease, Sac/val was more effective than Val in reducing not only cardiac but also aortic fibrosis (Suematsu et al., 2018). A reduced deposition of hyaluronic acid within the ligated LCA provides new therapeutic differences between ARNI and ARB. Targeting and/or modulation of hyaluronic acid has been a promising area for a translational research in vascular disease (Fischer, 2018; Sadowitz et al., 2012). Our results in injured arteries in mice warrant further studies for the better understanding of the pharmacodynamic and pharmacogenomic mechanisms of Entresto.
Even though mice in our experiments are pre‐hypertensive, data in hypertensive rats have also showed that Sac/val caused greater BP reduction than Val (Kusaka et al., 2015). We found that Sac/val was more effective in reducing BP, than Val in 129X1 mice, with higher levels of diastolic BP and mean arterial pressure than in B6 mice, at a baseline. A recent meta‐analysis of 12 studies (3,816 patients) confirmed better anti‐hypertensive efficacy for systolic and diastolic BP and an equal tolerability profile of Entresto compared with Val or olmesartan (Zhao et al., 2017). Previously reported endothelial dysfunction in aortas of 129X1 and 129P3/J sub‐strains of mice might be critical for the greater therapeutic effects of Sac/val in 129X1 mice (Ryan et al., 2002). Our findings in mice revealed a potential role of inherited genes in variation of pharmacodynamics between Sac/val and Val on injured arteries and BP responses. Carotid intima‐media thickness was unchanged in patients treated with calcium channel blockers with/without candesartan, despite significant reduction in BP (Ichihara, Kaneshiro, Takemitsu, Sakoda, & Itoh, 2006). The most striking finding was the significant reduction of not only the intimal thickening but also the smaller size of the ligated LCA after Sac/val‐P or Val‐P in B6 mice. Strain‐dependent diversity in vascular responses to Sac/val in mice could provide an explanation on reduced risks of coronary outcomes after Entresto compared with enalapril in PARADIGM‐HF (Mogensen et al., 2017). A greater protection against pathological remodelling after Entresto compared with olmesartan was also reported in the PARAMETER study (Prospective Comparison of Angiotensin Receptor Neprilysin Inhibitor With Angiotensin Receptor Blocker Measuring Arterial Stiffness in the Elderly) recently (Williams et al., 2017). Importantly, Val‐P and Sac/val‐P had no effect on LCA stiffness despite lesser carotid thickening in B6 mice. In contrast, Sac/val‐P had no effect on intimal thickening but significantly reduced LCA stiffness and fibrosis in 129X1 mice. These vascular changes in 129X1 mice corresponded to a greater BP‐lowering effects of Sac/val, compared with B6 mice. Further research is required to assess effectiveness of Entresto by measuring arterial stiffness as a surrogate marker of arterial pathology in patients.
One experimental caveat could be related to the levels of systolic BP in our estimations of the Young's modulus in the LCA using a recently reported method (Korshunov et al., 2017). Ultrasound RF data were collected in anaesthetized mice (under isoflurane the BP is lower), while BP measured in conscious mice. Unfortunately, it was technically not possible to measure BP in anaesthetized mice, in our experimental protocol. A higher mortality of 129X1 compared with B6 mice was another challenge in our experiments. However, our findings are similar to that in articles on poor healing in 129S6/SvEv mice (Masinde et al., 2006; van den Borne et al., 2009). We observed 40–70% survival of 129X1 mice, which is better than 33% survival of the 129S6/SvEv mice after myocardial infarction (van den Borne et al., 2009). Although, we have not compared pharmacokinetic profiles of Sac/val and Val, we found the same levels of CYP2C9 in both mouse strains. Besides, Novartis Pharmaceuticals determined the dose and the frequency of administration of Sac/val and Val in B6 mice (not shown), which is similar to dosing of Entresto in healthy human subjects (Ayalasomayajula et al., 2017).
In this study, we demonstrated that treating 129X1 mice with an ARNI, Sac/val, is more effective than treating them with an ARB, Val, to decrease BP, carotid stiffness, and carotid fibrosis after injury. We discovered that the inhibition of both hyalurononglucosaminidase activity and the carotid deposition of hyaluronic acid are likely to be the therapeutic advantages of treatment with Sac/val rather than Val, in 129X1 mice. Our results showed a genetic diversity of responses to arterial injury and the superiority of Sac/val over Val, which could be important factors in the choice of treatment in a patient.
CONFLICT OF INTEREST
V.A.K. received a research support LCZ696BUSNC16T from Novartis Pharmaceuticals Corp.
AUTHOR CONTRIBUTIONS
V.A.K. performed conceptualization and project administration. B.Q. and V.A.K. performed the experiments. B.Q., A.F., R.A., M.P.S., and V.A.K. analysed the data. B.Q., A.F., and V.A.K. prepared the figures. M.M.D. and B.C.B. interpreted results of the experiments. V.A.K. drafted the manuscript. B.Q., A.F., R.A., M.P.S., M.M.D., B.C.B., and V.A.K. edited and revised the manuscript. B.Q., A.F., R.A., M.P.S., M.M.D., B.C.B., and V.A.K. approved final version of the manuscript.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design & Analysis, Immunoblotting and Immunochemistry, and Animal Experimentation, and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Table S1.
Surviving reads by Trimmomatic, a trimming tool for Illumina NGS data
Table S2. Upregulated genes in the ligated carotids in 129X1 versus B6 mice after Veh‐P regimen
Table S3. Downregulated genes in the ligated carotids in 129X1 versus B6 mice after Veh‐P regimen
Table S4. Upregulated genes in the ligated carotids in 129X1 versus B6 mice after Veh‐T regimen
Table S5. Downregulated genes in the ligated carotids in 129X1 versus B6 mice after Veh‐T regimen
Table S6. Upregulated genes in the ligated carotids in 129X1 versus B6 mice after Val‐P regimen
Table S7. Downregulated genes in the ligated carotids in 129X1 versus B6 mice after Val‐P regimen
Table S8. Upregulated genes in the ligated carotids in 129X1 versus B6 mice after Sac/val‐P regimen
Table S9. Downregulated genes in 129X1 versus B6 mice after Sac/val‐P regimen
Table S10. Upregulated genes in the ligated carotids in 129X1 versus B6 mice after Val‐T regimen
Table S11. Downregulated genes in the ligated carotids in 129X1 versus B6 mice after Val‐T regimen
Table S12. Upregulated genes in the ligated carotids in 129X1 versus B6 mice after Sac/val‐T regimen
Table S13. Downregulated genes in the ligated carotids in 129X1 vs. B6 mice after Sac/val‐T regimen
Table S14. Upregulated GO molecular functions in 129X1 versus B6 mice common across Sac/val, Val and Veh regimens
Table S15. Downregulated GO molecular functions in 129X1 versus B6 mice common across Sac/val, Val and Veh regimen
Table S16. Upregulated GO molecular functions in 129X1 versus B6 mice shared by Sac/val and Val regimens
Table S17. Downregulated GO molecular functions in 129X1 versus B6 mice shared between Sac/val and Val regimens
Table S18. Upregulated GO molecular functions in 129X1 versus B6 mice unique to Sac/val regimens
Table S19. Downregulated GO molecular functions in 129X1 versus B6 mice unique to Sac/val regimens
Table S20. Blood flow profiles in the ligated left carotid artery across the groups
Table S21. Blood flow profiles in the contralateral right carotid artery across the groups
Figure S1. A graphical representation of experimental outline. Mice were organized into 12 experimental groups based upon strain (C57BL/6 J, B6, or 129X1/SvJ, 129X1), compound (Veh, Val, or Sac/val) or treatment regimen (prophylactic or therapeutic). Mice subjected to blood pressure (BP) training between 9 and 2 days prior to interventions. Ultrasound (US) and BP were measured at 1‐week (5–7 days post ligation) and 2‐week (12–14 days post ligation) time points.
Figure S2. Post‐surgical survival rates of treated mice. Survival rates for mice receiving prophylactic (P‐) regimens (Veh, Val, or Sac/val) in B6 (A) and 129X1 mice (B). Survival rate after therapeutic (T‐) regimens in B6 (C) and 129X1 mice (D). Carotid ligation occurred on day 0. E. Body weights for mice receiving P‐regimens. F. Body weights for mice receiving Tregimen. Open bars indicate body weights at the baseline (time of the surgery, Day 0). Black bars represent 2‐week measurements. Values are mean ± SEM; *, p < 0.05 vs. Baseline. n, Number of animals per group.
Figure S3. Heatmaps show pairwise comparisons between gene expression in the ligated carotids in Veh‐treated 129X and B6 mice. Sample‐to‐sample distances were plotted for visualizing the variability within and between 129X1 and B6 mice after prophylactic (A) and therapeutic (B) regimens of Veh. Heatmaps show hierarchically clustered Euclidean distances between samples and a direction of gene expression: from lowest dark blue, to the highest – red color.3
Figure S4. A principal component analysis of gene expression in the ligated carotids in between 129X and B6 mice. The samples shown in the 2‐dimentional plane spanned by their first two principal components, which are the two components explaining most of the variance after prophylactic (A) and therapeutic (B) regimens. The individual carotid samples are colorcoded.
Figure S5. Hemodynamic changes after therapeutic (T‐) regimen of treatments in two inbred mouse strains. A. Systolic blood pressure (mmHg) of mice after T‐regimens of the compounds (Veh, Val, Sac/val). B. Diastolic blood pressure, mmHg. C. Mean arterial pressure, mmHg. D. Heart rate, beats/min. Open bars indicate parameters at the baseline. Black bars represent 2‐week measurements. Values are mean ± SEM; *, p < 0.05 vs. Baseline; †, p < 0.05 vs. Veh‐P, 2 weeks; ‡, p < 0.05 vs. B6 mice. n, Number of animals per group.
Figure S6. Similar metabolic responses to Sac/val or Val in inbred mice. A. Representative Western Blots for CYP2C9 and GAPDH in mouse livers. Quantification the ratios of CYP2C9 to GAPDH is shown in bar graphs. B. Plasma concentration of cGMP in ng/mL. Experimental groups (Veh, Val, or Sac/val) are listed on X‐axis. Black bars show mice after prophylactic (P‐) regimens. Grey bars show mice after therapeutic (T‐) regimen. Values are mean ± SEM; n, Number of animals per group.
Figure S7. Carotid artery remodeling after therapeutic (T‐) regimen of treatments in two inbred mouse strains. A. Representative 3‐dimensional (3D) reconstructions of the 2 mm‐length from bifurcation of left carotid artery (LCA) and right carotid artery (RCA) after T‐regimen of 4 Veh in B6 and 129X1 mice. B. Representative 3D carotids after T‐regimen of Val in B6 and 129X1 mice. C. Representative 3D carotids after T‐regimen of Sac/val in B6 and 129X1 mice. Black color shows lumen, yellow – intima, red – media, green – adventitia volume. D. Quantifications of the lumen volumes (top left), ratio of intima to media (top right), adventitia volume (bottom left) and external elastic lamina (EEL) volume (bottom right). Black bars represent ligated LCA. Open bars indicate RCA. Experimental groups (Veh, Val, or Sac/val) are listed on X‐axis. Values are mean ± SEM; *, p < 0.05 vs. B6; †, p < 0.05 vs. RCA. n, Number of animals per group.
Figure S8. Vein‐diagrams of upregulated and downregulated gene numbers in injured carotid artery in 129X1 vs. B6 mice after therapeutic (T‐) regimens. Open circles represent differentially expressed gene numbers Veh‐treated mice. Light grey circles – Val‐treated mice. Dark grey circles – Sac/val‐treated mice. The numbers in overlay areas show shared genes between the treatments.
Figure S9. Strain‐dependent differences in efficacy after therapeutic (T‐) Sac/val versus Val regimen in inhibiting of fibrosis in the injured carotid artery. A. A quantification of fibrosis within intima‐media area of the LCA, %. C. A quantification of fibrosis in adventitia of the LCA, %. Open bars indicate B6. Black bars represent 129X1 mice. Experimental groups (Veh, Val, or Sac/val) are listed on X‐axis. Values are mean ± SEM; *, p < 0.05 vs. B6; †, p < 0.05 vs. Sac/val. n, Number of animals per group.
ACKNOWLEDGEMENTS
This study was supported by the Grant LCZ696BUSNC16T from Novartis Pharmaceuticals Corporation. The authors would like to thank Michelle Zanche and Jason Myers (UR Genomics Research Center) for assistance with RNAseq, Orazio Silvano for help with mouse histology, and Dr Wendy Bates for veterinary support of experiments on mice.
Korshunov VA, Quinn B, Faiyaz A, et al. Strain‐selective efficacy of sacubitril/valsartan on carotid fibrosis in response to injury in two inbred mouse strains. Br J Pharmacol. 2019;176:2795–2807. 10.1111/bph.14708
REFERENCES
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Marrion, N. V. , Peters, J. A. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. British Journal of Pharmacology, 174, S17–S129. 10.1111/bph.13878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174, S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Animals . (2011). National Research Council: Guide for the Care and Use of Laboratory. Washington, DC: Animals National Academies Press. [Google Scholar]
- Ayalasomayajula, S. , Langenickel, T. , Pal, P. , Boggarapu, S. , & Sunkara, G. (2017). Clinical pharmacokinetics of sacubitril/valsartan (LCZ696): A novel angiotensin receptor‐neprilysin inhibitor. Clinical Pharmacokinetics, 56, 1461–1478. 10.1007/s40262-017-0543-3 [DOI] [PubMed] [Google Scholar]
- Batchu, S. N. , Hughson, A. , Gerloff, J. , Fowell, D. J. , & Korshunov, V. A. (2013). Role of Axl in early kidney inflammation and progression of salt‐dependent hypertension. Hypertension, 62, 302–309. 10.1161/HYPERTENSIONAHA.113.01382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchu, S. N. , Smolock, E. M. , Dyachenko, I. A. , Murashev, A. N. , & Korshunov, V. A. (2015). Autonomic dysfunction determines stress‐induced cardiovascular and immune complications in mice. Journal of the American Heart Association, 4 10.1161/JAHA.115.001952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchu, S. N. , Xia, J. , Ko, K. A. , Doyley, M. M. , Abe, J. , Morrell, C. N. , & Korshunov, V. A. (2015). Axl modulates immune activation of smooth muscle cells in vein graft remodeling. American Journal of Physiology Heart and Circulatory Physiology, 309, H1048–H1058. 10.1152/ajpheart.00495.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, B. J. , Davis, R. C. , Civelek, M. , Orozco, L. , Wu, J. , Qi, H. , … Lusis, A. J. (2015). Genetic architecture of atherosclerosis in mice: A systems genetics analysis of common inbred strains. PLoS Genetics, 11, e1005711 10.1371/journal.pgen.1005711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries, E. M. , Lammers, L. A. , Achterbergh, R. , Klumpen, H. J. , Mathot, R. A. , Boelen, A. , & Romijn, J. A. (2016). Fasting‐induced changes in hepatic P450 mediated drug metabolism are largely independent of the constitutive androstane receptor CAR. PLoS ONE, 11, e0159552 10.1371/journal.pone.0159552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar, R. , Domrachev, M. , & Lash, A. E. (2002). Gene expression omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Research, 30, 207–210. 10.1093/nar/30.1.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, J. W. (2018). Role of hyaluronan in atherosclerosis: Current knowledge and open questions. Matrix Biology, 78‐79, 324–336. 10.1016/j.matbio.2018.03.003 [DOI] [PubMed] [Google Scholar]
- Gerloff, J. , & Korshunov, V. A. (2012). Immune modulation of vascular resident cells by Axl orchestrates carotid intima‐media thickening. The American Journal of Pathology, 180, 2134–2143. 10.1016/j.ajpath.2012.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara, A. , Kaneshiro, Y. , Takemitsu, T. , Sakoda, M. , & Itoh, H. (2006). Benefits of candesartan on arterial and renal damage of non‐diabetic hypertensive patients treated with calcium channel blockers. American Journal of Nephrology, 26, 462–468. 10.1159/000096581 [DOI] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korshunov, V. A. , & Berk, B. C. (2003). Flow‐induced vascular remodeling in the mouse: A model for carotid intima‐media thickening. Arteriosclerosis, Thrombosis, and Vascular Biology, 23, 2185–2191. 10.1161/01.ATV.0000103120.06092.14 [DOI] [PubMed] [Google Scholar]
- Korshunov, V. A. , & Berk, B. C. (2004). Strain‐dependent vascular remodeling: The “Glagov phenomenon” is genetically determined. Circulation, 110, 220–226. 10.1161/01.CIR.0000134958.88379.2E [DOI] [PubMed] [Google Scholar]
- Korshunov, V. A. , Massett, M. P. , Carey, R. M. , & Berk, B. C. (2004). Role of angiotensin‐converting enzyme and neutral endopeptidase in flow‐dependent remodeling. Journal of Vascular Research, 41, 148–156. 10.1159/000077144 [DOI] [PubMed] [Google Scholar]
- Korshunov, V. A. , Schwartz, S. M. , & Berk, B. C. (2007). Vascular remodeling: Hemodynamic and biochemical mechanisms underlying Glagov's phenomenon. Arteriosclerosis, Thrombosis, and Vascular Biology, 27, 1722–1728. 10.1161/ATVBAHA.106.129254 [DOI] [PubMed] [Google Scholar]
- Korshunov, V. A. , Wang, H. , Ahmed, R. , Mickelsen, D. M. , Zhou, Q. , Yan, C. , & Doyley, M. M. (2017). Model‐based vascular elastography improves the detection of flow‐induced carotid artery remodeling in mice. Scientific Reports, 7, 12081 10.1038/s41598-017-12321-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krittanawong, C. , & Kitai, T. (2017). Pharmacogenomics of angiotensin receptor/neprilysin inhibitor and its long‐term side effects. Cardiovascular Therapeutics, 35 10.1111/1755-5922.12272 [DOI] [PubMed] [Google Scholar]
- Kuleshov, M. V. , Jones, M. R. , Rouillard, A. D. , Fernandez, N. F. , Duan, Q. , Wang, Z. , … Ma'ayan, A. (2016). Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Research, 44, W90–W97. 10.1093/nar/gkw377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaka, H. , Sueta, D. , Koibuchi, N. , Hasegawa, Y. , Nakagawa, T. , Lin, B. , … Kim‐Mitsuyama, S. (2015). LCZ696, angiotensin II receptor‐neprilysin inhibitor, ameliorates high‐salt‐induced hypertension and cardiovascular injury more than valsartan alone. American Journal of Hypertension, 28, 1409–1417. 10.1093/ajh/hpv015 [DOI] [PubMed] [Google Scholar]
- Masinde, G. L. , Li, R. , Nguyen, B. , Yu, H. , Srivastava, A. K. , Edderkaoui, B. , … Mohan, S. (2006). New quantitative trait loci that regulate wound healing in an intercross progeny from DBA/1J and 129x 1/SvJ inbred strains of mice. Functional & Integrative Genomics, 6, 157–163. 10.1007/s10142-005-0004-1 [DOI] [PubMed] [Google Scholar]
- McMurray, J. J. , Packer, M. , Desai, A. S. , Gong, J. , Lefkowitz, M. P. , Rizkala, A. R. , … PARADIGM‐HF Investigators and Committees (2014). Angiotensin‐neprilysin inhibition versus enalapril in heart failure. The New England Journal of Medicine, 371, 993–1004. 10.1056/NEJMoa1409077 [DOI] [PubMed] [Google Scholar]
- Miyoshi, T. , Nakamura, K. , Miura, D. , Yoshida, M. , Saito, Y. , Akagi, S. , … Ito, H. (2018). Effect of LCZ696, a dual angiotensin receptor neprilysin inhibitor, on isoproterenol‐induced cardiac hypertrophy, fibrosis, and hemodynamic change in rats. Cardiology Journal. 10.5603/CJ.a2018.0048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen, U. M. , Kober, L. , Kristensen, S. L. , Jhund, P. S. , Gong, J. , Lefkowitz, M. P. , … PARADIGM‐HF Investigators and Committees (2017). The effects of sacubitril/valsartan on coronary outcomes in PARADIGM‐HF. American Heart Journal, 188, 35–41. 10.1016/j.ahj.2017.02.034 [DOI] [PubMed] [Google Scholar]
- Ryan, M. J. , Didion, S. P. , Davis, D. R. , Faraci, F. M. , & Sigmund, C. D. (2002). Endothelial dysfunction and blood pressure variability in selected inbred mouse strains. Arteriosclerosis, Thrombosis, and Vascular Biology, 22, 42–48. 10.1161/hq0102.101098 [DOI] [PubMed] [Google Scholar]
- Sadowitz, B. , Seymour, K. , Gahtan, V. , & Maier, K. G. (2012). The role of hyaluronic acid in atherosclerosis and intimal hyperplasia. The Journal of Surgical Research, 173, e63–e72. 10.1016/j.jss.2011.09.025 [DOI] [PubMed] [Google Scholar]
- Seki, T. , Goto, K. , Kansui, Y. , Ohtsubo, T. , Matsumura, K. , & Kitazono, T. (2017). Angiotensin II receptor‐neprilysin inhibitor sacubitril/valsartan improves endothelial dysfunction in spontaneously hypertensive rats. Journal of the American Heart Association, 6 10.1161/JAHA.117.006617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolock, E. M. , Burke, R. M. , Wang, C. , Thomas, T. , Batchu, S. N. , Qiu, X. , … Korshunov, V. A. (2014). Intima modifier locus 2 controls endothelial cell activation and vascular permeability. Physiological Genomics, 46, 624–633. 10.1152/physiolgenomics.00048.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolock, E. M. , Ilyushkina, I. A. , Ghazalpour, A. , Gerloff, J. , Murashev, A. N. , Lusis, A. J. , & Korshunov, V. A. (2012). A genetic locus on mouse chromosome 7 controls elevated heart rate. Physiological Genomics, 44, 689–698. 10.1152/physiolgenomics.00041.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon, S. D. , Claggett, B. , Desai, A. S. , Packer, M. , Zile, M. , Swedberg, K. , … McMurray, J. J. (2016). Influence of ejection fraction on outcomes and efficacy of sacubitril/valsartan (LCZ696) in heart failure with reduced ejection fraction: The Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM‐HF) trial. Circulation Heart Failure, 9, e002744. [DOI] [PubMed] [Google Scholar]
- Solomon, S. D. , Zile, M. , Pieske, B. , Voors, A. , Shah, A. , Kraigher‐Krainer, E. , … McMurray, J. J. V. (2012). The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: A phase 2 double‐blind randomised controlled trial. Lancet, 380, 1387–1395. 10.1016/S0140-6736(12)61227-6 [DOI] [PubMed] [Google Scholar]
- Suematsu, Y. , Jing, W. , Nunes, A. , Kashyap, M. L. , Khazaeli, M. , Vaziri, N. D. , & Moradi, H. (2018). LCZ696 (sacubitril/valsartan), an angiotensin‐receptor neprilysin inhibitor, attenuates cardiac hypertrophy, fibrosis, and vasculopathy in a rat model of chronic kidney disease. Journal of Cardiac Failure, 24, 266–275. 10.1016/j.cardfail.2017.12.010 [DOI] [PubMed] [Google Scholar]
- van den Borne, S. W. , van de Schans, V. A. , Strzelecka, A. E. , Vervoort‐Peters, H. T. , Lijnen, P. M. , Cleutjens, J. P. , … Blankesteijn, W. M. (2009). Mouse strain determines the outcome of wound healing after myocardial infarction. Cardiovascular Research, 84, 273–282. 10.1093/cvr/cvp207 [DOI] [PubMed] [Google Scholar]
- von Lueder, T. G. , Wang, B. H. , Kompa, A. R. , Huang, L. , Webb, R. , Jordaan, P. , … Krum, H. (2015). Angiotensin receptor neprilysin inhibitor LCZ696 attenuates cardiac remodeling and dysfunction after myocardial infarction by reducing cardiac fibrosis and hypertrophy. Circulation Heart Failure, 8, 71–78. 10.1161/CIRCHEARTFAILURE.114.001785 [DOI] [PubMed] [Google Scholar]
- Wachter, R. , Viriato, D. , Klebs, S. , Grunow, S. S. , Schindler, M. , Engelhard, J. , … Bruce Wirta, S. (2018). Early insights into the characteristics and evolution of clinical parameters in a cohort of patients prescribed sacubitril/valsartan in Germany. Postgraduate Medicine, 130, 308–316. 10.1080/00325481.2018.1442090 [DOI] [PubMed] [Google Scholar]
- Williams, B. , Cockcroft, J. R. , Kario, K. , Zappe, D. H. , Brunel, P. C. , Wang, Q. , & Guo, W. (2017). Effects of sacubitril/valsartan versus olmesartan on central hemodynamics in the elderly with systolic hypertension: The PARAMETER study. Hypertension, 69, 411–420. 10.1161/HYPERTENSIONAHA.116.08556 [DOI] [PubMed] [Google Scholar]
- Zhao, Y. , Yu, H. , Zhao, X. , Ma, R. , Li, N. , & Yu, J. (2017). The effects of LCZ696 in patients with hypertension compared with angiotensin receptor blockers: A meta‐analysis of randomized controlled trials. Journal of Cardiovascular Pharmacology and Therapeutics, 22, 447–457. 10.1177/1074248417693379 [DOI] [PubMed] [Google Scholar]
- Zile, M. R. , Jhund, P. S. , Baicu, C. F. , Claggett, B. L. , Pieske, B. , Voors, A. A. , … Prospective Comparison of ARNI With ARB on Management of Heart Failure With Preserved Ejection Fraction (PARAMOUNT) Investigators (2016). Plasma biomarkers reflecting profibrotic processes in heart failure with a preserved ejection fraction: Data from the prospective comparison of ARNI with ARB on management of heart failure with preserved ejection fraction study. Circulation Heart Failure, 9 10.1161/CIRCHEARTFAILURE.115.002551 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Surviving reads by Trimmomatic, a trimming tool for Illumina NGS data
Table S2. Upregulated genes in the ligated carotids in 129X1 versus B6 mice after Veh‐P regimen
Table S3. Downregulated genes in the ligated carotids in 129X1 versus B6 mice after Veh‐P regimen
Table S4. Upregulated genes in the ligated carotids in 129X1 versus B6 mice after Veh‐T regimen
Table S5. Downregulated genes in the ligated carotids in 129X1 versus B6 mice after Veh‐T regimen
Table S6. Upregulated genes in the ligated carotids in 129X1 versus B6 mice after Val‐P regimen
Table S7. Downregulated genes in the ligated carotids in 129X1 versus B6 mice after Val‐P regimen
Table S8. Upregulated genes in the ligated carotids in 129X1 versus B6 mice after Sac/val‐P regimen
Table S9. Downregulated genes in 129X1 versus B6 mice after Sac/val‐P regimen
Table S10. Upregulated genes in the ligated carotids in 129X1 versus B6 mice after Val‐T regimen
Table S11. Downregulated genes in the ligated carotids in 129X1 versus B6 mice after Val‐T regimen
Table S12. Upregulated genes in the ligated carotids in 129X1 versus B6 mice after Sac/val‐T regimen
Table S13. Downregulated genes in the ligated carotids in 129X1 vs. B6 mice after Sac/val‐T regimen
Table S14. Upregulated GO molecular functions in 129X1 versus B6 mice common across Sac/val, Val and Veh regimens
Table S15. Downregulated GO molecular functions in 129X1 versus B6 mice common across Sac/val, Val and Veh regimen
Table S16. Upregulated GO molecular functions in 129X1 versus B6 mice shared by Sac/val and Val regimens
Table S17. Downregulated GO molecular functions in 129X1 versus B6 mice shared between Sac/val and Val regimens
Table S18. Upregulated GO molecular functions in 129X1 versus B6 mice unique to Sac/val regimens
Table S19. Downregulated GO molecular functions in 129X1 versus B6 mice unique to Sac/val regimens
Table S20. Blood flow profiles in the ligated left carotid artery across the groups
Table S21. Blood flow profiles in the contralateral right carotid artery across the groups
Figure S1. A graphical representation of experimental outline. Mice were organized into 12 experimental groups based upon strain (C57BL/6 J, B6, or 129X1/SvJ, 129X1), compound (Veh, Val, or Sac/val) or treatment regimen (prophylactic or therapeutic). Mice subjected to blood pressure (BP) training between 9 and 2 days prior to interventions. Ultrasound (US) and BP were measured at 1‐week (5–7 days post ligation) and 2‐week (12–14 days post ligation) time points.
Figure S2. Post‐surgical survival rates of treated mice. Survival rates for mice receiving prophylactic (P‐) regimens (Veh, Val, or Sac/val) in B6 (A) and 129X1 mice (B). Survival rate after therapeutic (T‐) regimens in B6 (C) and 129X1 mice (D). Carotid ligation occurred on day 0. E. Body weights for mice receiving P‐regimens. F. Body weights for mice receiving Tregimen. Open bars indicate body weights at the baseline (time of the surgery, Day 0). Black bars represent 2‐week measurements. Values are mean ± SEM; *, p < 0.05 vs. Baseline. n, Number of animals per group.
Figure S3. Heatmaps show pairwise comparisons between gene expression in the ligated carotids in Veh‐treated 129X and B6 mice. Sample‐to‐sample distances were plotted for visualizing the variability within and between 129X1 and B6 mice after prophylactic (A) and therapeutic (B) regimens of Veh. Heatmaps show hierarchically clustered Euclidean distances between samples and a direction of gene expression: from lowest dark blue, to the highest – red color.3
Figure S4. A principal component analysis of gene expression in the ligated carotids in between 129X and B6 mice. The samples shown in the 2‐dimentional plane spanned by their first two principal components, which are the two components explaining most of the variance after prophylactic (A) and therapeutic (B) regimens. The individual carotid samples are colorcoded.
Figure S5. Hemodynamic changes after therapeutic (T‐) regimen of treatments in two inbred mouse strains. A. Systolic blood pressure (mmHg) of mice after T‐regimens of the compounds (Veh, Val, Sac/val). B. Diastolic blood pressure, mmHg. C. Mean arterial pressure, mmHg. D. Heart rate, beats/min. Open bars indicate parameters at the baseline. Black bars represent 2‐week measurements. Values are mean ± SEM; *, p < 0.05 vs. Baseline; †, p < 0.05 vs. Veh‐P, 2 weeks; ‡, p < 0.05 vs. B6 mice. n, Number of animals per group.
Figure S6. Similar metabolic responses to Sac/val or Val in inbred mice. A. Representative Western Blots for CYP2C9 and GAPDH in mouse livers. Quantification the ratios of CYP2C9 to GAPDH is shown in bar graphs. B. Plasma concentration of cGMP in ng/mL. Experimental groups (Veh, Val, or Sac/val) are listed on X‐axis. Black bars show mice after prophylactic (P‐) regimens. Grey bars show mice after therapeutic (T‐) regimen. Values are mean ± SEM; n, Number of animals per group.
Figure S7. Carotid artery remodeling after therapeutic (T‐) regimen of treatments in two inbred mouse strains. A. Representative 3‐dimensional (3D) reconstructions of the 2 mm‐length from bifurcation of left carotid artery (LCA) and right carotid artery (RCA) after T‐regimen of 4 Veh in B6 and 129X1 mice. B. Representative 3D carotids after T‐regimen of Val in B6 and 129X1 mice. C. Representative 3D carotids after T‐regimen of Sac/val in B6 and 129X1 mice. Black color shows lumen, yellow – intima, red – media, green – adventitia volume. D. Quantifications of the lumen volumes (top left), ratio of intima to media (top right), adventitia volume (bottom left) and external elastic lamina (EEL) volume (bottom right). Black bars represent ligated LCA. Open bars indicate RCA. Experimental groups (Veh, Val, or Sac/val) are listed on X‐axis. Values are mean ± SEM; *, p < 0.05 vs. B6; †, p < 0.05 vs. RCA. n, Number of animals per group.
Figure S8. Vein‐diagrams of upregulated and downregulated gene numbers in injured carotid artery in 129X1 vs. B6 mice after therapeutic (T‐) regimens. Open circles represent differentially expressed gene numbers Veh‐treated mice. Light grey circles – Val‐treated mice. Dark grey circles – Sac/val‐treated mice. The numbers in overlay areas show shared genes between the treatments.
Figure S9. Strain‐dependent differences in efficacy after therapeutic (T‐) Sac/val versus Val regimen in inhibiting of fibrosis in the injured carotid artery. A. A quantification of fibrosis within intima‐media area of the LCA, %. C. A quantification of fibrosis in adventitia of the LCA, %. Open bars indicate B6. Black bars represent 129X1 mice. Experimental groups (Veh, Val, or Sac/val) are listed on X‐axis. Values are mean ± SEM; *, p < 0.05 vs. B6; †, p < 0.05 vs. Sac/val. n, Number of animals per group.


