Abstract
Acute gastroenteritis is common infectious disease in community in adults. This work represents an update of ‘Clinical guideline for the diagnosis and treatment of gastrointestinal infections’ that was developed domestically in 2010. The recommendation of this guideline was developed regarding the following; epidemiological factors, test for diagnosis, the indications of empirical antibiotics, and modification of antibiotics after confirming pathogen. Ultimately, it is expected to decrease antibiotic misuse and prevent antibiotic resistance.
Keywords: Infectious diarrhea, Traveler's diarrhea, Antibiotic
Introduction
Gastroenteritis refers to infection in the stomach and intestines, and most cases of acute gastroenteritis present as acute-onset diarrhea. Diarrhea is usually defined as passage of abnormally liquid or unformed stools at an increased amount and frequency. When diarrhea is caused by a source of infection and is accompanied by nausea, vomiting, and abdominal pain, it is referred to as infectious diarrhea. However, the microorganism causing the infection is rarely confirmed in clinic. Diarrhea is defined as acute if it lasts for 14 days or less, which is the case for most infectious diarrhea. In patient samples collected through ‘Sentinels in acute infectious diarrhea surveillance’ in Korea conducted by the Korea Centers for Disease Control and Prevention (KCDC), bacterial pathogens were isolated from 11.5 - 23.7% of samples between 2012 and 2016. In 2017, bacteria tested in the surveillance project (Salmonella spp., Escherichia coli, Shigella spp., Vibrio parahaemolyticus, Vibrio cholerae, Campylobacter spp., Clostridium perfringens, Staphylococcus aureus, Bacillus cereus, Listeria monocytogenes, and Yersinia enterocolitica) were isolated in 1,376 of 9,344 samples collected at 70 participating institutions, thus at a rate of 14.7%, which showed that bacteria do not account for a high number of cases of acute diarrhea. In general, acute gastroenteritis improves spontaneously and does not require antibiotic treatment. Inappropriate use of antibiotics may cause antibiotic-associated diarrhea or other complications and may also lead to antibiotic resistance in the long term. Although the Korean Society for Antimicrobial Therapy published ‘Clinical guideline for the diagnosis and treatment of gastrointestinal infections' in 2010, updates are required to reflect recent changes. Therefore, this guideline was developed in order to provide clinical recommendations based on the newest evidence on empirical antibiotic therapy for suspected acute gastroenteritis, which is commonly seen in clinic, and on targeted antibiotic treatment for cases with confirmed bacterial growth, with an ultimate aim to decrease antibiotic misuse and to prevent the rise of antibiotic-resistant bacterial strains.
Development of clinical guideline
1) Committee on clinical guideline
The committee comprised one chairperson (Yang Ree Kim, College of Medicine, The Catholic University of Korea) and six committee members (Youn Jeong Kim, College of Medicine, The Catholic University of Korea; Ki-Ho Park, Kyung Hee University School of Medicine; Seung Soon Lee, Hallym University College of Medicine; Eun Jung Lee, Soonchunhyang University College of Medicine; Hyo-Jin Lee, College of Medicine, The Catholic University of Korea; and Sung Kwan Hong, CHA University College of Medicine) recommended by the Korean Society for Antimicrobial Therapy and the Korean Society of Infectious Diseases, one member (Byoung Wook Bang, Inha University College of Medicine) recommended by the Korean Society of Gastroenterology, one member (Joonhong Park, College of Medicine, The Catholic University of Korea) recommended by the Korean Society of Clinical Microbiology, and one expert in clinical guidelines (Dong-Ah Park, National Evidence-Based Healthcare Collaborating Agency).
2) Target and scope of the guideline
The present guideline is for community-acquired infectious diarrhea and traveler's diarrhea in adults with the exception of Clostridioides difficile infection and immune-suppressed patients. Moreover, the guideline focused on the use of antibiotics in treatment. Although antidiarrheal agents and probiotics were included, intravenous hydration was excluded. The guideline was designed so that various specialists, resident physicians, government officials, and general practitioners treating acute gastroenteritis patients at hospitals and clinics of various sizes can easily refer to the guideline.
3) Selection of key questions
The committee selected a total of 10 key questions, and questions on intervention were organized based on the populations of interest, intervention, comparison and outcome (PICO) format.
4) Literature search and selection of clinical guidelines
To search for guidelines on antibiotic use in acute gastroenteritis published after 2010, ‘acute gastroenteritis’, ‘acute colitis’, ‘infectious colitis’, ‘infectious diarrhea’, ‘travelers’ diarrhea’, ‘food-borne’, and ‘water-borne’ were used as the keywords for search; ‘guideline’ and ‘recommendation’ were used as keywords to search for guidelines and were combined with other keywords through AND. The search was conducted on MEDLINE and EMBASE, which are international electronic databases, on KoreaMed and KMbase, which are Korean electronic databases, and on NGC, GIN, and KoMGI, which are databases on clinical practice guidelines. Two committee members independently selected clinical guidelines and came to an agreement. First, unrelated literature was excluded based on a review of titles and abstracts, and second, guidelines that satisfied the selection criteria were selected. The exclusion criteria were established based on a mutual agreement (Fig. 1).
Figure 1. Search strategies and details for each database.
NGC, national guideline clearinghouse; GIN, guidelines international network.
5) Evaluation of clinical guidelines and review of recency
For the four clinical guidelines selected through the aforementioned process, two committee members (per guideline) evaluated the quality of the guidelines through K-AGREE II and calculated the scores for each domain (Table 1). The guidelines that had scores above 50% in Domain 3, Rigor of Development, were selected. The clinical guideline on gastrointestinal diseases developed in 2010 did not have a high score in Rigor of Development; however, as it is the only guideline developed in Korea, it was used for adaptation. After reviewing the recency of the guidelines selected for adaptation, additional evidence was collected on PubMed and KoreaMed for each key question.
Table 1. Score for each domain in quality evaluation.
| Domain | Evaluative criteria | Guideline Aa | Guideline Bb | Guideline Cc | Guideline Dd |
|---|---|---|---|---|---|
| 1 | Scope and Purpose | 96% | 78% | 69% | 79% |
| 2 | Stakeholder Involvement | 57% | 70% | 52% | 57% |
| 3 | Rigor of Development | 87% | 63% | 73% | 52% |
| 4 | Clarity of Presentation | 93% | 100% | 100% | 99% |
| 5 | Applicability | 47% | 22% | 39% | 15% |
| 6 | Editorial Independence | 94% | 100% | 94% | 48% |
aShane AL, Mody RK, Crump JA, Tarr PI, Steiner TS, Kotloff K, Langley JM, Wanke C, Warren CA, Cheng AC, Cantey J, Pickering LK. 2017 Infectious Diseases Society of America clinical practice guidelines for the diagnosis and management of infectious diarrhea. Clin Infect Dis 2017;65:e45-80.
bRiddle MS, Connor BA, Beeching NJ, DuPont HL, Hamer DH, Kozarsky P, Libman M, Steffen R, Taylor D, Tribble DR, Vila J, Zanger P, Ericsson CD. Guidelines for the prevention and treatment of travelers' diarrhea: a graded expert panel report. J Travel Med 2017;24:S63-80.
cRiddle MS, DuPont HL, Connor BA. American College of Gastroenterology(ACG) clinical guideline: diagnosis, treatment, and prevention of acute diarrheal infections in adults. Am J Gastroenterol 2016;111:602-22.
dThe Korean Society of Infectious Diseases, Korean Society for Chemotherapy, The Korean Society of Clinical Microbiology. Clinical guideline for the diagnosis and treatment of gastrointestinal infections. Infect Chemother 2010;42:323-61.
6) Preparation of the guideline and determination of the grade of recommendation
For each key question, the initial draft of the guideline included the actual guideline, grade of recommendation, and background. The committee members prepared the initial draft based on tables comparing the four selected guidelines and relevant evidence for each key question and also considered evidence that was used in review of recency. After the initial draft was prepared, the grade of recommendation and the background for the recommendations were described. The overall quality of evidence used for recommendations for each key question was evaluated as ‘high’, ‘moderate’, ‘low’, and ‘very low’. When the key question had no supporting evidence, when evidence could be assumed based on expert agreement, and when recommendations were absolutely necessary despite a lack of evidence, the level of evidence of the recommendations was categorized as ‘expert opinion’. The grade of recommendation was evaluated as ‘strong recommendation (strong)’ or ‘weak recommendation (weak)’ upon consideration of the balance of desirable and undesirable outcome, quality of evidence, belief in patient value and preference, cost of treatment, and use of resources. The committee held meetings to discuss and reach agreement on the recommendations and grade of recommendation.
(1) Grade of recommendation
① Strong: It is best that most or all individuals receive the services outlined in the recommendation. The benefit clearly outweighs the cost or risk, or the cost or risk clearly outweighs the benefit.
② Weak: It may not be best for all individuals to receive the services outlined in the recommendation. The decision should be made based on patient value, preference, and circumstances. The level of evidence is low, or there is no clear difference in risks and benefits.
(2) Level of evidence
① High: Almost no other study will change the level of trust in the estimates of the effects.
② Moderate: Other studies may influence the committee's trust in the estimates of the effects, and the level of trust may also change.
③ Low: Other studies may significantly influence the committee's trust in the estimates of the effects, and the level of trust may also change.
④ Very low: The committee does not trust the calculated estimates of the effects.
⑤ Expert opinion: Although there is no supporting evidence, the committee has determined through official expert meetings that the recommendation is clinically appropriate at present.
7) Review by external experts
Through a meeting held on January 11, 2019, opinions on the guideline was gathered from physicians with their own clinical practice and specialists in infectious diseases, gastroenterology, and clinical microbiology. The guideline was also reviewed and approved by the Korean Academy of Family Medicine, Korean Society of Infectious Diseases, Korean Society of Gastroenterology, Korean Society of Clinical Microbiology, and Korean Society for Antimicrobial Therapy.
8) Limitations and future tasks of the guideline
The guideline was developed based on Korean and international clinical practice guidelines and recent literature available at the time of development. If new drugs are developed or if significant changes are observed in antibiotic resistance of pathogenic bacteria, the guideline may be updated.
9) Support
The guideline was supported as a Policy Research Project by the KCDC in 2018 (research project number 2018-E2803-00). The committee members who participated in developing this guideline were not influenced by any means by government organizations, academic societies, pharmaceutical companies, or other for-profit institutions.
Summary of guideline
Recommendations for each key question
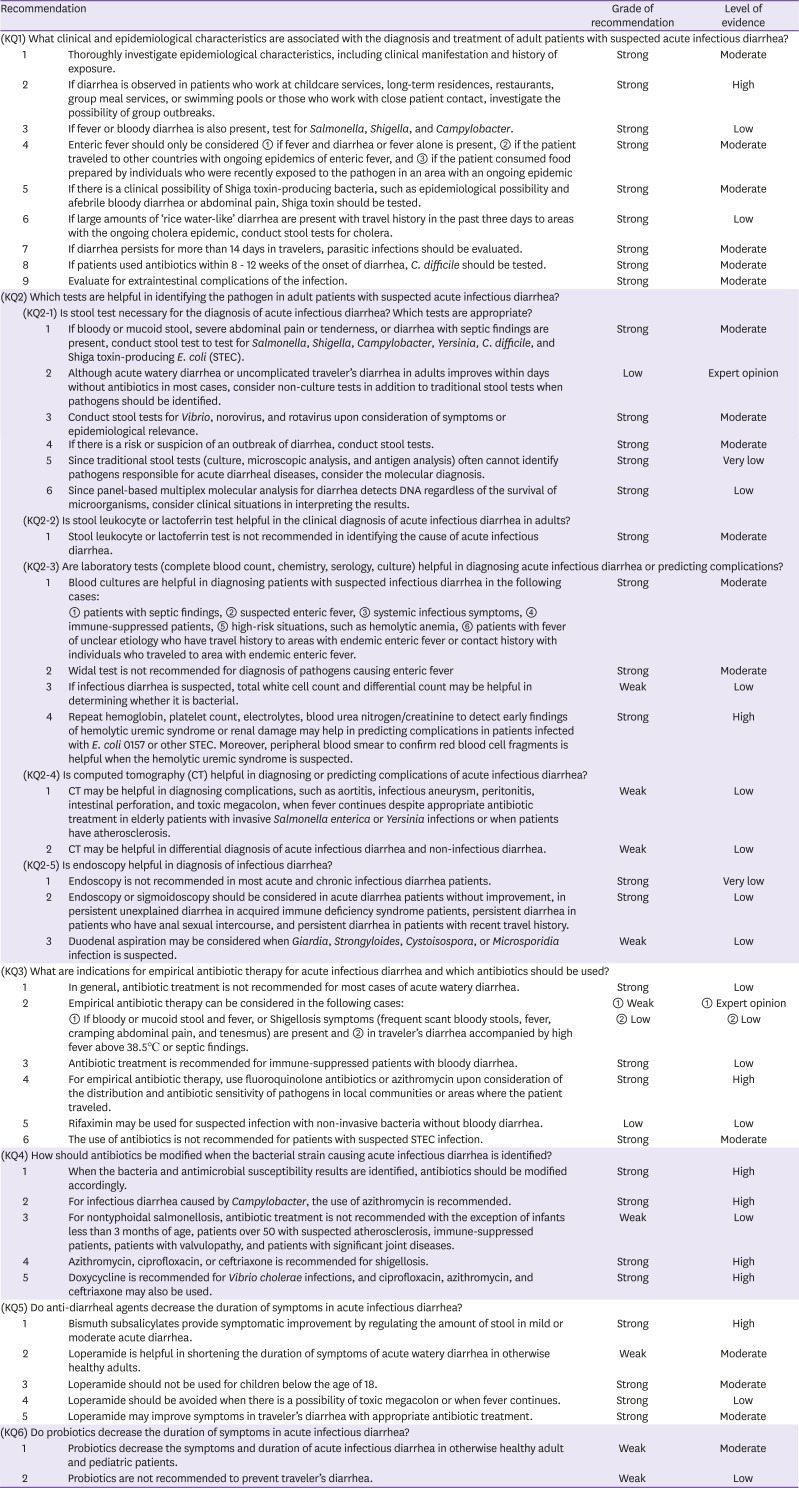
KQ1. What clinical and epidemiological characteristics are associated with the diagnosis and treatment of adult patients with suspected acute infectious diarrhea?
Infectious diarrhea has different causes and incidence in different countries based on the level of public health, lifestyle, and diet. Korea has a surveillance system on gastrointestinal infections characterized by vomiting and diarrhea at 196 surveillance institutions including tertiary hospitals, hospitals with more than 200 beds, and public hospitals. Of the 15,717 pathogens isolated in 2017, 9,276 cases were viral (59.0%), most of which were caused by norovirus and rotavirus, and 6,373 were bacterial (40.5%) caused by Salmonella, Clostridium perfringens, and Campylobacter; 68 were caused by protozoa (0.4%), most of which were caused by Giardia lamblia [1]. In the United States, food-borne outbreaks were caused by norovirus in most cases, followed by Salmonella, between 2009 and 2015 [2]. Enteric fever includes typhoid fever caused by Salmonella enterica subspecies enterica serovar Typhi (Salmonella typhi) and paratyphoid fever caused by Salmonella enterica subspecies enterica serovar Paratyphi (Salmonella paratyphi) A, B, and C. Enteric fever is most common in Central Asia and South East Asia, and is also observed in other Asian countries, Africa, Latin America, and Oceania [3]. The most common serotype of Salmonella in Korea between 1998 and 2007 were Salmonella typhi, Salmonella enterica subspecies enterica serovar Enteritidis (Salmonella nteritidis), and Salmonella enterica subspecies enterica serovar Typhimurium (Salmonella typhimurium) [4]. Salmonella causes food- or water-borne gastroenteritis in Korea. Although its incidence is on a decreasing trend, typhoid fever introduced from other countries has increased owing to increases in travels to other countries and foreign nationals living in Korea [5].
Since possible pathogens can be estimated based on epidemiological characteristics in patients with suspected acute infectious diarrhea (Table 2), food consumption (undercooked meat, eggs, shellfish, and milk), consumption of unsterilized water, contact with pets, contact with other infected individuals, history of stay at group facility, travel history, underlying diseases, sexual history, and occupation should be confirmed. Vibrio spp. and norovirus are common causes of diarrhea after consumption of uncooked seafood or shellfish, and diarrhea after consumption of uncooked meat or poultry may be caused by Shiga toxin-producing Escherichia coli (STEC) (beef), C. perfringens (beef and poultry), Salmonella (poultry), Campylobacter (poultry), Yersinia (pork and pork intestine), Staphylococcus aureus (poultry). When patients consumed unpasteurized milk, their diarrhea may be accountable to Salmonella, Campylobacter, Yersinia enterocolitica, S. aureus toxin, Cryptosporidium, or STEC, and Salmonella or Shigella contamination is common in eggs. Water can cause infectious diseases directly through consumption or indirectly through contamination of food or dishes [2]. Consumption of unsterilized water may lead to Campylobacter, Cryptosporidium, Giardia, Shigella, Salmonella, or STEC infection, and Cryptosporidium or other water-borne infections are possible after swimming at pools. In Korea, there was an outbreak of acute diarrhea in 67 patients that used a pool in 2008; in six patients with severe diarrhea, norovirus was identified in three patients. Since norovirus with a similar RNA sequence was also detected in samples of groundwater, the outbreak was reported to have been caused by contaminated groundwater [6]. Diarrhea in prisons can be accounted for by norovirus, C. difficile, Shigella, Cryptosporidium, Giardia, Salmonella, STEC, and rotavirus, and diarrhea in childcare services may have been caused by rotavirus, Cryptosporidium, Giardia, Shigella, or STEC. C. difficile may be accountable if the patient has recent history of antibiotic use. Infectious diarrhea is caused by different common bacteria in patients of different ages; for infants of 6 - 18 months, rotavirus is common, whereas nontyphoidal Salmonella is common for patients younger than 3 months or patients older than 50 with atherosclerosis. Shigella should be considered first for patients aged 1 - 7 years, and Campylobacter should be considered for young adults. Traveler's diarrhea is a common disease associated with travelling and is observed in 30 - 70% of travelers depending on the area and season; it is most commonly caused by E. coli, Campylobacter jejuni, Shigella, and Salmonella. South East Asia, Central Asia, India, Africa, Mexico, and Latin America are high-risk areas of traveler's diarrhea.
Table 2. Epidemiologic factors associated with pathogens of diarrhea.
| Epidemiological factors | Possible pathogens | |
|---|---|---|
| Food-related | Food at hotel or restaurant | Norovirus, nontyphoidal Salmonella, Clostridium perfringens, Bacillus cereus, Staphylococcus aureus, Campylobacter, ETEC, STEC, Listeria, Shigella, Cyclospora cayetanensis, Cryptosporidium |
| Unpasteurized milk | Salmonella, Campylobacter, Yersinia enterocolitica, S. aureus toxin, Cryptosporidium, STEC, Brucella (goat milk products), Mycobacterium bovis, Coxiella burnetii | |
| Raw or uncooked meat or poultry | STEC (meat), C. perfringens (meat, poultry), Salmonella (poultry), Campylobacter (poultry), Yersinia (pork, pork intestine), S. aureus (poultry), Trichinella (pork, wild animal meat) | |
| Fruits or vegetables | STEC, nontyphoidal Salmonella, Cyclospora, Cryptosporidium, Norovirus, Hepatitis A, Listeria monocytogenes | |
| Uncooked eggs | Salmonella, Shigella | |
| Shellfish | Vibrio, Norovirus, Hepatitis A, Plesiomonas | |
| Exposure or contact | Consumption of unsterilized water | Campylobacter, Cryptosporidium, Giardia, Shigella, Salmonella, STEC, Plesiomonas shigelloides |
| Swimming at a pool | Cryptosporidium | |
| Prisons | Norovirus, C. difficile, Shigella, Cryptosporidium, Giardia, STEC, Rotavirus | |
| Childcare services | Rotavirus, Cryptosporidium, Giardia, Shigella, STEC | |
| Recent antibiotic use | C. difficile, multi-drug-resistant Salmonella | |
| Travel history to areas with poor public health | Escherichia coli (enteroaggregative, enterotoxigenic, enteroinvasive), Shigella, Salmonella Typhi, nontyphoidal Salmonella, Campylobacter, Vibrio cholerae,Entamoeba histolytica, Giardia, Blastocystis, Cyclospora, Cystoisospora, Cryptosporidium | |
| Contact with pets that have diarrhea | Campylobacter, Yersinia | |
| Contact with pig stool | Balantidium coli | |
| Contact with poultry | Non-typhoidal Salmonella | |
| Visits to farms or zoos | STEC, Cryptosporidium, Campylobacter | |
ETEC, enterotoxigenic E. coli; STEC, Shiga toxin-producing E. coli.
Although causative bacteria may not cause significant differences in clinical manifestations of acute diarrhea, possible pathogens may be considered based on characteristic symptoms (Table 3). Salmonella, Campylobacter, Yersinia, or Shigella should be considered first if persistent fever and systemic symptoms are present [7]. Yersinia has symptoms similar to those of acute appendicitis, thus necessitating differential diagnosis [7,8]. Rotavirus and norovirus often cause watery diarrhea, and Shigella, C. jejuni, Salmonella, STEC, and enteroinvasive E. coli (EIEC) often cause bloody diarrhea [7]. For food-related outbreaks, the possible pathogen can be estimated based on the incubation period. Short incubation periods of 1 - 6 hours are estimated to have been caused by consumption of toxins, so S. aureus or Bacillus cereus producing emetic toxin is suspected. C. perfringens or B. cereus producing diarrheal toxin are suspected for incubation periods of 8 - 16 hours, and Enterotoxigenic E. coli (ETEC), Salmonella, Shigella, and Vibrio cholerae may be suspected for incubation periods of 16 - 72 hours [7,8]. If there is a travel history to areas with cholera epidemics with large amounts of ‘rice water-like’ diarrhea, the possibility of cholera should be considered. Since 1990, around 10 cases of cholera have been reported annually in Korea; in 2017, all five cases were introduced from other countries (four from the Philippines and one from India) [9].
Table 3. Clinical findings associated with pathogens of diarrhea.
| Clinical findings | Possible pathogens |
|---|---|
| Bloody diarrhea | STEC, Shigella, Salmonella, Campylobacter, Entamoeba histolytica, non-cholera Vibrio, Yersinia, Balantidium coli, Plesiomonas |
| Chronic diarrhea | Cryptosporidium, Giardia lamblia, Cyclospora cayetanensis, Cystoisospora belli, Entamoeba histolytica |
| Abdominal pain | STEC, Salmonella, Shigella, Campylobacter, Yersinia, Non-cholera Vibrio, Clostridioides difficile |
| Severe abdominal pain and bloody diarrhea, mild or no fever | STEC, Salmonella, Shigella, Campylobacter, Yersinia enterocolitica |
| Abdominal pain with fever (similar to appendicitis) | Yersinia enterocolitica, Yersinia pseudotuberculosis |
| Nausea and vomiting lasting ≤24 hours | S. aureus toxin or Bacillus cereus (emetic toxin) |
| Vomiting, 2–3 days of non-bloody diarrhea | Norovirus |
STEC, Shiga toxin-producing E. coli.
STEC is highly toxic and can cause a hemolytic uremic syndrome in young children and elderly patients. Moreover, it is important in the perspective of public health as it spreads easily. It can cause infections in small numbers below 102 colonies, so infections may arise easily secondary to contaminated food or environment [7]. Although STEC O157:H7 is the most common serotype that causes infections in humans worldwide, non-O157 STEC can also cause symptoms. In 2011, there were outbreaks of enteritis caused by STEC O104:H4 in Germany and France; of the 3,816 cases in Germany, 22% had hemolytic uremic syndrome [10,11]. According to 20 years of epidemiological investigation of STEC O157:H7 between 1982 and 2002 in the United States, hemolytic uremic syndrome was found in 4% of 8,598 cases, with mortality of 0.5% [12]. Although research on STEC has been lacking in Korea, a previous report suggested that STEC was identified in 0.19% of 17,148 diarrhea patients in Kwangju between 2004 and 2018 [13]. Diarrhea appears approximately 2 - 12 days after consumption of STEC, and symptoms vary between mild and bloody diarrhea, with around 90% of patients experiencing bloody stool [14]. The symptoms are often accompanied by abdominal pain and generally start as non-bloody diarrhea and progress to be bloody 1-3 days later; bloody diarrhea is more common in STEC O157:H7 infections than in non-O157 STEC [15,16]. Hemolytic uremic syndrome may arise in 5 - 13 days of diarrhea.
Although most cases of traveler's diarrhea improve spontaneously, 10% of patients may have persistent diarrhea for several weeks to months. Here, parasitic infections, the most common of which is Giardia, should be considered [17]. When afebrile bloody diarrhea and abdominal pain are present after traveling to areas with epidemics of bacteria that produce Shiga toxin, STEC infection can be suspected [18].
Extraintestinal complications (Table 4) of enteritis, such as the following, require caution: Yersinia, Campylobacter, Salmonella, and Shigella may cause erythema nodosum; Campylobacter may cause Guillain-Barre syndrome; STEC or Shigella dysenteriae serotype 1 may cause hemolytic uremic syndrome; Salmonella, Shigella, Campylobacter, and Yersinia may cause reactive arthritis, commonly referred to as Reiter's syndrome, or intestinal perforation; and Salmonella and Yersinia may cause aortitis or osteomyelitis. Postinfectious irritable bowel syndrome is also a well-known complication of infectious enteritis. This should be considered when patients present with chronic diarrhea or abdominal pain after infectious diarrhea or traveler's diarrhea [19,20]; the condition is characterized by persistent gastrointestinal symptoms even after loss of infectious causes, owing to persistent, permanent changes to gastrointestinal functions after infectious enteritis. Although the symptoms improve in most cases within one year, they sometimes persist for several years, in which case assessment by a gastroenterologist is necessary. A study reported that irritable bowel syndrome developed in 9.2% of patients with bacterial enteritis confirmed through cultures within three months and in 12.3% of the patients within six months [21].
Table 4. Extraintestinal complications associated with pathogens of diarrhea.
| Clinical findings | Possible pathogens |
|---|---|
| Postinfection irritable bowel syndrome | Campylobacter, Salmonella, Shigella, STEC, Giardia |
| Hemolytic uremic syndrome | STEC, Shigella dysenteriae serotype 1 |
| Erythema nodosum | Yersinia, Campylobacter, Salmonella, Shigella |
| Guillain-Barre syndrome | Campylobacter |
| Reactive arthritis (Reiter's syndrome) | Salmonella, Shigella, Campylobacter, Yersinia, rarely Giardia, Cyclospora cayetanensis |
| Intestinal perforation | Salmonella, Campylobacter, Yersinia, Entamoeba histolytica |
| Aortitis, osteomyelitis | Salmonella, Yersinia |
STEC, Shiga toxin-producing E. coli.
Since other epidemiological data on infectious diarrhea are lacking outside of the sentinel surveillance of gastrointestinal infections in Korea, more Korean research is warranted in this regard.
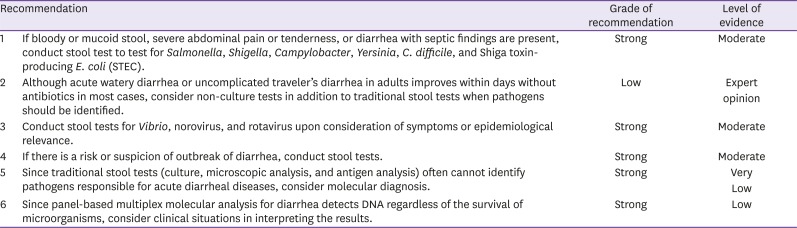
KQ2. Which tests are helpful in identifying the pathogen in adult patients with suspected acute infectious diarrhea?
KQ2-1. Is the stool test necessary for diagnosis of acute infectious diarrhea? Which tests are appropriate?
In general, acute watery diarrhea improves spontaneously without particular treatment. Stool tests are necessary when pathogens should be confirmed from a public health perspective due to risks of further progression of the disease or risks of outbreaks.
Since it is difficult to identify responsible pathogens based on clinical symptoms of infectious diarrhea, in vitro diagnostic tests should be conducted on stool samples to identify the pathogens. In vitro tests of microorganisms include bacterial and viral culture, parasite/parasitic egg smears, enzyme-based immunological assays such as viral antigen test (norovirus and rotavirus), parasitic antigen test (Giardia and Entamoeba histolytica), and bacterial toxin test (C. difficile), as well as recently introduced molecular microbiological tests. Various pathogens can cause acute infectious diarrhea, and in vitro tests are limited in terms of the complexity and cost. Therefore, only highly pathogenic bacteria, such as Salmonella, Shigella, Campylobacter, Yersinia, C. difficile, STEC, and Vibrio et al., are selectively cultured, and only certain viruses, such as norovirus and rotavirus, are tested in enzyme-based immunological assays; here, although enzyme-based immunological assays take a short amount of time, they have relatively low sensitivity and specificity [22].
For usual bacterial cultures, fresh stool is seeded onto culture media to detect Salmonella, Shigella, and C. jejuni. Acute diarrhea caused by Y. enterocolitica, C. difficile, Vibrio, or STEC is tested additionally when there is clinical suspicion. To detect Y. enterocolitica, samples are seeded on cefsulodin-irgasan-novobiocin (CIN) medium for culture at room temperature; samples are seeded on thiosulfate citrate bile salt sucrose (TCBS) medium for Vibrio and on sorbitol-MacConkey medium for STEC. When diarrhea persists for more than two weeks, Giardia is often responsible; for diagnosis, protozoa is confirmed in stool smear or cyst antigens are confirmed through immunological assays. Bacteria that cause acute diarrhea through toxins include ETEC, STEC, Clostridium botulinum, C. difficile, B. cereus, and S. aureus. STEC and C. difficile can also be tested through commercialized immunological test or molecular microbiological kits.
Fresh stool is preferred for bacterial tests for acute diarrhea, and rectal swabs may also be used. Rectal swabs are transported in modified Stuart transport medium. All samples should be delivered to the laboratory within two hours as delays in delivery may lead to drops in stool pH, thus suppressing the growth of some bacteria including Shigella. If timely delivery to the laboratory is difficult, the samples may be refrigerated for a short period of time and transported on ice. If samples should be delivered to over long distances to contracted testing institutions, the samples should be deep-frozen on dry ice (−70°C).
Panel-based multiplex molecular analysis for diarrhea employs polymerase chain reaction (PCR), which is widely used for molecular microbiological analyses, to use DNA from samples to simultaneously test for various pathogens causing diarrhea, such as bacteria, viruses, and parasites, and often has a higher sensitivity compared to conventionally used culture tests [23,24]. Therefore, it is useful in detecting pathogens causing acute infectious diarrhea, which are often not found easily through conventional stool culture [25,26]. According to a previous report, when multiplex molecular analysis and conventional diagnostic test were compared for detection of viruses, bacteria, and parasites in 1,758 stool samples collected from 1,516 patients, molecular analysis identified pathogens in 530 samples (30%) whereas conventional test identified pathogens in only 324 samples (18%) [22]. Another study comparing multiplex molecular analysis and conventional tests (culture to identify bacteria and electron microscopy to identify viruses) reported that molecular analysis identified pathogenic viruses in many samples that were negative in electron microscopy and multiplex molecular analysis had high sensitivity to many bacteria that were not detected in culture, including enteropathogenic E. coli (EPEC hereafter), enteroaggregative E. coli (EAEC hereafter), and non-O157 STEC. Overall, multiplex molecular analysis (60/135, 44.4%) identified pathogens in more than double of cases identified by conventional tests (24/135, 17.8%) [23].
Recently, microfluidic devices that enable sample pre-processing, mixing, isolation, and analysis to be performed on one chip have been developed and have been applied as nucleic acid-based point-of-care assays, where the high sensitivity and specificity of nucleic acid amplification are maintained with simultaneous nucleic acid extraction within short amounts of time [27,28]; these assays have been very useful in early diagnosis of infectious diarrhea.
However, since molecular tests identify the presence of DNA from microorganisms, they cannot distinguish between live and dead bacteria. Therefore, the tests may come out as positive even after treatment, and pathogenic colonies or normal gut flora may also lead to positive results. For these reasons, clinical situations should be considered when interpreting the results. Further research is also required on the cost-effectiveness and South Korean data on the utility of panel-based multiplex molecular analysis.
KQ2-2. Is stool leukocyte or lactoferrin test helpful in clinical diagnosis of acute infectious diarrhea in adults?
Most acute inflammatory diarrhea is caused by Campylobacter, C. difficile, enterohemorrhagic E. coli (EHEC hereafter), EIEC, Salmonella, Shigella, and Yersinia, and non-inflammatory diarrhea is caused by Clostridium food poisoning, ETEC, Staphylococcus, Vibrio cholerae, viruses (norovirus and rotavirus), and parasites (Giardia and Cryptosporidium) [8]. Most pathogens causing non-inflammatory diarrhea cause diarrhea through toxins but do not cause inflammation in intestinal mucosa; therefore, leukocytes or occult blood is rarely found in stool. Although stool leukocyte or lactoferrin test is not widely used in acute diarrhea patients in Korea, they are screening tests used for diagnosis of intestinal inflammation abroad. Since neutrophils in stool denature with time, the tests should be conducted in a timely manner. If stool leukocyte test is positive, it is highly likely that the diarrhea is of inflammatory origin, and the test is often negative in viral, parasitic, or toxin-caused diarrhea. However, stool leukocytes are only occasionally observed in inflammatory diarrhea, are not uniformly distributed, and have limited sensitivity. Moreover, although stool smears are used for differential diagnosis of invasive infections, the smears often result in false positives or negatives. Lactoferrin does not denature during transport or pre-processing and can thus be used as an alternative marker to stool leukocytes. However, since lactoferrin is also present in inflammatory enteritis of non-infectious etiologies, differential diagnosis from infectious inflammatory diarrhea is necessary [29], and it is not used commonly in clinical laboratories.
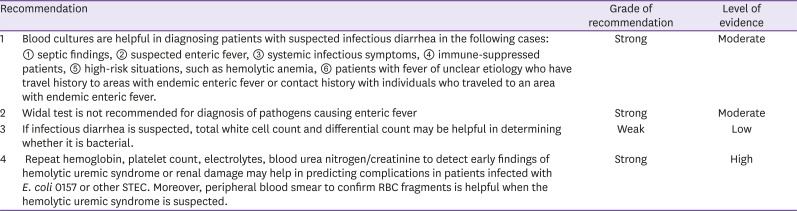
KQ2-3. Are laboratory tests (CBC, chemistry, serology, culture) helpful in diagnosing acute infectious diarrhea or predicting complications?
When infectious diarrhea is suspected, blood culture may be helpful in identifying the pathogen in patients with septic findings, patients with suspected enteric fever, and suppressed patients with fever [30,31]. Non-typhoidal Salmonella, Campylobacter, Shigella, Listeria, non-cholera Vibrio, and Yersinia may cause invasive infections and infectious diarrhea in many immune-suppressed patients, and blood culture may be employed to identify the pathogen and test for antibiotic sensitivity [32,33,34,35,36]. In order to confirm bacteremia, cultures of blood samples drawn from different sites should be conducted in 2-3 repeats. It is advisable to collect 20ml of samples, and the cultures should be done prior to antibiotic use [37]. Recently developed automated blood culture instruments, such as BacT/ALERT 3D automated microbial detection system, perform automated reads of each culture bottle at regular, short intervals to generate rapid test results; these instruments also have very high sensitivity at 95 - 98%.
The Widal test detects agglutinin, which responds to Salmonella O (somatic) antigens and H (flagella) antigens, for rapid serological diagnosis of enteric fever. Since it is cheap and simple, it was widely used previously, but it is no longer recommended due to its low specificity and sensitivity to enteric fever [30,38]. The Widal test results should be interpreted upon consideration of the past history of enteric fever, vaccination history, and antigen levels in healthy individuals in community [39]. Increased vaccination against enteric fever and other infections by S. enterica may decrease the specificity of Widal test, and cross-reaction may also be observed in non-Salmonella infections (e.g., malaria, dengue fever, and brucellosis) in areas with endemics of enteric fever [38,40]. There also are significant deviations in agglutinin levels in healthy populations in community; this is because the level may change with time and vary depending on how endemic enteric fever is in a given area [30].
Peripheral total and differential white cell count may be helpful in diagnosing whether infectious diarrhea is of bacterial, viral, or parasitic etiology. When infectious diarrhea is caused by bacteria, total leukocyte and neutrophil count often increase. In bacterial sepsis, total leukocyte and platelet count may decrease below the normal range. The leukemoid reaction may be observed in shigellosis. If infectious diarrhea is of viral etiology, the total white cell count may be within normal range with increases in lymphocyte fraction, and eosinophil count may increase in parasitic infections. Monocytes may increase in intracellular pathogenic infections such as Salmonella infections.
Since hemolytic uremic syndrome arises with time in infectious diarrhea, single complete blood count (CBC) is not enough for evaluation of its risks. Hemoglobin levels close to normal ranges may indicate dehydration. If platelet count decreases for 1 - 14 days from the onset of diarrhea, the risk of hemolytic uremic syndrome increases. If platelet count increases or stabilizes in patients in recovery, CBC monitoring can be discontinued. Patients with increasing serum creatinine, blood pressure, and body fluid volume should be closely monitored, and treatment for acute renal failure should be considered [41].
KQ2-4. Is computed tomography (CT) helpful in diagnosing or predicting complications of acute infectious diarrhea?
Although aortitis and infectious aneurysm are very rare, they often have unfavorable prognosis and are caused by Gram-positive bacteria including Staphylococcus, Enterococcus, and Streptococcus pneumoniae. However, they also may occur as complications of acute infectious diarrhea; Salmonella infections may lead to abdominal aortitis, and the incidence is high in individuals at high risks of atherosclerosis. CT enables rapid diagnosis of aortitis, aortic dissection, and hematoma of vascular walls [42]. Yersinia infection may cause complications, including bacteremia, mesenteric lymphadenitis, endocarditis, and infectious aneurysm, and these complications are common in patients with diabetes, chronic liver disease, poor nutrition status, and tumor, as well as in elderly patients. When bacteria are identified in blood cultures, CT may be helpful in diagnosis of aortitis [35,43]. Infectious abdominal aneurysm has been reported following Campylobacter infection, particularly Campylobacter fetus infection. Although this is a very rare condition, there is a high risk of rupture. Therefore, early diagnosis is very important for prognosis, and surgical treatment is necessary along with antibiotic treatment [44].
Acute diarrhea may also be present in inflammatory bowel diseases and ischemic enteritis, and CT may help in the differential diagnosis of these. On radiological findings, hyperplasia of bowel walls may lead to ‘empty lumen sign’ in infectious diarrhea, as well as relatively less ‘fat stranding’ compared to inflammatory bowel disease or ischemic enteritis. ‘Fat stranding’, ‘comb’ sign, fistula, or abscess may be found in inflammatory bowel diseases. Ischemic enteritis often invades the sigmoid colon or splenic flexure, and mesenteric infiltration is a major radiological finding. These CT findings may help in differential diagnosis of the cause of acute diarrhea [45]. Different changes in the intestinal wall thickness caused by inflammation may be observed depending on the cause. If the infection invades the small intestine, the intestinal wall may be normal on CT or may only show mild edema. Infection by E. coli O157 or enteritis caused by C. difficile infection is characterized by severe hyperplasia of colon wall on CT [46,47,48].
KQ2-5. Is endoscopy helpful in diagnosis of infectious diarrhea?
Although acute infectious diarrhea is common in healthy individuals, most cases do not require tests or treatment as they recover spontaneously within short period. Likewise, endoscopy to evaluate the cause of diarrhea is also not recommended [49]. Diarrhea is categorized into acute (less than 14 days), persistent (14 - 29 days), and chronic (30 days or longer) [50], and endoscopy is recommended for acute diarrhea without improvement, persistent diarrhea of unclear etiology in AIDS patients, and persistent diarrhea associated with anal sexual intercourse [29]. Endoscopy may also be helpful in persistent diarrhea after traveling to tropical or subtropical regions.
Sigmoidoscopy is sufficient for differential diagnosis of acute diarrhea. Although colonoscopy does not play a significant role in the diagnosis of acute infectious diarrhea and is thus not recommended, it may be considered if other conditions, such as colon cancer, are suspected. Even when colonoscopy is used, strong laxatives should be avoided whenever possible, and the test should be done with light enema. Endoscopy may be used to confirm inflammation state of colon mucosa and to differentially diagnose various types of enteritis through biopsy at different sites [51,52]; endoscopy is particularly useful in diagnosis of CMV enteritis and C. difficile enteritis [53]. Moreover, intestinal juice may be aspirated during endoscopy to obtain useful information for differential diagnosis of enteritis. A previous study reported that bacterial culture of intestinal juice aspirated during endoscopy and stool culture had a 91.2% concordance rate, thus suggesting that intestinal juice aspiration may be useful in the diagnosis [54]. In general, endoscopy is more useful for differential diagnosis of chronic diarrhea than acute diarrhea, and it is particularly helpful for diagnosis of Giardiasis, celiac disease, Crohn's disease, Whipple's disease, and eosinophilic gastroenteritis [53]. Since acute infectious diarrhea is mostly caused by lower gastrointestinal tract infections, gastroscopy is not recommended for this; however, gastroscopy may still be useful for a subset of patients. If trophozoite is found in duodenal biopsy and aspiration, Giardiasis can be diagnosed [55,56], and duodenal aspiration is also useful for suspected Strongyloides, Cystoisospora, or Microsporidia infection [57].
KQ3. What are indications for empirical antibiotic therapy for acute infectious diarrhea and which antibiotics should be used?
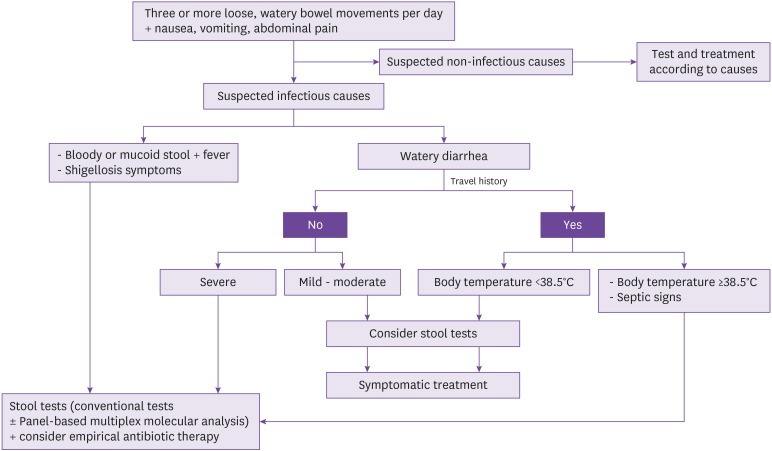
Acute watery diarrhea is often viral in etiology (norovirus, rotavirus, and adenovirus). Even when bacterial in etiology, the symptoms often improve spontaneously without treatment, and treatment does not necessarily shorten the duration of symptoms. Considering the side effects and cost of antibiotics as well as antibiotic resistance, antibiotic treatment does not offer much benefit [58]. Therefore, antibiotic treatment is not recommended with the exception of special cases, including immune-suppressed patients. Diarrhea can be classified as mild, moderate, and severe. Mild diarrhea is defined as bearable and is characterized by three or less loose bowel movements per day; patients can still travel or engage in other activities as scheduled. Moderate diarrhea is characterized by four or more bowel movements per day, and patients travel or activity plans are interfered by diarrhea. Severe diarrhea is defined as six or more bowel movements per day, and it interferes with daily activities and prevents planned trips or other activities. All bloody diarrhea is defined as severe [59]. When empirical antibiotic therapy was used for moderate traveler's diarrhea, the treatment was found to decrease the duration of diarrhea by 1.5 days on average [58,60,61], and by 16 - 30 hours for severe traveler's diarrhea [58].
Salmonella, Campylobacter, Shigella, and STEC are the most common pathogenic bacteria causing acute bloody diarrhea. Multiple randomized controlled studies demonstrated that empirical antibiotic therapy in these patients lead to 1-day reduction in the duration of symptoms compared to placebo treatment. However, antibiotic treatment increases the time for excretion of Salmonella, and can also cause the excretion of fluoroquinolone-resistant Campylobacter. Therefore, considering the benefits and risks of treatment, empirical antibiotic therapy is not recommended for most patients except immune-suppressed patients and those with severe infections. Although no evidence supporting that antibiotic use is clearly beneficial could be found, this guideline considered clinical environment in South Korea as well as expert opinions for the following recommendation: antibiotic use can be considered if bloody or mucoid stool and fever, or Shigellosis symptoms (frequent scant bloody diarrhea fever, cramping abdominal pain, and tenesmus) are present and in traveler's diarrhea accompanied by high fever above 38.5°C or septic findings (Fig. 2).
Figure 2. Algorithm for treatment of infectious diarrhea.
- Mild: Diarrhea is bearable, and the patient is capable of travelling or other activities as planned.
- Moderate: Diarrhea interferes planned travels or other activities.
- Severe: Diarrhea interferes with daily activities and prevents planned travels or other activities.
Selection of antibiotics for empirical antibiotic therapy of acute infectious diarrhea should consider the distribution and antibiotic sensitivity of pathogens in local communities or areas where the patient traveled. Although fluoroquinolone antibiotics, including ciprofloxacin and levofloxacin, have been recommended for first-line therapy, resistance against these antibiotics has increased recently. Moreover, fluoroquinolone antibiotics have risks of serious side effects, such as ligament inflammation, ligament rupture, peripheral neuropathy, and central nervous system side effects, and thus require caution.
In some areas, macrolides, such as azithromycin, is recommended due to increased resistance of Campylobacter to fluoroquinolone. In Europe in 2012, Campylobacter infections were three times more common than nontyphoidal Salmonella infections [62], and ciprofloxacin resistance of Campylobacter was reported to be as high as 44% in some European countries [63]. Fluoroquinolone resistance of Campylobacter has also been reported to be high in Mexico (56%) and Thailand (>92%) [64,65]. Considering these, macrolides including azithromycin may be considered for empirical antibiotic therapy in areas where Campylobacter is common and has high resistance to fluoroquinolone.
According to the KCDC's analysis of 3,526 samples isolated from infectious diarrhea patients in 2014, Salmonella species accounted for 13.5%, and Campylobacter species accounted for 6.1% [66]. Moreover, 29% (63/218) of Campylobacter have been reported to be resistant to fluoroquinolones although this report was made from a single institution [67]. Therefore, the percentage of Campylobacter and increase in fluoroquinolone resistance should be considered in infectious diarrhea in South Korea, and the use of macrolides, such as azithromycin, should also be considered.
Since rifaximin is a non-absorbable rifamycin derivative, it is relatively safe. It shortened the duration of symptoms compared to placebo in a randomized controlled trial [68], and its effects were comparable to those of fluoroquinolones [69,70]. Rifaximin is often effective against E. coli and less effective against invasive bacteria, such as Campylobacter, Salmonella, and Shigella. In particular, resistance is an issue in Campylobacter. Therefore, rifaximin is not recommended in areas where invasive bacteria are common or in patients with suspected infection with invasive bacteria (bloody diarrhea).
In studies employing animal models and in vitro models of STEC infection, fluoroquinolone and trimethoprim-sulfamethoxazole (TMP/SMX) antibiotics were associated with increased secretion of Shiga toxin, but fosfomycin, azithromycin, and rifaximin did not increase the excretion of Shiga toxin [71,72,73]. Recent meta-analyses of STEC patients did not show a significant correlation between antibiotic use and hemolytic uremic syndrome [74,75]. However, when the analysis was repeated only on studies with low risk of bias and appropriate definition of the hemolytic uremic syndrome, the risk of hemolytic uremic syndrome doubled when antibiotics were used [75]. Therefore, the use of antibiotics is not recommended for patients with suspected or confirmed STEC infections.
For empirical antibiotic therapy, single administration or three-day regimen is recommended for most uncomplicated cases. However, a previous study suggested that five-day regimen, rather than single administration or three-day regimen, is more effective in gastroenteritis caused by Shigella dysenteriae [76].
KQ4. How should antibiotics be modified when the bacterial strain causing acute infectious diarrhea is identified?
Although multiple randomized controlled trials showed significant decreases in the duration of symptoms of Campylobacter gastroenteritis with antibiotic treatment, the degree of significance was not great. A meta-analysis showed that treatment with fluoroquinolone or macrolide shortened the duration of symptoms by 1.32 days [77]. Azithromycin was more effective in decreasing the excretion of bacteria than ciprofloxacin in diarrhea observed in American soldiers deployed to Thailand; this finding seems to have been related to the high Campylobacter prevalence and fluoroquinolone resistance in the area [78]. Since antibiotic use increases recurrence and prolongs bacterial excretion in nontyphoidal salmonellosis, it is not recommended in most cases [79]. According to a randomized controlled trial on pediatric patients with Salmonella enteritis, bacteriological recurrence was observed in 53% of cases treated with ampicillin or amoxicillin, but no recurrence was found in the placebo control group. Moreover, 38% of patients with bacteriological recurrence had symptomatic recurrence [79]. This seems to be because antibiotic treatment in this population of patients damages the gut flora. In a meta-analysis on 767 patients in 12 studies, antibiotic treatment did not yield significant benefit in symptomatic improvement and duration in adult nontyphoidal salmonellosis patients who were otherwise healthy [80]. In cases where there is a high risk of bacteremia or high risks of complications from gastrointestinal infection, antibiotics may be used. When the KCDC analyzed 219 bacterial strains of nontyphoidal Salmonella clinically isolated between 2006 and 2008, the resistance to ampicillin, nalidixic acid, ciprofloxacin, and TMP/SMX was 49%, 50%, <1%, and 8%, respectively. The use of azithromycin, ciprofloxacin, or ceftriaxone is recommended for shigellosis. Although TMP/SMX or ampicillin may be used when isolated bacteria are sensitive, a Korean study reported a high resistance rate. In 67 strains of Shigella sonnei isolated in Jeollanam-do in 1999 - 2000, the resistance to trimethoprim, sulfonamide, nalidixic acid, and ampicillin was 100%, 99%, 70%, and 49%, respectively, but no resistance to cefotaxime or ciprofloxacin was observed [81].
KQ5. Do anti-diarrheal agents decrease the duration of symptoms in acute infectious diarrhea?
Antidiarrheal agents include intestinal motility inhibitors, adsorbents, and intestinal secretion inhibitors. Intestinal secretion or motility inhibitors may be helpful in decreasing the frequency or duration of diarrhea for symptomatic improvement of acute infectious diarrhea patients with moderate symptoms [82].
Bismuth subsalicylates, which are intestinal secretion inhibitors, decrease the frequency of diarrhea and improve nausea and abdominal pain within 24 hours of treatment in acute diarrhea patients. They are effective in improving the symptoms of traveler's diarrhea and are particularly helpful in preventing symptoms in ETEC enteritis [83,84]. Coformulations with bismuth subnitrate are available in Korea for acute diarrhea patients. Racecadotril is an enkephalinase-specific inhibitor, which is a secretion inhibitor that decreases diarrhea without influence on intestinal motility. It is effective in diarrhea in pediatric patients and had similar effects as loperamide in acute diarrhea in adults [85,86,87].
Loperamide inhibits intestinal motility to decrease the movement of intestinal contents and promote the absorption, thereby decreasing diarrhea. It also suppresses the secretion of intestinal mucosal secretion, which contributes to decreasing diarrhea [88,89]. It shortens the duration by 1 day, and decreases amount and frequency of watery diarrhea in otherwise healthy adults. Meta-analyses have reported that side effects outweigh treatment effects in pediatric patients, especially those younger than 3, patients with poor nutrition, patients with moderate or severe dehydration, patients with systemic symptoms, and patients with bloody diarrhea. Side effects include intestinal obstruction, abdominal distension, lethargy, and even death [90]. Loperamide may be used with antibiotics for fast symptomatic improvement of traveler's diarrhea. Although the treatment effects are not clear, it helps to decrease the symptoms and is known to be relatively safe with few reports of side effects. When a meta-analysis compared single use of antibiotics and combined use of loperamide (4 mg administration initially, additional administration of 2 mg with each episode of diarrhea, up to 16 mg per day), clinical improvement was greater in the combined treatment group 24 hours after the start of treatment, and the duration of diarrhea was also shorter in the combined treatment group after treatment. The treatment failure rate was also lower in the combined treatment group. In terms of the safety, the two groups did not differ significantly without any major side effects. When the effects of loperamide in traveler's diarrhea was compared to those of bismuth subsalicylates, the effects were greater in the group receiving loperamide, and there was no significant difference in side effects [61,91,92]. When loperamide is used in acute diarrhea, constipation may arise. When used in severe intestinal inflammation, megacolon can arise, and the duration of symptoms may increase. Therefore, the use of loperamide should be avoided when there is a possibility of megacolon or when fever persists from severe inflammatory responses. In particular, for enteritis caused by C. difficile or C. perfringens, loperamide should not be used as it may lead to toxic megacolon or intestinal expansion. The side effects were more common when loperamide was used on its own without appropriate antibiotic treatment [93,94,95].
Adsorbents, such as kaolin, pectin, charcoal, and attapulgite, do not decrease the frequency or duration of diarrhea and, thus, are not recommended in infectious diarrhea [96,97].
KQ6. Do probiotics decrease the duration of symptoms in acute infectious diarrhea?
The World Health Organization defined probiotics as live microorganisms that are beneficial for the host when administered in adequate amounts [98]. Theoretically, the administration of beneficial bacteria in acute infectious diarrhea suppresses the proliferation of harmful bacteria and thus treats or prevents acute infectious diarrhea. Many studies have been conducted on probiotics in pediatric patients with acute bacterial diarrhea, and most studies found that probiotics were effective in decreasing the duration and frequency of acute infectious diarrhea [98,99]. In a Cochrane analysis of 63 studies on 8,014 patients with acute infectious diarrhea, most of whom were pediatric patients, the groups receiving probiotics had diarrhea for one day less on average compared to control groups, and the frequency of bowel movements on day 2 of administration was 0.8/day less in the probiotics groups than in the control groups. Lactobacillus GG and Saccharomyces boulardii were the most commonly used probiotics in these studies [100]. However, since less studies have been conducted on adult patients than on pediatric patients with acute infectious diarrhea, it is difficult to draw conclusion on the effects in adults. Moreover, as many probiotics studies had different definition of diarrhea, cause of diarrhea, interpretation of results, methods, and probiotic strains, it is difficult to compare their effects [101]. According to research conducted on adults, when Enterococcus SF 68 was administered per os in 211 patients, the duration of diarrhea was 1.69 days in the group receiving the treatment and 2.81 days in the control group. At day 4 of treatment, diarrhea resolved in all patients in the treatment group whereas it persisted in 15.2% of patients in the control group; in other words, probiotics were found to decrease the duration of acute infectious diarrhea [102]. Similarly, when Enterococcus SF 68 was administered in 123 adult patients with acute diarrhea, diarrhea improved in 87.2% of patients in the treatment group on day 4 of treatment, compared to 59.5% improvement observed in the control group, and no side effects of probiotics were reported [103]. However, Mitra AK et al. administered enterococcus SF 68 to 183 acute bacterial diarrhea patients for 3 days and observed no decrease in the duration and frequency of bowel movement [104]. Although probiotics have been covered by the public health insurance in South Korea for pediatric patients with acute infectious diarrhea, antibiotic-associated diarrhea, and necrotizing enteritis since 2011, the same coverage does not apply for adults (notice number 2011-74). Probiotics are known to be safe with very little side effects, but caution is still required as cases of fungemia or bacteremia from probiotics have been reported in immune-suppressed patients [105].
It is difficult to conclude that certain probiotic strains are superior to others in acute infectious diarrhea. Since the effects of probiotics are strain-specific, it is also difficult to apply the findings of a study to other closely related species [106]. Moreover, research on dose-dependent effects is lacking, and the results indicated no correlation between dose and treatment effects [107]. However, in five out of six studies where different doses of probiotics were administered, dose-dependent increases in probiotics in stool were observed, indicating a recovery in stool [107]. Research is also lacking in comparison of single and combined therapy in acute infectious diarrhea [108].
Although research has been conducted to evaluate acute infectious diarrhea in adults in the context of recent travels, it is difficult to interpret the results due to different research environments, variability in probiotic strains, and short follow-up duration [12]. Moreover, two meta-analyses showed preventive efficacy against traveler's diarrhea, the prophylactic use of probiotics is not recommended due to insufficient evidence [109,110]. (Table 5)(Table 6)
Table 5. Empirical antibiotics in acute infectious diarrhea.
| Antibiotic | Dose | Duration |
|---|---|---|
| Ciprofloxacin | 500 mg PO twice daily or | 3 days |
| 500 mg PO once daily | 3 days | |
| 750 mg PO | Single dose | |
| Levofloxacin | 500 mg PO | 3 days |
| Azithromycin | 500 mg PO | 3 days |
| 1,000 mg PO | Single dose | |
| Rifaximin | 200 mg PO three times daily | 3 days |
PO, per os.
Table 6. Recommended antibiotics by pathogen.
| Pathogen | First-line antibiotics | Second-line antibiotics |
|---|---|---|
| Campylobacter | Azithromycin | Ciprofloxacinb |
| Non-typhoidal Salmonella | Usually not indicateda | NA |
| Salmonella enterica Typhi or Paratyphi | Ceftriaxone or ciprofloxacin | Ampicillinb, TMP/SMXb, or azithromycin |
| Shigella | Azithromycin, ciprofloxacinb, or ceftriaxone | TMP/SMXb or ampicillinb |
| Vibrio cholerae | Doxycycline | Ciprofloxacin, azithromycin, or ceftriaxone |
| Non-choleraic Vibrio | Noninvasive disease: usually not indicated | Noninvasive disease: usually not indicated |
| Invasive disease: ceftriaxone + doxycycline | Invasive disease: TMP/SMX + aminoglycoside |
aCeftriaxone, ciprofloxacin, TMP/SMX, or amoxicillin may be used when there is a risk of invasive infections.
bHas a high risk of resistance in South Korea and may be used based on sensitivity test results. Caution is required when sensitivity is unknown (e.g., only positive PCR results).
NA, not available; TMP/SMX, trimethoprim-sulfamethoxazole.
Footnotes
Conflict of Interest: No conflicts of interest.
References
- 1.Korea Centers for Disease Control and Prevention (KCDC) Infectious diseases surveillance yearbook, 2017. [Accessed 20 December 2018]. Available at: http://www.cdc.go.kr/npt/biz/npp/portal/nppPblctDtaMain.do? pblctDtaSeAt=1.
- 2.Dewey-Mattia D, Manikonda K, Hall AJ, Wise ME, Crowe SJ. Surveillance for foodborne disease outbreaks-United States, 2009-2015. MMWR Surveill Summ. 2018;67:1–11. doi: 10.15585/mmwr.ss6710a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004;82:346–353. [PMC free article] [PubMed] [Google Scholar]
- 4.Kim S. Salmonella serovars from foodborne and waterborne diseases in Korea, 1998-2007: total isolates decreasing versus rare serovars emerging. J Korean Med Sci. 2010;25:1693–1699. doi: 10.3346/jkms.2010.25.12.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoo S, Pai H, Byeon JH, Kang YH, Kim S, Lee BK. Epidemiology of Salmonella enterica serotype typhi infections in Korea for recent 9 years: trends of antimicrobial resistance. J Korean Med Sci. 2004;19:15–20. doi: 10.3346/jkms.2004.19.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koh SJ, Cho HG, Kim BH, Choi BY. An outbreak of gastroenteritis caused by norovirus-contaminated groundwater at a waterpark in Korea. J Korean Med Sci. 2011;26:28–32. doi: 10.3346/jkms.2011.26.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Korean Society of Infectious Diseases, Korean Society for Chemotherapy, The Korean Society of Clinical Microbiology. Clinical guideline for the diagnosis and treatment of gastrointestinal infections. Infect Chemother. 2010;42:323–361. [Google Scholar]
- 8.DuPont HL. Clinical practice. Bacterial diarrhea. N Engl J Med. 2009;361:1560–1569. doi: 10.1056/NEJMcp0904162. [DOI] [PubMed] [Google Scholar]
- 9.Korea Centers for Disease Control and Prevention (KCDC) Infectious disease portal. [Accessed 20 December 2018]. Available at: http://www.cdc.go.kr/npt/biz/npp/ist/bass/bassDissStatsMain.do.
- 10.Wu CJ, Hsueh PR, Ko WC. A new health threat in Europe: Shiga toxin-producing Escherichia coli O104:H4 infections. J Microbiol Immunol Infect. 2011;44:390–393. doi: 10.1016/j.jmii.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Frank C, Werber D, Cramer JP, Askar M, Faber M, an der Heiden M, Bernard H, Fruth A, Prager R, Spode A, Wadl M, Zoufaly A, Jordan S, Kemper MJ, Follin P, Müller L, King LA, Rosner B, Buchholz U, Stark K, Krause G HUS Investigation Team. Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N Engl J Med. 2011;365:1771–1780. doi: 10.1056/NEJMoa1106483. [DOI] [PubMed] [Google Scholar]
- 12.Rangel JM, Sparling PH, Crowe C, Griffin PM, Swerdlow DL. Epidemiology of Escherichia coli O157:H7 outbreaks, United States, 1982-2002. Emerg Infect Dis. 2005;11:603–609. doi: 10.3201/eid1104.040739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim MJ, Kim SH, Kim TS, Kee HY, Seo JJ, Kim ES, Park JT, Chung JK, Lee J. Identification of shiga toxin-producing E. coli isolated from diarrhea patients and cattle in Gwangju area, Korea. J Bacteriol Virol. 2009;39:29–39. [Google Scholar]
- 14.Mody RK, Luna-Gierke RE, Jones TF, Comstock N, Hurd S, Scheftel J, Lathrop S, Smith G, Palmer A, Strockbine N, Talkington D, Mahon BE, Hoekstra RM, Griffin PM. Infections in pediatric postdiarrheal hemolytic uremic syndrome: factors associated with identifying shiga toxin-producing Escherichia coli . Arch Pediatr Adolesc Med. 2012;166:902–909. doi: 10.1001/archpediatrics.2012.471. [DOI] [PubMed] [Google Scholar]
- 15.Croxen MA, Law RJ, Scholz R, Keeney KM, Wlodarska M, Finlay BB. Recent advances in understanding enteric pathogenic Escherichia coli . Clin Microbiol Rev. 2013;26:822–880. doi: 10.1128/CMR.00022-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tarr PI, Gordon CA, Chandler WL. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet. 2005;365:1073–1086. doi: 10.1016/S0140-6736(05)71144-2. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention (CDC) Traveler's health: travelers' diarrhea. [Accessed 20 December 2018]. Available at: https://wwwnc.cdc.gov/travel/yellowbook/2018/the-pre-travel-consultation/travelers-diarrhea.
- 18.Slutsker L, Ries AA, Greene KD, Wells JG, Hutwagner L, Griffin PM. Escherichia coli O157:H7 diarrhea in the United States: clinical and epidemiologic features. Ann Intern Med. 1997;126:505–513. doi: 10.7326/0003-4819-126-7-199704010-00002. [DOI] [PubMed] [Google Scholar]
- 19.Mutsch M, Pitzurra R, Hatz C, Steffen R. Post-infectious sequelae of travelers' diarrhea: irritable bowel syndrome. J Travel Med. 2014;21:141–143. doi: 10.1111/jtm.12094_1. [DOI] [PubMed] [Google Scholar]
- 20.Connor BA. Sequelae of traveler's diarrhea: focus on postinfectious irritable bowel syndrome. Clin Infect Dis. 2005;41(Suppl 8):S577–S586. doi: 10.1086/432956. [DOI] [PubMed] [Google Scholar]
- 21.Koh SJ, Lee DH, Lee SH, Park YS, Hwang JH, Kim JW, Jeong SH, Kim N, Im JP, Kim JS, Jung HC. Incidence and risk factors of irritable bowel syndrome in community subjects with culture-proven bacterial gastroenteritis. Korean J Gastroenterol. 2012;60:13–18. doi: 10.4166/kjg.2012.60.1.13. [DOI] [PubMed] [Google Scholar]
- 22.McAuliffe GN, Anderson TP, Stevens M, Adams J, Coleman R, Mahagamasekera P, Young S, Henderson T, Hofmann M, Jennings LC, Murdoch DR. Systematic application of multiplex PCR enhances the detection of bacteria, parasites, and viruses in stool samples. J Infect. 2013;67:122–129. doi: 10.1016/j.jinf.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Amrud K, Slinger R, Sant N, Desjardins M, Toye B. A comparison of the Allplex™ bacterial and viral assays to conventional methods for detection of gastroenteritis agents. BMC Res Notes. 2018;11:514. doi: 10.1186/s13104-018-3645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cybulski RJ, Jr, Bateman AC, Bourassa L, Bryan A, Beail B, Matsumoto J, Cookson BT, Fang FC. Clinical impact of a multiplex gastrointestinal polymerase chain reaction panel in patients with acute gastroenteritis. Clin Infect Dis. 2018;67:1688–1696. doi: 10.1093/cid/ciy357. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Y, Zhu X, Hou H, Lu Y, Yu J, Mao L, Mao L, Sun Z. Characteristics of diarrheagenic Escherichia coli among children under 5 years of age with acute diarrhea: a hospital based study. BMC Infect Dis. 2018;18:63. doi: 10.1186/s12879-017-2936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thakur N, Jain S, Changotra H, Shrivastava R, Kumar Y, Grover N, Vashistt J. Molecular characterization of diarrheagenic Escherichia coli pathotypes: association of virulent genes, serogroups, and antibiotic resistance among moderate-to-severe diarrhea patients. J Clin Lab Anal. 2018;32:e22388. doi: 10.1002/jcla.22388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim SH, Shin JH. Point-of-care diagnostics for infectious diseases: present and future. Korean J Med. 2018;93:181–187. [Google Scholar]
- 28.Ramanan P, Bryson AL, Binnicker MJ, Pritt BS, Patel R. Syndromic panel-based testing in clinical microbiology. Clin Microbiol Rev. 2018;31:pii:e00024–17. doi: 10.1128/CMR.00024-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shane AL, Mody RK, Crump JA, Tarr PI, Steiner TS, Kotloff K, Langley JM, Wanke C, Warren CA, Cheng AC, Cantey J, Pickering LK. 2017 Infectious Diseases Society of America clinical practice guidelines for the diagnosis and management of infectious diarrhea. Clin Infect Dis. 2017;65:e45–80. doi: 10.1093/cid/cix669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parry CM, Hien TT, Dougan G, White NJ, Farrar JJ. Typhoid fever. N Engl J Med. 2002;347:1770–1782. doi: 10.1056/NEJMra020201. [DOI] [PubMed] [Google Scholar]
- 31.Bhan MK, Bahl R, Bhatnagar S. Typhoid and paratyphoid fever. Lancet. 2005;366:749–762. doi: 10.1016/S0140-6736(05)67181-4. [DOI] [PubMed] [Google Scholar]
- 32.Crump JA, Medalla FM, Joyce KW, Krueger AL, Hoekstra RM, Whichard JM, Barzilay EJ Emerging Infections Program NARMS Working Group. Antimicrobial resistance among invasive nontyphoidal Salmonella enterica isolates in the United States: National Antimicrobial Resistance Monitoring System, 1996 to 2007. Antimicrob Agents Chemother. 2011;55:1148–1154. doi: 10.1128/AAC.01333-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Angulo FJ, Swerdlow DL. Bacterial enteric infections in persons infected with human immunodeficiency virus. Clin Infect Dis. 1995;21(Suppl 1):S84–S93. doi: 10.1093/clinids/21.supplement_1.s84. [DOI] [PubMed] [Google Scholar]
- 34.Keddy KH, Sooka A, Crowther-Gibson P, Quan V, Meiring S, Cohen C, Nana T, Sriruttan C, Seetharam S, Hoosen A, Naicker P, Elliott E, Haffejee S, Whitelaw A, Klugman KP Group for Enteric, Respiratory, and Meningeal Disease Surveillance in South Africa (GERMS-SA) Systemic shigellosis in South Africa. Clin Infect Dis. 2012;54:1448–1454. doi: 10.1093/cid/cis224. [DOI] [PubMed] [Google Scholar]
- 35.Cover TL, Aber RC. Yersinia enterocolitica. N Engl J Med. 1989;321:16–24. doi: 10.1056/NEJM198907063210104. [DOI] [PubMed] [Google Scholar]
- 36.Haq SM, Dayal HH. Chronic liver disease and consumption of raw oysters: a potentially lethal combination--a review of Vibrio vulnificus septicemia. Am J Gastroenterol. 2005;100:1195–1199. doi: 10.1111/j.1572-0241.2005.40814.x. [DOI] [PubMed] [Google Scholar]
- 37.Lee A, Mirrett S, Reller LB, Weinstein MP. Detection of bloodstream infections in adults: how many blood cultures are needed? J Clin Microbiol. 2007;45:3546–3548. doi: 10.1128/JCM.01555-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olopoenia LA, King AL. Widal agglutination test - 100 years later: still plagued by controversy. Postgrad Med J. 2000;76:80–84. doi: 10.1136/pmj.76.892.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.House D, Wain J, Ho VA, Diep TS, Chinh NT, Bay PV, Vinh H, Duc M, Parry CM, Dougan G, White NJ, Hien TT, Farrar JJ. Serology of typhoid fever in an area of endemicity and its relevance to diagnosis. J Clin Microbiol. 2001;39:1002–1007. doi: 10.1128/JCM.39.3.1002-1007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waddington CS, Darton TC, Pollard AJ. The challenge of enteric fever. J Infect. 2014;68(Suppl 1):S38–S50. doi: 10.1016/j.jinf.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 41.Holtz LR, Neill MA, Tarr PI. Acute bloody diarrhea: a medical emergency for patients of all ages. Gastroenterology. 2009;136:1887–1898. doi: 10.1053/j.gastro.2009.02.059. [DOI] [PubMed] [Google Scholar]
- 42.Lopes RJ, Almeida J, Dias PJ, Pinho P, Maciel MJ. Infectious thoracic aortitis: a literature review. Clin Cardiol. 2009;32:488–490. doi: 10.1002/clc.20578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karachalios G, Bablekos G, Karachaliou G, Charalabopoulos AK, Charalabopoulos K. Infectious endocarditis due to Yersinia enterocolitica. Chemotherapy. 2002;48:158–159. doi: 10.1159/000064923. [DOI] [PubMed] [Google Scholar]
- 44.Hagiya H, Matsumoto M, Furukawa H, Murase T, Otsuka F. Mycotic abdominal aortic aneurysm caused by Campylobacter fetus: a case report and literature review. Ann Vasc Surg. 2014;28:1933.e7–1933.14. doi: 10.1016/j.avsg.2014.06.072. [DOI] [PubMed] [Google Scholar]
- 45.Plastaras L, Vuitton L, Badet N, Koch S, Di Martino V, Delabrousse E. Acute colitis: differential diagnosis using multidetector CT. Clin Radiol. 2015;70:262–269. doi: 10.1016/j.crad.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 46.Horiki N, Maruyama M, Fujita Y, Suzuki Y, Tanaka T, Imoto I, Adachi Y. CT evaluation of infectious colitis. Nihon Shokakibyo Gakkai Zasshi. 2002;99:925–934. [PubMed] [Google Scholar]
- 47.Macari M, Balthazar EJ. CT of bowel wall thickening: significance and pitfalls of interpretation. AJR Am J Roentgenol. 2001;176:1105–1116. doi: 10.2214/ajr.176.5.1761105. [DOI] [PubMed] [Google Scholar]
- 48.Thoeni RF, Cello JP. CT imaging of colitis. Radiology. 2006;240:623–638. doi: 10.1148/radiol.2403050818. [DOI] [PubMed] [Google Scholar]
- 49.Riddle MS, DuPont HL, Connor BA. ACG clinical guideline: diagnosis, treatment, and prevention of acute diarrheal infections in adults. Am J Gastroenterol. 2016;111:602–622. doi: 10.1038/ajg.2016.126. [DOI] [PubMed] [Google Scholar]
- 50.DuPont HL. Acute infectious diarrhea in immunocompetent adults. N Engl J Med. 2014;370:1532–1540. doi: 10.1056/NEJMra1301069. [DOI] [PubMed] [Google Scholar]
- 51.Surawicz CM, Belic L. Rectal biopsy helps to distinguish acute self-limited colitis from idiopathic inflammatory bowel disease. Gastroenterology. 1984;86:104–113. [PubMed] [Google Scholar]
- 52.Waye JD. Differentiation of inflammatory bowel conditions by endoscopy and biopsy. Endoscopy. 1992;24:551–554. doi: 10.1055/s-2007-1010543. [DOI] [PubMed] [Google Scholar]
- 53.ASGE Standards of Practice Committee. Shen B, Khan K, Ikenberry SO, Anderson MA, Banerjee S, Baron T, Ben-Menachem T, Cash BD, Fanelli RD, Fisher L, Fukami N, Gan SI, Harrison ME, Jagannath S, Lee Krinsky M, Levy M, Maple JT, Lichtenstein D, Stewart L, Strohmeyer L, Dominitz JA. The role of endoscopy in the management of patients with diarrhea. Gastrointest Endosc. 2010;71:887–892. doi: 10.1016/j.gie.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 54.Barbut F, Beaugerie L, Delas N, Fossati-Marchal S, Aygalenq P, Petit JC Infectious Colitis Study Group. Comparative value of colonic biopsy and intraluminal fluid culture for diagnosis of bacterial acute colitis in immunocompetent patients. Clin Infect Dis. 1999;29:356–360. doi: 10.1086/520215. [DOI] [PubMed] [Google Scholar]
- 55.Goka AK, Rolston DD, Mathan VI, Farthing MJ. The relative merits of faecal and duodenal juice microscopy in the diagnosis of giardiasis. Trans R Soc Trop Med Hyg. 1990;84:66–67. doi: 10.1016/0035-9203(90)90386-s. [DOI] [PubMed] [Google Scholar]
- 56.Wahnschaffe U, Ignatius R, Loddenkemper C, Liesenfeld O, Muehlen M, Jelinek T, Burchard GD, Weinke T, Harms G, Stein H, Zeitz M, Ullrich R, Schneider T. Diagnostic value of endoscopy for the diagnosis of giardiasis and other intestinal diseases in patients with persistent diarrhea from tropical or subtropical areas. Scand J Gastroenterol. 2007;42:391–396. doi: 10.1080/00365520600881193. [DOI] [PubMed] [Google Scholar]
- 57.Goka AK, Rolston DD, Mathan VI, Farthing MJ. Diagnosis of Strongyloides and hookworm infections: comparison of faecal and duodenal fluid microscopy. Trans R Soc Trop Med Hyg. 1990;84:829–831. doi: 10.1016/0035-9203(90)90098-y. [DOI] [PubMed] [Google Scholar]
- 58.De Bruyn G, Hahn S, Borwick A. Antibiotic treatment for travellers' diarrhoea. Cochrane Database Syst Rev. 2000:CD002242. doi: 10.1002/14651858.CD002242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Riddle MS, Connor BA, Beeching NJ, DuPont HL, Hamer DH, Kozarsky P, Libman M, Steffen R, Taylor D, Tribble DR, Vila J, Zanger P, Ericsson CD. Guidelines for the prevention and treatment of travelers' diarrhea: a graded expert panel report. J Travel Med. 2017;24(suppl_1):S57–S74. doi: 10.1093/jtm/tax026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Libman M CATMAT. Summary of the Committee to Advise on Tropical Medicine and Travel (CATMAT) statement on travellers' diarrhea. Can Commun Dis Rep. 2015;41:272–284. doi: 10.14745/ccdr.v41i11a03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DuPont HL, Ericsson CD, Farthing MJ, Gorbach S, Pickering LK, Rombo L, Steffen R, Weinke T. Expert review of the evidence base for self-therapy of travelers' diarrhea. J Travel Med. 2009;16:161–171. doi: 10.1111/j.1708-8305.2009.00300.x. [DOI] [PubMed] [Google Scholar]
- 62.Bartels C, Beaute J, Fraser G, de Jong B, Urtaza J, Nicols G, Niskanen T, Palm D, Robesyn E, Severi E, Tavoschi L, Varela Santos C, Van Walle I, Warns-Petit E, Westrell T, Whittaker R. Annual epidemiological report 2014: food- and waterborne diseases and zoonoses. Stockholm: ECDC; 2014. [Google Scholar]
- 63.Eurosurveillance editorial team. European Union Summary Report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food 2012 published. Euro Surveill. 2014;19:20748. doi: 10.2807/ese.19.12.20748-en. [DOI] [PubMed] [Google Scholar]
- 64.Zaidi MB, McDermott PF, Campos FD, Chim R, Leon M, Vazquez G, Figueroa G, Lopez E, Contreras J, Estrada-Garcia T. Antimicrobial-resistant Campylobacter in the food chain in Mexico. Foodborne Pathog Dis. 2012;9:841–847. doi: 10.1089/fpd.2012.1127. [DOI] [PubMed] [Google Scholar]
- 65.Serichantalergs O, Pootong P, Dalsgaard A, Bodhidatta L, Guerry P, Tribble DR, Anuras S, Mason CJ. PFGE, Lior serotype, and antimicrobial resistance patterns among Campylobacter jejuni isolated from travelers and US military personnel with acute diarrhea in Thailand, 1998-2003. Gut Pathog. 2010;2:15. doi: 10.1186/1757-4749-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim NO, Jung SM, Na HY, Chung GT, Yoo CK, Seong WK, Hong S. Enteric bacteria isolated from diarrheal patients in Korea in 2014. Osong Public Health Res Perspect. 2015;6:233–240. doi: 10.1016/j.phrp.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cho IJ, Yim J, Lee Y, Kim MS, Seo Y, Chung HS, Yong D, Jeong SH, Lee K, Chong Y. Trends in isolation and antimicrobial susceptibility of enteropathogenic bacteria in 2001-2010 at a Korean tertiary care hospital. Ann Clin Microbiol. 2013;16:45–51. [Google Scholar]
- 68.Infante RM, Ericsson CD, Jiang ZD, Ke S, Steffen R, Riopel L, Sack DA, DuPont HL. Enteroaggregative Escherichia coli diarrhea in travelers: response to rifaximin therapy. Clin Gastroenterol Hepatol. 2004;2:135–138. doi: 10.1016/s1542-3565(03)00322-7. [DOI] [PubMed] [Google Scholar]
- 69.Riddle MS, Connor P, Fraser J, Porter CK, Swierczewski B, Hutley EJ, Danboise B, Simons MP, Hulseberg C, Lalani T, Gutierrez RL, Tribble DR TrEAT TD Study Team. Trial Evaluating Ambulatory Therapy of Travelers' Diarrhea (TrEAT TD) study: a randomized controlled trial comparing 3 single-dose antibiotic regimens with loperamide. Clin Infect Dis. 2017;65:2008–2017. doi: 10.1093/cid/cix693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.DuPont HL, Jiang ZD, Ericsson CD, Adachi JA, Mathewson JJ, DuPont MW, Palazzini E, Riopel LM, Ashley D, Martinez-Sandoval F. Rifaximin versus ciprofloxacin for the treatment of traveler's diarrhea: a randomized, double-blind clinical trial. Clin Infect Dis. 2001;33:1807–1815. doi: 10.1086/323814. [DOI] [PubMed] [Google Scholar]
- 71.Ochoa TJ, Chen J, Walker CM, Gonzales E, Cleary TG. Rifaximin does not induce toxin production or phage-mediated lysis of shiga toxin-producing Escherichia coli . Antimicrob Agents Chemother. 2007;51:2837–2841. doi: 10.1128/AAC.01397-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ohara T, Kojio S, Taneike I, Nakagawa S, Gondaira F, Tamura Y, Gejyo F, Zhang HM, Yamamoto T. Effects of azithromycin on shiga toxin production by Escherichia coli and subsequent host inflammatory response. Antimicrob Agents Chemother. 2002;46:3478–3483. doi: 10.1128/AAC.46.11.3478-3483.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang X, McDaniel AD, Wolf LE, Keusch GT, Waldor MK, Acheson DW. Quinolone antibiotics induce Shiga toxin-encoding bacteriophages, toxin production, and death in mice. J Infect Dis. 2000;181:664–670. doi: 10.1086/315239. [DOI] [PubMed] [Google Scholar]
- 74.Safdar N, Said A, Gangnon RE, Maki DG. Risk of hemolytic uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 enteritis: a meta-analysis. JAMA. 2002;288:996–1001. doi: 10.1001/jama.288.8.996. [DOI] [PubMed] [Google Scholar]
- 75.Freedman SB, Xie J, Neufeld MS, Hamilton WL, Hartling L, Tarr PI Alberta Provincial Pediatric Enteric Infection Team (APPETITE) Nettel-Aguirre A, Chuck A, Lee B, Johnson D, Currie G, Talbot J, Jiang J, Dickinson J, Kellner J, MacDonald J, Svenson L, Chui L, Louie M, Lavoie M, Eltorki M, Vanderkooi O, Tellier R, Ali S, Drews S, Graham T, Pang XL. Shiga toxin-producing Escherichia coli infection, antibiotics, and risk of developing hemolytic uremic syndrome: A meta-analysis. Clin Infect Dis. 2016;62:1251–1258. doi: 10.1093/cid/ciw099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bennish ML, Salam MA, Khan WA, Khan AM. Treatment of shigellosis: III. Comparison of one- or two-dose ciprofloxacin with standard 5-day therapy. A randomized, blinded trial. Ann Intern Med. 1992;117:727–734. doi: 10.7326/0003-4819-117-9-727. [DOI] [PubMed] [Google Scholar]
- 77.Ternhag A, Asikainen T, Giesecke J, Ekdahl K. A meta-analysis on the effects of antibiotic treatment on duration of symptoms caused by infection with Campylobacter species. Clin Infect Dis. 2007;44:696–700. doi: 10.1086/509924. [DOI] [PubMed] [Google Scholar]
- 78.Kuschner RA, Trofa AF, Thomas RJ, Hoge CW, Pitarangsi C, Amato S, Olafson RP, Echeverria P, Sadoff JC, Taylor DN. Use of azithromycin for the treatment of Campylobacter enteritis in travelers to Thailand, an area where ciprofloxacin resistance is prevalent. Clin Infect Dis. 1995;21:536–541. doi: 10.1093/clinids/21.3.536. [DOI] [PubMed] [Google Scholar]
- 79.Nelson JD, Kusmiesz H, Jackson LH, Woodman E. Treatment of Salmonella gastroenteritis with ampicillin, amoxicillin, or placebo. Pediatrics. 1980;65:1125–1130. [PubMed] [Google Scholar]
- 80.Onwuezobe IA, Oshun PO, Odigwe CC. Antimicrobials for treating symptomatic non-typhoidal Salmonella infection. Cochrane Database Syst Rev. 2012;11:CD001167. doi: 10.1002/14651858.CD001167.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oh JY, Yu HS, Kim SK, Seol SY, Cho DT, Lee JC. Changes in patterns of antimicrobial susceptibility and integron carriage among Shigella sonnei isolates from southwestern Korea during epidemic periods. J Clin Microbiol. 2003;41:421–423. doi: 10.1128/JCM.41.1.421-423.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Spruill WJ, Wade WE. Diarrhea, constipation, and irritable bowel syndrome. 7th ed. New York: McGraw Hill Medical; 2007. pp. 617–623. [Google Scholar]
- 83.DuPont HL, Sullivan P, Pickering LK, Haynes G, Ackerman PB. Symptomatic treatment of diarrhea with bismuth subsalicylate among students attending a Mexican university. Gastroenterology. 1977;73:715–718. [PubMed] [Google Scholar]
- 84.DuPont HL. Bismuth subsalicylate in the treatment and prevention of diarrheal disease. Drug Intell Clin Pharm. 1987;21:687–693. doi: 10.1177/106002808702100901. [DOI] [PubMed] [Google Scholar]
- 85.Primi MP, Bueno L, Baumer P, Berard H, Lecomte JM. Racecadotril demonstrates intestinal antisecretory activity in vivo. Aliment Pharmacol Ther. 1999;13(Suppl 6):3–7. doi: 10.1046/j.1365-2036.13.s6.3.x. [DOI] [PubMed] [Google Scholar]
- 86.Salazar-Lindo E, Santisteban-Ponce J, Chea-Woo E, Gutierrez M. Racecadotril in the treatment of acute watery diarrhea in children. N Engl J Med. 2000;343:463–467. doi: 10.1056/NEJM200008173430703. [DOI] [PubMed] [Google Scholar]
- 87.Wang HH, Shieh MJ, Liao KF. A blind, randomized comparison of racecadotril and loperamide for stopping acute diarrhea in adults. World J Gastroenterol. 2005;11:1540–1543. doi: 10.3748/wjg.v11.i10.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schiller LR, Santa Ana CA, Morawski SG, Fordtran JS. Mechanism of the antidiarrheal effect of loperamide. Gastroenterology. 1984;86:1475–1480. [PubMed] [Google Scholar]
- 89.Stoll R, Ruppin H, Domschke W. Calmodulin-mediated effects of loperamide on chloride transport by brush border membrane vesicles from human ileum. Gastroenterology. 1988;95:69–76. doi: 10.1016/0016-5085(88)90292-2. [DOI] [PubMed] [Google Scholar]
- 90.Li ST, Grossman DC, Cummings P. Loperamide therapy for acute diarrhea in children: systematic review and meta-analysis. PLoS Med. 2007;4:e98. doi: 10.1371/journal.pmed.0040098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Riddle MS, Arnold S, Tribble DR. Effect of adjunctive loperamide in combination with antibiotics on treatment outcomes in traveler's diarrhea: a systematic review and meta-analysis. Clin Infect Dis. 2008;47:1007–1014. doi: 10.1086/591703. [DOI] [PubMed] [Google Scholar]
- 92.Johnson PC, Ericsson CD, DuPont HL, Morgan DR, Bitsura JA, Wood LV. Comparison of loperamide with bismuth subsalicylate for the treatment of acute travelers' diarrhea. JAMA. 1986;255:757–760. [PubMed] [Google Scholar]
- 93.DuPont HL, Hornick RB. Adverse effect of lomotil therapy in shigellosis. JAMA. 1973;226:1525–1528. [PubMed] [Google Scholar]
- 94.Bos J, Smithee L, McClane B, Distefano RF, Uzal F, Songer JG, Mallonee S, Crutcher JM. Fatal necrotizing colitis following a foodborne outbreak of enterotoxigenic Clostridium perfringens type A infection. Clin Infect Dis. 2005;40:e78–e83. doi: 10.1086/429829. [DOI] [PubMed] [Google Scholar]
- 95.Koo HL, Koo DC, Musher DM, DuPont HL. Antimotility agents for the treatment of Clostridium difficile diarrhea and colitis. Clin Infect Dis. 2009;48:598–605. doi: 10.1086/596711. [DOI] [PubMed] [Google Scholar]
- 96.DuPont HL, Ericsson CD, DuPont MW, Cruz Luna A, Mathewson JJ. A randomized, open-label comparison of nonprescription loperamide and attapulgite in the symptomatic treatment of acute diarrhea. Am J Med. 1990;88:20S–3S. doi: 10.1016/0002-9343(90)90271-e. [DOI] [PubMed] [Google Scholar]
- 97.Portnoy BL, DuPont HL, Pruitt D, Abdo JA, Rodriguez JT. Antidiarrheal agents in the treatment of acute diarrhea in children. JAMA. 1976;236:844–846. [PubMed] [Google Scholar]
- 98.Dinleyici EC PROBAGE Study Group. Vandenplas Y. Lactobacillus reuteri DSM 17938 effectively reduces the duration of acute diarrhoea in hospitalised children. Acta Paediatr. 2014;103:e300–5. doi: 10.1111/apa.12617. [DOI] [PubMed] [Google Scholar]
- 99.Freedman SB, Ali S, Oleszczuk M, Gouin S, Hartling L. Treatment of acute gastroenteritis in children: an overview of systematic reviews of interventions commonly used in developed countries. Evid Based Child Health. 2013;8:1123–1137. doi: 10.1002/ebch.1932. [DOI] [PubMed] [Google Scholar]
- 100.Allen SJ, Martinez EG, Gregorio GV, Dans LF. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010:CD003048. doi: 10.1002/14651858.CD003048.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shane AL, Cabana MD, Vidry S, Merenstein D, Hummelen R, Ellis CL, Heimbach JT, Hempel S, Lynch SV, Sanders ME, Tancredi DJ. Guide to designing, conducting, publishing and communicating results of clinical studies involving probiotic applications in human participants. Gut Microbes. 2010;1:243–253. doi: 10.4161/gmic.1.4.12707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Buydens P, Debeuckelaere S. Efficacy of SF 68 in the treatment of acute diarrhea. A placebo-controlled trial. Scand J Gastroenterol. 1996;31:887–891. doi: 10.3109/00365529609051997. [DOI] [PubMed] [Google Scholar]
- 103.Wunderlich PF, Braun L, Fumagalli I, D'Apuzzo V, Heim F, Karly M, Lodi R, Politta G, Vonbank F, Zeltner L. Double-blind report on the efficacy of lactic acid-producing Enterococcus SF68 in the prevention of antibiotic-associated diarrhoea and in the treatment of acute diarrhoea. J Int Med Res. 1989;17:333–338. doi: 10.1177/030006058901700405. [DOI] [PubMed] [Google Scholar]
- 104.Mitra AK, Rabbani GH. A double-blind, controlled trial of bioflorin (Streptococcus faecium SF68) in adults with acute diarrhea due to Vibrio cholerae and enterotoxigenic Escherichia coli. Gastroenterology. 1990;99:1149–1152. doi: 10.1016/0016-5085(90)90638-h. [DOI] [PubMed] [Google Scholar]
- 105.Kochan P, Chmielarczyk A, Szymaniak L, Brykczynski M, Galant K, Zych A, Pakosz K, Giedrys-Kalemba S, Lenouvel E, Heczko PB. Lactobacillus rhamnosus administration causes sepsis in a cardiosurgical patient--is the time right to revise probiotic safety guidelines? Clin Microbiol Infect. 2011;17:1589–1592. doi: 10.1111/j.1469-0691.2011.03614.x. [DOI] [PubMed] [Google Scholar]
- 106.Rijkers GT, Bengmark S, Enck P, Haller D, Herz U, Kalliomaki M, Kudo S, Lenoir-Wijnkoop I, Mercenier A, Myllyluoma E, Rabot S, Rafter J, Szajewska H, Watzl B, Wells J, Wolvers D, Antoine JM. Guidance for substantiating the evidence for beneficial effects of probiotics: current status and recommendations for future research. J Nutr. 2010;140:671S–676S. doi: 10.3945/jn.109.113779. [DOI] [PubMed] [Google Scholar]
- 107.Ouwehand AC. A review of dose-responses of probiotics in human studies. Benef Microbes. 2017;8:143–151. doi: 10.3920/BM2016.0140. [DOI] [PubMed] [Google Scholar]
- 108.Chapman CM, Gibson GR, Rowland I. Health benefits of probiotics: are mixtures more effective than single strains? Eur J Nutr. 2011;50:1–17. doi: 10.1007/s00394-010-0166-z. [DOI] [PubMed] [Google Scholar]
- 109.McFarland LV. Meta-analysis of probiotics for the prevention of traveler's diarrhea. Travel Med Infect Dis. 2007;5:97–105. doi: 10.1016/j.tmaid.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 110.Sazawal S, Hiremath G, Dhingra U, Malik P, Deb S, Black RE. Efficacy of probiotics in prevention of acute diarrhoea: a meta-analysis of masked, randomised, placebo-controlled trials. Lancet Infect Dis. 2006;6:374–382. doi: 10.1016/S1473-3099(06)70495-9. [DOI] [PubMed] [Google Scholar]