Abstract
Mesozooplankton communities in marine ecosystems are mainly influenced by both anthropogenic pollutants (e.g. nutrients and heavy metals) and natural variables (e.g. temperature, salinity and geographic distance). To achieve a deeper understanding of the effects of anthropogenic pollutants on mesozooplankton communities, we analyzed the community structure of mesozooplankton from 91 stations representing five typical estuarine regions in the Bohai Sea and assessed the relative importance of anthropogenic pollutants and natural variables by using multiple statistical approaches. Cd was identified as the leading pollutant for observed community variation among the five regions, followed by NH4-N and COD. Redundancy analysis (RDA) model demonstrated that mesozooplankton communities were largely determined by both anthropogenic pollutants and natural variables, and the indicator species of mesozooplankton also varied when responding to different factors. Variance partitioning analysis showed both anthropogenic pollutants and natural variables posed significant influences (ANOVA, P < 0.05) on the mesozooplankton community structure, but the explanatory power of anthropogenic pollutants overrode the natural variables. These observations highlighted the importance of anthropogenic pollutants in the shifts of zooplankton structures among different regions. Our results obtained in this study provided new insights into the mechanism of the influence of anthropogenic pollutants on mesozooplankton communities in estuarine areas.
Subject terms: Animal physiology, Environmental impact
Introduction
The estuarine ecosystems, which link the sea with freshwater habitats, are among the most ecologically and social-economically important globally1,2. However, this ecotone have been seriously threatened, particularly in the past half century, by anthropogenic activities, such as massive release of chemical pollutants3. The increasing pollution has caused significantly negative effects, such as the losses of marine biodiversity and disturbance of ecological function4. Hence, it is crucial to comprehensively study the influence of anthropogenic pollutants on inhabitant organism communities, and the understanding has been considered as great potential to monitor, manage and protect marine biodiversity5–7.
The variation of marine communities can be driven by multiple factors such as natural variables and anthropogenic pollutants8–10. Mounting evidence demonstrated that natural variables including temperature, salinity and geographic distance play key roles in shaping species richness and abundance10–14. Simultaneously, anthropogenic pollutants, such as nutrients and heavy metals, could also influence biodiversity and geographical distribution of marine communities. For example, many anthropogenic pollutants such as ammonia nitrogen and cadmium can pose toxic effects on plankton and fish, thus decreasing the survival rates of sensitive species and reducing species diversity15–18. When exploring the influence of anthropogenic pollutants on marine communities, we should also consider the impact of natural variables, which may mask the ecological influence of anthropogenic pollutants6–10.
Zooplankton inhabiting coastal ecosystems are key components in the food webs, acting an important trophic link between the marine primary producers and higher trophic levels3,19. The crucial role in marine food webs indicates that decline in biodiversity of zooplankton could decrease the survival rates of higher trophic organisms such as fish20, and eventually pose far-reaching consequences for the marine food webs21. Moreover, zooplankton assemblages consist of species highly sensitive to environmental pollutants such as nutrients and heavy metals, and their variation could be attributed to different levels of pollution in marine systems, such as estuarine systems19,22,23 and coastal systems24,25, also making them good indicators to explore ecological effects of anthropogenic pollutants. Available studies mainly focused on ecological effects of environmental variables and hydrological processes on zooplankton community structure26,27. However, natural variables and anthropogenic pollutants may act synergistically, additively or antagonistically28–33, suggesting that it is essential to distinguish respective effects of these two categories of factors.
The Bohai Sea, which is a semi-closed inner sea located in the northeast China, has been impacted by serious pollution caused by anthropogenic activities. The coastal regions support various industries derived from extremely rapid urbanization and industrialization in the past decades, and such rapid development along the coastal regions has caused severe pollution problems to Bohai Sea, especially for the coastal estuaries. The pollution levels varied among the coastal estuaries of Bohai Sea, especially for heavy metal pollutions3,34–37. For example, Jinzhou Bay, which is surrounded by one of the old industrial bases in China, was found with the highest concentrations of Cd, the pollution levels gradually decreased along Jinzhou Bay, Luanhekou Estuary, Laizhou Bay, Shuangtaizi Estuary and Bohai Bay36. Thus, the environmental gradients along the coastal bays or estuaries provide premise conditions for the exploration of ecological response of zooplankton under anthropogenic stress.
In this study, we aimed to: (1) characterize the community structure of mesozooplankton in the typical Bohai estuarine regions, (2) identify the key factors responsible for local and regional community structure variation, and further (3) explore the respective ecological roles of anthropogenic pollutants and natural variables on community structure.
Results
Environmental features
Generally, the average value of all environmental factors showed significant difference (P < 0.05) among the five regions (Fig. 1; Table 1). Tests based on nonmetric multidimensional scaling (NMDS) and the analysis of similarity (ANOSIM) also provided similar results (Stress = 0.14, Global R = 0.409, P = 0.001; Fig. 2A), supporting significant environmental gradients among the five regions.
Figure 1.
Sampling locations of zooplankton communities along the coastal bays or estuaries of Bohai Sea, China. All maps are made by ArcGIS version 10.0 (ESRI Company, USA).
Table 1.
Average value and standard deviation and ANOVA test for each environmental factor recorded and sampling dates and coordinate in the five coastal regions of Bohai Sea.
| Environmental parameters | Jinzhou Bay | Luanhe Estuary | Bohai Bay | Laizhou Bay | Yellow River Estuary | F | p value |
|---|---|---|---|---|---|---|---|
| Sampling dates | 28/08/2015 | 13/08/2015 | 4/08/2015 | 11/08/2015 | 9/08/2015 | ||
| Coordinate |
120.9797–121.0789 N 40.7681–40.7800 E |
119.2917–119.5900 N 39.4417–39.7833 E |
117.7667–118.0667 N 38.6569–39.0967 E |
119.0500–119.8300 N 36.7500–37.5000 E |
119.0833–119.5167 N 37.4564–38.0333 E |
||
| Temperature (°C) | 27.76 ± 0.13b | 27.08 ± 0.90b | 28.51 ± 0.0.28a | 29.16 ± 0.62a | 27.08 ± 1.07b | 30.90 | <0.000 |
| Salinity | 29.52 ± 0.79b | 30.79 ± 0.46a | 30.71 ± 0.63a | 29.33 ± 1.53b | 28.09 ± 1.79c | 18.31 | <0.001 |
| COD (mg/L) | 1.34 ± 0.29b | 1.36 ± 0.13b | 2.15 ± 0.51a | 2.05 ± 0.51a | 1.57 ± 0.63b | 14.02 | <0.001 |
| Dissolved oxygen (mg/L) | 6.32 ± 0.22b | 7.71 ± 1.05a | 6.56 ± 1.30b | 7.04 ± 1.25ab | 7.33 ± 1.58ab | 3.91 | 0.006 |
| Suspended matter (mg/L) | 9.87 ± 3.26b | 20.43 ± 2.05ab | 22.02 ± 2.83a | 14.80 ± 8.15b | 25.74 ± 14.82a | 10.02 | <0.001 |
| PO4-P (mg/L) | 0.0208 ± 0.0355a | 0.0076 ± 0.0035b | 0.0121 ± 0.0047ab | 0.006 ± 0.005b | 0.0048 ± 0.004b | 3.84 | 0.006 |
| NO2-N (mg/L) | 0.0404 ± 0.0317b | 0.0124 ± 0.0051b | 0.0745 ± 0.05a | 0.0082 ± 0.0091c | 0.0615 ± 0.0182ab | 26.39 | <0.001 |
| NO3-N (mg/L) | 0.2256 ± 0.2633b | 0.0946 ± 0.0184b | 0.1897 ± 0.1219b | 0.5849 ± 0.6757a | 0.1349 ± 0.0427b | 7.41 | <0.001 |
| NH4-N (mg/L) | 0.043 ± 0.0318b | 0.0358 ± 0.0134b | 0.1226 ± 0.0575a | 0.0745 ± 0.0616b | 0.1588 ± 0.0813a | 17.82 | <0.001 |
| Chlorophyll-a (μg/L) | 4.1854 ± 1.7692ab | 5.6525 ± 5.0847ab | 6.726 ± 5.8175a | 4.0186 ± 1.8437ab | 2.6585 ± 5.1404b | 2.58 | 0.041 |
| pH | 8.15 ± 0.10a | 8.07 ± 0.09b | 8.00 ± 0.05b | 8.08 ± 0.06ab | 8.01 ± 0.07b | 12.06 | <0.001 |
| As (mg/L) | 0.0061 ± 0.0006a | 0.0012 ± 0.0004c | 0.0023 ± 0.0003bc | 0.0026 ± 0.0013b | 0.0017 ± 0.0006c | 95.01 | <0.001 |
| Hg (mg/L) | 0.0001 ± 0a | 0 ± 0c | 0 ± 0c | 0.0001 ± 0b | 0.0001 ± 0b | 38.70 | <0.001 |
| Cu (mg/L) | 0.0034 ± 0.0016ab | 0.0033 ± 0.0009ab | 0.0045 ± 0.0026a | 0.0031 ± 0.0008b | 0.0016 ± 0.0006c | 10.72 | <0.001 |
| Pb (mg/L) | 0.0025 ± 0.0007a | 0.0002 ± 0.0001c | 0.0018 ± 0.0017ab | 0.0011 ± 0.0009b | 0.0011 ± 0.0005b | 15.58 | <0.001 |
| Cd (mg/L) | 0.0007 ± 0.0001a | 0.0004 ± 0.0001b | 0.0002 ± 0.0001c | 0 ± 0.0001d | 0.0001 ± 0c | 127.10 | <0.001 |
| Zn (mg/L) | 0.0243 ± 0.0023b | 0.0132 ± 0.0029b | 0.0112 ± 0.0084b | 0.1724 ± 0.0624a | 0.0151 ± 0.0046b | 117.60 | <0.001 |
COD represented chemical oxygen demand.
Different superscript letters indicate significant difference within a row.
Figure 2.
The results of nonmetric multidimensional scaling ordination (NMDS) of environmental variables at each sampling location (A) and zooplankton communities at each sampling location (B).
Specifically, the concentrations of As, Hg, Pb and Cd were the highest in Jinzhou Bay among the five regions and significantly differed from those in other regions (Table 1). While, the concentration of Zn in Laizhou Bay was significantly higher than that in the rest four regions. The concentration of Cu in Jinzhou Bay, Luanhe Estuary and Bohai Bay was significantly higher than that in Laizhou Bay and Yellow River Estuary. One organic pollutant indicator, COD, in Bohai Bay and Laizhou Bay were significantly higher than that in Yellow River Estuary, Luanhe Estuary and Jinzhou Bay. Luanhe Estuary and Bohai Bay showed significantly higher salinity than that in Jinzhou Bay, Laizhou Estuary and Yellow River Estuary. The concentration of NH4-N in Yellow River Estuary and Bohai Bay was significantly higher than that in the rest three regions. While, the concentration of NO3-N in Laizhou Bay was highest and significant difference was observed between Laizhou Bay and the other regions (Table 1).
Zooplankton community structure
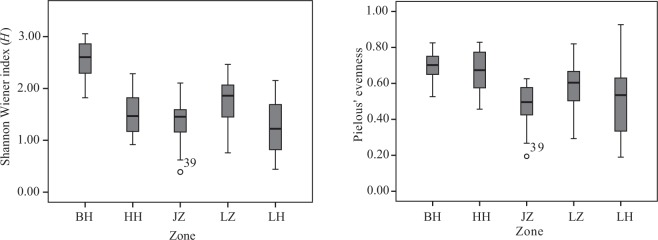
A total of 43 mesozooplankton species were identified in the five regions across all 91 sampling stations (Table 2). The mesozooplankton communities were dominated by Paracalanus parvus (26.94%), Acartia bifilosa (25.30%), Oithona similis (11.50%) and A. pacifica (9.21%). When the four diversity index (Species richness, Shannon-Wiener index, Pielous’ evenness and Simpson diversity index) within each region were subjected to the non-parametric Mann-Whitney U test, we found significant difference between regions (P < 0.05 for most pairs, Table 2). In addition, a coincident result was also observed based on statistical percentiles, as there appeared different median values and 25th and 75th percentiles, such as for Shannon-Wiener index and Pielous’ evenness (Fig. 3).
Table 2.
Occurrence, density (ind.m−3, Mean ± SD) and diversity index of the zooplankton species at each sampling zone in Bohai Sea (Augest 2015).
| Species | Bohai Bay | Yellow River Estuary | Jinzhou Bay | Luanhe Estuary | Laizhou Bay | Average |
|---|---|---|---|---|---|---|
| Paracalanus parvus | 2861.9 ± 2893 | 30382.8 ± 2077.2 | 742.4 ± 804.8 | 2419 ± 1479.3 | 7049.1 ± 9506.5 | 3170.5 ± 5061.6 |
| Acartia bifilosa | 9240.4 ± 12850.4 | 0 ± 0 | 0 ± 0 | 2410 ± 7677.9 | 1489.3 ± 3985.4 | 2977.4 ± 8070.4 |
| Oithona similis | 1283.9 ± 1430.1 | 13263.4 ± 1093.7 | 2970.5 ± 1826.4 | 1703.7 ± 2835.9 | 404.6 ± 689.5 | 1353.5 ± 1952.1 |
| Acartia pacifica | 3397.6 ± 7151.4 | 20158.7 ± 3781.6 | 8.4 ± 8 | 84.3 ± 158.3 | 444 ± 666.8 | 1084.3 ± 3870.1 |
| Oikopleura dioica | 1032.3 ± 1245.7 | 0 ± 0 | 0.8 ± 2.9 | 3098.9 ± 6277.6 | 0 ± 0 | 1044.3 ± 3477.6 |
| Acartia hongi | 0 ± 0 | 44710.7 ± 3930.4 | 264.4 ± 284.5 | 0 ± 0 | 0 ± 0 | 526.2 ± 1924.1 |
| Aidanosagitta crassa | 1468.1 ± 1176.9 | 2985.9 ± 270.9 | 150.2 ± 96.3 | 234.1 ± 159.9 | 93.1 ± 117.3 | 456.5 ± 780.4 |
| Ditrichocorycaeus affinis | 1085.9 ± 1975.2 | 2788.9 ± 339.7 | 0 ± 0 | 85 ± 97.4 | 65.2 ± 135.6 | 305.3 ± 1012.4 |
| Centropages dorsispinatus | 1069.1 ± 1305.9 | 810 ± 103.3 | 0 ± 0 | 8.1 ± 35.7 | 216.6 ± 441.9 | 291.2 ± 761.4 |
| Noctiluca scintillans | 0 ± 0 | 22723.7 ± 3449 | 0 ± 0 | 0 ± 0 | 18.2 ± 78.9 | 253.5 ± 1509.1 |
| Microsetella norvegica | 349.9 ± 739.6 | 0 ± 0 | 0 ± 0 | 119.9 ± 415.3 | 1.3 ± 5.7 | 108.8 ± 422.7 |
| Labidocera euchaeta | 372.4 ± 721.9 | 256.1 ± 50 | 2.1 ± 4 | 0 ± 0 | 0 ± 0 | 84.9 ± 366 |
| Parvocalanus crassirostris | 0 ± 0 | 0 ± 0 | 406.9 ± 357.5 | 0 ± 0 | 0.8 ± 3.6 | 53.8 ± 186.5 |
| Pseudodiaptomus marinus | 38.5 ± 109.2 | 0 ± 0 | 0 ± 0 | 2.2 ± 10.2 | 1.2 ± 5.4 | 9.3 ± 52.8 |
| Eirene ceylonensis | 21.9 ± 22 | 0 ± 0 | 2.7 ± 3.9 | 11 ± 18 | 0 ± 0 | 8.1 ± 16.1 |
| Eirene tenuis | 30.3 ± 23.4 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 6.7 ± 16.6 |
| Labidocera bipinnata | 26.6 ± 70.3 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.1 ± 0.3 | 5.9 ± 34.2 |
| Corycaeus japonicus | 0 ± 0 | 0 ± 0 | 42.1 ± 104.2 | 0 ± 0 | 0 ± 0 | 5.6 ± 39.1 |
| Tortanus spinicaudatus | 23.9 ± 40.1 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 5.3 ± 20.9 |
| Pleurobrachia globos | 0 ± 0 | 0 ± 0 | 0 ± 0 | 17.8 ± 37.9 | 0 ± 0 | 4.7 ± 20.7 |
| Obelia dichotoma | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 17.1 ± 55.3 | 3.6 ± 25.7 |
| Calanus sinicus | 9.3 ± 19.9 | 0 ± 0 | 0 ± 0 | 0.9 ± 2.5 | 4.2 ± 12.8 | 3.1 ± 11.4 |
| Hydractinia minima | 5 ± 10.3 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 1.1 ± 5.2 |
| Acanthomysis longirostris | 0 ± 0 | 0 ± 0 | 5.7 ± 5.5 | 0 ± 0 | 0 ± 0 | 0.8 ± 2.7 |
| Oithona brevicornis | 0 ± 0 | 0 ± 0 | 0 ± 0 | 2.3 ± 11.1 | 0 ± 0 | 0.6 ± 5.7 |
| Centropages mcmurrichi | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 2.5 ± 11 | 0.5 ± 5.0 |
| Evadne tergestina | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 2.5 ± 10.8 | 0.5 ± 4.9 |
| Acetes chinensis | 2.3 ± 6.8 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.5 ± 3.3 |
| Mysis relicta | 2.2 ± 9.8 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.5 ± 4.6 |
| Clytia hemisphaerica | 1.9 ± 5.8 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.4 ± 2.8 |
| Penilia avirostris | 0 ± 0 | 0 ± 0 | 0 ± 0 | 1.4 ± 6.6 | 0 ± 0 | 0.4 ± 3.4 |
| Labidocera bipinnata | 0 ± 0 | 0 ± 0 | 0 ± 0 | 1.4 ± 4.2 | 0 ± 0 | 0.4 ± 2.2 |
| Clytia globosa | 1.5 ± 3.6 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.3 ± 1.8 |
| Eirene menoni | 1.4 ± 3.1 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.3 ± 1.5 |
| Ectopleura dumortieri | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.5 ± 1.1 | 0 ± 0 | 0.1 ± 0.6 |
| Themisto gracilipes | 0 ± 0 | 0 ± 0 | 0.8 ± 2.9 | 0 ± 0 | 0 ± 0 | 0.1 ± 1.0 |
| Podocoryne minina | 0 ± 0 | 0 ± 0 | 0.8 ± 1.6 | 0 ± 0 | 0 ± 0 | 0.1 ± 0.6 |
| Erythrops minuta | 0.5 ± 2 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.1 ± 1.0 |
| Centropages tenuiremis | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.4 ± 1.4 | 0 ± 0 | 0.1 ± 0.7 |
| Schmackeria poplesia | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.4 ± 1.6 | 0.1 ± 0.7 |
| Labidocera pavo | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.3 ± 1.3 | 0 ± 0 | 0.1 ± 0.6 |
| Sarsia nipponica | 0.3 ± 1.1 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.1 ± 0.5 |
| Tortanus derjuginii | 0 ± 0 | 0 ± 0 | 0.4 ± 0.9 | 0 ± 0 | 0 ± 0 | 0.1 ± 0.3 |
| Species richness | 1.28 ± 0.25a | 0.50 ± 0.16c | 0.76 ± 0.23bc | 0.82 ± 0.18b | 0.64 ± 0.24c | |
| Shannon-Wiener index | 2.57 ± 0.32a | 1.52 ± 0.40c | 1.35 ± 0.48c | 1.76 ± 0.43b | 1.26 ± 0.55c | |
| Peilou’s evenness | 0.70 ± 0.08a | 0.66 ± 0.12ab | 0.47 ± 0.13c | 0.59 ± 0.13b | 0.50 ± 0.21bc | |
| Simpson diversity index | 0.77 ± 0.07a | 0.55 ± 0.13bc | 0.47 ± 0.17c | 0.60 ± 0.15b | 0.44 ± 0.20c |
Different superscript letters indicate significant difference (P < 0.05) within a column.
Figure 3.
Boxplots of Shannon-Wiener index and Pielou’s evenness of mesozooplankton in the five regions. Boxes, central bars and solid lines indicate the interquartile range, the median and the data range, respectively. The outliers are circles lying outside 1.5 times the interquartile range.
The zooplankton communities showed large variation along the environmental gradient based on NMDS and ANOVA analyses (Stress = 0.2, global R = 0.691 with P = 0.001; Fig. 2B). Further analyses based on similarity percentage analysis (SIMPER) and pairwise global tests showed zooplankton communities were significantly different among regions (Table S1). The species dissimilarity between regions ranged from 41.23 (global R = 0.662, P = 0.001, Bohai Bay versus Luanhe Estuary) to 65.92 (global R = 0.99, P = 0.001, Bohai Bay versus Jinzhou Bay), and the average value was 54.95 (Table S1). In addition, the species contribution to the dissimilarity among regions largely varied. For example, the contribution of P. parvus, a widely distributed species, ranged from 2.16% (Bohai Bay versus Luanhe Estuary) to 11.43% (Laizhou Bay versus Jinzhou Estuary) and A. hongi, another common species, ranged from 4.78% (Bohai Bay versus Jinzhou Bay) to 18.31% (Yellow River Estuary versus Laizhou Bay).
On the other hand, at the intra-regional level, we found a high level of species similarity. The species similarity ranged from 58.26 (Laizhou Bay) to 76.85 (Bohai Bay) with the average value of 65.50 (Table 3). The species contribution to the similarity within each region largely varied, for example, from 6.75% (Jinzhou Bay) to 18.42% (Yellow River Estuary) for P. parvus (Table 3).
Table 3.
Results of the similarity percentage (SIMPER) analysis for each region in Bohai Sea.
| Species | Average Abundance (individual/m3) | Average Similarity (%) | Contribution (%) | Cumulative Contribution (%) | |
|---|---|---|---|---|---|
| Zone Bohai Average similarity:76.85 | Acartia bifilosa | 9240.43 | 10.53 | 13.71 | 13.71 |
| Paracalanus parvus | 2861.91 | 9.24 | 12.02 | 25.73 | |
| Aidanosagitta crassa | 1468.15 | 8.65 | 11.25 | 36.98 | |
| Acartia pacifica | 3397.64 | 8.50 | 11.06 | 48.05 | |
| Oithona similis | 1283.91 | 8.32 | 10.82 | 58.87 | |
| Oikopleura dioica | 1032.29 | 7.82 | 10.18 | 69.05 | |
| Centropages dorsispinatus | 1069.06 | 5.61 | 7.30 | 76.35 | |
| Microsetella norvegica | 349.93 | 4.53 | 5.89 | 82.24 | |
| Ditrichocorycaeus affinis | 1085.89 | 3.47 | 4.52 | 86.76 | |
| Labidocera euchaeta | 372.38 | 3.41 | 4.44 | 91.19 | |
| Zone Yellow River Average similarity:59.64 | Paracalanus parvus | 1898.92 | 18.42 | 30.89 | 30.89 |
| Acartia hongi | 2794.42 | 15.25 | 25.57 | 56.45 | |
| Aidanosagitta crassa | 186.62 | 9.66 | 16.19 | 72.65 | |
| Oithona similis | 828.96 | 8.52 | 14.29 | 86.94 | |
| Ditrichocorycaeus affinis | 174.30 | 3.63 | 6.08 | 93.02 | |
| Zone Jinzhou Average similarity:64.39 | Oithona similis | 2970.53 | 23.62 | 36.68 | 36.68 |
| Aidanosagitta crassa | 150.21 | 11.73 | 18.21 | 54.89 | |
| Parvocalanus crassirostris | 406.94 | 8.78 | 13.64 | 68.53 | |
| Paracalanus parvus | 742.43 | 6.75 | 10.48 | 79.02 | |
| Acartia hongi | 264.40 | 4.82 | 7.49 | 86.50 | |
| Acartia pacifica | 8.39 | 3.71 | 5.76 | 92.27 | |
| Zone Luanhe Average similarity:68.34 | Paracalanus parvus | 2418.99 | 17.87 | 26.15 | 26.15 |
| Oithona similis | 1703.75 | 11.60 | 16.97 | 43.12 | |
| Aidanosagitta crassa | 234.07 | 11.25 | 16.47 | 59.58 | |
| Acartia bifilosa | 2410.00 | 11.04 | 16.16 | 75.74 | |
| Oikopleura dioica | 3098.90 | 7.43 | 10.87 | 86.61 | |
| Ditrichocorycaeus affinis | 85.04 | 3.12 | 4.57 | 91.18 | |
| Zone Laizhou Average similarity:58.26 | Acartia bifilosa | 1489.25 | 13.68 | 23.48 | 23.48 |
| Paracalanus parvus | 7049.09 | 13.47 | 23.13 | 46.61 | |
| Aidanosagitta crassa | 93.09 | 11.16 | 19.16 | 65.76 | |
| Oithona similis | 404.60 | 8.17 | 14.02 | 79.78 | |
| Acartia pacifica | 444.03 | 7.00 | 12.01 | 91.79 |
Explanatory variables for observed community structure
A total of 17 environmental factors and 18 spatial vectors transformed from geographic coordinate values for sampling stations were considered as possible drivers for structuring observed zooplankton community. Based on forward selection, 13 key factors showed significant influence on the observed community structure (P < 0.05, Table S2), including nine anthropogenic pollutants (Cd, NH4-N, COD, As, Hg, DO, NO2-N, Cu and NO3-N) and four natural variables (two spatial variables (V1and V2), salinity and temperature). The selected factors were used for the construction of parsimonious RDA model, which was globally significant (P = 0.001) with 49.52% of adjusted R2, and the first two axis (RDA1 and RDA2) explained 23.96% and 13.21% of total variations of mesozooplankton communities, respectively (Fig. 4). For anthropogenic pollutants, Cd was the largest contributor in affecting zooplankton community structure, followed by NH4-N, COD, As, Hg, DO, NO2-N, Cu and NO3-N (Fig. 4, Table S2). For example, one high abundance species O. similis showed positive correlation with Cd (Fig. 4, Table 2). For natural variables, V2 was the leading contributor of variation in zooplankton community structure, followed by salinity, V1 and temperature (Fig. 4, Table S2). Specifically, O. similis, P. crassirostris and A. longirostris were negatively correlated with V2, whereas P. parvus was positively correlated with V1 (Fig. 4). In addition, we detected different influence of anthropogenic pollutants on mesozooplankton. For example, the abundance of O. similis, P. crassirostris and Acanthomysis longirostris were mainly affected by Cd and As, whereas both heavy metals posed no obvious influence on A. bifilosa (Fig. 4). Indeed, the abundance of A. bifilosa was mainly affected by COD, whereas COD had no obvious influence on P. parvus (Fig. 4). Spearson correlation analyses also confirmed this pattern: the abundance of O. similis was positively correlated with the concentration of Cd (rho = 0.336, P = 0.01, Fig. S1) and A. bifilosa was positively correlated with COD (rho = 0.301, P = 0.04, Fig. S1).
Figure 4.

The ordination plot based on redundancy analysis of zooplankton communities. The RDA model (type 2 scaling) explains 49.52% of total variation of mesozooplankton communties with a significant influence (P = 0.001). Only the first five top species (1–5 in red color) with the highest contributions to the RDA model are listed.
To explore the relative roles of anthropogenic pollutants and natural variables in structuring zooplankton community, variance partitioning was performed for explanatory variables based on nine anthropogenic pollutants (Cd, NH4-N, COD, As, Hg, DO, NO2-N, Cu and NO3-N) and four natural variables (V1, V2, salinity and temperature). The results showed that the shared explained percentage between anthropogenic pollutants and natural variables was 25.7%. Anthropogenic pollutants alone explained 13.9% of the total variation of community structure when excluding the influence of natural variables. Conversely, natural variables alone explained 9.8% of the total variations of community structure when removing the anthropogenic pollutant influence (Fig. 5).
Figure 5.
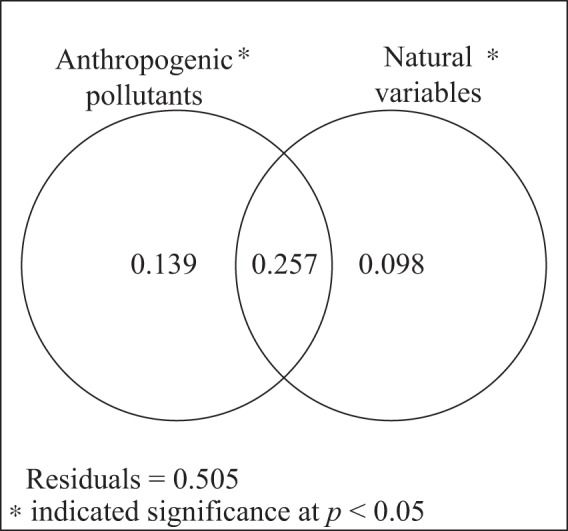
Venn diagram for two explanatory variables. The results of variance partitioning analysis to assess the response of anthropogenic pollutants and natural variables to community structure.
Discussion
The present study showed that the marine water quality varied largely among the five regions, ranging from very poor for both Jinzhou Bay and Bohai Bay, due to sewage pollution, to good in farming locations such as Luanhe Estuary. Those observations highlighted the differences in their surrounding land-use. A high level of heavy metal pollution was noted in Jinzhou Bay which was characterized with a high level of Cd, As and Hg pollutions in this investigation, mainly due to wastewater discharge by surrounding mining industries in the past century36. The organic pollutant concentrations in marine water columns were found to be generally high for Bohai Bay, with high COD being found in the Bohai Bay sampling stations because of sewage discharge from the big cities (Beijing and Tianjin)38,39. Whereas the opposite water quality states was observed in Yellow River Estuary, as shown in Fig. 4. Our observations are in agreement with recent studies which have demonstrated that the differences in land-use largely contribute to changes in pollutant variables in aquatic systems29,36,40. Hence, the understanding of anthropogenic pollutions induced by different land-use would provide insights into the management of aquatic resource.
Our results clearly showed that both anthropogenic pollutants and natural variables play a crucial role in structuring variation of mesozooplankton communities in the Bohai Sea, the influence of anthropogenic pollutants on mesozooplankton communities overrode that of natural variables. Our RDA model showed that the variations of mesozooplankton communities were in line with the changes in anthropogenic pollutants and natural variables among the five regions, with the effects of anthropogenic pollutants and natural variables being integrated into overall resultant mesozooplankton communities, which was in agreement with recent studies28,29. Some species may be sensitive to anthropogenic pollutants, some species may be influenced by natural variables and some other species can be affected by both factors. Those findings were consistent with previous studies in other aquatic systems such as lake, river and marine systems, they have demonstrated that the species‘ responses to environmental factors varied largely19,22,23,33,41–45. Thus, different mesozooplankton species responded differently to anthropogenic pollutants and natural variables in the current study, suggesting some species of mesozooplankton communities at different sampling locations can be used as reliable ecological indicators to reflect local environmental pollutions. For example, O. similis is a cosmopolitan species, their abundance was positively correlated with the concentration of Cd in the coastal regions of Bohai Sea (O. similis-Cd, rho = 0.321, P = 0.01). High levels of Cd concentrations were frequently reported in Jinzhou Bay36,46, where large numbers of O. similis were detected. Whereas, low concentrations of Cd were detected in Bohai Bay and Luanhe Estuary, where few O. similis were recorded. Our results indicated that O. similis showed high tolerance to Cd pollution and have potential to be a bioindicator of Cd contamination. This founding was in agreement with other studies, they demonstrated that the genera Oithona such as O. nana could be used as an indicator of anthropogenic pollution47,48. Oithona was characterized with flexible diet, shorter life cycle and higher reproduction rate compared to other mesozooplankton48,49, those physiology features could partly explain its higher tolerance to anthropogenic pollutions.
In addition, A. bifilosa showed positive response to COD concentration (A. bifilosa-COD, rho = 0.301, P = 0.04), indicating the variation of A. bifilosa abundance may mirror the fluctuation of COD in marine coastal regions. Indeed, COD contents can reflect the concentrations of anthropogenic organic matters, which can provide aquatic animals with foods and often promote the proliferation and growth of some species of zooplankton communities50. Moreover, the abundance of P. parvus was negatively correlated with NH4-N concentration (Fig. 4), which was also selected by forward selection as an anthropogenic pollutant with significant influences on mesozooplankton communities. High concentration of NH4-N can decrease the rates of growth and survival for copepod species51. Hence, it is expected that NH4-N toxicity can cause similar negative influence on P. parvus. However, other factors such as high trophic predators may affect the determinate of bioindicator species, and species may show different tolerant abilities to toxic pollutants under different environments29, more studies based on different trophic levels and toxicological validations under laboratory conditions should be performed to verify those findings in the field.
In the current study, Cd was identified as the leading anthropogenic pollutant structuring zooplankton communities in coastal regions of Bohai Sea. Whereas inorganic nitrogen was considered as the main driver for observed zooplankton structure in Tangshan Bay52. Moreover, total phosphate, NH4-N and Mg2+ were identified as the leading factors in Chaobai river, Beiyun river and Fuyang river of China, respectively41,43,44. Our results combined with other findings revealed that different leading anthropogenic pollutants were recognized in different ecosystems, even in different regions of the same ecosystems. Those findings indicated that the leading anthropogenic pollutants for observed structure of zooplankton community may be regional-specific, suggesting more works should be performed to clarify driving mechanism of zooplankton structure in regional scale.
Zooplankton community diversity within each region is traditionally used to reflect local environmental conditions53,54. Our study showed that Bohai Bay and Jinzhou Bay were both heavily polluted by anthropogenic pollutants. However, two Bays showed large variations in diversity index in the current study. Bohai Bay was mainly suffered from organic pollution, which may pose toxic effects on some species but also may provide more food resources to support more species55,56, thus resulted in high species diversity. Jinzhou Bay was mainly polluted by heavy metals, which may only have negative effects on aquatic species, leading to decline of mesozooplankton diversity. Those observations suggested zooplankton community diversity may be not an ideal index to mirror local anthropogenic pollutions, whereas maybe an ideal index to reflect local organic pollutions. Alternatively, the shifts of mesozooplankton community composition in major groups between regions, such as the variation of bioindicator species, may be good indexes to represent local anthropogenic pollutions, as have been revealed at other communities such as benthic communities29,57,58.
Traditionally, natural variables including hydrological processes, temperature and salinity are often considered as the major determinants of community structures, and other variables including pollutions played less importance30–32. For example, the identification of V2 as leading natural factor indicated that spatial variables such as ocean current may play a crucial role in driving spread of some species, including O. similis, P. crassirostris and A. longirostris, and eventually affect local community structures. However, variance partitioning in our study showed that the explained percentage of community variation by pollutants alone was larger (13.9%) than that by natural variables (9.8%) alone, indicating that anthropogenic pollutants contributed larger than natural variables to the variation of zooplankton community in the coastal regions of Bohai Sea. In the open seas, natural variables such as ocean current and water temperature may play major roles in structuring zooplankton structure59,60. Whereas in semi-closed seas such as Bohai Sea, both higher pollution levels36,46 and weaker ocean currents61 may lead to the observed distribution patterns of mesozooplankton communities in the present study. Thus, the relative roles of anthropogenic pollutants and natural variables in shaping zooplankton structure may largely depend on the relative strengths of both factors. In addition, about quarter of total variations of zooplankton community structure was simultaneously explained by anthropogenic pollutants and natural variables, suggesting strong colinearity between two types of factors exist in the coastal regions of Bohai Sea. Strikingly, P. parvus showed positive correlation with V1, dispersal alone cannot explain this observation. One reasonable reason may be that V1 such as ocean current-dispersal largely shaped unmeasured variables or biological processes, which may be actual factor affecting P. parvus. Those observations indicated natural variables such as salinity and hydrological processes in this study may affect degradation and dispersal of pollutants6,46, highlighting the necessity of excluding the influence of natural variables when exploring the ecological effects of anthropogenic pollutants on zooplankton composition in the field. This idea is especially applicable for marine systems at coastal waters because of complexities of hydrological processes and obvious gradients of natural variables.
In conclusion, our study clearly showed that the mesozooplankton communities among the five regions varied significantly along the environmental gradients. Multiple analyses identified that both anthropogenic pollutants and natural variables were major factors driving mesozooplankton communities in the coastal marine system. Cd was identified the leading anthropogenic pollutants factor structuring mesozooplankton community, followed by Hg, COD, NH4-N, As, Zn, NO2-N. The species responses to those environmental factors varied largely and mainly depended on organism taxa, suggesting some species can be used as potential bioindicators of environmental pollutants. Further analyses showed that anthropogenic pollutants still played a major role with significant influence on the mesozooplankton community even after removing the natural variable influence, highlighting the necessity of considering negative effects of anthropogenic pollutants on coastal ecosystems in environmental management and monitoring programs. Methodologically, our results emphasized the importance of excluding influence of natural variables including hydrological processes, temperature and salinity when exploring the ecological effects of anthropogenic pollutants on plankton community structure, especially at coastal waters. However, this study was only performed on mesozooplankton that were adequately identified based on morphological features, other zooplankton such as microzooplankton have not been test on this issues. More works on different trophic levels should be carried out using feasible molecular-based methods such as metabarcoding-based identification approach62.
Material and Methods
Study region and sampling stations
This study focused on five important estuarine areas of the Bohai Sea. The sampling stations mainly distribute in the shallow coastal areas, the water depth of sampling stations is between 2.5 m and 17.0 m. The water column is generally mixed homogeneously due to strong tidal mixing. Neither thermocline nor halocline was observed in the sampling stations of this study because the summertime stratification of the water column mainly occurs in the deep basins (25~35 m depth) in the central Bohai Sea63. A total of 91 sample stations were set up over the coastal area of Bohai Sea (Fig. 1), including Jinzhou Bay (12 stations), Luanhe Estuary (24 stations), Bohai Bay (20 stations), Yellow River Estuary (16 stations) and Laizhou Bay (19 stations). Jinzhou Bay, located in northwest of Liaodong Gulf, is a semi-closed shallow water area. Six rivers including Lianshan River, Wuli River, Lao River, Cishan River, Zhouliu River and Tashan River flow into Jinzhou Bay. It is famous as an old industrial base, and become one of the most polluted coastal area in China. Luanhekou Estuary is located on the northwest coast of Bohai Sea with water depths less than 20 m. Freshwater and sediment discharges have decreased greatly since the 1980s due to large dams and reservoirs built along the Luanhe River. Bohai Bay is located on the west of Bohai Sea, near the city of Tianjin and Beijing. Bohai Bay is a typical semi-enclosed coastal area and has limited water exchange with the ocean. Large quantities of industrial and domestic wastewater discharges flow into Bohai Bay from rivers of Beijing-Tianjin. The western coast of Bohai Bay locates the Tianjin Ports, the 10th largest port in the cargo throughout in the world. Yellow River Estuary is located in the southwestern part of Bohai Sea, the end of the second largest river (Yellow River) of the world in terms of sediment load. Yellow River Estuary is characterized with high concentration of Ammonia nitrogen64. Laizhou Bay is located on the southern part of Bohai Sea, accounting for up to 10% of the total area. It’s a semi-closed shallow area with average water depth less than 10 m. There are more than a dozen of rivers running into the Laizhou Bay, among which Yellow River, Xiaoqinghe River and Wei River are the most important. All samples were collected in the August, 2015 (Table 1).
Zooplankton sampling and enumeration
Mesozooplankton samples were quantitatively collected in each sampling station. Specifically, we firstly measured water depth for each sampling station and collected mesozooplankton samples using a plankton net (505 μm mesh size, 50 cm mouth diameter) by towing vertically from 2 m above the bottom to the surface with a speed of 0.5–0.8 m/s. The filtered water volume (m3) was measured using the rope length multiplied by the mouth area (0.2 m2). The samples were collected and preserved immediately in 5% formaldehyde. In the laboratory, all individuals (zooplankton larvae were not included) were identified into species and enumerated. The abundance (ind./m3) of each species was calculated as the number of individuals divided by the filtered water volume. In cases when the mean is presented, the standard deviation was provided (mean ± SD).
Environmental variable sampling and analysis
Surface seawater samples were collected with a 5 L Niskin bottles from 0.5 m below the surface at each station. The seawater salinity and temperature was measured in situ with a multiparameter sensor YSI6600, and pH values were determined with a pH meter. The seawater for dissolved oxygen (DO) analysis was collected with a tube reaching the bottom of bottle until the water overflowed. Suspended matter samples were filtered through pre-weighted Whatman GF/F fiber filters (25 mm). The samples for metal determination were filtered immediately through Whatman GF/F fiber filter (0.45 mm), and then acidified with 10% HNO3, placed in an ice box and transported to the laboratory. Concentrations of NO3-N, NO2-N, NH4-N and PO4-P in seawater were determined according to the methods described by Grasshoff et al.65. DO was determined using the Winkler titration method. Chlorophyll-a (Chl-a) was determined by filtering 100–200 mL seawater onto GF/F fiber filter by a cascading filtering device under low vacuum pressure. After extraction with 90% acetone, Chl-a was determined by a Turner Design fluorometer (TD Trilogy). The concentrations of dissolved heavy metals were determined using the inductively coupled plasma mass spectrometry (ICP-MS, Thermo X series) for Cd, Pb, Zn and Cu, while the content of Hg and As was determined using the atomic fluorescence spectrometer (AFS-920).
Spatial variables
Besides environmental variables, the variations on the spatial distribution of aquatic communities are traditionally correlated with geographical distances between sampling stations43,44,66. The spatial distances were generated based on Cartesian coordinates and Euclidian distance matrix, which were transformed from longitude and latitude among the sampling stations. In detail, the longitude and latitude were converted to Cartesian coordinates using the geoXY function available in the SoDa packages in R software v.3.4.167. Then, an Euclidian distance matrix on this Cartesian coordinates was computed using the dist function and PCNM (Principal Coordinates of Neighbor Matrices) analysis (permutations = 1000) was performed on this matrix using PCNM function implemented in the PCNM package. The method of PCNM68,69 can effectively model spatial structure in biological communities among sampling stations70 and has been increasingly used in various groups including bacteria and phytoplankton71,72. In this study, we attempted to apply the method of PCNM to mesozooplankton in order to understand the effects of spatial variables on mesozooplankton community. The number of PCNM variables formed is always dependent on the number of sampling stations and their spatial relations. At last, a total of 18 PCNM vectors (V1-V18) showing positive spatial autocorrelation were formed and used as spatial variables for subsequent redundancy analysis (RDA) and forward selection. In detail, the first PCNM vectors indicate spatial relations among sampling stations at a large scale (e.g. between sampling stations across regions) and the last PCNM vectors represent spatial relations among a small scale (e.g. between sampling stations in the same region).
Statistics analysis
In order to separate the effects of anthropogenic pollutants on mesozooplankton communities from natural environmental factors, the 17 environmental variables together with 18 spatial variables were reclassified into two groups: natural variables (temperature, salinity, and spatial variables) and anthropogenic pollutants (COD, suspend matters, DO, Chl-a, pH, PO4, NO2-N, NO3-N, NH4-N, As, Hg, Cu, Pb, Cd, and Zn). The average value and standard deviation for each environmental variable and study location were calculated. One-way ANOVAs were used to compare means of environmental variables among study locations, after testing for homogeneity of variances (Levene’s test, P < 0.05) and normality of distribution (Shapiro-Wilk test, P < 0.05) using Paleontological Statistics (PAST) version 3.0173. Significant ANOVAs (P < 0.05) were followed by Tukey HSD post hoc analysis to identify differences between study locations using PAST version 3.01.
Before statistical analyses, all measured environmental factors (except for pH) and mesozooplankton data were log10 (x + 1) transformed to improve normality. To characterize distribution patterns of zooplankton, the composition and abundance of zooplankton were analyzed using non-parametric multivariate methods implemented in PRIMER 5.074. The abundance of zooplankton between regions was compared using nonmetric multidimensional scaling (NMDS) and the analysis of similarity (ANOSIM), which is based on Bray-Curtis distance and rank dissimilarity. The major species driving distribution patterns of zooplankton assemblages at both inter regions and intra regions were identified using similarity percentage analysis (SIMPER) with a cutoff of 90% contributions. The NMDS, ANOSIM and SIMPER analyses were performed using PRIMER 5.074
To recognize the major factors responsible for observed zooplankton community structure, we performed the linear ordination method of RDA, which was chosen mainly based on a preliminary detrended correspondence analysis (DCA) on zooplankton community. The DCA showed that the longest length of gradient (3.03) was shorter than four, indicating that the majority of taxa showed a linear response to explanatory variables75. To avoid multicollinearity problems and construct parsimonious RDA model, which has been proved to have greater predictive power for the relationship between zooplankton communities and explanatory variables76, we conducted forward selection to select significant explanatory variables including environmental factors and spatial variables using the forward.sel function (ANOVAS; 1000 permutations) in packfor package in R, which simultaneously taken account for significance (P < 0.05) and adjusted R2 of the global RDA model with all available explanatory variables77. To verify the correlations obtained from RDA analysis, additional Spearman correlation analysis was also performed.
To evaluate the ecological effects of anthropogenic pollutons on mesozooplankton structures, variance partitioning and partial redundancy analysis (pRDA) were performed to estimate explained percentage of the significant anthropogenic pollutions and natural variables selected by forward selection. Variance analyses (ANOVAS; 1000 permutations) were performed to test the significance of RDA and pRDA. Those analyses including RDA, pRDA, ANOVA and DCA analyses were computed using vegan package in R software.
Supplementary information
Acknowledgements
This study was supported by grants from National Natural Science Foundation of China (31572595, 41576149) and Marine Ecology Status Monitoring and Assessment (Marine Environment Monitoring Environmental Monitoring and Assessment Operational Program) (2018-J-10). Many thanks for the comments from the editor and the reviewers.
Author Contributions
H.L. conceived the study. Y.G., Q.Y., X.W., A.Z. and H.L. designed the experiment. Y.G. and H.L. conducted the experiments and analyzed the data. Y.G., Q.Y. and H.L. wrote the manuscript. All authors reviewed and commented on the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-46047-5.
References
- 1.Cowen RK, Sponaugle S. Larval dispersal and marine population connectivity. Ann. Rev. Mar. Sci. 2009;1:443–466. doi: 10.1146/annurev.marine.010908.163757. [DOI] [PubMed] [Google Scholar]
- 2.Levin LA, et al. The Function of Marine Critical Transition Zones and the Importance of Sediment Biodiversity. Ecosystems. 2001;4:430–451. doi: 10.1007/s10021-001-0021-4. [DOI] [Google Scholar]
- 3.Zhang R, et al. Antibiotics in the offshore waters of the Bohai Sea and the Yellow Sea in China: Occurrence, distribution and ecological risks. Environ. Pollut. 2013;174:71–77. doi: 10.1016/j.envpol.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Palmer MA, et al. Linkages between Aquatic Sediment Biota and Life Above Sediments as Potential Drivers of Biodiversity and Ecological Processes. Bioscience. 2000;50:1062–1075. doi: 10.1641/0006-3568(2000)050[1062:LBASBA]2.0.CO;2. [DOI] [Google Scholar]
- 5.Johnston EL, Roberts DA. Contaminants reduce the richness and evenness of marine communities: a review and meta-analysis. Environ. Pollut. 2009;157:1745–1752. doi: 10.1016/j.envpol.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Cardoso PG, Raffaelli D, Lillebø AI, Verdelhos T, Pardal MA. The impact of extreme flooding events and anthropogenic stressors on the macrobenthic communities’. dynamics. Estuar. Coast. Shelf S. 2008;76:553–565. doi: 10.1016/j.ecss.2007.07.026. [DOI] [Google Scholar]
- 7.Dolbeth M, et al. Anthropogenic and natural disturbance effects on a macrobenthic estuarine community over a 10-year period. Mar. Pollut. Bull. 2007;54:576–585. doi: 10.1016/j.marpolbul.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Garcia MD, Sonia Barria de Cao M. Anthropogenic pollution along the coast of a temperate estuary: effects on tintinnid assemblages. Hydrobiologia. 2018;809:201–219. doi: 10.1007/s10750-017-3465-z. [DOI] [Google Scholar]
- 9.Xie Y, et al. Ecogenomic responses of benthic communities under multiple stressors along the marine and adjacent riverine areas of northern Bohai Sea, China. Chemosphere. 2017;172:166–174. doi: 10.1016/j.chemosphere.2016.12.121. [DOI] [PubMed] [Google Scholar]
- 10.Elliott M, Quintino V. The estuarine quality paradox, environmental homeostasis and the difficulty of detecting anthropogenic stress in naturally stressed areas. Mar. Pollut. Bull. 2007;54:640–645. doi: 10.1016/j.marpolbul.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Telesh IV, Khlebovich VV. Principal processes within the estuarine salinity gradient: A review. Mar. Pollut. Bull. 2010;61:149–155. doi: 10.1016/j.marpolbul.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Baliarsingh SK, Srichandan S, Lotliker AA, Kumar TS, Sahu KC. Zooplankton Distribution in Coastal Water off Gopalpur, North-Western Bay of Bengal. J. Ocean U. China. 2018;17:879–889. doi: 10.1007/s11802-018-3414-0. [DOI] [Google Scholar]
- 13.Fanjul A, et al. Zooplankton seasonality across a latitudinal gradient in the Northeast Atlantic Shelves Province. Cont. Shelf Res. 2018;160:49–62. doi: 10.1016/j.csr.2018.03.009. [DOI] [Google Scholar]
- 14.Kodama T, et al. Spatial variations in zooplankton community structure along the Japanese coastline in the Japan Sea: influence of the coastal current. Ocean Sci. 2018;14:355–369. doi: 10.5194/os-14-355-2018. [DOI] [Google Scholar]
- 15.Azarbad H, et al. Microbial community structure and functioning along metal pollution gradients. Environ. Toxicol. Chem. 2013;32:1992–2002. doi: 10.1002/etc.2269. [DOI] [PubMed] [Google Scholar]
- 16.Dong Y, Rosenbaum RK, Hauschild MZ. Assessment of Metal Toxicity in Marine Ecosystems: Comparative Toxicity Potentials for Nine Cationic Metals in Coastal Seawater. Environ. Sci. Technol. 2016;50:269–278. doi: 10.1021/acs.est.5b01625. [DOI] [PubMed] [Google Scholar]
- 17.Finn RN. The physiology and toxicology of salmonid eggs and larvae in relation to water quality criteria. Aquat. Toxicol. 2007;81:337–354. doi: 10.1016/j.aquatox.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 18.Schram E, et al. The impact of elevated water ammonia and nitrate concentrations on physiology, growth and feed intake of pikeperch (Sander lucioperca) Aquaculture. 2014;420:95–104. doi: 10.1016/j.aquaculture.2013.10.027. [DOI] [Google Scholar]
- 19.Fernández-Severini MD, Hoffmeyer MS, Marcovecchio JE. Heavy metals concentrations in zooplankton and suspended particulate matter in a southwestern Atlantic temperate estuary (Argentina) Environ. Monit. Assess. 2013;185:1495–1513. doi: 10.1007/s10661-012-3023-0. [DOI] [PubMed] [Google Scholar]
- 20.Cross AD, Beauchamp DA, Moss JH, Myers KW. Interannual Variability in Early Marine. Growth, Size-Selective Mortality, and Marine Survival for Prince William Sound Pink Salmon. Mar. Coast. Fish. 2009;1:57–70. [Google Scholar]
- 21.Frederiksen M, Edwards M, Richardson AJ, Wanless S. From Plankton to Top Predators: Bottom-up Control of a Marine Food Web across Four Trophic Levels. J. Anim. Ecol. 2006;75:1259–1268. doi: 10.1111/j.1365-2656.2006.01148.x. [DOI] [PubMed] [Google Scholar]
- 22.Uriarte L, Villate F. Effects of pollution on zooplankton abundance and distribution in two estuaries of the Basque coast (Bay of Biscay) Mar. Pollut. Bull. 2004;49:220–228. doi: 10.1016/j.marpolbul.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Uriarte I, Villate F. Differences in the abundance and distribution of copepods in two estuaries of the Basque coast (Bay of Biscay) in relation to pollution. J. Plankton Res. 2005;27:863–874. doi: 10.1093/plankt/fbi059. [DOI] [Google Scholar]
- 24.Bermejo R, Fuente GDL, Ramírez-Romero E, Vergara JJ, Hernández I. Spatial variability and response to anthropogenic pressures of assemblages dominated by a habitat forming seaweed sensitive to pollution (northern coast of Alboran Sea) Mar. Pollut. Bull. 2016;105:255–264. doi: 10.1016/j.marpolbul.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 25.Du P, et al. Responses of mesozooplankton communities to different anthropogenic activities in a subtropical semi-enclosed bay. J. Mar. Biol. Assoc. UK. 2017;98:1–14. [Google Scholar]
- 26.Estrada R, et al. Late-summer zooplankton community structure, abundance, and distribution in the Hudson Bay system (Canada) and their relationships with environmental conditions, 2003–2006. Prog. Oceanogr. 2012;101:121–145. doi: 10.1016/j.pocean.2012.02.003. [DOI] [Google Scholar]
- 27.Sofia Dutto M, Lopez Abbate MC, Biancalana F, Berasategui AA, Hoffmeyer MS. The impact of sewage on environmental quality and the mesozooplankton community in a highly eutrophic estuary in Argentina. ICES J. Mar. Sci. 2012;69:399–409. doi: 10.1093/icesjms/fsr204. [DOI] [Google Scholar]
- 28.Araujo AV, Dias CO, Bonecker SL. Differences in the structure of copepod assemblages in four tropical estuaries: importance of pollution and the estuary hydrodynamics. Mar. Pollut. Bull. 2017;115:412–420. doi: 10.1016/j.marpolbul.2016.12.047. [DOI] [PubMed] [Google Scholar]
- 29.Devercelli M, Scarabotti P, Mayora G, Schneider B, Giri F. Unravelling the role of determinism and stochasticity in structuring the phytoplanktonic metacommunity of the Parana River floodplain. Hydrobiologia. 2016;764:139–156. doi: 10.1007/s10750-015-2363-5. [DOI] [Google Scholar]
- 30.Drira Z, Bel Hassen M, Ayadi H, Aleya L. What factors drive copepod community distribution in the Gulf of Gabes, Eastern Mediterranean Sea? Environ. Sci. Pollut. Res. Int. 2014;21:2918–2934. doi: 10.1007/s11356-013-2250-4. [DOI] [PubMed] [Google Scholar]
- 31.Morse RE, Friedland KD, Tommasi D, Stock C, Nye J. Distinct zooplankton regime shift patterns across ecoregions of the U.S. Northeast continental shelf Large Marine Ecosystem. J. Marine Syst. 2017;165:77–91. doi: 10.1016/j.jmarsys.2016.09.011. [DOI] [Google Scholar]
- 32.Descroix A, Harvey R, Galbraith PS. Macrozooplankton community patterns driven by water circulation in the St.~Lawrence marine system, Canada. Mar. Ecol. Prog. Ser. 2005;302:103–119. doi: 10.3354/meps302103. [DOI] [Google Scholar]
- 33.Moritz C, et al. Disentangling the role of connectivity, environmental filtering, and spatial structure on metacommunity dynamics. Oikos. 2013;122:1401–1410. [Google Scholar]
- 34.Zhang W, et al. Microplastic pollution in the surface waters of the Bohai Sea, China. Environ. Pollut. 2017;231:541. doi: 10.1016/j.envpol.2017.08.058. [DOI] [PubMed] [Google Scholar]
- 35.Peng Z, Song JM, Yuan HM. Persistent organic pollutant residues in the sediments and mollusks from the Bohai Sea coastal areas, North China: an overview. Environ. Int. 2009;35:632–646. doi: 10.1016/j.envint.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 36.Li H, et al. Comprehensive large-scale investigation and assessment of trace metal in the coastal sediments of Bohai Sea. Mar. Pollut. Bull. 2018;129:126–134. doi: 10.1016/j.marpolbul.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Chen S, Xia T. Environmental risk assessment of heavy metals in Bohai Sea, North China. Procedia Environ. Sci. 2010;2:1632–1642. doi: 10.1016/j.proenv.2010.10.174. [DOI] [Google Scholar]
- 38.Yi W, et al. Fate of DDT-related compounds in Bohai Bay and its adjacent Haihe Basin, North China. Mar. Pollut. Bull. 2005;50:439–445. doi: 10.1016/j.marpolbul.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 39.Hu J, et al. Occurrence of trace organic contaminants in Bohai Bay and its adjacent Nanpaiwu River, North China. Mar. Chem. 2005;95:1–13. doi: 10.1016/j.marchem.2004.06.004. [DOI] [Google Scholar]
- 40.Naifar I, et al. Spatial distribution and contamination assessment of heavy metals in marine sediments of the southern coast of Sfax, Gabes Gulf, Tunisia. Mar. Pollut. Bull. 2018;131:53–62. doi: 10.1016/j.marpolbul.2018.03.048. [DOI] [PubMed] [Google Scholar]
- 41.Peng H, Xiong W, Zhan A. Fine-scale environmental gradients formed by local pollutants largely impact zooplankton communities in running water ecosystems. Aquat. Biol. 2018;27:43–53. doi: 10.3354/ab00695. [DOI] [Google Scholar]
- 42.Xiong W, et al. Determinants of community structure of zooplankton in heavily polluted river ecosystems. Sci. Rep. 2016;6:22043. doi: 10.1038/srep22043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiong W, et al. Zooplankton community structure along a pollution gradient at fine geographical scales in river ecosystems: The importance of species sorting over dispersal. Mol. Ecol. 2017;26:4351–4360. doi: 10.1111/mec.14199. [DOI] [PubMed] [Google Scholar]
- 44.Yang Y, et al. Geographical distribution of zooplankton biodiversity in highly polluted running water ecosystems: Validation of fine-scale species sorting hypothesis. Ecol. Evol. 2018;8:4830–4840. doi: 10.1002/ece3.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beisner BE, Peres-Neto PR, Lindström ES, Barnett A, Longhi ML. The role of environmental and spatial processes in structuring lake communities from bacteria to fish. Ecology. 2006;87:2985–2991. doi: 10.1890/0012-9658(2006)87[2985:TROEAS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 46.Gao X, Zhou F, Chen CT. Pollution status of the Bohai Sea: an overview of the environmental quality assessment related trace metals. Environ. Int. 2014;62:12–30. doi: 10.1016/j.envint.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 47.Serranito B, Aubert A, Stemmann L, Rossi N, Jamet JL. Proposition of indicators of anthropogenic pressure in the bay of Toulon (mediterranean sea) based on zooplankton time-series. Cont. Shelf Res. 2016;121:3–12. doi: 10.1016/j.csr.2016.01.016. [DOI] [Google Scholar]
- 48.Etilé RND, et al. Spatio-temporal variations of the zooplankton abundance and composition in a West African tropical coastal lagoon (Grand-Lahou, Côte d’Ivoire) Hydrobiologia. 2008;624:171–189. doi: 10.1007/s10750-008-9691-7. [DOI] [Google Scholar]
- 49.Castellani C, Irigoien X, Harris RP, Lampitt RS. Feeding and egg production of Oithona similis in the North Atlantic. Mar. Ecol. Prog. Ser. 2005;288:173–182. doi: 10.3354/meps288173. [DOI] [Google Scholar]
- 50.Kulikova TP, Syarki MT. Effect of Anthropogenic Eutrophication on Zooplankton Distribution in Kondopoga Bay of Lake Onega. Water Resour. 2004;31:85–91. doi: 10.1023/B:WARE.0000013578.96202.93. [DOI] [Google Scholar]
- 51.Jepsen PM, Andersen CVB, Schjelde J, Hansen BW. Tolerance of un-ionized ammonia in live feed cultures of the calanoid copepod Acartia tonsa Dana. Aquac. Res. 2015;46:420–431. doi: 10.1111/are.12190. [DOI] [Google Scholar]
- 52.Li, Z. W. & Cui, L. T. Environmental control of zooplankton community structure in Tangshan Bay, China. Chinese Joural of Applied Ecology28, 3797–3804 (in Chinese with English Abstract) (2017). [DOI] [PubMed]
- 53.Fileman ES, Fitzgeorge-Balfour T, Tarran GA, Harris RP. Plankton community diversity from bacteria to copepods in bloom and non-bloom conditions in the Celtic Sea in spring. Estuar. Coast. Shelf S. 2011;93:403–414. doi: 10.1016/j.ecss.2011.05.009. [DOI] [Google Scholar]
- 54.Mukherjee B, Nivedita M, Mukherjee D. Plankton diversity and dynamics in a polluted eutrophic lake, Ranchi. J. Environ. Biol. 2010;31:827–839. [Google Scholar]
- 55.Karlsson J, et al. Terrestrial organic matter support of lake food webs: Evidence from lake metabolism and stable hydrogen isotopes of consumers. Limnol. Oceanogr. 2012;57:1042–1048. doi: 10.4319/lo.2012.57.4.1042. [DOI] [Google Scholar]
- 56.Otake T, Nogami K, Maruyama K. Dissolved and particulate organic matter as possible food sources for eel leptocephali. Mar. Ecol. Prog. Ser. 1993;92:27–34. doi: 10.3354/meps092027. [DOI] [Google Scholar]
- 57.Schmidt TS, Soucek DJ, Cherry DS. Integrative assessment of benthic macroinvertebrate community impairment from metal-contaminated waters in tributaries of the Upper Powell River, Virginia, USA. Environ. Toxicol. Chem. 2010;21:2233–2241. doi: 10.1002/etc.5620211030. [DOI] [PubMed] [Google Scholar]
- 58.Jiang Y, Yang EJ, Min JO, Kang SH, Lee SH. Using pelagic ciliated microzooplankton communities as an indicatorfor monitoring environmental condition under impact of summersea-ice reduction in western Arctic Ocean. Ecol. Indic. 2013;34:380–390. doi: 10.1016/j.ecolind.2013.05.026. [DOI] [Google Scholar]
- 59.Hays GC, Richardson AJ, Robinson C. Climate change and marine plankton. Trends Ecol. Evol. 2005;20:337–344. doi: 10.1016/j.tree.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 60.Arashkevich EG, et al. Mesozooplankton in the open Black Sea: Regional and seasonal characteristics. J. Marine Syst. 2014;135:81–96. doi: 10.1016/j.jmarsys.2013.07.011. [DOI] [Google Scholar]
- 61.Wang Z, Sheng D, Chen C, Soares CG. Long-term characteristics and extreme parameters of currents and sea levels in the Bohai Sea based on 20-year numerical hindcast data. Nat. Hazards. 2015;76:1603–1624. doi: 10.1007/s11069-014-1560-2. [DOI] [Google Scholar]
- 62.Zhan A, et al. High sensitivity of 454 pyrosequencing for detection of rare species in aquatic communities. Methods Ecol. Evol. 2013;4:558–565. doi: 10.1111/2041-210X.12037. [DOI] [Google Scholar]
- 63.Zhou F, et al. Circulations associated with cold pools in the Bohai Sea on the Chinese continental shelf. Cont. Shelf Res. 2017;137:25–38. doi: 10.1016/j.csr.2017.02.005. [DOI] [Google Scholar]
- 64.Liu SM, et al. Impacts of human activities on nutrient transports in the Huanghe (Yellow River) estuary. J. Hydrol. 2012;430-431:103–110. doi: 10.1016/j.jhydrol.2012.02.005. [DOI] [Google Scholar]
- 65.Grasshoff, K., Kremling, K. & Ehrhardt, M. Methods of Seawater Analysis, 3rd Edition. Wiley-VCH, New York (2007).
- 66.Benestan L, et al. Seascape genomics provides evidence for thermal adaptation and current-mediated population structure in American lobster (Homarus americanus) Mol. Ecol. 2016;25:5073–5092. doi: 10.1111/mec.13811. [DOI] [PubMed] [Google Scholar]
- 67.R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing (2015).
- 68.Borcard D, Legendre P. All-scale spatial analysis of ecological data by means of principal coordinates of neighbour matrices. Ecol. Model. 2002;153:51–68. doi: 10.1016/S0304-3800(01)00501-4. [DOI] [Google Scholar]
- 69.Borcard D, Legendre P, Avois-Jacquet C, Tuomisto H. Dissecting the spatial structure of ecological data at multiple scales. Ecology. 2004;85:1826–1832. doi: 10.1890/03-3111. [DOI] [Google Scholar]
- 70.Dray S, Legendre P, Peres-Neto PR. Spatial modelling: a comprehensive framework for principal coordinate analysis of neighbour matrices (PCNM) Ecol. Model. 2006;196:483–493. doi: 10.1016/j.ecolmodel.2006.02.015. [DOI] [Google Scholar]
- 71.Vilmi A, Karjalainen SM, Nokela T, Tolonen K, Heino J. Unravelling the drivers of aquatic communities using disparate organismal groups and different taxonomic levels. Ecol. Indic. 2016;60:108–118. doi: 10.1016/j.ecolind.2015.06.023. [DOI] [Google Scholar]
- 72.Brind’Amour A, Boisclair D, Legendre P, Borcard D. Multiscale spatial distribution of a littoral fish community in relation to environmental variables. Limnol. Oceanogr. 2005;50:465–479. doi: 10.4319/lo.2005.50.2.0465. [DOI] [Google Scholar]
- 73.Hammer Ø, Harper DAT, Ryan PD. PAST-Palaeontological Statistics Software package for education and data analysis. Palaeontol. Electron. 2001;4:1–9. [Google Scholar]
- 74.Clarke, K. R., Gorley, R. N. PRIMER v5: User Manual/Tutorial. Plymouth: PRIMER-E Ltd. Available at, http://www.primer-e.com/ (2001).
- 75.Lepš, J. & Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO 4.5. (Cambridge University Press, 2003).
- 76.Gauch HG. Prediction, Parsimony and Noise. Am. Sci. 1993;81:468–478. [Google Scholar]
- 77.Datry T, et al. Metacommunity patterns across three Neotropical catchments with varying environmental harshness. Freshwater Biol. 2016;61:277–292. doi: 10.1111/fwb.12702. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.