Highlights
-
•
A deep knowledge of ocular microbiome could be the key to manage eye disorders.
-
•
Culture-based techniques and molecular analysis often lead to incomplete or biased results.
-
•
Genomics and bioinformatics are novel fields in ophthalmology, with great potential.
-
•
With NGS, it will be possible to identify microbes. But, many challenges still remain.
Keywords: Metagenomics, Ocular infection, NGS (next generation sequencing), Ocular surface, Microbiome
Abstract
Metagenomic analysis was originally associated with the studies of genetic material from environmental samples. But, with the advent of the Human Microbiome Project, it has now been applied in clinical practices. The ocular surface (OS) is the most exposed part of the eye, colonized by several microbial communities (both, OS and environmental) that contribute to the maintenance of the physiological state. Limited knowledge has been acquired on these microbes due to the limitations of conventional diagnostic methods. Emerging fields of research are focusing on Next Generation Sequencing (NGS) technologies to obtain reliable information on the OS microbiome. Currently only pre-specified pathogens can be detected by conventional culture-based techniques or Polymerase Chain Reaction (PCR), but there are conditions to state whether metagenomics could revolutionize the diagnosis of ocular diseases. The aim of this review is to provide an updated overview of the studies involving NGS technology for OS microbiome.
1. Introduction
Human body is populated with an enormous variety of bacteria, archaea, fungi and viruses, which form a commensal, symbiotic and pathogenic community collectively known as human microbiome [1,2]. The estimated amount of microorganisms is in the order of trillions, at least 10 times more than the number of human cells [1,2]. This evidence has increased the interest of the scientific community to understand the role of these microorganisms in a day-to-day life. A foundation step was represented by the Human Microbiome Project (HMP), launched in 2008 by the United States National Institutes of Health, with the aim to reveal and characterize the microbial populations of five main body areas, i.e. gut, mouth, nose, skin and urogenital tract [3,4]. In the same year, the European Commission granted a project called MetaHIT, focused on understanding the correlations between human intestinal microbiota and some disorders, in particular inflammatory bowel disease and obesity (http://www.metahit.eu). Both research programs would have never been possible without the availability of up-to-date knowledge and technology, in particular, metagenomics. Metagenomics refers to the genomic analysis of microorganisms’ populations, based on the development of Next Generation Sequencing (NGS) technology, which overcome the need to separate the genomes or to culture the microbes. Ideally, NGS can detect all the microorganisms present in a clinical sample, producing huge amounts of sequencing data that need to be decoded [5], and has the potential to improve diagnostic yield, as it is inherently unbiased and hypothesis-free [6]. Metagenomics has already shown its efficiency by providing a correlation between the changes in gut microbiota that has found to be associated with several diseases like cancer, obesity, asthma, atherosclerosis and diabetes [[7], [8], [9], [10]].
Researches are also aiming at examining the microbiome of other body areas to understand if microbiome and diseases could be correlated. One of these areas is the ocular surface (OS), the part of the eye that is exposed to the environment. Ocular microbiome could play a key role in the maintaining the physiology of the eye and its alteration could be correlated with eye disorders, for example infections [11]. Thus, Shestopalov et al. started the Ocular Microbiome Project to understand and correlate the ocular microbiome with ocular disorders [12].
The aim of this article is to review the published literature on the application of metagenomics in ophthalmology, especially the knowledge of the OS microbial communities, and the potential clinical relevance of the NGS approach in the diagnosis of OS disorders.
Could metagenomics be the driving force in acquiring deep knowledge of the OS disorders and its effective diagnosis, similar to what has been already found for other body areas?
2. The rise of metagenomics
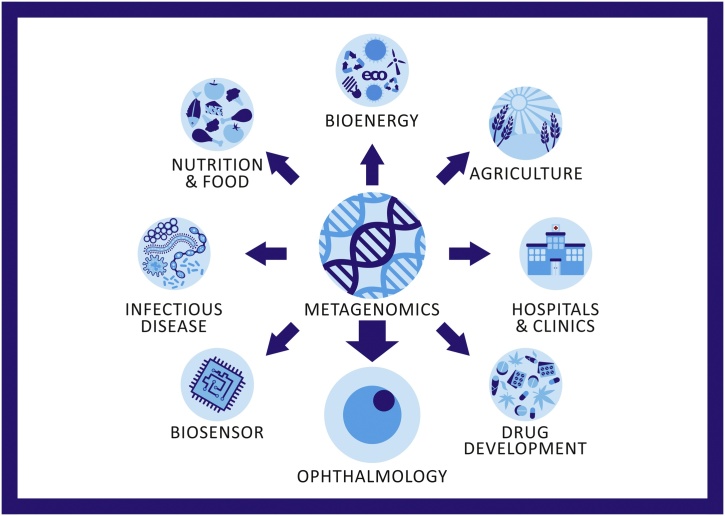
Metagenomics represents a relatively new discipline in genomic analysis. It was used initially for detecting the environmental samples [13] however, it has been recently found to be a useful tool in various areas, including medicine and clinical practices (Fig. 1). Evolution of NGS technology, also known as high-throughput sequencing or third-sequencing generation, allowed its adoption by producing massive sequencing data. Indeed, the previous Sanger sequencing method was a low-throughput approach based on dideoxynucleotide chain termination. Although it was a suitable tool to sequence specific genes or fragments, but as was too laborious and expensive, it was difficult to investigate complex samples due to its sequencing speed, which was only a few thousand nucleotides per week.
Fig. 1.
Schematic representation of different areas of Metagenomics.
The application of NGS allows sequencing from thousands to millions of nucleic acid segments simultaneously in a single run. Thus, allowing to decode important large genomes, such as the human genome. This also allows full taxonomical profiling (“who is it?”) and compare the functions (“what is it doing?”) of microbial communities from different areas, in a short span of time compared to the Sanger method [14].
After sample collection and nucleic acid extraction (DNA or RNA), the term NGS is generically used to indicate the two main sequencing methods: the marker gene sequencing approach (also called targeted-amplicon sequencing) and the shotgun approach. In the former, amplicons from a single conserved gene are produced by Polymerase Chain Reaction (PCR) (library preparation and cluster generation). The conserved gene that is most commonly used is the 16S rRNA, because it is ubiquitous and formed from constant and variable regions that allow the definition of taxa [15]. In addition, a universal target for eukaryotic organisms is the 18S rRNA gene. Moreover, internal transcribed spacer (ITS) regions of rRNA operons are frequently used to identify fungal species [16]. Targeted-amplicon sequencing is used mainly in microbiome analysis with taxonomic purposes. In the shotgun approach, instead, the sequencing is performed across random fragments of all DNA in a given sample and can be used also in case of unknown microbial target. Both methods have advantages and disadvantages. Targeted-amplicon sequencing is usually used to characterize a particular microbial group in a sample, while shotgun is the only possible approach for the identification of previously uncharacterized microbes [17]. Both can be used to detect pathogens, even if sometimes one type of technique is more appropriate than the other. For example, if the etiological agent is suspected to be a virus, shotgun metagenomics is warranted [16], while in the case of a low biomass sample, marker gene metagenomics may be able to sequence the infectious agent more adequately [16].
However, metagenomics does not only sequence, but also interprets the retrieved data and perhaps this is the most critical step. Indeed, the high-throughput capabilities of NGS approach leads to an exponential accumulation of sequence data that need to be interpreted. Hence the requirement to develop increasingly appropriate bioinformatics tools, i.e. specific bioinformatics algorithms that transform raw sequence of NGS signal outputs in suitable and organized information. Complex and computationally expensive data analysis processes are therefore required [5].
3. OS microbiome: before the NGS adoption
The eye is characterized by an external surface made up of mucosal tissues. It is exposed to the environment and it is the first defense line of the eye, which is subjected to constant challenges. Instead the inner part is sterile, with a highly efficient blood-retinal barrier [18]. The ocular surface (OS) consists of conjunctiva, cornea, and tear film. It is the site of possible disorders such as dry eye, local or systemic inflammations (for example blepharitis, conjunctivitis or keratoconjunctivitis), autoimmune diseases and infections.
In 1907 Axenfeld first attempted to characterize the ocular microbiome [19]. Since then many studies have been reported [20,21], but it is still not enough. A deep knowledge of the OS microbiome (exact composition, function, etc.) could be highly relevant for diagnostic purposes or to establish correlations with other diseases, thus understanding the specific causative agent.
Conventional culture-based techniques are still currently used for microbial identification but, due to their limitations, high performance diagnostic techniques are being developed and implemented (PCR and, mostly, NGS). Indeed, with culture-based methods, the incubation and inoculation of the clinical specimens (usually in high volumes) have to be performed on a range of appropriate media. The sensitivity is found to be relatively poor (yield of 40–70%) [22,23] and the probability of false-positive results could be significant [24]. Results could also be biased due to the fast-growing microorganisms, which can be easily cultivated on a standard media [11]. Moreover, studies comparing conventional culture techniques and molecular analysis have shown very often, that results obtained by these methods are incomplete or biased by false-positive data, thus highlighting the limitations of traditional culture-based techniques in terms of sensitivity and reproducibility [21,[25], [26], [27], [28], [29]]. Graham et al. studied the bacterial population of 91 subjects classified as normal or dry eye [30]. The overall bacterial genera identified by culture-based techniques led to the identification of a smaller number of organisms compared to that obtained with PCR and DNA sequencing. Indeed, only Coagulase negative staphylococci and Bacillus sp were identified by culture method while other 8 genera were found by DNA sequencing, including 5 uncultured bacterial genera.
For these reasons, a comprehensive analysis of the microbial diversity is usually possible only through the support of innovative technologies like NGS.
4. OS microbiome: what is currently known?
The microbiome is considered crucial in maintaining the homeostasis of the OS. The impairments are evident in conditions such as keratoprosthesis, antibiotic exposure, infectious states and dry eye [31,32]. As the ocular microbiome and its functions still remain relatively unknown [33], the continuous improvements in NGS technologies could lead to an in-depth characterization of the OS microbial communities and hence serve as a useful tool for the diagnosis of ocular disease [11], specifically ocular infections. One of the main limitations in the diagnosis of ocular disorders has always been the difficulty in obtaining large amount of samples, due to the delicate anatomy and the eye size. NGS-based approach would allow characterization of all the genomic content, even when the available sample size is limited [34,35], sometimes even less than 2 ng/μl of extracted DNA [12].
A first starting point, identified by Huang et al., is the necessity to discriminate the commensal microbiota from transient, to understand their distribution and to better recognize the infective eye diseases that arise from commensal flora or from an increased virulence of transient flora [36]. The increased virulence of the resident pathogens may be the cause of some diseases, as reported for post-cataract surgery endophthalmitis in which the inflammation was due to the patient’s own conjunctival flora introduced into the anterior chamber during the surgical practice [37].
There is no general consensus amongst the scientists as some suggests that age and gender could play an important role in shaping the OS microbiome and the others don’t. In a study involving 90 subjects (48 young and 42 old adults) metagenomic shotgun sequencing was carried out [38]. Samples were obtained from the inferior bulbar conjunctiva and were analyzed with the aim to search the microbiome profile differences. A diversity of bacterial communities between males and females and even a more significant variation in bacterial composition between young and old adults was found. OS microbiome therefore seems to be a complex area of research, however, due to novel techniques like metagenomics, new opportunities and results are being decoded, although many challenges still needs to overcome.
4.1. Conjunctiva and metagenomics
In 2011, a first pilot study involving 4 subjects was conducted with an aim to explore the bacterial diversity of a healthy human conjunctiva using 16S rRNA gene amplicon based approach [39]. This study revealed an unpredicted diverse microbial community (average of 221 phylotypes per individual microbiome, classified into 5 phyla and 59 distinct genera), with some ubiquitous genera that included bacteria known as commensal, environmental and opportunistic pathogens.
In 2016, using the same NGS technology to analyze 31 conjunctival samples, Huang et al. identified a high microbial diversity, classified into 25 phyla and 526 distinct genera, providing a framework to investigate the potential roles played by diverse microbiota and the complex host-microbiome and microbiome-microbiome interactions [36].
In 2016, Doan et al. applied three complementary techniques (traditional culture, 16S rDNA deep sequencing and BRiSK, a metagenomic detection method for DNA-based life forms) to better characterize microbes and other DNA-based communities in conjunctival samples of 107 healthy volunteers [25]. They found a relatively consistent correlation between individuals, principally formed by Straphylococci, Diphtheroids, Propinibacteria and Streptococci. Interestingly, in 65% of all conjunctival samples tested, they found Torque Teno Virus, a small DNA anellovirus usually correlated with several intraocular inflammatory conditions (such as endophthalmitis). This work proved that viruses and microbial pathogens could populate the OS without necessarily causing disease. The reason of their presence is yet unknown.
In 2017, the conjunctiva of 45 healthy subjects were sampled at three time points over three months and processed using culture-dependent and culture-independent methods (16S rRNA gene sequencing) with the aim of understanding whether the microbial communities of the OS change over time [29]. This study investigated the relationship between microbiome and OS disorders. They concluded that every single individual has some constant taxa in OS during an entire life, which were indicated as “individual-specific or minimal core microbiomes” [29]. Analyzing these studies, a rich and various OS microbiome would emerge.
Nevertheless, a contradictory theory doubts that the OS harbors hundreds of genera of bacteria. The results of the sequencing could be biased due to the presence of environmental contaminants or reagents. Salter et al. proved this theory by showing that the presence of contaminants in extraction kits and laboratory reagents could impact results obtained from sequence-based approaches [40]. This event is particularly evident in low biomass samples, similar to the ocular surface. Hence, concurrent sequencing of negative control samples is strongly advised [40]. A method validation could thus be one of the major challenges to overcome in order to allow the introduction of NGS in the clinical practice.
4.2. Ocular surface microenvironment and dry eye
Dry eye (DE) is a multifactorial disease of the OS and the tear film. The common symptoms are discomfort, visual disturbance and tear film instability with potential damage to the OS and inflammation [41]. In 2016, Watters et al. investigated the ocular microbiome in patients with DE due to meibomian gland dysfunction (MGD). MGD is a condition in which the glands that make the lipid layer of the tears are not functional, allowing the water phase of the tears to dry out, leading to a DE condition [42]. Although this prospective observational study had several limitations, mainly the use of conventional culture techniques, the authors associated the presence of some bacteria, such as Straphylococcus aureus, with DE, thus establishing an OS microbiome-DE correlation.
Zegans et al. hypothesized the possibility of treating inflammatory diseases caused by OS microbiome dysregulation through the introduction of appropriate commensal microbes [43]. Indeed, for gut, a microbiome transplantation resulted as a successful treatment strategy in the management of Clostridium difficile infection [44]. However, no similar evidence has been found for the eye. A deeper knowledge of changes in the microbiome associated with inflammatory diseases could be a good starting point for development of new treatment strategies for restoring OS homeostasis.
4.3. OS microbiome and contact lens wearers
Shin et al. investigated the correlation between the contact lens wearers to the microbiome changes [33]. They analyzed the bacterial communities of the conjunctiva and the skin under the eye of 20 subjects (9 lenses wearers and 11 non-lenses wearers) by a 16S rRNA gene-base sequencing technique. They found that the OS microbiota of lens wearers is different from that of non-wearers, and that the lens wearers have the conjunctival microbiota more similar to that of the skin. Particularly, they reported that lens wearers had a higher abundance of Methylobacterium, Lactobacillus, Acinetobacter, Pseudomonas and a lower abundance of Haemophilus, Streptococcus, Staphylococcus and Corynebacterium compared to non-lens wearers. However, they recommended further research to demonstrate that the microbiome structure provides less protection against pathogens [33]. Additionally, they reported that anesthetic eye drops used before sampling reduced the detected ocular microbiota diversity, thus biasing the results obtained.
In another study, conjunctival microbial communities in contact lens wearers (soft contact lens and orthokeratology lens) and non-contact lens wearers were compared using an amplicon sequencing approach [31,32]. Microbiome in the two groups was found relatively similar, though differences between orthokeratology lens wearers and non-wearers were greater than those in the soft contact lens group.
By examining these two studies [[31], [32], [33]], Boost et al. concluded that the use of contact lenses has a fairly small impact on the microbiome changes of healthy subjects when compared to the proteome [20] (i.e. the whole set of proteins expressed by an organism). Nevertheless, this “fairly small impact” has been possible to detect only with the use of NGS (possibly not achievable with conventional techniques). Moreover, the use of contact lenses has been associated with an increased susceptibility and risk of infection, such as microbial keratitis [20], therefore it should be diagnosed and treated relatively.
4.4. OS and antibiotics
Antibiotics are normally used to kill the pathogens. Broad-spectrum antibiotics or a cocktail of drugs (antibiotics, antivirals, antifungals) are generally empirically administered especially in those cases where mixed diagnoses occur. However, the antibiotics must be chosen carefully and therefore knowing the causative microorganism could be highly desirable. Overuse of antibiotics for OS may increase resistance and the ocular microbial communities could be damaged, resulting in a greater susceptibility of the OS [36]. In an animal study, the corneas of gentamycin-treated mice (corneas with reduced commensals – reduction induced by antibiotic treatment) had more severe susceptibility to P. aeruginosa-induced keratitis compared to control mice (corneas with commensals – untreated mice) [21]. This study showed that the ocular ubiquitous microbiota contributed to the resistance towards infection. The loss of OS microbiota homeostasis could be dangerous. A specific therapy is possible only when the exact nature of the etiological agent is known. Additionally, the microbial communities that play a protective function for the eye has to be preserved. World Health Organization is urging all countries to plan actions to reduce the misuse of antibiotics and minimize the increase in resistance (http://www.who.int/antimicrobial-resistance/en/) towards antibiotics. Moreover, a targeted therapy could decrease the cost and time of treatment, leading to a social and economic impact. Lastly, a promising application of NGS could be the characterization of antibiotic resistance directly from clinical samples in order to find the best therapeutic approach [45].
4.5. Metagenomics in OS diagnostics
Few studies have been performed to understand if metagenomics could be a reliable diagnostic tool in management of eye infection and OS diseases. The premises are interesting. Even if not directly related to the OS, a recent study by Gonzales et al. showed the practical use of metagenomics in ophthalmology [46]. They studied two cases of intraocular lymphoproliferative disorders using a metagenomic deep sequencing approach. It was possible to detect the infectious pathogen that drive intraocular lymphomas as well as common and rare cancer associated mutations, using small amounts of aqueous fluid as samples.
A Chinese woman in New York with erythema, swelling in her body and red sore eyes did not have a proper diagnosis using conventional methods [47]. However, with an unbiased metagenomic approach using DNA samples obtained from her eyes and subcutaneous tissue, it was possible to establish that the patient suffered from Malayan filariasis.
Li et al. evaluated 16 cases of infectious keratitis and 4 controls in formalin-fixed corneas by NGS to identify pathogens from corneal specimens [28]. They were able to establish the feasibility of using metagenomics to investigate bacteria, fungi, amoeba, and viruses associated with pathogenic corneal infections.
Doan et al. showed that metagenomic DNA sequencing is highly concordant, but better than pathogen-directed PCR [26]. This conclusion was obtained analyzing 31 PCR-positive samples and 36 PCR-negative samples by DNA sequencing.
In a recent study, Kirstahler et al. examined vitreous samples from patients with endophthalmitis following cataract surgery or intravitreal injection [48]. They designed the experiment by comparing two DNA isolation procedures and then carried out metagenomic analysis. For 12 out of 14 patients, the dominant etiologic agent was identified: Staphylococcus epidermidis for six patients, Enterococcus faecalis for two patients, Serratia marcescens for one patient, Paenibacillus spp. for one patient and Staphylococcus hominis for one patient. Therefore, they concluded that genomic-based analyses of human ocular body fluid specimens can provide actionable information related to infectious disease management. In addition, they established that it is possible to identify antibiotic-resistant genes through the analysis of whole genome sequences [48].
Perhaps the most representative result that links metagenomics to the diagnosis of OS infections was obtained by Doan et al. in a study involving patients with uveitis [6,25]. They examined 6 intraocular fluid samples – 5 from subjects with known diagnoses and one from a subject with bilateral chronic uveitis without a known etiology. Using metagenomic deep sequencing approach, diagnoses-confirming results were obtained and, more importantly, the identification of the causative agent of chronic uveitis (unknown etiology) was possible. In this study, NGS has proven to be a highly effective technology, especially when the other conventional methods failed.
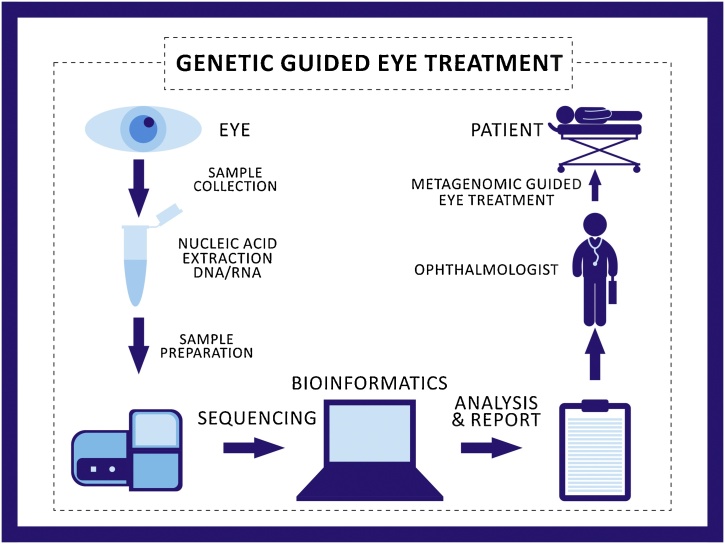
One of the most crucial limitations of using metagenomics data is that the downstream bioinformatics data reveals extensive results. This generates huge amount of data, which becomes difficult for a clinician to record and analyze on a routine basis. For the clinicians, however, it would be more appropriate to have a decoded result to understand the exact OS disorder etiology. Only the development of advanced bioinformatics tools that could allow processing exclusively the required data with the resistance profile could potentially transform metagenomics as a practical solution and a widespread technique. The use of metagenomics in an ophthalmic practice has thus been speculatively illustrated in Fig. 2.
Fig. 2.
The process towards genetic guided treatment in the field of ophthalmology. The figure indicates different procedures of sample collection, nucleic acid extraction, sample preparation, sequencing, bioinformatics, analysis and report writing, indication to the eye surgeon for specific drug usage for specific microorganism and metagenomics guided eye treatment on the patient. Being highly specific and cost effective, metagenomics could be potentially used in ophthalmology in the near future.
5. Conclusions and future perspectives
The ocular microbiome contributes to the normal physiological state of the eye and any alteration disturbs the homeostasis, leading to disorders. The current trend is to find new target specific diagnostic approaches leading to new therapies, as the traditional techniques are only limited to the detection of pre-specified pathogens. Currently, in case of ocular infections, a widespread possibility is to take a corneal scrape and use it for culture-based or PCR investigations. However, often there are no results (more than 50% of all presumed intraocular infections do not have an identified pathogen) [23]. Meanwhile, patients undergo a broad-spectrum therapy based on empirical treatment. The implementation of metagenomics in a clinical setting appears to be promising [49] as NGS could detect all the agents present in a clinical sample [50], with absolute numbers without any hypothesis. We anticipate that if technologies like NGS becomes a standard diagnostic assay, it will be easier to identify key microbes of the disease (even if these are rare pathogens). Of all the disorders previously analyzed, ocular infections represent a significant example, as they could be treated with appropriate and targeted therapies. However, many challenges still remain. Sequencing data need to be decoded by advanced bioinformatics tools to correctly interpret the information found. Furthermore, a validation of the method is necessary to avoid interferences by the extraction kits and the laboratory reagents. Eventually, it is desirable that randomized clinical trials, able to show the beneficial effect of metagenomics, are developed.
A first workable strategy to design a clinical trial could be the combined use of NGS with traditional approaches, to have a cross-control between various techniques and confirmed results, although this may not be enough. Data interpretation costs have to be significantly reduced (currently the estimated overall cost is of several hundreds to thousands of dollars per sample analyzed) [51] and data interpretation times shortened. It seems too early to expect routine use of NGS, however, it definitely has potential to overcome the traditional diagnostic methods in the very near future. No cases of randomized clinical trials have been reported so far however, it is hoped to be available soon. Chiu et al. hypothesize that over the next 5 years, prospective clinical trial data evaluating the utility and cost-effectiveness of metagenomics will become available [51].
Recently, we used next-generation sequencing, mainly 16S and 18S, for the detection of microorganisms present in human donor corneal preservation medium. We found that metagenomics technique provides complete taxonomic profiling unlike the conventional methods. Although all the genomic material of the organisms was detected, it was difficult to find live and active microbes that could have affected the preservation media. We also noted that the costs and the turn-around time need to be reduced significantly, and the detection of viable organisms would be necessary to introduce this technology in routine clinical practice [52], as currently, the NGS technique detects all the genomic material available in the sample. One of the major limitations of next-generation sequencing for the OS is that OS harbors hundreds of bacterial genera. There are chances that mostly the sequencing results would represent environmental contaminants. This is true for low biomass samples, such as conjunctival samples. However, similar to Shestopalov et al. [12] we have also found that only <2 ng/μL volume of the sample could be enough to obtain a full genome sequencing result. However, it has been noted that sampling and the technology in itself needs a higher degree of standardization before putting it into clinical practice.
Further investigations of the OS microbiome and continuous use of NGS technologies for diagnostic purposes are required to consolidate the method and introduce it into the clinical practice, preferably according to validated and standardized protocols.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests.
Acknowledgements
The authors thank Mrs. Jaini Parekh for the illustration of Fig. 1, Fig. 2. The authors thank the European Society of Cataract & Refractive Surgeons for the META-COR grant (PI: Davide Borroni), funded through the ESCRS clinical research trial funding programme 2017-2018.
References
- 1.The Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The NIH HMP Working Group, Peterson J., Garges S. The NIH human microbiome project. Genome Res. 2009;19:2317–2323. doi: 10.1101/gr.096651.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gevers D., Knight R., Petrosino J.F. The human microbiome project: a community resource for the healthy human microbiome. PLoS Biol. 2012;10 doi: 10.1371/journal.pbio.1001377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turnbaugh P.J., Ley R.E., Hamady M. The human microbiome project: exploring the microbial part of ourselves in a changing world. Nature. 2007;449:804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roy S., LaFramboise W.A., Nikiforov Y.E. Next-generation sequencing informatics: challenges and strategies for implementation in a clinical environment. Arch. Pathol. Lab. Med. 2016;140:958–975. doi: 10.5858/arpa.2015-0507-RA. [DOI] [PubMed] [Google Scholar]
- 6.Doan T., Wilson M.R., Crawford E.D. Illuminating uveitis: metagenomic deep sequencing identifies common and rare pathogens. Genome Med. 2016;8:90. doi: 10.1186/s13073-016-0344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohtani N. Microbiome and cancer. Sem Immunopathol. 2015;37:65–72. doi: 10.1007/s00281-014-0457-1. [DOI] [PubMed] [Google Scholar]
- 8.SanMiguel A., Grice E.A. Interactions between host factors and the skin microbiome. Cell. Mol. Life Sci. 2015;72:1499–1515. doi: 10.1007/s00018-014-1812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanz Y., Olivares M., Moya-Pérez Á. Understanding the role of gut microbiome in metabolic disease risk. Pediatr. Res. 2015;77:236–244. doi: 10.1038/pr.2014.170. [DOI] [PubMed] [Google Scholar]
- 10.Viaud S., Daillère R., Boneca I.G. Gut microbiome and anticancer immune response: really hot Sh*t! Cell Death Differ. 2015;22:199–214. doi: 10.1038/cdd.2014.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu L.J., Liu J. Human microbiota and ophthalmic disease. Yale J. Biol. Med. 2016;89:325–330. [PMC free article] [PubMed] [Google Scholar]
- 12.Shestopalov V.I., Antonopoulos D.A., Miller D. Metagenomic analysis of bacterial community at the human conjunctiva. Invest. Ophthalmol. Vis. Sci. 2010;51:2409. [Google Scholar]
- 13.Riesenfeld C.S., Schloss P.D., Handelsman J. Metagenomics: genomic analysis of microbial communities. Annu. Rev. Genet. 2004;38:525–552. doi: 10.1146/annurev.genet.38.072902.091216. [DOI] [PubMed] [Google Scholar]
- 14.Kim D., Hofstaedter C.E., Zhao C. Optimizing methods and dodging pitfalls in microbiome research. Microbiome. 2017;5:52. doi: 10.1186/s40168-017-0267-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tringe S.G., Hugenholtz P. A renaissance for the pioneering 16S rRNA gene. Curr. Opin. Microbiol. 2008;11:442–446. doi: 10.1016/j.mib.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Forbes J.D., Knox N.C., Ronholm J. Metagenomics: the next culture-independent game changer. Front. Microbiol. 2017;8:1069. doi: 10.3389/fmicb.2017.01069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Browne H.P., Forster S.C., Anonye B.O. Culturing of’ unculturable’ human microbiota reveals novel taxa and extensive sporulation. Nature. 2016;533:543–546. doi: 10.1038/nature17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caspi R.R. Immunology of the eye–inside and out. Int. Rev. Immunol. 2013;32:1–3. doi: 10.3109/08830185.2012.750138. In this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linhart R.W. The effect of the eye patch on organisms of the conjunctival sac. Am. J. Ophthalmol. 1950;33:1280–1282. doi: 10.1016/0002-9394(50)91001-4. [DOI] [PubMed] [Google Scholar]
- 20.Boost M., Cho P., Wang Z. Disturbing the balance: effect of contact lens use on the ocular proteome and microbiome. Clin. Exp. Optom. 2017;100:459–472. doi: 10.1111/cxo.12582. [DOI] [PubMed] [Google Scholar]
- 21.Kugadas A., Christiansen S.H., Sankaranarayanan S. Impact of microbiota on resistance to ocular pseudomonas aeruginosa-induced keratitis. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Endophthalmitis Vitrectomy Study Group, Results of the Endophthalmitis Vitrectomy Study A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch. Ophthalmol. 1995;113:1479–1496. [PubMed] [Google Scholar]
- 23.Taravati P., Lam D., Van Gelder R.N. Role of molecular diagnostics in ocular microbiology. Curr. Ophthalmol. Rep. 2013;1:10. doi: 10.1007/s40135-013-0025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laupland K.B., Valiquette L. The changing culture of the microbiology laboratory. Can. J. Infect. Dis. Med. Microbiol. 2013;24:125–128. doi: 10.1155/2013/101630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doan T., Akileswaran L., Andersen D. Paucibacterial microbiome and resident DNA virome of the healthy conjunctiva. Invest. Ophthalmol. Vis. Sci. 2016;57:5116–5126. doi: 10.1167/iovs.16-19803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doan T., Acharya N.R., Pinsky B.A. Metagenomic DNA sequencing for the diagnosis of intraocular infections. Ophthalmology. 2017;124:1247–1248. doi: 10.1016/j.ophtha.2017.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eguchi H., Hotta F., Kuwahara T. Diagnostic approach to ocular infections using various techniques from conventional culture to next-generation sequencing analysis. Cornea. 2017;36(Suppl. 1):S46–S52. doi: 10.1097/ICO.0000000000001338. [DOI] [PubMed] [Google Scholar]
- 28.Li Z., Breitwieser F.P., Lu J. Identifying corneal infections in formalin-fixed specimens using next generation sequencing. Invest. Ophthalmol. Vis. Sci. 2018;59:280–288. doi: 10.1167/iovs.17-21617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozkan J., Nielsen S., Diez-Vives C. Temporal stability and composition of the ocular surface microbiome. Sci. Rep. 2017;7:9880. doi: 10.1038/s41598-017-10494-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graham J.E., Moore J.E., Jiru X. Ocular pathogen or commensal: a PCR-based study of surface bacterial flora in normal and dry eyes. Invest. Ophthalmol. Vis. Sci. 2007;48:5616–5623. doi: 10.1167/iovs.07-0588. [DOI] [PubMed] [Google Scholar]
- 31.Zhang H., Zhao F., Hutchinson D.S. Conjunctival microbiome changes associated with soft contact lens and orthokeratology lens wearing. Invest. Ophthalmol. Vis. Sci. 2017;58:128–136. doi: 10.1167/iovs.16-20231. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X., Jeyalatha M.V., Qu Y. Dry eye management: targeting the ocular surface microenvironment. Int. J. Mol. Sci. 2017;18:1398. doi: 10.3390/ijms18071398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin H., Price K., Albert L. Changes in the eye microbiota associated with contact lens wearing. MBio. 2016;7:e00198–16. doi: 10.1128/mBio.00198-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hedegaard J., Thorsen K., Lund M.K. Next-generation sequencing of RNA and DNA isolated from paired fresh-frozen and formalin-fixed paraffin-embedded samples of human cancer and normal tissue. PLoS One. 2014;9 doi: 10.1371/journal.pone.0098187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yozwiak N.L., Skewes-Cox P., Stenglein M.D. Virus identification in unknown tropical febrile illness cases using deep sequencing. PLoS Negl. Trop. Dis. 2012;6:e1485. doi: 10.1371/journal.pntd.0001485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang Y., Yang B., Li W. Defining the normal core microbiome of conjunctival microbial communities. Clin. Microbiol. Infect. 2016;22:643. doi: 10.1016/j.cmi.2016.04.008. e7-643.e12. [DOI] [PubMed] [Google Scholar]
- 37.Speaker M.G., Milch F.A., Shah M.K. Role of external bacterial flora in the pathogenesis of acute postoperative endophthalmitis. Ophthalmology. 1991;98:639–649. doi: 10.1016/s0161-6420(91)32239-5. [DOI] [PubMed] [Google Scholar]
- 38.Wen X., Miao L., Deng Y. The influence of age and sex on ocular surface microbiota in healthy adults. Invest. Ophthalmol. Vis. Sci. 2017;58:6030–6037. doi: 10.1167/iovs.17-22957. [DOI] [PubMed] [Google Scholar]
- 39.Dong Q., Brulc J.M., Iovieno A. Diversity of bacteria at healthy human conjunctiva. Invest. Ophthalmol. Vis. Sci. 2011;52:5408–5413. doi: 10.1167/iovs.10-6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salter S.J., Cox M.J., Turek E.M. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol. 2014;12:87. doi: 10.1186/s12915-014-0087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.The definition and classification of dry eye disease: report of the definition and classification subcommittee of the international Dry Eye WorkShop. Ocul. Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 42.Watters G.A., Turnbull P.R., Swift S. Ocular surface microbiome in meibomian gland dysfunction. Clin. Exp. Ophthalmol. 2017;45:105–111. doi: 10.1111/ceo.12810. [DOI] [PubMed] [Google Scholar]
- 43.Zegans M.E., Van Gelder R.N. Considerations in understanding the ocular surface microbiome. Am. J. Ophthalmol. 2014;158:420–422. doi: 10.1016/j.ajo.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gianotti R.J., Moss A.C. Fecal microbiota transplantation: from clostridium difficile to inflammatory bowel disease. Gastroenterol. Hepatol. 2017;13:209–213. [PMC free article] [PubMed] [Google Scholar]
- 45.Stefan C.P., Koehler J.W., Minogue T.D. Targeted next-generation sequencing for the detection of ciprofloxacin resistance markers using molecular inversion probes. Sci. Rep. 2016;6:25904. doi: 10.1038/srep25904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gonzales J., Doan T., Shantha J.G. Metagenomic deep sequencing of aqueous fluid detects intraocular lymphomas. Br. J. Ophthalmol. 2018;102:6–8. doi: 10.1136/bjophthalmol-2017-311151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao D., Yu Q., Wang G. Diagnosis of a malayan filariasis case using a shotgun diagnostic metagenomics assay. Parasit. Vectors. 2016;9:86. doi: 10.1186/s13071-016-1363-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kirstahler P., Bjerrum S.S., Friis-Møller A. Genomics-based identification of microorganisms in human ocular body fluid. Sci. Rep. 2018;8:4126. doi: 10.1038/s41598-018-22416-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Forbes J.D., Knox N.C., Peterson C.L., Reimer A.R. Highlighting clinical metagenomics for enhanced diagnostic decision-making: a step towards wider implementation. Comput. Struct. Biotechnol. J. 2018;16:108–120. doi: 10.1016/j.csbj.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Graf E.H., Simmon K.E., Tardif K.D. Unbiased detection of respiratory viruses by use of RNA sequencing-based metagenomics: a systematic comparison to a commercial PCR Panel. J. Clin. Microbiol. 2016;54:1000–1007. doi: 10.1128/JCM.03060-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chiu C.Y., Miller S.A. Clinical metagenomics. Nat. Rev. Genet. 2019;20:341–355. doi: 10.1038/s41576-019-0113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parekh M., Borroni D., Romano V. Next-generation sequencing for the detection of microorganisms present in human donor corneal preservation medium. BMJ Open Ophthalmol. 2019;4 doi: 10.1136/bmjophth-2018-000246. e000246. [DOI] [PMC free article] [PubMed] [Google Scholar]