Abstract
Breast cancer dormancy is an underlying challenge toward targeting and controlling metastatic recurrence and disease progression. Development of engineered, well-defined in vitro models is necessary to systematically recapitulate tumor dormancy and investigate potential therapeutic strategies. Toward this end, a set of sixteen hydrogel formulations with varying degrees of adhesivity and crosslink density was developed for encapsulation, three-dimensional (3D) culture, and phenotypic assessment of MDA-MB-231 breast cancer cells. The hydrogel adhesivity was regulated by incorporation of RGDS peptide conjugated to acrylate poly(ethylene glycol) (PEG-RGDS) and the crosslink density by incorporation of N-vinyl pyrrolidinone (NVP). Here, we present data concerning the characterization of hydrogel properties (PEG-RGDS incorporation, hydrogel crosslink density, and hydrogel diffusivity as a function of NVP concentration) and phenotypic metrics (viability, early apoptosis, proliferation, metabolic activity, viable cell density, and morphological features) of encapsulated MDA-MB-231s over 15 days in culture. Interpretation of this data can be found in a research article titled “Tunable Hydrogels for Controlling Phenotypic Cancer Cell States to Model Breast Cancer Dormancy and Reactivation” (Pradhan et al., 2019) [1].
Keywords: Cancer, Dormancy, Metastasis, Relapse, Hydrogel, Tissue engineering
Specifications Table
| Subject area | Biology |
| More specific subject area | Biomaterials, Tissue Engineering, Cancer |
| Type of data | Graphs, figures |
| How data was acquired | Gel Permeation Chromatography: Waters, aqueous column Fluorescence and Phase Contrast Microscopy: Zeiss AxioObserver Z1 inverted fluorescent microscope equipped with structured illumination and a Hamamatsu ORCA-Flash 4.0LT camera Microwell Plate Fluorescence Assay: Plate Reader, Biotek Synergy Confocal microscopy: Zeiss LSM 710 |
| Data format | Raw and analyzed |
| Experimental factors | Cancer cells were cultured within sixteen hydrogel formulations with varying matrix adhesivity and crosslink density for 15 days. |
| Experimental features | Phenotypic cell metrics corresponding to viability, viable cell density, proliferation, early apoptosis and 3D morphology were assessed and hydrogel formulations were classified into groups supporting specific phenotypic cancer states. |
| Data source location | University of Delaware, Newark, DE, USA |
| Data accessibility | Data is available from the corresponding author upon reasonable request. |
| Related research article | Tunable Hydrogels for Controlling Phenotypic Cancer Cell States to Model Breast Cancer Dormancy and Reactivation. Shantanu Pradhan, John H. Slater, Biomaterials 2019 (In press) [1] |
Value of the data
|
1. Data
The dataset in this article describes the cellular characteristics of cancer cells cultured within engineered hydrogels with varying adhesivity and crosslink density. Fig. 1 describes the physical and biochemical characterization of the hydrogels. Fig. 2 describes the cellular metrics (viability, early apoptosis proliferation, viable cell density, and metabolic activity) assessed in hydrogels with varying concentrations of PEG-RGDS and NVP. Fig. 3 describes the comparative assessment of metabolic activity on a per viable cell basis in different hydrogel formulations over the course of 15 days. Fig. 4 describes 3D morphological features (area, diameter, aspect ratio, circularity, and roundness) of cell clusters within different hydrogel formulations. Fig. 5 displays representative phase contrast images of cells cultured within different hydrogel formulations on day 15 post-encapsulation.
Fig. 1.
Hydrogel Characterization. (A) Gel permeation chromatography analysis of the conjugation of peptide sequences (PQ and RGDS) with PEG-SVA. (B) Normalized fluorescence intensities of Alexa Fluor 488 labeled PEG-RGDS (PEG-RGDS-AF488) in PEG-PQ hydrogels at varying concentrations of NVP. Blue, red and green bars represent measured fluorescence intensities in hydrogels pre-crosslinking, post crosslinking but pre-rinsing, and post-rinsing respectively. (C) Calculated conjugation efficiencies of PEG-RGDS into PEG-PQ hydrogels with varying NVP concentrations. * indicates p < 0.05. (B–C) Values represent mean ± standard deviation; n = 4 hydrogels per condition. (D) Representative fluorescent images of methacryloxyethyl thiocarbamoyl rhodamine B labeled PEG-PQ hydrogels with varying NVP concentrations incubated with 100 μg/mL collagenase IV at various time points. Control represents hydrogels in PBS buffer with no collagenase. Scale bar = 500 μm. Plot of ln(1-Mt/M∞) versus time for diffusion of (E) 3 kDa dextran and (F) 150 kDa dextran in PEG-PQ hydrogels with varying NVP concentrations. Correlation coefficients of fitted trendlines for each group are listed.
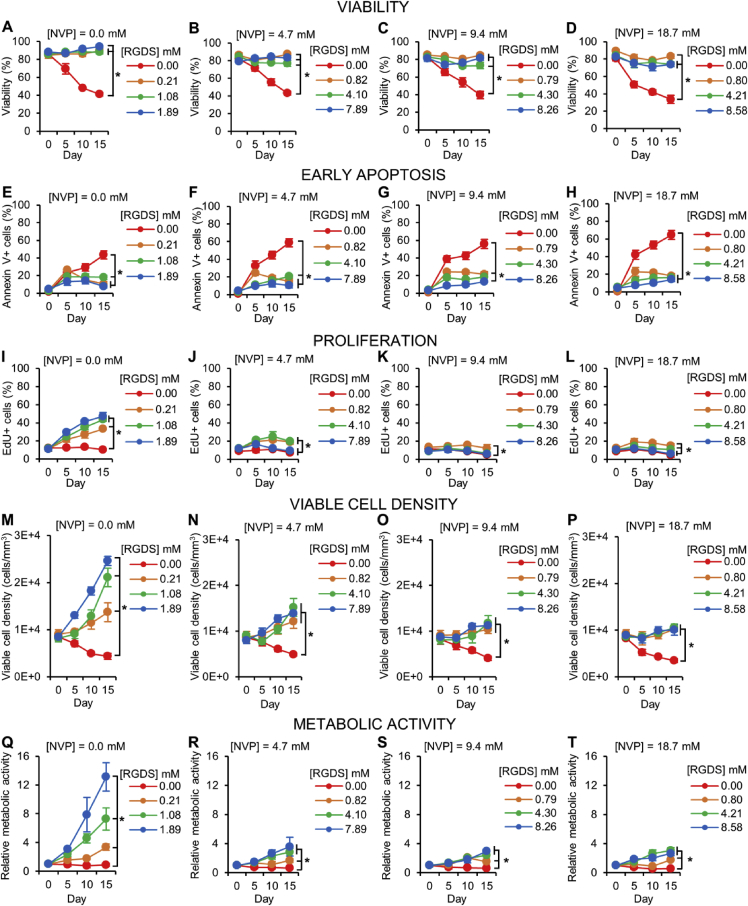
Fig. 2.
Dormancy-Related Cellular Metrics. Quantification of (A–D) cell viability, (E–H) early apoptosis, (I–L) proliferation, (M-P) viable cell density, and (Q–T) relative metabolic activity (normalized to day 0 values) of MDA-MB-231 cells in hydrogels with varying NVP and PEG-RGDS concentrations over 15 days in culture. Values represent mean ± standard deviation. * indicates p < 0.05. (A–P) n = 6 z-stacks from 3 hydrogels per condition. (Q–T) n = 5 hydrogels per condition.
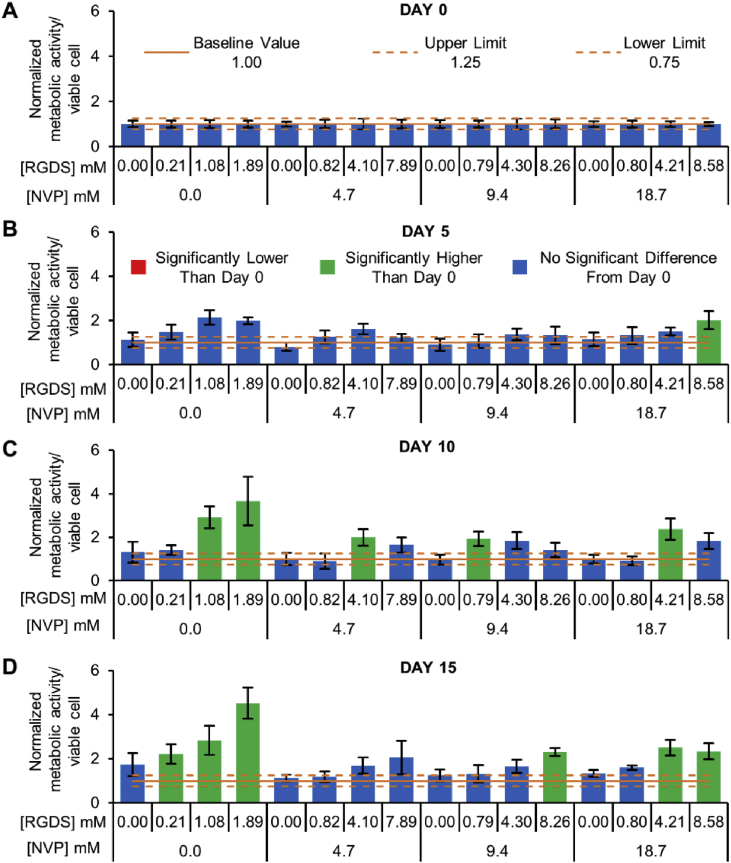
Fig. 3.
Cellular Metabolic Activity. (A–D) Metabolic activity per viable cell within hydrogel formulations with varying NVP and PEG-RGDS concentrations on days 0, 5, 10 and 15. The baseline value (solid orange line) represents the mean of all groups on day 0. The upper and lower limit (dashed orange lines) represent the deviation/noise in the day 0 measurements. Blue bars denote no significant change compared to day 0 values for the corresponding groups. Green bars denote significantly higher values compared to corresponding group values on day 0 respectively. Values represent mean ± standard deviation. p < 0.05; n = 5 hydrogels per condition.
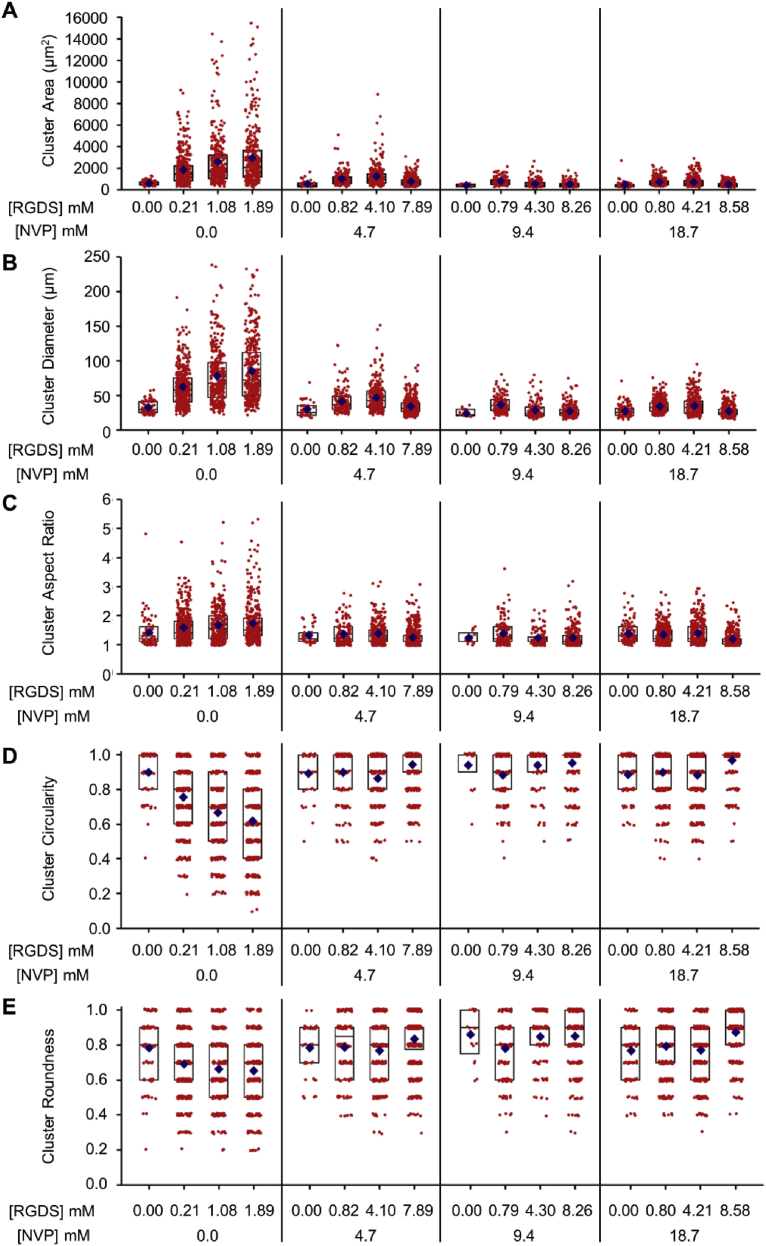
Fig. 4.
Cell Cluster Morphometric Analysis. Quantification of cell cluster (A) area, (B) diameter, (C) aspect ratio, (D) circularity, and (E) roundness within PEG-PQ hydrogels with varying PEG-RGDS and NVP concentrations after 15 days in culture. Red points denote individual clusters and blue diamonds represent mean of each group. Black rectangles represent upper quartile, median, and lower quartile of each group. n = 5 z-stacks from 3 hydrogels per condition.
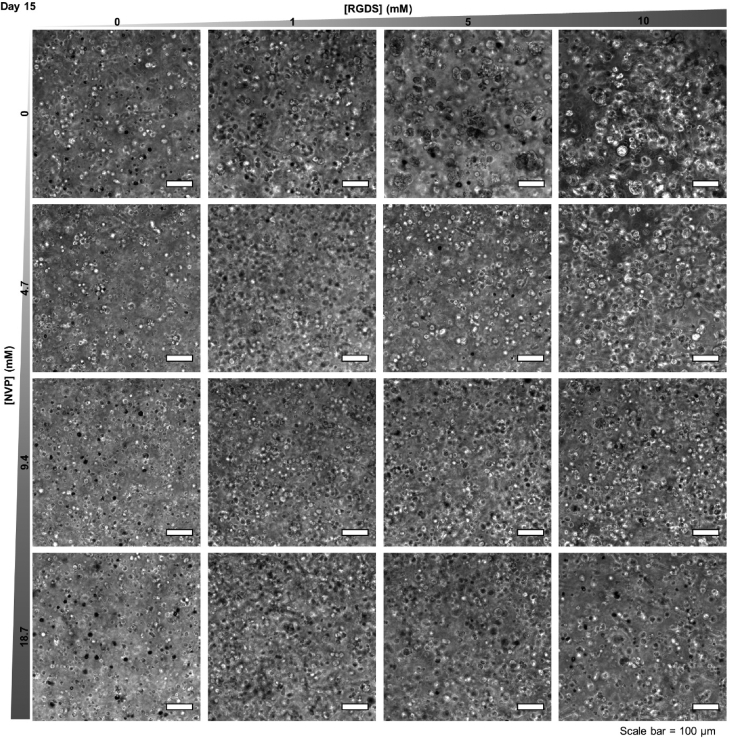
Fig. 5.
Cancer Cell/Cluster Morphology. Representative maximum intensity z-projections from 3D phase contrast image stacks of MDA-MB-231 cells encapsulated within PEG-PQ hydrogels of varying PEG-RGDS and NVP concentrations after 15 days in culture. Scale bar = 100 μm.
2. Experimental design, materials, and methods
2.1. Macromer synthesis and characterization
PEG-PQ and PEG-RGDS macromers were synthesized as described previously [1]. Gel permeation chromatography (GPC) was employed to verify successful PEG-peptide conjugation. PEG-SVA, PEG-PQ and PEG-RGDS were dissolved in deionized (DI) water, allowed to incubate at room temperature for 24 hours, filtered through a 0.22 μm filter, and injected through the port of the GPC fitted with an aqueous column. The refractive index of the eluates was monitored over time and the relative intensity obtained for each sample was normalized to 1.00. The elution time corresponding to the peak intensity was compared and used to quantify conjugation of the synthesized products.
The concentration of PEG-RGDS incorporated within the PEG-PQ hydrogels as a function of NVP concentration (0.0, 4.7, 9.4, 18.7 mM) in the prepolymer solution was quantified via fluorescence imaging and analysis. Alexa Fluor® 488 succinimidyl ester was conjugated to PEG-RGDS to form PEG-RGDS-488 which was added to the prepolymer solution at a concentration of 0.5 mM. The concentration of PEG-RGDS was adjusted to keep the total concentration of RGDS moieties constant at 1, 5, or 10 mM. Fluorescent images of the prepolymer solution prior to crosslinking, immediately post-crosslinking, and after overnight incubation in PBS were acquired. A GFP filter cube (excitation: 450–490 nm, emission: 500–550 nm) was used with an excitation intensity of 20 mW/cm2 and acquisition time of 100 ms. A replicate of 4 hydrogels were measured for each condition. The fluorescence intensities of the prepolymer solution were measured via FIJI software (NIH, version 1.52n), subtracted from background noise, and normalized to 1.00 for each condition. The RGDS incorporation efficiency was obtained by dividing the fluorescence intensity of the hydrogels post-rinsing in PBS by that obtained immediately post-crosslinking. The assumptions made for quantifying conjugation efficiency were described previously [1].
The degradability of PEG-PQ hydrogels as a function of NVP concentration was evaluated using methacryloxyethyl thiocarbamoyl rhodamine B (PolyFluor® 570) as a fluorescent tracer conjugated to the hydrogel backbone. 1 mM PolyFluor® 570 was mixed with PEG-PQ prepolymer solutions containing 0.0, 4.7, 9.4, or 18.7 mM NVP and hydrogels were crosslinked and allowed to incubate overnight in PBS as described previously [1]. Warm collagenase IV (100 μg/mL) in PBS was added to hydrogels and imaged at 15 min intervals over 3 hours. A rhodamine filter cube (excitation: 538–562 nm, emission: 570–640 nm) was used with an excitation intensity of 27 mW/cm2 and acquisition time of 100 ms. Hydrogels with 0.0 mM NVP incubated in PBS (without collagenase) were used as a control to account for photobleaching of the fluorophore during time-lapse image acquisition. A replicate of 3 hydrogels was used for each condition. Hydrogel degradation was quantified in FIJI software by subtracting background fluorescence intensity from the hydrogel fluorescence intensity.
The diffusivity in PEG-PQ hydrogels as a function of NVP concentration was quantified using 3 kDa and 150 kDa fluorescein isothiocyanate (FITC)-dextran diffusing from the hydrogel over time. Crosslinked hydrogels with 4.7, 9.4, or 18.7 mM NVP were incubated in FITC-dextran for 48 hours at 4 °C to reach equilibrium. Swollen samples were transferred to well plates containing DI water and samples containing the diffused FITC-dextran were collected every 15 minutes for 4 hours. The fluorescence intensity was measured using a plate reader (Biotek Synergy, Excitation: 490 nm, Emission: 525 nm) until no change was observed. An equal volume of DI water was added at each collection step. Fluorescence intensity values were normalized to the total intensity of released FITC-dextran over the experimental time course. Analysis of diffusivity was conducted as described previously [1], [2].
2.2. Assessment of cellular metrics
Cell viability, early apoptosis, and proliferation of cells cultured within different hydrogel formulations were evaluated using the Live/Dead® cell viability kit (Invitrogen), CF568 Annexin V stain (Biotium), and the Click-It® EdU Imaging Kit (Invitrogen) respectively according to the supplier's protocol and through quantification of fluorescently labeled cells using FIJI software [1]. Viable cell density was quantified by dividing the number of viable cells in each z-stack by the volume of the corresponding z-stack. Metabolic activity of cells was measured using Alamar Blue assay (Thermo Fisher) [1]. The cellular metabolic activity (on a per viable cell basis) was evaluated by dividing the relative metabolic rate for cells in each hydrogel formulation by the viable cell density and normalized to day 0 values (average of 1.00).
Cell cluster morphometric analysis was conducted by labeling encapsulated cells in hydrogel formulations with Alexa Fluor 568 phalloidin (Invitrogen) and Hoechst 33342 and via image analysis of confocal z-stacks using FIJI software as described earlier [1]. Cell clusters were manually traced and the projected area, Feret diameter, aspect ratio, circularity, and roundness quantified.
2.3. Statistical analysis
All statistical analyses were conducted using Minitab 18 Statistical Software (Minitab Inc.). Normality of distribution and equality in variance among groups were evaluated. Assuming equal sample size of compared groups, one-way analysis of variance (ANOVA) with Tukey's family error rate of 5% was used to evaluate statistical significance between multiple groups. In the case of unequal variance, the Games-Howell post-hoc test was employed following the ANOVA analysis. Unless otherwise indicated, p < 0.05 was considered statistically significant.
Acknowledgments
This work was supported by funding form the National Institutes of Health/National Cancer Institute IMAT Program (R21CA214299) and the W. M. Keck Foundation (15A00396). The authors would like to acknowledge Dr. Shuang Liu (Xinqiao Jia research group) for help with GPC analysis. Confocal microscopy was provided by the Bio-Imaging Center in the Delaware Biotechnology Institute, supported with grants from the NIH-NIGMS (P20 GM103446), the NSF (IIA-1301765) and the State of Delaware.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Pradhan Shantanu, Slater John H. Tunable hydrogels for controlling phenotypic cancer cell states to model breast cancer dormancy and reactivation. Biomaterials. 2019;215:119177. doi: 10.1016/j.biomaterials.2019.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pradhan S., Hassani I., Seeto W.J., Lipke E.A. PEG-fibrinogen hydrogels for three-dimensional breast cancer cell culture. J. Biomed. Mater. Res. A. 2017;105:236–252. doi: 10.1002/jbm.a.35899. [DOI] [PubMed] [Google Scholar]