Graphical abstract
Keywords: CaCO3 precipitation, Bio-cement, Microalgae, Optimization, Waste management
Highlights
-
•
CKD with microalgae sp. Chlorella kessleri is investigated for maximum bio-cement yields.
-
•
A predictive quadratic model was developed for CaCO3 yield with R2 value of c.a. 92%.
-
•
Low temperature and high pH were found to be important parameters in RSM study.
-
•
Under optimal set, a maximum of 96% Ca was extracted experimentally from CKD.
-
•
FTIR, XRD and EDS analysis confirmed the produced bio-cement compound.
Abstract
The main aim of this study was to maximize bio-cement (CaCO3) production through a waste feedstock of cement kiln dust (CKD) as a source of calcium by deployment of microalgae sp. Chlorella kessleri. The effect of process parameters such as temperature, pH and time-intervals of microalgae cultivation, were set as criteria that ultimately subscribe to a process of optimization. In this regard, a single factor experiments integrated with response surface methodology (RSM) via central composite design (CCD) was considered. A quadratic model was developed to predict the maximum CaCO3 yield. A ceiling of 25.18 g CaCO3 yield was obtained at an optimal set of 23 °C, pH of 10.63 and day-9 of microalgae culture. Under these optimized conditions, maximum 96% calcium was extracted from CKD. FTIR, XRD and EDS analyses were conducted to characterize the CaCO3 precipitates. Compressive modes of mechanical testing seemed to hold conventional cement complimented by CaCO3 co-presence markedly superior to mere cement performance as far as compressive strength is concerned. The latter criterion exhibited further increase in correspondence with rise in cement to bio-cement ratio. This investigative endeavour at hand offers a simple pivotal platform on the basis of which a scale-up of microalgae-infested bio-cement production might be facilitated in conjunction with the added benefit of alleviation in environmental pollution through cement waste utilization.
1. Introduction
Cement manufacturing is one of the leading process industries with respect to total annual productions and global market share. It uses naturally occurring limestone, a non-renewable raw material, and converts it into cements through calcination processes. During the calcination/conversion process and unit operations, it generates huge amount of cement kiln dust (CKD), which ultimately requires proper collection, management and disposal. Typically, 15–20 tons of CKD is generated per 100 tons of cement produced [[1], [2], [3]]. The calcination is an energy intensive process, thus consumes significant amount of fuels. As a result, the cement industry imposes substantial burden on worldwide carbon-footprint by direct and indirect CO2 emissions. Estimation shows that the cement industry contributes to nearly 5% of global CO2 emissions [1]. Having said that, the ever-growing urban inhabitance, the intimately associated enhancement in quality of living and emanating growth in energy demand collectively contribute towards the exhaustion of resources and generates additional wastes.
On a positive note, breakthrough scientific developments have rendered waste of relevance to the cement industry still beneficial as a resource for specific applications. Cement kiln dust (CKD) is a heterogeneous alkaline wastes that comprises amongst other problematic species, minute granules remnant of feedstock with variable extents of scorching. CKD is infamous for being dubbed a surplus of impending and grave detriment should it not be handled with utmost caution by virtue of its corrosive nature, and the looming damage it imposes to skin, eye and respiratory tracts [1]. Conventionally, CKD is used in land reclamation. It can also serve as a source of Ca in mineral carbonation [2,3]. However, its abundant availability and favorable physicochemical characteristics makes it a suitable material for more valued bio-cement production.
Bio-cement is an aggregate of calcite nanoparticles forming point-to-point contacts cementing the sand particles. Their very existence is facilitated in coincidence with high pH, elevated calcium cation and carbonate anion infestations [4]. Bio-cement has many advantages over conventional cement, such as shorter preparation time and suitability for in-situ process. Its production is also energetically more efficient as compared to conventional cement production, given it requires ambient temperature as opposed to traditional high temperature (1000 ⁰C) processes. Therefore, bio-cement production has a potential of contributing to not only energy savings but also to the minimization CO2 emissions efforts [5]. In applications, bio-cement can remediate cracks in building materials, rocks of different strengths development and regain strength within a month. It also enhances the durability of bricks by increasing compressive strength and reducing their permeability [4]. Due to the after mentioned facts, bio-cement emerges as a breakthrough product emanating from ongoing promising developments on the bioprocessing technology conventionally dubbed as bio-cementation or Microbial Induced Calcite Precipitation (MICP). In addition to the bio-cement production, the MICP has also been investigated in wide breadth of applications, including calcium extraction from waste-water, soil remediation (ridding it from ground-water metal infestation), removal of polychlorinated biphenyl, atmospheric CO2 sequestration, and biodegradation of pollutants/radionuclides [[6], [7], [8]].
Along with different methods, various forms of microorganisms, which include bacteria and microalgae, or other photosynthetic microorganisms have been used to produce bio-cement. CO2 might be found chiefly contributing in metallic processes pertinent to photosynthetic microorganisms (Eq. 1). The latter, in turn, acts in a dynamic equilibrium amongst HCO3− and CO32- anions as laid out in Eq. 2 [[9], [10], [11]]. Via photosynthesis, microalgae utilize CO2 and/or HCO3−, and release OH− causing an increase in the pH as described in Eq. 3 [12]. Photosynthetic microalgae precipitate calcium carbonate when they live in environment saturated with calcium ions and carbonates [13]. At high pH, negative functional groups in the algae cell wall are exposed allowing the positive charges ions to attach. Therefore, in the presence of calcium ions, they will attach to the cell wall attracting carbonate to bind forming calcium carbonate nuclei as described at chemical reaction in Eq. 4.
| (1) |
| (2) |
| (3) |
| (4) |
Bio-cement formation by means of adopting an agglomerate of bio-silica. The blend in concern can be obtained through combustion of organic residues with Portland cement. As a matter of fact, Si-rich rice ash in addition to tropical vegetation make for strong, resilient biocement. Al-Thawadi, & Cord-Ruwisch [4] used bacteria called Bacillus sp. MCP11 (DSM 23,526) to develop an industrially suitable cost-effective microbial process to produce urease active cells. They investigated the effects of produced urease active cells as a catalyst to produce bio-cement. Ariyanti [14] identified three main groups of microorganisms and their pathways for induced CaCO3 precipitation in analogy to that accustomed for photosynthetic microorganism as the likes of, cyanobacteria and microalgae; sulphate reducing bacteria; and specific classes of microorganism contributing in the nitrogen cycle. They have also discussed the feasibility study of using microalgae for bio-cement production.
One can conclude from the above discussion that the appropriate balance of the process parameters is at the core of piercing the bounds of CaCO3 productivity using microalgae cultivation approaches. Most of the experimental studies reported in open literature considered trial-and-error or single factor experimental design (i.e. through allowing an entire set of parameters to be held fixed whilst a unique contributing factor lends itself for manipulation and hence critique thereupon) to optimize the experimental parameters. However, both the single parameter and the trial-and-error approach require large number of experiments and longer period time for process optimization. More importantly, the approach in concern falls short of addressing the multifaceted nature of interactions amongst governing factors and in doing so fails to unveil the true optimal conditions ultimately sought [15,16]. In this regard, central composite design (CCD), one of the response surface methodologies (RSMs), can adequately address the shortcomings of the trial-and-error and single parameter approaches [17]. Briefly, RSM is the most well-known statistical, fully randomized and bias free method in finding the optimum conditions using quadratic regression models. RSM has been used widely for optimization and modelling in different aspects including bio-synthesis materials such as biodiesel, bio-cement and various bacterial/microalgal media for high yield such as lipases, chitinases, xylanases, keratinases, lipid productivity including CO2 biofixation [[18], [19], [20], [21], [22], [23], [24], [25]]. Dhami et al. [21] optimized the medium components using central composite design (CCD) to heighten urease yield and explore its influence on MICP thereafter. Hossain et al. [20] used fractional factorial design (FFD) of the investigation in conjunction with the RSM utilizing a batch reactor to determine the optimum conditions necessary to produce biodiesel from spent cooking oil. The RSM is also used to find optimal microalgal growth and CO2 biofixation rates [19,22,23]. Several studies reported the influence of cultivation conditions on microalgal lipid productivity (LP) using RSM approach [26]. Kiran et al [27] used RSM alongside CCD aiming for improved biomass and lipid yield of microalgae (Oocystis sp.) by seeking the optimization of nutrient levels (namely, nitrate and phosphate) and temperature. The latter was sought in isolation from domestic wastewater. As far as bio-cement synthesis process is concerned, a few studies have been found related to the usage of RSM.
As CaCO3 is widely used raw material in many industries and it has numerous applications, particularly for geotechnical aspects so it is highly important to study the precipitation of CaCO3 either by chemical treatment or through microbial process. The prime motive underpinning this investigative endeavour is to study the maximum yield of calcium carbonate precipitates, which is formed through CKD using microalgae. In preliminary experiments (single factor design), several parameters that affect the precipitation were studied such as temperature, pH, algae cells to waste ratio and algae cultivation time. RSM with CCD approach was used for optimizing the conditions (e.g. temperature and pH) to maximize the yield of precipitates formed by MICP using microalgae, Chlorella kessleri. The quadratic regression model developed from the CCD was validated against the experimental data. To the best of knowledge of the present authors, no preceding work have reported in the literature that satisfyingly attests to beneficial attempts towards the optimization of CaCO3 precipitation from CKD by means of deploying response surface methodology (RSM) in synergy with central composite design (CCD).
2. Materials and methods
2.1. Materials
Cement kiln dust (CKD) sample (particle size of 75–150 μm) along with wt% composition was investigated with the permission of Falcon Cement Factory, Kingdom of Bahrain. The cement sample contains approximately 78.26% CKD, which primarily comprises lime (51.12%), silica (17.10%), iron (3.38%), alumina (3.29%), magnesium oxide (1.67%) and some traces of other oxides (Na2O, SO3, H2O) with the remainder prone to being flared on incineration. Analytical grade acetic acid (99.8% pure) used in this study was received from Sigma Aldrich.
2.2. Algae species and growth circumstances
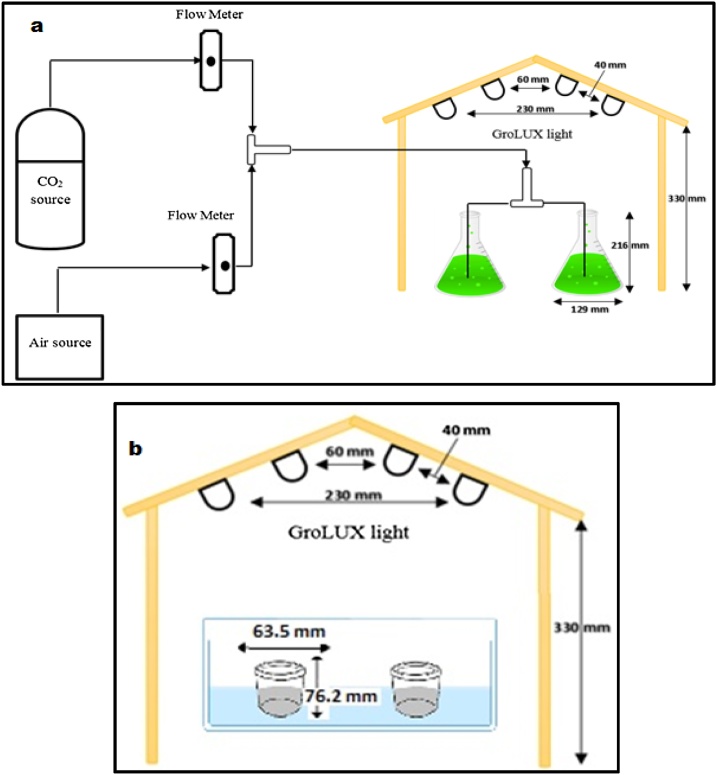
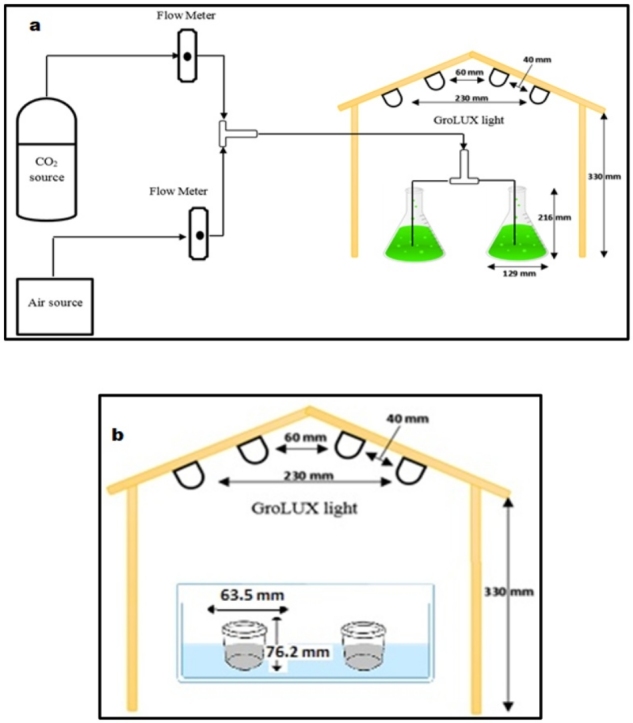
Access to microalgae species, Chlorella kessleri (UTEX-2229), was rendered possible through culture collection in collaboration with University of Texas, USA. The nurturing environment used for algae growth is formally termed ‘Bold's Basal Medium’ (BBM). PYREX 1 L Erlenmeyer flasks were utilized as batch photobioreactors to accommodate Algae species whilst being cultured, as presented in Fig. 1a. A restricted working volume to 500 mL was made to host an initial algal concentration of 2.2 × 107 cells mL−1 (obtained using hemocytometer) consistently throughout all cultivation conditions. An average light intensity of 65 μmol m-2s-1 (measured through a LI-250 Light Meter) was achieved by means of a cluster of four Grolux fluorescent bulbs placed over the reactors from the ceiling of an encompassing wooden frame. The reactors in concern soak in a temperature-controlled water bath. A mixture of 96% ambient air and 4% carbon dioxide was consistently pumped to the cultures. For each set of experiments, the cultures cycle was set for eleven days. The cultivation time of days 5, 7 and 9 (e.g., early, middle and end of log phase algae culture, respectively) were used separately for production of bio-cement.
Fig. 1.
Schematic diagram of the experimental setup used to: a) cultivate Chlorella kessleri and b) produce bio-cement.
2.3. Quantification of algae biomass
For measurement and growth investigations, the product samples (1 mL each) were collected every day. An UV-spectrophotometer (UNICAM) was deployed in an effort to provide a quantified perspective on ‘optical density’ at the wake of adequate dilution at 690 nm. Meanwhile, a biomass-optical densities standard curve (Supplementary Fig. S-1) was utilized to allow for biomass (g/L) determination. Biomass values (g/L) were found after vacuum filtration of an appreciable quantity of sample (10 mL) at every three days, with eventual drying at 55 °C for 24 h.
2.4. Preliminary study of calcium carbonate precipitate
One-factor-at-a-time, OFAT strategy (one factor is varied while keeping the other factors at their constant level) was used for preliminary study of biocement (calcium carbonate precipitates) production. For this, a solution was made by mixing 50 gm of waste sample in 100 mL of de-ionized water in a container. To maintain the weight ratio, 28 mL (≈25 gm) of algae solution was added. In order to maintain the pH, acetic acid was also added as a buffer. The solution was well mixed and kept under the four grolux fluorescent tubes. A schematic of the experimental setup for bio-cement production is shown in Fig. 1b. The precipitates were harvested upon the conclusion of every 24 h over a timespan of three consecutive days. Wet samples fresh out of the culture medium were weighed and kept overnight to dry in an oven at 105 °C. Similarly, other sets of experiments were performed by changing the parameters such as algae culture dwelling durations (5, 7 and 9 days), temperature (25, 30 and 35ᵒC) and pH (7, 9, 10 and 12).
2.5. Sample characterization
2.5.1. FTIR analysis
For the identification of calcium carbonate precipitates through its functional group, FTIR spectrometer was used. For that purpose, FTIR Alpha T model was adopted. Before carrying out the analysis, the ATR diamond surface was rinsed with ethanol, immediately after 2–3 mg of precipitate sample was placed on the surface in concern. The FTIR instrument was conveyed the acquired raw data to a computer where a built-in OPUS 7.0 software can be utilized. It was in the wake of the 4th runs that the spectrograms were displayed on OPUS interface lending itself readily towards being processed. The spectrums were obtained in correspondence with a broad range of wavelengths and absorbance intensities. The peaks were critiqued and ultimately identified on the basis of bonding and functional group pattern.
2.5.2. XRD analysis
The crystalline phase of calcite nanoparticles were analyzed by powder X-ray diffraction (XRD) method using Rigaku Ultima VI diffractometer equipped with Cu-radiation source (k = 1.5418 Å) from 2 = 20° up 70°, with step size of 0.04° and counting time of 2.5 s. Rietveld refinements were carried out using PDXL program available in association with the diffractometer.
2.5.3. EDX analysis
Energy dispersive X-ray (EDX) analysis was conducted with the aid of a Bruker BSD detector with a resolution of 127 eV whilst maintaining recommended count levels at 5–6 Kcps through optimization of beam alignment and maximizing probe current without compromising resolution.
2.5.4. Compressive strength test
The compressive strengths endured by the synergistic system of precipitates combined with conventional cement at different proportions were determined and ultimately compared with the strength of conventional cement. Different strands of specimens were prepared by experimenting with varying ratios of conventional cement in conjunction with biocement each time. For conventional cement a standard procedure was deployed. The latter was manifested in the adoption of three specimens of the same dimension (50 × 50 x 50 mm), wherein 75 ml of water was used to complete a cement to sand ratio of 1:2. The specimens were allowed to dry for 12 h and then left for curing in water for 3 days. The statistical span aimed at exploring experimental scatter in compressive performance was conducted such that three discrete measurements were obtained from three specimens of each condition prepared for which a typical average value was identified. Analogously, specimens of different conventional cement-to-biocement ratios (1:1, 2:1 and 3:1) were further prepared and investigated with a fixed sand and water mixture. For each ratio, triplicate experiments for the compressive strength were carried out and average value for each specimen was calculated.
2.6. Statistical modeling
Design of experiments is a methodical statistical technique with the prime aim of optimizing the outcomes (whether, through maximization or minimization) in light of the process feed of influencing factors [17]. As far as the investigative endeavour at hand is concerned, a response surface methodology with central composite design (CCD) was deployed to optimize two important input factors, cultivation temperature and pH on bio-cement yield. A total of 13 experimental runs or treatments (with 4 factorial points, 4 axial or star points, and 2 center points) were conducted. The interspacing separating a star points from a center point is given by α = 2n/4 (for two factors, α = ±1.414). The behavioural layout underpinning the process is described by Eq. 5, which includes the full spectrum of terms of interaction irrespective of what each term’s particular significance might be.
| (5) |
where, y is the predicted response, xi is the coded variables, βo is the intercept term, βi is the linear effect, βii is the squared effect and βij is the interaction effect.
For statistical scrutiny, the true variables/factors Zi were coded as xi in accordance with Eq. 6:
| (6) |
where, xi is the coded value of the true variable, ; is the value of at the center point and is the step change.
Table 1 lists the treatment with the range and levels of two independent variables (cultivation temperature and pH) that are studied. A non-biased take on the approach adopted was ensured by maintaining random handling of all treatments pursued. A regression equation or model was developed for biocement yield. The developed model was used to determine the optimum collaborative roles amongst the full extent of independent variables involved via a prediction profile plot.
Table 1.
Central composite design (CCD) for optimizing temperature and pH with experimental data and model prediction.
| No. of runs | Temperature (x1) coded level | pH (x2) coded level | Temperature actual level | pH actual level | CaCO3 precipitate (g) |
||
|---|---|---|---|---|---|---|---|
| Experimental data | Model prediction | Error (%) | |||||
| 1 | 0 | −1.4142 | 29 | 6.37 | 3.29 | 2.25 | 31.64 |
| 2 | 1.41421 | 0 | 35 | 8.5 | 18.62 | 15.22 | 18.28 |
| 3 | 1 | 1 | 33 | 10 | 16.9 | 20.35 | 20.40 |
| 4 | −1 | 1 | 25 | 10 | 11.28 | 12.43 | 10.17 |
| 5 | −1.41421 | 0 | 23 | 8.5 | 4.91 | 4.76 | 3.07 |
| 6 | 1 | −1 | 33 | 7 | 10.91 | 13.32 | 22.06 |
| 7 | 0 | 0 | 29 | 8.5 | 6.6 | 6.60 | 0.00 |
| 8 | 0 | 0 | 29 | 8.5 | 6.7 | 6.60 | 1.49 |
| 9 | −1 | −1 | 25 | 7 | 6.34 | 6.45 | 1.68 |
| 10 | 0 | 1.41421 | 29 | 10.62 | 27.3 | 24.79 | 9.20 |
2.7. Statistical significance
ANOVA (Analysis of variance) was deployed to ascertain statistical significance. A p-value < 0. 05 was dubbed statistically significant at 5% level while 0.05<p-value < 0.1 was also labelled significant at 10% level. All experiments were conducted such that duplicates are persistently available. Minitab (version 17.0) software was utilized throughout to serve all regression and graphical analysis purposes.
3. Results and discussions
3.1. Growth profile of microalgae sp. Chlorella kessleri
To characterize the time-resolved growth phase, it is imperative to realise the growth pattern of microalgae species Chlorella kessleri in BBM. The population of Chlorella kessleri grown in a batch photo-bioreactor containing BBM is shown in the Supplementary Fig. S-2. One can see from this figure that for 3 h incubation, the growth of Chlorella kessleri was fairly sluggish. The absorbance then continued to increase until day 9 before reaching to a plateau. For preliminary experiments, the samples were obtained at days 5, 7 and 9, respectively and used separately for bio-cement production since these particular time intervals all resided within logarithmic growth phase and a consistent behaviour of algal growth was documented.
3.2. Preliminary study on bio-cement production
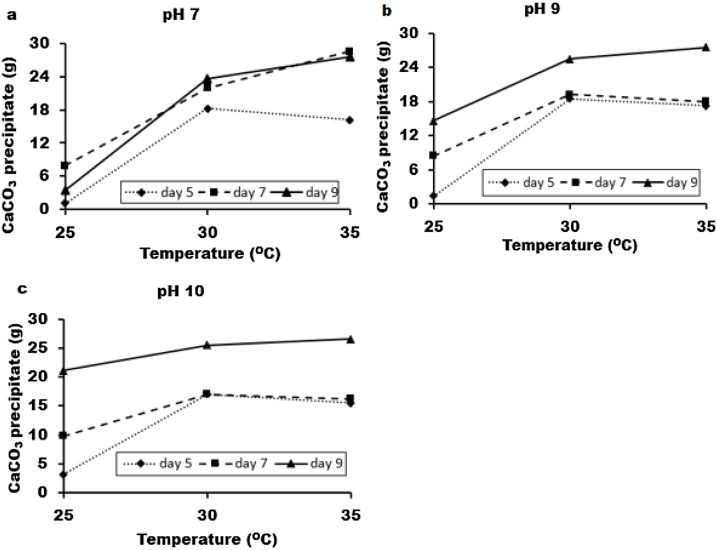
Prior to parametric study, one-factor-at-a-time, OFAT strategy or single factor experiments was used to screen important environmental factors for bio-cement production. It was along this line that three process variables such as reaction mixture pH, temperature, and the time-intervals of microalgae cultivation were considered. The amount of CaCO3 precipitate was collected and weighed-in every 24 h for 3 days. Fig. 2 depicts the effect of temperature, pH and the dwelling periods of microalgae culture on CaCO3 yield. At a fixed value of pH 7, the effect of temperature and microalgae growth period on the CaCO3 yield is shown in Fig. 2a. Similarly, results at pH 9 and 10 are presented in Fig. 2b and c, respectively. The results indicated that all these factors are critical if bio-cement production was to be optimized. The amount of CaCO3 precipitate obtained by using day-9 algae is comparatively higher for all cases and keeps soring towards greater heights with rise in temperature. Whereas the precipitate in the wake of deploying day-5 and day-7 algae cultivation, might commence with an increase, however, soon succumbs to a noticeable descend towards lower realms (except circumstance where pH = 7). The likely rationale in justifying such observations might reside in the increase of cell number with more active and matured cells. As such increase biomass at this stage may increase to the bio-cementation reaction. Therefore, day-9 algae culture was considered as the optimum condition for calcium carbonate precipitation and thereby it was not included for parametric study. In addition, results indicated that temperature and pH are important parameters for CaCO3 production, which agrees with that of literature [4]. Thus, to maximize the CaCO3 yield, these two factors were optimized further by parametric study.
Fig. 2.
The CaCO3 precipitation with different temperature, pH and microalgae growth period. The initial pH for reaction mixture is (a) 7, (b) 9 and (c) 10, respectively.
The images of calcite or CaCO3 precipitation through activities of microalgae is shown in Supplementary Fig. S-3. Two types of precipitate patterns were observed such as granular shape (Fig. S-3a) and crystalline form with sharp edges (Fig. S-3b). Usually, at the beginning, the granular shape calcite was formed (until 2 days) and later it was converted into sharp edged crystalline shape. However, the detail underlying mechanism of this patterns is still unclear. Therefore, it is required to explore further in this field.
3.3. Statistical scrutiny in parametric investigation
3.3.1. Parity plot and analysis of variance (ANOVA)
The experimental data obtained from the standard run of CCD matrix is shown in Table 1. A pragmatic model was subjected to critique utilizing ANOVA at a certainty level of 95%. A forecast model addressing bio-cement yield pertinent to this investigation might be expressed as in Eq. 7 with the aid of CCD.
| (7) |
where, is the CaCO3 yield (g), in the reaction temperature (oC) and in the pH of the reaction mixture.
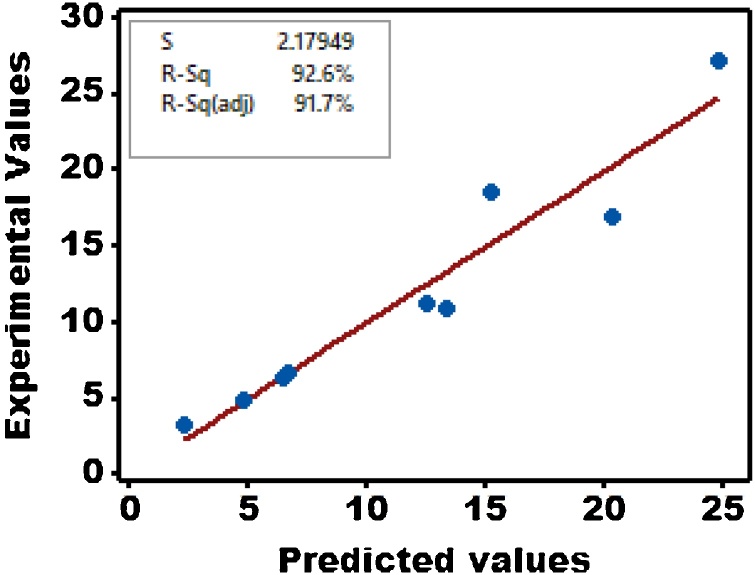
Fig. 3 shows the parity plot, which might be presumed as a graphical manifestation of the interdependency amongst graphically shows the relationship between anticipated and actual experimental values of relevance to bio-cement yield in this investigation. Nonetheless, a threshold value of 0.75 should be surpassed by R2 values in the parity plot if the empirical model put forth here might, at all, be validated considering the nature of the experimental design in consideration. Ultimately, a revised R2 value pertinent to anticipated bio-cement yield might be found in the vicinity of 0.917; that is 91.7% of the scatter in the details might be accounted for by the model, whilst the latter fails to address a mere 8.3% of variability. As such, Eq. 7 might be taken as a model that is adequately qualified to describe the effects imposed by influential factors on the ultimate response.
Fig. 3.
Parity plot for bio-cement production.
As far as statistical scrutiny is concerned, it is somewhat preordained that a model be validated against the null hypothesis. This revolves around setting all regression coefficients to zero. The validity of a model in ANOVA analyses might be dealt with via the p-value, which is a common indicator for this purpose. Having said that, the null hypothesis might only seize to be valid should the p-value of relevance to the model be rendered ≤ 0.10. All in all, it is the coefficients that exhibit inferior p-value or relatively elevated F-value that are deemed relatively more pronounced in influencing the significance of the model. Table 2 outlines p- and F-values corresponding to each discrete term constituting Eq. 7. The gist of the collective set of data examined thus far testifies to the significance of the 95% confidence level arrived at from a statistical perspective. The linear term for pH (x2) might be perceived as one that contributes the most to bio-cement yield with p-value amounting to 0.018. The immediate successors in influence to the latter might be presumed to be the linear term of reaction temperature (x2) (p-value = 0.061)) alongside the quadratic term of pH (x2) (p-value 0.07). The aforementioned terms stand out as significant influencers if bio-cement yield is to be ultimately manipulated/optimized. Hence, the empirical model put forth in this investigation might offer adequate forecast on bio-cement production.
Table 2.
Analysis of variance (ANOVA) for bio-cement yield.
| Term | Coefficient | SE Coefficient | F-Value | p-Value |
|---|---|---|---|---|
| Model | – | – | 6.27 | 0.08 |
| Constants | 3.8378 | 2.887 | – | 0.276 |
| Block | 2.7623 | 1.415 | 3.81 | 0.146 |
| x1 | 3.6974 | 1.258 | 8.63 | 0.061 |
| x2 | 6.0152 | 1.275 | 22.25 | 0.018 |
| x1*x1 | 4.4564 | 2.074 | 4.61 | 0.121 |
| x2*X2 | 4.8402 | 1.747 | 7.68 | 0.07 |
| x1*x2 | 0.2625 | 1.78 | 0.02 | 0.892 |
3.3.2. Effect of environmental factors on bio-cement yield
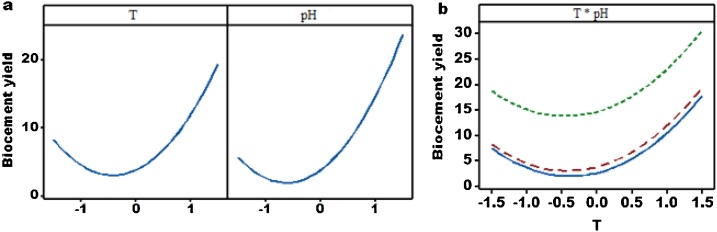
In an effort to assess the influence of environmental factors (e.g., T, and pH) on response, bio-cement yield, main effect and interaction plots were produced and presented as displayed in Fig. 4. It is accustomed that various extents of the influencing factors trigger different intensities of responses. It is important to reveal that the factor has no effect on the response if the line is parallel to the x-axis. The steeper slope of the line entails, the higher degree of the main effect. Fig. 4a shows that both factors are important for bio-cement production.
Fig. 4.
Main effect (a) and interaction (b) plots for bio-cement yield.
An interaction plot is a visual image of the interplay of two input parameters, temperature and pH on bio-cement yield. It is important to note that if the lines are parallel, there is no interaction within the factors. However, if the difference in slope between the lines is higher, there is a greater magnitude of interaction. The data in Fig. 4b indicates that the bio-cement yield decreases with the levels to a minimum value and then increases with the levels. Such trend implies that both operating factors (e.g., T, and pH) have significant impact on the bio-cement production. Further, T and pH have both positive and negative impacts on the response. However, the interaction effect among factors (e.g., T and pH) was absence or low on the bio-cement yield, which agrees with the ANOVA results in Table 2 (p-value = 0.892).
3.3.3. Optimization of bio-cement yield
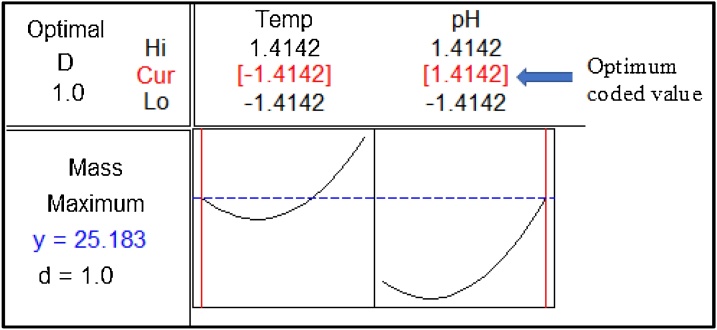
A single optimal set of environmental conditions to maximize bio-cement yield was determined by using response optimizer plot, which is generated via Statistical software, Minitab®. Fig. 5 shows the response optimizer plot for bio-cement yield. The optimum coded values for T and pH are -0.1.4142 and 1.4142, respectively. The true predicted values (calculated using Eq. 7) for these coded values are 23 of temperature and 10.62 of pH, resulting the maximum calcium carbonate yield of 25.183 g. Bio-cement production was carried out for three times at optimized conditions, and the average value on bio-cement yield was calculated. The predicted and experimental bio-cement yields were reported as 26.2 and 27.1 g, respectively, with less than 5% error. Thus, the optimized conditions for the process is verified.
Fig. 5.
Response optimizer plot for bio-cement yield. Here, d and D are individual and overall desirability functions of the response, respectively (ideal value of d or D = 1). Hi, Lo and Cur are high, low and current factors level (coded values) settings, respectively.
3.4. Characterization of calcium carbonate precipitates
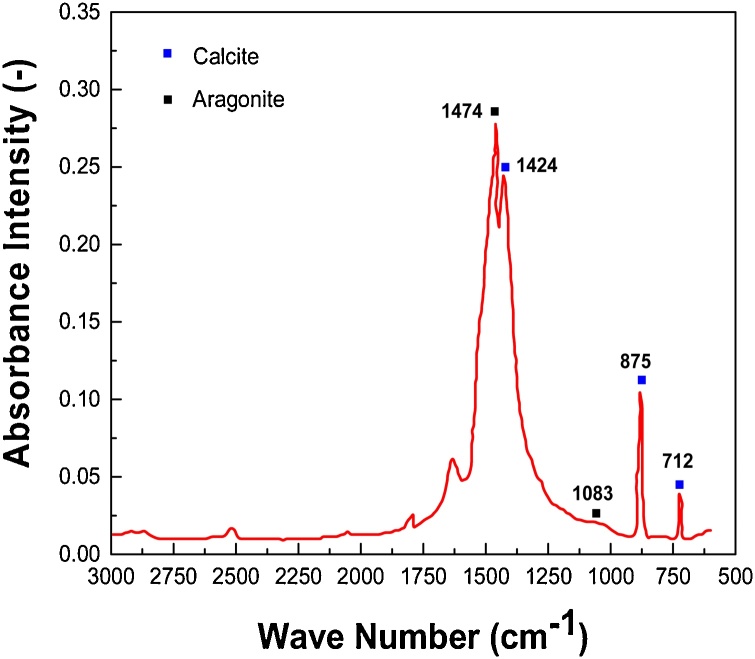
The precipitates produced for the bio-cement study was characterized by FTIR, EDX, XRD and compressive strength tests. For the identification of bio-cement precipitate i.e. limestone (calcite and aragonite), FTIR analytical technique was used. From analysis, corresponding spectrograms were obtained for calcite and aragonite showing wavelengths and absorbance intensities of different functional groups. The vibrational bands at 1474 cm−1, 1424 cm−1, 875 cm−1 and 712 cm−1 confirmed that sample consists of both aragonite and calcite. The sharp peak at 1474 cm−1 belongs to aragonite (prismatic crystal nature i.e. form of limestone) however, the sharp peaks at 1424 cm−1, 875 cm−1 and 712 cm−1 are characteristic of calcite as shown in Fig. 6. The band at 1083 cm−1 enables to determine the contents of calcite in aragonite and of aragonite in calcite. The smaller peak at 2515 cm−1 probably indicates a C–O stretch whereas the pack of two to three peaks between 2800 cm−1 and 3000 cm−1 represent a C–H stretching indicating that traces of hydrocarbons are present in the sample. The observed functional group peaks of the precipitates formed were then compared with the literature indicating that precipitates formed are composed of CaCO3 [28,29].
Fig. 6.
FTIR analysis of produced precipitates.
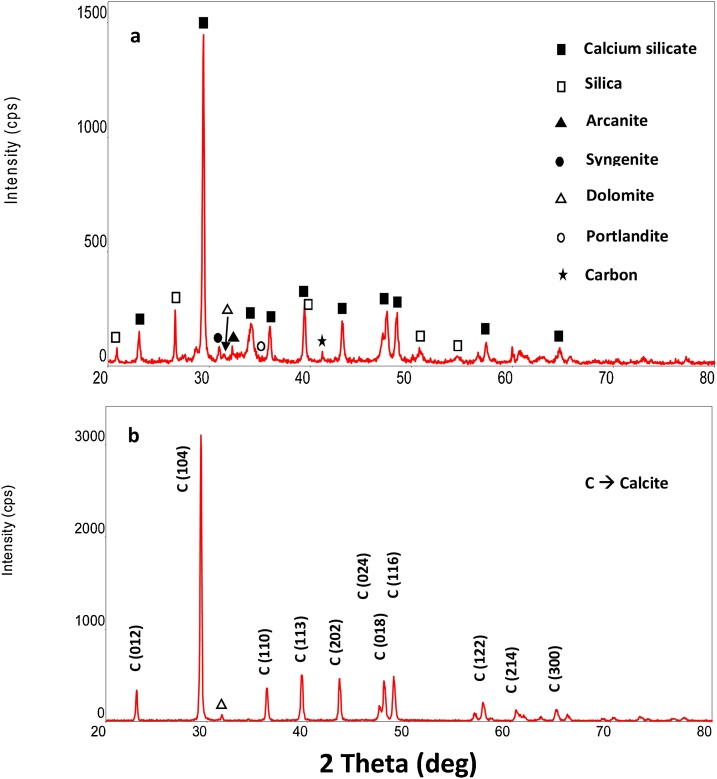
XRD analysis shows that CKD is amorphous in nature and sample contains mainly silica (SiO2) and calcium which exists in the form of calcium silicate (CaSiO3). Black and hollow square symbols on the respective peaks represent the presence of calcium and silica compounds as shown in Fig. 7a. In addition, analysis also shows the presence of arcanite (▲), sungenite (●), dolomite (Δ) and portlandite (○) phases. Peaks shown in Fig. 7b clearly indicate the precipitation of pure calcite nanoparticles with crystalline size of 58.3 nm in the presence of micro algae of day 9 (log phase) at 25 °C and pH 7.2. The crystalline size was estimated by employing a well-known equation named Debye-Scherrer Eq. (8) which is given as follows [30]:
| (8) |
where, D is the crystalline size, λ is the wavelength of x-ray with value of 1.5418 Å for CuKα, K is a crystalline shape factor of which the approximate value is 0.9, θ is the Bragg’s angle and β denotes the full width at half maximum intensity.
Fig. 7.
XRD pattern of (a) CKD sample (b) precipitate produced using microalgae.
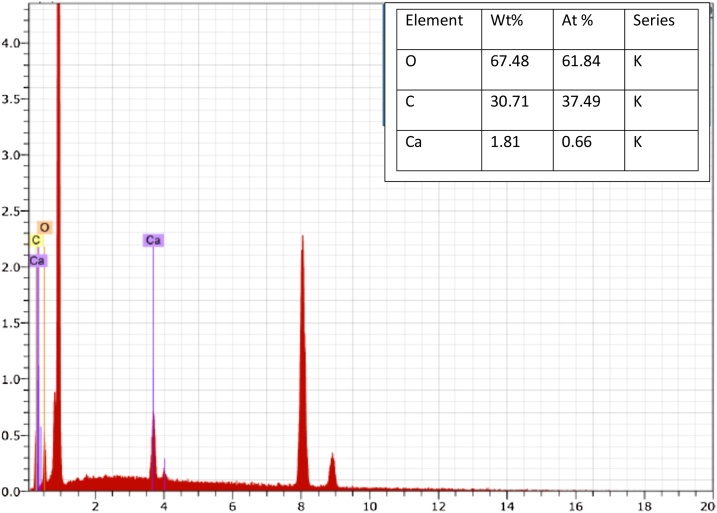
In addition, the silicate particles were not part of the crystals, as they precipitated at the bottom of the bioreactor. Some additional peaks are also observed which are attributed to impurities formed during the precipitation. However, at higher pH 9.0 and pH 10, tiny peaks of dolomite (CaMg(CO3)2) were also observed indicating that at higher pH, magnesium also started precipitating and started incorporating with calcium forming dolomite. EDX of the precipitates was also performed as shown in Fig. 8. Similar to the XRD, EDX data clearly indicate the CaCO3 precipitates which are formed during the microbial reaction at pH 7 and 25 °C at day 9. The embedded table in Fig. 8 shows the Wt% and At% of each element of the precipitates produced indicating that the precipitates produced by microbial process consist of calcium, carbon and oxygen i.e. CaCO3.
Fig. 8.
EDX pattern of precipitate produced using microalgae.
It is well known that the compressive strength is the maximum compressive stress at which a given solid material can sustain without having any fracture by applying gradual load on the material. The compression strength test of the produced biocement combining with the conventional cement for different ratios were also determined. For each ratio of conventional cement to biocement, the average compressive strength was calculated. The compressive strength increases with the increase of cement to biocement ratio as shown in Table 3. The values of all compressive strength in the presence of biocement were higher than that of the conventional cement (16 N/mm2) alone. It clearly indicates that at 1:1 ratio of cement to biocement, strength is found to be 20 N/mm2 and it increases at higher ratios i.e. 34 N/mm2 and 42 N/mm2 at ratios of 2:1 and 3:1, respectively. Thus, the compression strength test indicates that the overall strength increases with the supplement of CaCO3 precipitate. The probable reason for this is that the precipitates may propagate continuously in the mortar sample and give strength to the cemented material by filling the pores in the specimen. In addition, compression strength may be enhanced by increasing the compression of the cemented material and the presence of dead weight to decrease the void volume [4]. The strength with pure biocement could not be calculated as the specimens were found to be unstable during curing process.
Table 3.
Effect of conventional cement to biocement ratio on the compression strength.
| Cement / biocement ratio | Compressive strength (N/mm2) |
|---|---|
| 0:1 | – |
| 1:0 | 16 |
| 1:1 | 20 |
| 2:1 | 34 |
| 3:1 | 42 |
4. Conclusions
Optimization of bio-cement production from cement kiln dust using microalgae was carried out to estimate the effects of the operating parameters (i.e. reaction temperature, reaction initial pH, time-intervals of microalgae culture) on calcium carbonate yield. Preliminary data indicated that all these process parameters are important for bio-cement production. A statistical RSM with CCD approach was introduced for advanced optimization of reaction temperature and reaction initial pH. As such, a nonlinear model was generated to predict the maximum CaCO3 yield. The maximum CaCO3 yield of 25.18 g was obtained at 23 °C, pH of 10.62 with day-9 microalgae culture. Under optimal values, a maximum of 96% calcium from CKD was used for CaCO3 yield. The production of bio-cemented product was confirmed by using several analytical tools (e.g., FTIR, EDS and XRD). The compression test indicated that the strength of the conventional cement supplemented with CaCO3 precipitate was comparatively higher than that of conventional cement alone and it increases with the increase of cement to bio-cement ratio. The anticipated data agreed well with experimental values. Thus, the optimization study of microalgae’s involvement in bio-cement production could lead the expedition of this process in construction engineering as well as mitigation of environmental pollution by waste utilization.
Acknowledgements
The authors would like to gratefully acknowledge the support provided by King Abdulaziz City for Science and Technology (KACST) through the Science & Technology Unit at King Fahd University of Petroleum & Minerals (KFUPM) for funding this work through project No. KACST A-T-32-62 as part of the National Science, Technology and Innovation Plan.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2019.e00356.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Bobicki E.R., Liu Q., Xu Z., Zeng H. Carbon capture and storage using alkaline industrial wastes. Prog. Energy Combust. Sci. 2012;38:302–320. [Google Scholar]
- 2.Katsuyama Y., Yamasaki A., Iizuka A., Fujii M., Kumagai K., Yanagisawa Y. Development of a process for producing high-purity calcium carbonate (CaCO3) from waste cement using pressurized CO2. Environ. Prog. 2005;24:162–170. [Google Scholar]
- 3.Kashef-Haghighi S., Ghoshal S. CO2 sequestration in concrete through accelerated carbonation curing in a flow-through reactor. Ind. Eng. Chem. Res. 2010;49:1143–1149. [Google Scholar]
- 4.Al-Thawadi S., Cord-Ruwisch R. Calcium carbonate crystals formation by ureolytic bacteria isolated from Australian soil and sludge. J. Adv. Sci. Eng. Res. 2012;2:12–26. [Google Scholar]
- 5.Reddy S.R.L., Manjusha A., Arun Kumar M. Bio cement – an eco friendly construction material. Int. J. Curr. Eng. Technol. 2015;55:2277–4106. [Google Scholar]
- 6.Wang J., Ersan Y.C., Boon N., De Belie N. Application of microorganisms in concrete: a promising sustainable strategy to improve concrete durability. Appl. Microbiol. Biotechnol. 2016;100:2993–3007. doi: 10.1007/s00253-016-7370-6. [DOI] [PubMed] [Google Scholar]
- 7.Dhami N.K., Reddy M.S., Mukherjee A. Bacillus megaterium mediated mineralization of calcium carbonate as biogenic surface treatment of green building materials. World J. Microbiol. Biotechnol. 2013;29:2397–2406. doi: 10.1007/s11274-013-1408-z. [DOI] [PubMed] [Google Scholar]
- 8.De Muynck W., De Belie N., Verstraete W. Microbial carbonate precipitation in construction materials: a review. Ecol. Eng. 2010;36:118–136. [Google Scholar]
- 9.Abreu A.P., Fernandes B., Vicente A.A., Teixeira J., Dragone G. Mixotrophic cultivation of Chlorella vulgaris using industrial dairy waste as organic carbon source. Bioresour. Technol. 2012;118:61–66. doi: 10.1016/j.biortech.2012.05.055. [DOI] [PubMed] [Google Scholar]
- 10.Chiu S.Y., Kao C.Y., Tsai M.T., Ong S.C., Chen C.H., Lin C.S. Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresour. Technol. 2009;100:833–838. doi: 10.1016/j.biortech.2008.06.061. [DOI] [PubMed] [Google Scholar]
- 11.Chiu S.Y., Kao C.Y., Tsai M.T., Ong S.C., Chen C.H., Lin C.S. Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresour. Technol. 2009;100:833–838. doi: 10.1016/j.biortech.2008.06.061. [DOI] [PubMed] [Google Scholar]
- 12.Heath C.R., Leadbeater B.C.S., Callow M.E. Effect of inhibitors on calcium carbonate deposition mediated by freshwater algae. J. Appl. Phycol. 1995;7:367–380. [Google Scholar]
- 13.Santomauro G., Baier J., Huang W., Pezold S., Bill J. Formation of calcium carbonate polymorphs induced by living microalgae. J. Biomater. Nanobiotechnol. 2012;03:413–420. [Google Scholar]
- 14.Ariyanti D., ayani Hadiyanto N.A.H. Feasibility of {using} {microalgae} for {biocement} {production} through {biocementation} J. Bioprocess. Biotechnol. 2012;2 [Google Scholar]
- 15.Achal V., Mukherjee A., Reddy M.S. Microbial concrete: way to enhance the durability of building structures. J. Mater. Civ. Eng. 2011;23:730–734. [Google Scholar]
- 16.Rathi P., Goswami V.K., Sahai V., Gupta R. Statistical medium optimization and production of a hyperthermostable lipase from Burkholderia cepacia in a bioreactor. J. Appl. Microbiol. 2002;93:930–936. doi: 10.1046/j.1365-2672.2002.01780.x. [DOI] [PubMed] [Google Scholar]
- 17.Niu B., Wang H., Wang J., Tan L. Multi-objective bacterial foraging optimization. Neurocomputing. 2013;116:336–345. [Google Scholar]
- 18.Hossain S.M.Z., Alnoaimi A., Razzak S.A., Ezuber H., Al-Bastaki N., Safdar M., Alkaabi S., Hossain M.M. Multiobjective optimization of microalgae (Chlorella sp.) growth in a photobioreactor using Box-Behnken design approach. Can. J. Chem. Eng. 2018;96:1903–1910. [Google Scholar]
- 19.Hossain S.M.Z., Hossain M.M., Razzak S.A. Optimization of CO2Biofixation by Chlorella vulgaris using a tubular photobioreactor. Chem. Eng. Technol. 2018;41:1313–1323. [Google Scholar]
- 20.Zakir Hossain S.M., Sultana N., Irfan M.F., Elkanzi E.M.A., Al-Aali Y.A.M., Taha A., Manirul Haque S. Optimization of biodiesel production from spent palm cooking oil using fractional factorial design combined with the response surface methodology. Am. J. Appl. Sci. 2016;13:1255–1263. [Google Scholar]
- 21.Dhami N.K., Alsubhi W.R., Watkin E., Mukherjee A. Bacterial community dynamics and biocement formation during stimulation and augmentation: implications for soil consolidation. Front. Microbiol. 2017;8 doi: 10.3389/fmicb.2017.01267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dhami N.K., Alsubhi W.R., Watkin E., Mukherjee A. Bacterial community dynamics and biocement formation during stimulation and augmentation: implications for soil consolidation. Front. Microbiol. 2017;8 doi: 10.3389/fmicb.2017.01267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anjos M., Fernandes B.D., Vicente A.A., Teixeira J.A., Dragone G. Optimization of CO2bio-mitigation by Chlorella vulgaris. Bioresour. Technol. 2013;139:149–154. doi: 10.1016/j.biortech.2013.04.032. [DOI] [PubMed] [Google Scholar]
- 24.Kazeem M.A., Hossain S.M.Z., Hossain M.M., Razzak S.A. Application of central composite design to optimize culture conditions of Chlorella vulgaris in a batch photobioreactor: an efficient modeling approach. Chem. Prod. Process Model. 2018 [Google Scholar]
- 25.Rajendran A., Selvaraj V., Thangavelu V. Statistical optimization and kinetic modeling of xylanase production by Arthrobacter sp. Asia-Pac. J. Chem. Eng. 2008;3:347–353. [Google Scholar]
- 26.Ramnani P., Gupta R. Optimization of medium composition for keratinase production on feather by Bacillus licheniformis RG1 using statistical methods involving response surface methodology. Biotechnol. Appl. Biochem. 2004;40:191. doi: 10.1042/BA20030228. [DOI] [PubMed] [Google Scholar]
- 27.Binnal P., Babu P.N. Statistical optimization of parameters affecting lipid productivity of microalga Chlorella protothecoides cultivated in photobioreactor under nitrogen starvation. South African J. Chem. Eng. 2017;23:26–37. [Google Scholar]
- 28.Kiran B., Pathak K., Kumar R., Deshmukh D. Statistical optimization using Central Composite Design for biomass and lipid productivity of microalga: a step towards enhanced biodiesel production. Ecol. Eng. 2016;92:73–81. [Google Scholar]
- 29.Jacob D.E., Wirth R., Agbaje O.B.A., Branson O., Eggins S.M. Planktic foraminifera form their shells via metastable carbonate phases. Nat. Commun. 2017;8 doi: 10.1038/s41467-017-00955-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rani B.J., Ravina M., Saravanakumar B., Ravi G., Ganesh V., Ravichandran S., Yuvakkumar R. Ferrimagnetism in cobalt ferrite (CoFe2O4) nanoparticles. Nano-Struct. Nano-Objects. 2018;14:84–91. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.