Abstract
Background
General anesthesia with neuromuscular blockade may facilitate total shoulder arthroplasty but appears to increase risk of cerebral oxygen desaturation. Cerebral desaturation is undesirable and is a proxy for risk of stroke.
Purposes/Questions
This study tested the hypothesis that cerebral oxygen desaturation occurs frequently during general anesthesia with neuromuscular blockade and positive-pressure ventilation but does not occur with spontaneous ventilation. Correlations were sought among cerebral oxygen saturation, blood pressure, and cardiac index.
Methods
We designed a prospective, observational, cohort study to measure cerebral oxygenation in 25 patients during general anesthesia, both with and without positive-pressure ventilation. Patients undergoing elective shoulder arthroplasty in the sitting position received an arterial catheter, near-infrared spectroscopic measurement of cerebral oxygenation, and non-invasive cardiac output measurement. Moderate hypotension was allowed. Blood pressure was supported as needed with ephedrine or low-dose epinephrine (but avoiding phenylephrine). Hypercapnia (45 to 55 mmHg) was targeted during positive-pressure ventilation.
Results
No cerebral oxygen desaturations occurred, regardless of ventilation mode. Under positive-pressure ventilation, the median (interquartile range: Q1, Q3) cerebral oxygenation was 110% of baseline (104, 113), the mean arterial pressure was 62% of baseline (59, 69), and the cardiac index was 82% of baseline (71, 104). Cerebral oxygenation did not correlate with blood pressure or cardiac index but had moderate correlation with end-tidal carbon dioxide. No strokes occurred.
Conclusions
There were no signs of inadequate brain perfusion during general anesthesia using paralytic agents. Positive-pressure ventilation with moderate hypotension in the sitting position does not endanger patients, in the context of moderate hypercapnia and hemodynamic support using ephedrine or epinephrine.
Electronic supplementary material
The online version of this article (10.1007/s11420-018-9642-4) contains supplementary material, which is available to authorized users.
Keywords: cerebral oxygenation, shoulder arthroplasty, positive-pressure ventilation
Introduction
Surgeons often request general anesthesia (GA) with neuromuscular paralysis to facilitate total shoulder arthroplasty (TSA), but anesthesiologists may have concerns about the management of GA in the sitting position. In particular, GA may increase risk of complications such as stroke [14, 19]. The low incidence of stroke [5] makes it impractical to perform prospective studies with stroke as an endpoint. Cerebral desaturation event (CDE) incidence, determined by cerebral oximetry [15], is often used as a proxy for risk of stroke. Rates of CDEs can be as high as 80% during sitting-position GA [14]. The incidence of CDEs during GA has been reported at 57%, compared to 0% CDEs during nerve block with sedation [7]. The studies that demonstrate a higher incidence of CDE with GA used positive-pressure ventilation (PPV). The primary goal of the current study is to determine whether or not PPV is a risk factor for CDE. This is an important question because if PPV makes patients more likely to have a stroke, then surgeons and anesthesiologists could limit duration or frequency of patient exposure to PPV.
It is also important to consider the management of blood pressure in the sitting position. Surgeons may request a lowering of the blood pressure, but anesthesiologists may worry about the safety of hypotension. Many authorities recommend avoiding even mild hypotension [4, 19]. However, at some centers, hypotension is utilized without obvious difficulty in the sitting position [6, 9, 11, 16, 26, 27]. PPV may increase the hypotension often associated with GA in the sitting position. The association between GA and CDEs could be due to GA-associated hypotension, since hypotension is a potential cause of cerebral ischemia [2, 10, 22]. The best way to determine adequacy of organ perfusion is debated. Mean arterial pressure (MAP) is often advocated, but cardiac index (CI) may be a better predictor of perfusion [8, 18, 24, 25]. For example, phenylephrine increases blood pressure but decreases cardiac output and regional cerebral oxygen saturation (SctO2) [12]. GA with PPV may promote cerebral desaturation by decreasing cardiac index. The sitting position is classically thought to cause a decrease in CI [20], but not all studies show a statistically significant decline [23].
Other reasons that PPV could impair cerebral blood flow include PPV-associated elevation of central venous pressure (thereby decreasing cerebral perfusion pressure) or PPV-associated hyperventilation. Avoiding hyperventilation was recently found to improve SctO2 among sitting-position shoulder arthroscopy patients [17].
Our primary study hypothesis was that CDEs occur frequently during GA with PPV but not during GA with spontaneous ventilation. We had three goals. First, we sought to determine whether PPV is associated with CDEs during GA in the sitting position. Second, we sought to determine whether decreases in blood pressure and/or decreases in CI correlate with CDE occurrence. Third, we sought to determine the incidence of stroke in this patient study group using the Canadian Neurological Scale. If CDEs are correlated with ventilation mode, blood pressure, or CI, it follows that patients could benefit from modification of these intra-operative risk factors.
Methods
We designed a prospective, observational, cohort study to determine the effects of spontaneous ventilation vs. PPV on CDE rates, MAP, and CI. Patients would receive GA via a laryngeal mask airway, with and without PPV. This single-center study was approved by the Institutional Review Board (IRB) of the Hospital for Special Surgery (an orthopedics teaching hospital) and registered at clinicaltrials.gov (#NCT02198183). Written informed consent was obtained from each patient. Anesthesiologists were blinded to CI and SctO2.
CDE rates were determined in each patient both with and without PPV, allowing patients to serve as their own controls. Moderate hypotension was allowed, but blood pressure was supported as needed with ephedrine or low-dose epinephrine. The primary outcome was the incidence of CDEs, defined as a reduction in SctO2 greater than 20% from baseline that lasts for a minimum of 90 s. The 90-s duration was used to reduce false positives. In a similar study that defined CDEs as a reduction in SctO2 greater than 20% for any duration, the median CDE duration was a physiologically questionable 30 s (one measurement time period), suggesting that many were artifacts [27]. Secondary outcomes included CI and MAP.
The inclusion criteria applied were patient age of 18 to 99 years, elective shoulder arthroplasty in the sitting position, participating surgeon, participating anesthesiologist, planned GA, planned interscalene nerve block, and planned arterial catheter. The exclusion criteria were contraindications to GA or regional anesthesia, indications for an endotracheal tube, body mass index (BMI) of 30 or more, ejection fraction of less than 50%, current stress test positive for ischemia, current signs or symptoms of myocardial ischemia, intolerance to study medications, lack of facility with English, or a history of transient ischemic attack, stroke, or significant pulmonary disease (restrictive or obstructive).
Height, weight, age, American Society of Anesthesiologists physical status, and coexisting diseases were recorded. Pre-operative neurologic screening used the Canadian Neurological Scale, an instrument validated for measurement of deficits from acute strokes; low scores indicate greater deficit [3]. This baseline was compared to the value obtained on the patient’s first post-operative day. Data were recorded and managed through password-protected electronic data capture tools hosted at the Clinical and Translational Science Center (CTSC) at Weill Cornell Medical College (New York, NY). Research Electronic Data Capture (REDCap) is a secure, web-based application that supports data capture for research studies.
Cerebral oxygenation was measured using the FORE-SIGHT ELITE™ Absolute Tissue Oximetry Monitor (CasMed, Branford, CT, USA). CI was determined with the NICOM™ Monitor (Cheetah Medical Inc., Newton, MA, USA). Pre-operatively, patients received FORE-SIGHT sensors on either side of the forehead and NICOM sensors bilaterally on the upper and lower quadrants of the back. Following sedation but before the induction of anesthesia, the electrodes were connected to their respective machines. Sensors were removed prior to transfer to the recovery room. SctO2 measurements were recorded every 2 s, MAP, pulse oximetry (SpO2), systolic blood pressure (SBP), and heart rate measurements every 1 s, and CI, CO, and stroke volume measurements every 30 s.
Participating patients received a standardized anesthetic: GA, interscalene nerve block, routine monitors, and an arterial catheter. Prior to surgery, sedation was administered and an ultrasound-guided interscalene nerve block (0.25% bupivacaine 20 cc, with preservative-free dexamethasone 3 mg/30 cc) performed. GA was induced with propofol. A laryngeal mask airway was placed. Anesthesia was maintained with propofol infusions, nitrous oxide (30 to 60%), and isoflurane (0.2 to 0.6%).
Blood pressure management goals were MAP value of over 60 mmHg and SBP of over 100 mmHg, using a radial arterial catheter transduced at the level of the external auditory meatus. Blood pressure goals were modified as clinically appropriate, depending on the patient’s comorbidities, baseline blood pressure, and surgical requests. The vasoactive therapies of choice were epinephrine infusions (4 μg/mL) or ephedrine boluses (5 mg/mL). If epinephrine or ephedrine were not tolerated (e.g., tachycardia, ectopy), the protocol allowed addition of a phenylephrine infusion (40 μg/mL). Phenylephrine was placed in a tertiary role because of concerns that alpha-adrenergic effects would reduce CI and SctO2 [12].
Spontaneous ventilation was used until surgical request for “relaxation,” when vecuronium was administered (0.06 mg/kg IV), and PPV was initiated (with a rate of 12 to 16 breaths/m, pressure inspired value of 10 to 15 cm H2O, end-tidal carbon dioxide goal of 45 to 55 mmHg). Paralysis was maintained as needed with additional vecuronium (1 to 2 mg, as needed).
Statistical Analysis
CDE rates in similar studies were 56.7 and 80.3% for patients undergoing PPV, but 0% with sedation and spontaneous ventilation [7, 14]. No previous studies were found that described CDE rates under GA with spontaneous ventilation. The study was powered to detect a slightly conservative 52% vs. 0% incidence of CDEs during PPV and spontaneous ventilation, respectively (Table 1). Using McNemar’s test for paired nominal data, 25 patients would provide 80% power at a two-sided alpha of 0.05 to detect this difference. Initially, it was planned to enroll 30 patients to account for exclusions and incomplete data. However, after enrollment of 20 patients, five had incomplete data due to instrument malfunction or use of an endotracheal tube. IRB approval was obtained to enroll up to 40 patients until the goal of 25 patients with usable data was reached. No data analysis was performed prior to completion of final enrollment; the decision to recruit additional patients was based on maintaining power.
Table 1.
Assumptions for power analysisa
| No PPV: 0% CDE PPV: 52% CDE |
CDE with PPV No. (%) | ||
|---|---|---|---|
| Yes | No | ||
| CDE with spontaneous ventilation. No. (%) | Yes | 0 (0%) | 0 (%) |
| No | 13 (52%) | 12 (48%) | |
PPV positive-pressure ventilation, CDE cerebral desaturation event
aExplanation of power analysis
Continuous variables are presented as means with standard deviations or medians with first and third quartiles, depending on the distribution of the data. Baseline was defined as the time between sedation start and laryngeal mask airway insertion. Apparent artifacts in the MAP, carbon dioxide, and SpO2 data were removed manually. When plausible invasive MAP values were not available, data were supplemented with non-invasive MAP values from the same patient. Hemodynamic measurements were compared between time periods (baseline, GA without PPV, GA with PPV) using paired t tests. The correlations between longitudinal measurements of pairs of hemodynamic variables were assessed via bivariate linear mixed modeling with an unstructured covariance structure. Correlation coefficients are presented as point estimates with 95% bootstrapped confidence intervals calculated from 1000 resamples. Correlation coefficients were calculated using one observation per minute to decrease computational burden. Categorical variables are presented as counts and percentages. The a priori data analysis plan dictated that the primary outcome (occurrence of at least one CDE) would be compared between GA without PPV and GA with PPV using McNemar’s test. However, with a 0% incidence of CDEs during both PPV and spontaneous ventilation, this test could not be performed. As an exploratory analysis, the incidence of CDEs was assessed using a more lenient definition (i.e., any length of time with greater than 20% reduction in SctO2 from baseline or any SctO2 reading less than 50%) both with and without patients excluded from the main analyses. Statistical analyses were performed using SAS, version 9.3 (SAS Institute, Cary, NC, USA). All statistical hypothesis tests were two-sided.
Results
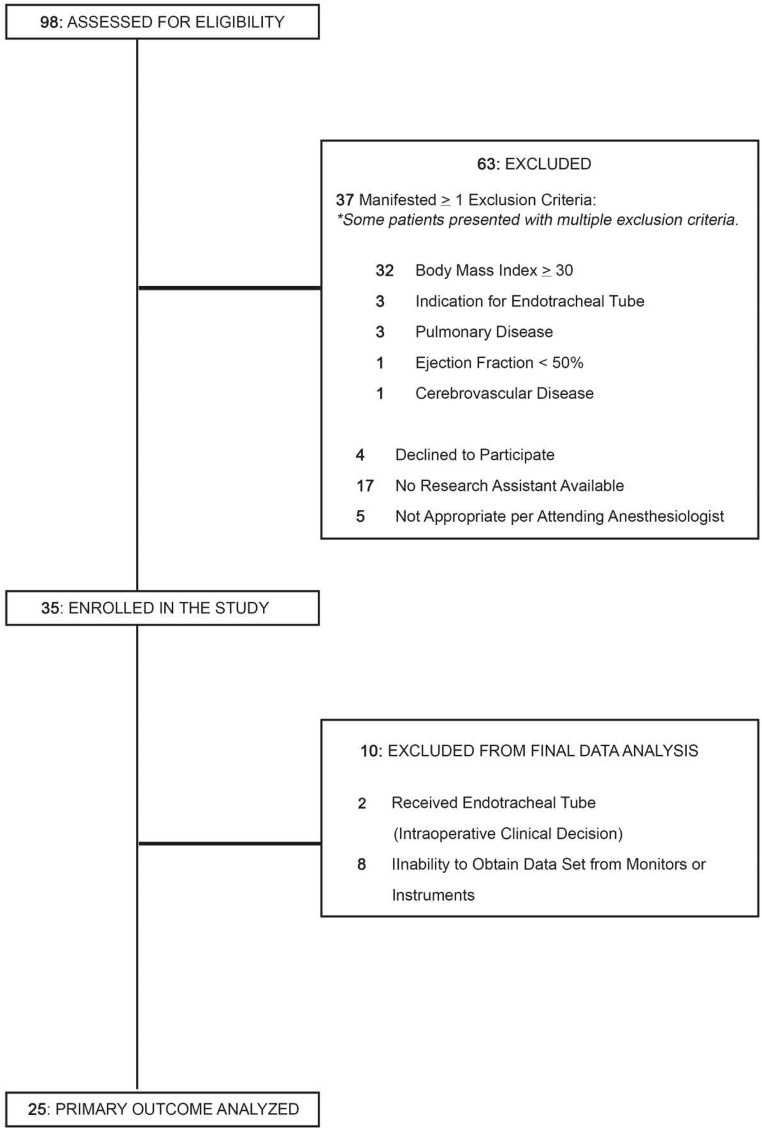
Thirty-five patients were enrolled (from July 2014 to May 2015), and 25 were included for data analysis (Fig. 1). Two patients were withdrawn due to the need for an endotracheal tube, which precluded spontaneous ventilation. Eight patients had problems with monitors or instruments, leading to their exclusion for inadequate data. Demographic characteristics of included patients are presented in Table 2, with baseline values for vital signs in Table 3. Most patients received vasoactive therapy with epinephrine and/or ephedrine, but eight did not require vasoactive therapy (Table 4). During GA without PPV, a median of 12% of the time was spent below the target MAP of 60 mmHg. During GA with PPV, a median of 11% of the time was spent below the target MAP.
Fig. 1.
Patient flowchart for prospective, cohort study. Of the 35 patients enrolled, 2 were withdrawn for placement of an endotracheal tube and 8 for monitor or instrument problems. Data were analyzed from 25 patients.
Table 2.
Patient characteristics
| Characteristics | Mean (SD) or number (%) |
|---|---|
| Age (years) | 67 (8) |
| Sex | |
| Male | 14 (56%) |
| Female | 11 (44%) |
| Height (cm) | 172 (10) |
| Weight (kg) | 76 (11) |
| BMI (kg/m2) | 26 (2) |
| Ethnicity | |
| Not Hispanic or Latino | 25 (100%) |
| Hispanic or Latino | 0 |
| Race | |
| Black | 1 (4%) |
| White | 24 (96%) |
| ASA physical status | |
| I | 0 |
| II | 22 (88%) |
| III | 3 (12%) |
| Smoking status | |
| Nonsmoker | 10 (40%) |
| Former smoker | 13 (52%) |
| Current smoker | 2 (8%) |
| Comorbidities | |
| CAD | 2 (8%) |
| Atrial fibrillation | 2 (8%) |
| Diabetes mellitus | 2 (8%) |
| Hypertension | 12 (48%) |
| COPD | 2 (8%) |
BMI body mass index, ASA American Society of Anesthesiologists, CAD coronary artery disease, COPD chronic obstructive pulmonary disease
Table 3.
Baseline dataa
| Characteristics | Mean (SD) |
|---|---|
| SpO2 (%) | 98 (2) |
| SctO2 (%) | 71 (4) |
| SBP (NIBP) (mmHg) | 140 (26) |
| MAP (NIBP) (mmHg) | 102 (18) |
| Cardiac output (L/min) | 5.6 (1.2) |
| Cardiac index (L/min/m2) | 3.0 (0.5) |
| Heart rate (beats/min) | 74 (11) |
| Stroke volume (mL) | 76 (19) |
SpO2 pulse oximetry, SctO2 regional cerebral tissue oxygen saturation, SBP systolic blood pressure, NIBP non-invasive blood pressure, MAP mean arterial pressure
aBaseline values were calculated as the average measurement between start of sedation and insertion of LMA
Table 4.
Intra-operative data
| Characteristics | Mean (SD) or number (%) |
|---|---|
| Anesthesia time (min) | 170 (23) |
| LMA time (min) | 134 (22) |
| PPV (min) | 72 (24) |
| Duration of hypotension (min, % of intra-operative time) | |
| GA without PPV | 8 (SD 10; 12%) |
| GA with PPV | 20 (SD 21; 25%) |
| ETCO2 (mmHg) | |
| GA without PPV | 44 ± 5 |
| GA with PPV | 45 ± 6 |
| Fluids | |
| Lactated Ringer’s (mL) | 1408 ± 227 |
| Other | 0 |
| EBL (mL) | 154 ± 52 |
| Vasoactive compounds | |
| Not administered | 8 (32%) |
| Administered | |
| Epinephrine only | 6 (24%) |
| Ephedrine only | 9 (36%) |
| Epinephrine + ephedrine | 2 (8%) |
ETCO2 end-tidal carbon dioxide, LMA laryngeal mask airway, GA general anesthesia, PPV positive-pressure ventilation, EBL estimated blood loss
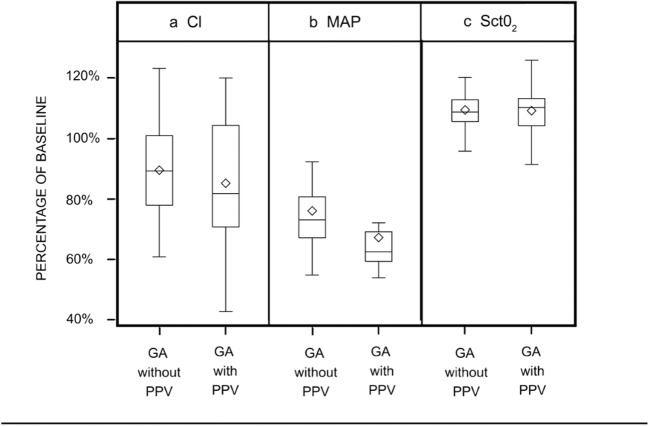
There were no CDEs under either spontaneous ventilation or PPV, using study criteria for CDE (more than 20% SctO2 reduction from baseline lasting 90 s or longer) (Table 5). The planned McNemar’s test could not be performed, given the 0% incidence. To explore the effect of the CDE definition, a post hoc sensitivity analysis was performed using a relaxed definition (any occurrence of more than 20% reduction in SctO2 from baseline or any SctO2 reading of less than 50%), analyzing all patients (whether included or excluded). One included patient had a 6-s desaturation during PPV. The short duration and abrupt resolution of this event is consistent with an unstable signal and does not seem to be an actual physiologic event. One excluded patient had a 30-s desaturation during spontaneous ventilation, also likely due to an unstable signal. Thus, the absence of CDEs was due neither to the exclusion of some patients for monitor problems nor to the use of a 90-s cut-off. Normalized composite data failed to show an effect of PPV on cerebral oxygenation (Fig. 2). Under PPV, the median (interquartile range: Q1, Q3) cerebral oxygenation was 110% of baseline (104, 113), the mean arterial pressure was 62% of baseline (59, 69), and the cardiac index was 82% of baseline (71, 104). Patient data were normalized to baseline and grouped into GA without and with PPV. The mean SctO2 values (with 95% confidence interval) were 0 (− 1 to 2) percentage points different during GA without PPV than during GA with PPV (p = 0.706). The MAP values were 9 (95% confidence interval, 6 to 12) percentage points higher during GA without PPV than during GA with PPV (p < 0.001). The CI index values were 4 (95%, − 2 to 11) percentage points higher during GA without PPV than during GA with PPV (p = 0.171).
Table 5.
Observed cerebral desaturation eventsa
| No PPV: 0% CDE PPV: 0% CDE |
CDE with PPV No. (%) | ||
|---|---|---|---|
| Yes | No | ||
| CDE with spontaneous ventilation. No. (%) | Yes | 0 (0%) | 0 (0%) |
| No | 0 (0%) | 0 (0%) | |
PPV positive-pressure ventilation, CDE cerebral desaturation event
aExplanation of power analysis
Fig. 2.
Composite analyses of physiologic data for all 25 patients. Data grouped into time periods of general anesthesia without positive-pressure ventilation (GA without PPV) and general anesthesia with positive-pressure ventilation (GA with PPV). All hemodynamic measurements normalized to baseline. CI cardiac index, MAP mean arterial pressure, SctO2 regional cerebral tissue oxygen saturation, GA general anesthesia, PPV positive-pressure ventilation.
Correlation analysis using mixed effects modeling showed that cerebral oxygen saturation was not influenced by MAP or CI (Table 6). Comparison of hemodynamic values (not normalized) to baseline showed that induction of GA decreased MAP and slightly decreased CI but increased SctO2. The differences in means (with 95% confidence intervals) were the following: MAP: GA without PPV, − 27 mmHg (− 33 to − 20), p < 0.001 vs. baseline; GA with PPV, − 36 mmHg (− 43 to − 29), p < 0.001 vs. baseline.
Table 6.
Correlation analysis
| Mixed effects modeling | Number | Correlation coefficienta (95% confidence interval) |
|---|---|---|
| SctO2 vs. MAP | 3215 | 0.03 (− 0.08, 0.14) |
| SctO2 vs. CI | 1674 | 0.19 (− 0.12, 0.34) |
| SctO2 vs. SpO2 | 3217 | 0.17 (− 0.11, 0.40) |
| SctO2 vs. ETCO2 | 3087 | 0.48 (0.32, 0.61) |
| MAP vs. CI | 3161 | 0.12 (0.03, 0.22) |
| MAP vs. CI (with epinephrine) | 1398 | 0.06 (− 0.06, 0.16) |
| MAP vs. CI (without epinephrine) | 1763 | 0.21 (0.04, 0.39) |
SpO2 pulse oximetry, SctO2 regional cerebral tissue oxygen saturation, ETCO2 end tidal carbon dioxide, MAP mean arterial pressure
aCorrelation coefficients can range from − 1 to 1, where − 1 indicates a perfect negative correlation and 1 indicates a perfect positive correlation. Correlation strength can be interpreted as follows:
0.00–0.19 = very weak; 0.20–0.39 = weak; 0.40–0.59 = moderate; 0.60–0.79 = strong; 0.80–1.0 = very strong
CI: GA without PPV, − 0.3 L/min/m2 (− 0.6 to − 0.1), p = 0.008; GA with PPV – 0.5 L/min/m2 (− 0.7 to − 0.2), p = 0.003.
SctO2: GA without PPV, 7% (5 to 9), p < 0.001; GA with PPV 6% (4 to 9), p < 0.001.
Very weak correlations were noted for the following: SctO2 vs. MAP, SctO2 vs. CI, SctO2 vs. SpO2, and MAP vs. CI. Moderate correlation was found for SctO2 vs. end-tidal carbon dioxide. As an exploratory post hoc analysis, subgroup analysis was performed for MAP vs. CI. Patients who received intra-operative epinephrine were analyzed separately from those who did not. Weak or very weak correlation was found in both groups.
The median change in stroke scores (post–pre) was 0 (0, 0) (interquartile range: Q1, Q3), indicating absence of stroke. No clinical indications of post-operative stroke were reported. No serious adverse events were reported to the IRB. All patients received a pre-operative Canadian Neurological Scale score of 7.5 or more and a post-operative score of 10 or more. Patients were followed until hospital discharge, typically 2 or 3 days after the operation.
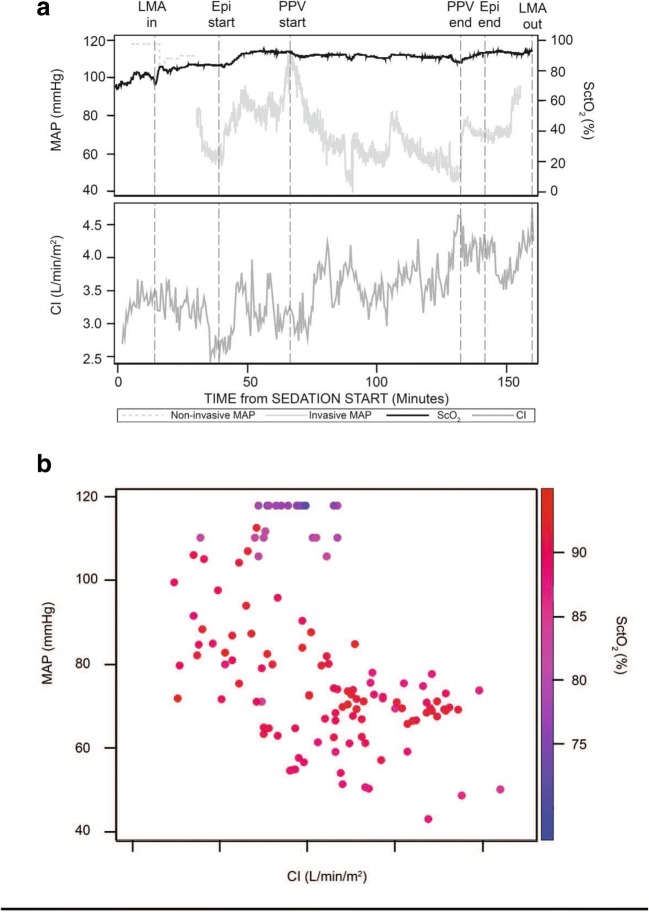
Data from a single typical operation are portrayed in Fig. 3, to illustrate both the clinical management and composite results. In this patient (Fig. 3a), SctO2 increased after the induction of GA, presumably due to decreased cerebral metabolism and increased fraction of inspired oxygen. MAP and CI fell after the induction of GA, but epinephrine increased both MAP and CI. There were no cerebral oxygen desaturations from baseline, even at MAP lower than 60 mmHg (Fig. 3b). The relationship between MAP, CI, and the SctO2 for this patient is depicted in color. Each data point represents 30 s, the minimum time interval for CI measurements. Although hypotension was common, hypotension was not apparently associated with low CI. Pre-induction values are clustered at the top of the figure, with high MAP but relatively low SctO2 values in the 70s.
Fig. 3.
a Relationship between cerebral oximetry, mean arterial blood pressure, and cardiac index over time. Data are from a single typical patient to provide an example of the clinical course. Events are indicated on the top horizontal axis: placement and removal of the airway, administration of epinephrine, and start and stop of positive-pressure ventilation. b Relationships among mean arterial blood pressure, cardiac index, and cerebral oximetry depicted as a heat map. Data provided are from a single, typical patient. Mean arterial blood pressure and cardiac index are plotted in graphic coordinates. Cerebral oximetry is represented by a color gradient. Each data point represents 30 s, the minimum time interval for cardiac index measurements. MAP mean arterial pressure, CI cardiac index, SctO2 regional cerebral tissue oxygenation, LMA laryngeal mask airway, Epi epinephrine, PPV positive-pressure ventilation.
Discussion
As predicted, CDEs did not occur with spontaneous ventilation, but contrary to previous publications and our study hypothesis, no CDEs were observed during GA with PPV. Because of concerns that hypotension may cause cerebral desaturation, the study also explored relationships between MAP, CI, and SctO2. GA was associated with a lower MAP and a slightly lower CI. SctO2 was elevated over baseline. Very weak correlations were found among MAP, CI, and SctO2. Thus, using this anesthesia protocol, within the described MAP ranges, changes in MAP were not associated with cerebral oxygen desaturation. No strokes were noted.
Study limitations include the use of SctO2 as a surrogate outcome for risk of stroke. SctO2 is frequently used in this fashion, in part because using stroke as an outcome would require a prohibitively large sample size. By placing the arterial line transducer at the external auditory meatus, the study essentially assumed the “waterfall physiology” of cerebral perfusion. This is the more conservative choice. A larger study with a broader range of patient comorbidities would likely generate more definitive safety conclusions. The power analysis, based on the literature, assumed 52% of patients with PPV would experience CDEs, but in fact, no CDEs were noted. Some patients enrolled into the study were not included in the primary analysis, mostly due to difficulties with monitors. However, exploratory analysis of all patients enrolled reached very similar conclusions. To protect patients and conform to our usual practices, the protocol employed measures intended to promote cerebral perfusion; different results may have been obtained with different protocols. The study protocol did not dictate a narrow range of acceptable blood pressures, and so it is possible that different results would be observed had the intra-operative blood pressures been more uniform.
That no CDEs were observed was unexpected. Reported rates of CDEs during sitting GA vary greatly: 18, 46, 57, and 71% [1, 7, 21, 24]. Advanced age and more extensive comorbidity burden are risk factors for CDEs in the sitting position [26, 27]. The CDE rate during sitting-position shoulder arthroscopy under nerve block plus sedation was 10% [27]. It was anticipated that arthroplasty patients would have a higher CDE rate than arthroscopy patients, because the latter tend to be younger, healthier patients, receiving brief non-invasive procedures without GA. Recently, a CDE rate of 56% was reported among sitting normotensive patients (MAP target, within 20% of baseline) with an end-tidal carbon dioxide level of 30 to 32 mmHg, but a 9% rate of CDEs occurred in the same patients with carbon dioxide levels of 40 to 42 mmHg [13]. During PPV in the current study, the CDE rate was 0 with relative hypercapnia (mean carbon dioxide levels, 46 mmHg) but also relative hypotension (mean MAP, 62% of baseline).
Moderate hypotension was allowed in this study. MAP values of over 60 and SBP levels of over 100 mmHg were allowed, using therapy with ephedrine or low-dose epinephrine. It has been suggested that hypotension in the sitting position could reduce cerebral perfusion, increase CDE rates, and increase cerebrovascular accident risk [13, 19]. Surgery in the beach-chair position is associated with diminished cerebral autoregulation [8]. In one study, the majority of CDEs occurred during MAPs more than 20% below baseline [13], but another study failed to find an association between blood pressure and cerebral desaturation [24]. Phenylephrine can be used to increase MAP [7, 13, 21, 24], but phenylephrine also decreases cardiac output and cerebral oxygenation [12]. Administration of phenylephrine in the sitting position increased MAP, decreased CI, and did not benefit SctO2, compared to control [23].
In conclusion, we found no evidence that PPV caused cerebral oxygen desaturation. No CDEs were observed under GA in the sitting position using the described protocol, contrary to many previous publications. Cerebral oxygenation did not correlate with MAP or CI. The results indicate that cerebral perfusion is adequate under GA (with or without PPV) during the use of moderate hypotension and moderate hypercapnia, with support of cardiac output using ephedrine or epinephrine.
Electronic Supplementary Material
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1225 kb)
(PDF 1224 kb)
(PDF 1224 kb)
Acknowledgments
We thank George Birch for his assistance with manuscript writing, editing, submission, and revision.
Funding
This study was funded by the Department of Anesthesiology—Research and Education Fund, Hospital for Special Surgery (New York, NY). The REDCap electronic data capture tools were funded by the Clinical and Translational Science Center Grant, National Center for Advancing Translational Science, National Institutes of Health (#UL1TR000457). CAS Medical Systems, Inc. (Branford, CT, USA) provided FORE-SIGHT cerebral oximetry monitors at no cost and probes at research cost for the study. Cheetah Medical, Inc. (Vancouver, WA, USA) provided NICOM hemodynamic monitor and probes at research cost.
Compliance with Ethical Standards
Conflict of Interest
Richard L. Kahn, MD, Yi Lin, MD, PhD, Enrique A. Goytizolo, MD, Michael A. Gordon, MD, Yuliya Gadulov, MD, Sean Garvin, MD, Kara Fields, MS, Amanda Goon, BA, and Isabel Armendi, BSc, declare that they have no conflicts of interest. Jacques T. YaDeau, MD, PhD, reports receiving grants and personal fees from Mallinckrodt, outside the submitted work. David M. Dines, MD, and Edward V. Craig, MD, MPH, both report receiving royalties and other fees from Zimmer Biomet, during the conduct of this study.
Human/Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013.
Informed Consent
Informed consent was obtained from all patients included in this study.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Footnotes
Level of Evidence: Level II
References
- 1.Aguirre J, Borgeat A, Trachsel T, Cobo Del Prado I, De Andres J, Buhler P. Cerebral oxygenation in patients undergoing shoulder surgery in beach chair position: comparing general to regional anesthesia and the impact on neurobehavioral outcome. Rev Esp Anestesiol Reanim. 2014;61(2):64–72. doi: 10.1016/j.redar.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Bijker JB, Persoon S, Peelen LM, et al. Intraoperative hypotension and perioperative ischemic stroke after general surgery. Anesthesiology. 2012;116(3):658–664. doi: 10.1097/ALN.0b013e3182472320. [DOI] [PubMed] [Google Scholar]
- 3.Cote R, Battista RN, Wolfson C, Boucher J, Adam J, Hachinski V. The Canadian Neurological Scale: validation and reliability assessment. Neurology. 1989;39(5):638–643. doi: 10.1212/WNL.39.5.638. [DOI] [PubMed] [Google Scholar]
- 4.Drummond JC. Stroke and intraoperative hypotension: to sleep, perchance to stroke—ay, there’s the rub. Anesth Analg. 2016;123(4):814–815. doi: 10.1213/ANE.0000000000001572. [DOI] [PubMed] [Google Scholar]
- 5.Friedman DJ, Parnes NZ, Zimmer Z, Higgins LD, Warner JP. Prevalence of cerebrovascular events during shoulder surgery and association with patient position. Orthopedics. 2009;32:256. doi: 10.3928/01477447-20091103-53. [DOI] [PubMed] [Google Scholar]
- 6.Gillespie R, Shishani Y, Streit J, et al. The safety of controlled hypotension for shoulder arthroscopy in the beach-chair position. J Bone Joint Surg Am. 2012;94:1284–1290. doi: 10.2106/JBJS.J.01550. [DOI] [PubMed] [Google Scholar]
- 7.Koh JL, Levin SD, Chehab EL, Murphy GS. Cerebral oxygenation in the beach chair position: a prospective study on the effect of general anesthesia compared with regional anesthesia and sedation. J Shoulder Elbow Surg. 2013;22(10):1325–1331. doi: 10.1016/j.jse.2013.01.035. [DOI] [PubMed] [Google Scholar]
- 8.Laflam A, Joshi B, Brady K, Yenokyan G, Brown C, Everett A, et al. Shoulder surgery in the beach chair position is associated with diminished cerebral autoregulation but no differences in postoperative cognition or brain injury biomarker levels compared with supine positioning: The Anesthesia Patient Safety Foundation Beach Chair Study. Anesth Analg. 2015;120(1):176–185. doi: 10.1213/ANE.0000000000000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JH, Min KT, Chun YM, Kim EJ, Choi SH. Effects of beach-chair position and induced hypotension on cerebral oxygen saturation in patients undergoing arthroscopic shoulder surgery. Arthroscopy. 2011;27(7):889–894. doi: 10.1016/j.arthro.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 10.Mazzon D, Danelli G, Poole D, Marchini C, Bianchin C. Beach chair position, general anesthesia and deliberated hypotension during shoulder surgery: a dangerous combination! Minerva Anestesiol. 2009;75(5):281–282. [PubMed] [Google Scholar]
- 11.McCulloch TJ, Liyanagama K, Petchell J. Relative hypotension in the beach-chair position: effects on middle cerebral artery blood velocity. Anaesth Intensive Care. 2010;38(3):486–491. doi: 10.1177/0310057X1003800312. [DOI] [PubMed] [Google Scholar]
- 12.Meng L, Cannesson M, Alexander BS, et al. Effect of phenylephrine and ephedrine bolus treatment on cerebral oxygenation in anaesthetized patients. Br J Anaesth. 2011;107(2):209–217. doi: 10.1093/bja/aer150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy GS, Szokol JW, Avram MJ, et al. Effect of ventilation on cerebral oxygenation in patients undergoing surgery in the beach chair position: a randomized controlled trial. Br J Anaesth. 2014;113:618–627. doi: 10.1093/bja/aeu109. [DOI] [PubMed] [Google Scholar]
- 14.Murphy GS, Szokol JW, Marymont JH, et al. Cerebral oxygen desaturation events assessed by near-infrared spectroscopy during shoulder arthroscopy in the beach chair and lateral decubitus positions. Anesth Analg. 2010;111(2):496–505. doi: 10.1213/ANE.0b013e3181e33bd9. [DOI] [PubMed] [Google Scholar]
- 15.Ono M, Zheng Y, Joshi B, Sigl JC, Hogue CW. Validation of a stand-alone near infrared spectroscopy system for monitoring cerebral autoregulation during cardiac surgery. Anesth Analg. 2013;116(1):198–204. doi: 10.1213/ANE.0b013e318271fb10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paul JE, Ling E, Lalonde C, Thabane L. Deliberate hypotension in orthopedic surgery reduces blood loss and transfusion requirements: a meta-analysis of randomized controlled trials. Can J Anaesth. 2007;54(10):799–810. doi: 10.1007/BF03021707. [DOI] [PubMed] [Google Scholar]
- 17.Picton P, Dering A, Alexander A, et al. Influence of ventilation strategies and anesthetic techniques on regional cerebral oximetry in the beach chair position. Anesthesiology. 2015;123(4):765–774. doi: 10.1097/ALN.0000000000000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pin-on P, Schroeder D, Munis J. The hemodynamic management of 5177 neurosurgical and orthopedic patients who underwent surgery in the sitting or “beach chair” position without incidence of adverse neurologic events. Anesth Analg. 2013;116(6):1317–1324. doi: 10.1213/ANE.0b013e31828446bb. [DOI] [PubMed] [Google Scholar]
- 19.Pohl A, Cullen DJ. Cerebral ischemia during shoulder surgery in the upright position: a case series. J Clin Anesth. 2005;17:463–469. doi: 10.1016/j.jclinane.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Porter JM, Pidgeon C, Cunningham AJ. The sitting position in neurosurgery: a critical appraisal. Br J Anaesth. 1999;82(1):117–128. doi: 10.1093/bja/82.1.117. [DOI] [PubMed] [Google Scholar]
- 21.Salazar D, Sears BW, Andre J, Tonino P, Marra G. Cerebral desaturation during shoulder arthroscopy: a prospective observational study. Clin Orthop Relat Res. 2013;471:4027–4034. doi: 10.1007/s11999-013-2987-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.So J, Shin WJ, Shim JH. A cardiovascular collapse occurred in the beach chair position for shoulder arthroscopy under general anesthesia—a case report. Korean J Anesthesiol. 2013;64(3):265–267. doi: 10.4097/kjae.2013.64.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soeding PF, Hoy S, Hoy G, Evans M, Royse CF. Effect of phenylephrine on the haemodynamic state and cerebral oxygen saturation during anesthesia in the upright position. Br J Anaesth. 2013;111(2):229–234. doi: 10.1093/bja/aet024. [DOI] [PubMed] [Google Scholar]
- 24.Triplet JJ, Lonetta CM, Levy JC, Everding NG, Moor MA. Cerebral desaturation events in the beach chair position: correlation of noninvasive blood pressure and estimated temporal mean arterial pressure. J Shoulder Elbow Surg. 2015;34:135–137. doi: 10.1016/j.jse.2014.06.041. [DOI] [PubMed] [Google Scholar]
- 25.Ya Deau JT. CORR Insights: hypotensive epidural anesthesia reduces blood loss in pelvic and sacral bone tumor resections. Clin Orthop Relat Res. 2017;475(3):641–642. doi: 10.1007/s11999-016-4900-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ya Deau JT, Casciano M, Liu SS, et al. Stroke, regional anesthesia in the sitting position, and hypotension: a review of 4169 ambulatory surgery patients. Reg Anesth Pain Med. 2011;123(4):765–774. doi: 10.1097/AAP.0b013e318228d54e. [DOI] [PubMed] [Google Scholar]
- 27.Ya Deau JT, Liu SS, Bang H, et al. Cerebral oximetry desaturation during shoulder surgery performed in a sitting position under regional anesthesia. Can J Anaesth. 2011;58(11):986–992. doi: 10.1007/s12630-011-9574-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1225 kb)
(PDF 1224 kb)
(PDF 1224 kb)