Abstract
Introduction
Epithelial–mesenchymal transition (EMT) induces the loss of cell–cell interactions in polarized epithelial cells and converts these cells to invasive mesenchymal-like cells. It is also involved in tissue fibrosis including that occurring in some ocular surface diseases such as pterygium and in subepithelial corneal fibrosis in limbal stem cell deficiency. Here, we examined the effects of the secretome of human adipose-derived mesenchymal stem cells (AdMSCs) on EMT in human corneal epithelial cells (CECs).
Methods
EMT was induced with transforming growth factor-β (TGF-β) in primary human CECs isolated from the human corneal limbus. The effects of the AdMSC secretome on EMT in these cells or stratified CEC sheets were analyzed by co-cultivation experiments with the addition of AdMSC conditioned-medium. The expression of EMT-related genes and proteins in CECs was analyzed. The superstructure of CECs was observed by scanning electron microscopy. Furthermore, the barrier function of CEC sheets was analyzed by measuring transepithelial electrical resistance (TER).
Results
The AdMSC secretome was found to suppress EMT-related gene expression and attenuate TGF-β-induced corneal epithelial dysfunction including the dissociation of cell–cell interactions and decreases in TER in constructed CEC sheets.
Conclusions
The secretome of AdMSCs can inhibit TGF-β-induced EMT in CECs. These findings suggest that this could be a useful source for the treatment for EMT-related ocular surface diseases.
Keywords: Human corneal epithelial cell(s), EMT, Human MSCs, MSC-conditioned medium (CM), TGF-β
Abbreviations: CEC(s), corneal epithelial cell(s); EMT, epithelial–mesenchymal transition; MSCs, mesenchymal stem cells; MSC-CM, MSC-conditioned medium; TGF-β, transforming growth factor-β
Highlights
-
•
Application of MSC secretome has potential as a cell-free therapy.
-
•
AdMSC secretome attenuates EMT-related expression in corneal epithelial cells (CECs).
-
•
AdMSC secretome mitigates TGF-β-induced inhibition of cell–cell interactions in CECs.
-
•
AdMSC secretome abrogates TGF-β-mediated barrier disruption in CEC sheets.
1. Introduction
Epithelial–mesenchymal transition (EMT) is a phenomenon in which polarized epithelial cells are transformed into mesenchymal- or myofibroblast-like cells. This process plays a critical role in embryonic development and tumor metastasis. It also contributes to tissue repair such as in wound healing [1]. However, a prolonged inflammatory state or excessive EMT leads to tissue fibrosis in organs such as the liver, lung, pancreas, and skin [2], [3], [4]. EMT also contributes to various eye diseases such as proliferative vitreoretinopathy and posterior capsule opacification, a complication of cataract surgery [5], [6], [7], [8]. It has been suggested that EMT is involved in ocular surface diseases such as pterygium [9], corneal subepithelial fibrosis [10], and Epstein–Barr virus (EBV)-associated keratitis [11]. Furthermore, when EMT occurs in corneal epithelial cells (CECs), it leads to disruptions in proliferation and the maintenance of homeostasis [12], [13]. Therefore, the suppression of EMT in the ocular surface might represent an effective treatment for EMT-related ocular surface diseases to maintain homeostasis. However, the detailed mechanism underlying EMT in these conditions has not been elucidated, and anti-EMT treatment for ocular surface diseases is not well established.
Mesenchymal stem cells (MSCs) are multipotent cells that can differentiate into three germ layer-lineages such as the mesodermal-lineage including osteoblasts, adipocytes, and chondrocytes [14]. MSCs also possess proliferative potential and therapeutic properties such as anti-inflammatory and immunomodulatory effects [15], [16]. Thus, these cells are a very useful source for cell-based therapies. Indeed, MSCs have been clinically applied for the treatment of various ailments such as graft-versus-host disease (GVHD) and cardiovascular, neurological, and autoimmune diseases [17]. Further, paracrine effects are thought to be responsible for the therapeutic effects of MSCs [18], which have been shown to suppress not only internal organ fibrosis such as visceral fibrosis, but also scaring and fibrosis on body surface tissues such as the skin, processes suggested to be mediated by EMT [19], [20], [21], [22], [23]. MSCs exist in various tissues. In particular, there are many reports of the effects of bone marrow-, adipose-, and umbilical cord-derived MSCs. Since Adipose tissue is easy to obtain, as compared to bone marrow, human adipose-derived MSCs (AdMSCs) are considered useful for practical applications of regenerative therapy [24].
Accordingly, the aim of this study was to examine the effects of the AdMSC secretome on EMT in the ocular surface by utilizing primary human corneal epithelial cells (CECs). This is the first report describing these effects.
2. Methods
2.1. Cell culture
Primary cultures of human CECs were established as previously described [25]. CECs were maintained with CnT-PR (CELLnTEC, Bern, Switzerland) with or without 20 ng/mL of recombinant human keratinocyte growth factor (KGF; Wako Pure Chemical Industries, Osaka, Japan) and 10 μM Y-27632 (Wako) for monolayer culture and DMEM:F12 (Life Technologies, Chesterfield, MO, USA) supplemented with 2% B-27 supplement, 20 ng/mL KGF, and 10 μM Y-27632 to reconstruct CEC sheets [26]. Recombinant human TGF-β1 used for induction of EMT into CECs was obtained from Peprotech (Rocky Hill, NJ, USA). Human AdMSCs were acquired from PromoCell (Heidelberg, Germany) and maintained with MSCGM-CD (Lonza) or Mesenchymal Stem Cell Growth Medium DXF (PromoCell). For the preparation of AdMSC-conditioned medium (CM), AdMSCs were cultured to 70–80% confluence in a T-75 flask. Subsequently, medium was changed to fresh medium. After 24–72 h of culture, the supernatant was collected, centrifuged at 300×g, and stored at −80 °C prior to use.
2.2. Co-culture of AdMSCs with TGF-β-treated CECs
Human CECs were seeded in 24-well plates at a density of 5 × 104 cells/well. After 24 h of incubation, they were cultured with CnT-PR containing 10 ng/ml of recombinant human TGF-β1. After 4 days of culture, the medium containing TGF-β1 was removed and changed to CnT-PR. Subsequently, 10,000 or 20,000 AdMSCs per insert were co-cultured with MSCGM-CD medium using cell culture inserts (BD Falcon, Franklin Lakes, NJ, USA) for 2 days.
2.3. Quantitative real-time reverse-transcriptase PCR (qRT-PCR)
Total RNA was extracted from cells using QIAzol reagent (QIAGEN, Valencia, CA, USA). SuperScript III First-Strand Synthesis System for qRT-PCR (Life Technologies) was then used to synthesize cDNA. qRT-PCR was performed using an ABI Prism 7500 Fast Sequence Detection System (Life Technologies) according to the manufacturer's instructions. TaqMan® MGB was used as described in Supplemental Table 1.
2.4. Immunofluorescence staining
Cells or cell sheets were fixed with 4% paraformaldehyde (PFA), washed three times with tris-buffered saline (TBS, TaKaRa Bio, Otsu, Japan), and incubated with TBS containing 5% donkey serum and 0.3% triton X-100 (Sigma–Aldrich, St. Louis, MO, USA) for 1 h. They were then incubated with primary antibodies (anti-VIM antibody, Abcam, ab92547, 1:200; anti-CLDN1 antibody, Life Technologies, 374900, 1:500) overnight at 4 °C and stained with Alexa Fluor®-conjugated secondary antibodies (Life Technologies) and Hoechst 33342 (Molecular Probes, Eugene, OR, USA). Stained samples were observed using an Axio Observer D1 and LSM710 (Carl Zeiss, Oberkochen, Germany). Images of three-dimensional CEC sheets were established using Zen software (Carl Zeiss).
2.5. Scanning electron microscopy (SEM)
After culturing hCECs on culture inserts, cells were fixed with 2% glutaraldehyde/2% PFA in phosphate-buffered saline (PBS) for 2 h at 4 °C. After washing three times with PBS, it was dehydrated with an ethanol series (30%, 50%, 70%, 80%, 90%, 95%, and 99.5% ethanol) and further treated with t-butyl alcohol. Cells on culture inserts were dried in a freeze-drying device (JFD-320; JEOL Ltd., Tokyo, Japan). Then, the bottom side of the membrane of the culture insert was adhered to the sampling stage with carbon tape. The surrounding membrane was cut off using disposable biopsy punches resulting in removal of the surrounding plastic container of the insert. The specimen pasted onto the sampling stage was subjected to platinum coating using an auto fine coater (FCL8 1600; JEOL) for 30 s. Then, the sample was observed by SEM (JSM-6510LA; JEOL) at 20 kV.
2.6. Measurement of transepithelial electrical resistance (TER)
Human CECs were seeded onto 12-well cell culture inserts. Electrical resistance was measured using the MilliCell ERS-2 (Millipore, Billerica, MA, USA) according to the manufacturer's instructions.
2.7. Statistical analysis
Data are expressed as the means ± SEM. Statistical analysis was performed based on the Dunnett's test and Steel test, and statistics were calculated using StatLight 2000 software (Yukms Co. Ltd., Tokyo, Japan).
3. Results
3.1. Co-culture with AdMSCs attenuates TGF-β1-induced EMT in CECs
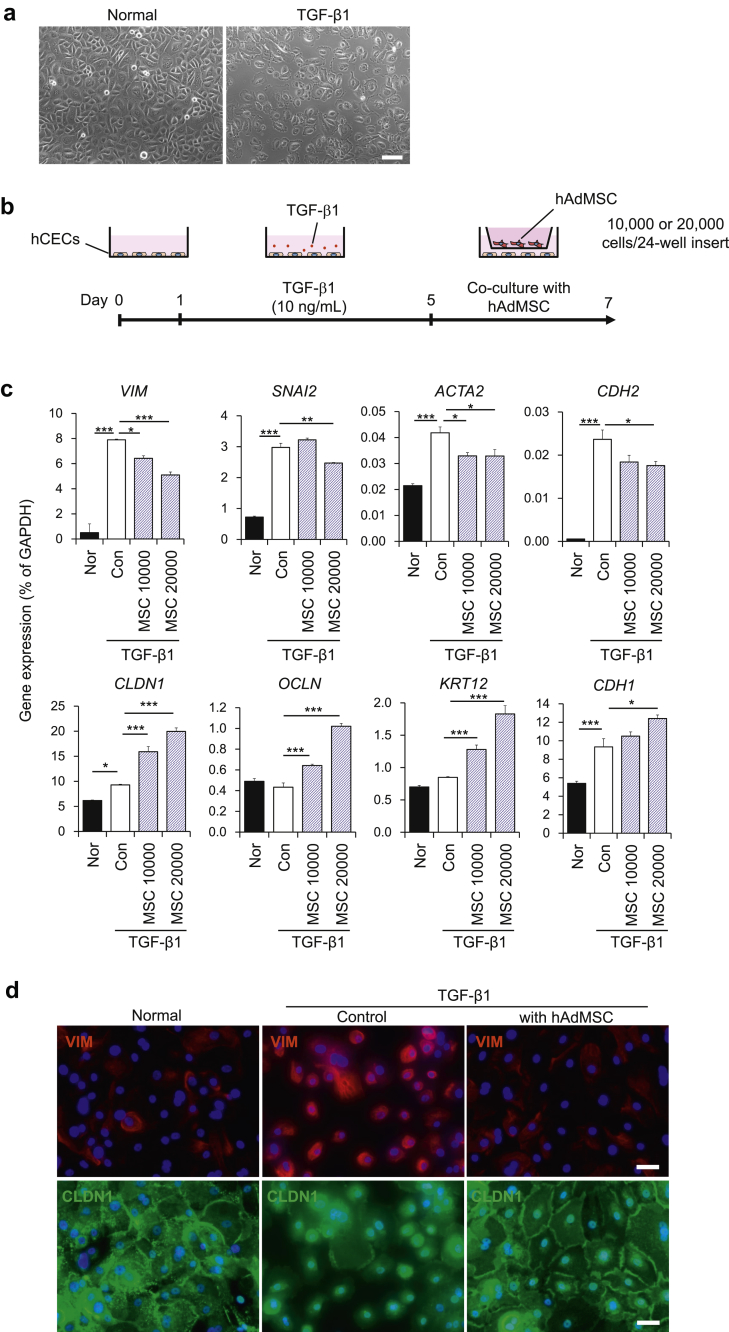
We first examined the effects of TGF-β1 on primary CECs isolated from the human cornea. Consistent with previous reports, TGF-β1 induced an EMT phenotype and abolished cell–cell interactions (Fig. 1a and Supplemental Fig. 1). Next, we investigated the effects of the AdMSC secretome on TGF-β1-induced EMT in CECs. After inducing EMT with TGF-β1, CECs were co-cultured with AdMSCs using cell culture inserts (Fig. 1b). For the non-TGFβ1-treated normal (Nor) and TGFβ1-treated control (Con) groups, the culture insert was not seeded with AdMSC, and only MSC maintenance medium was added. Co-culture with AdMSCs attenuated the TGF-β1-induced up-regulation of mesenchymal-related genes such as VIM, SNAI2, ACTA2, and CDH2 in CECs. Moreover, this also increased gene expression levels of epithelial genes such as CLDN1, OCLN, KRT12, and CDH1 (Fig. 1c). These effects on suppression of VIM and the increase of epithelial genes were dose-dependent (i.e., cell number-dependent). Immunostaining results also showed that TGF-β1-induced EMT phenotypes including increased expression of VIM and the mislocalization of CLDN1 between cells were abrogated by co-cultivation with AdMSCs (Fig. 1d). These results showed that the AdMSC secretome could attenuate TGF-β1-induced EMT in CECs.
Fig. 1.
Co-culture with mesenchymal stem cells (MSCs) attenuates TGF-β1-induced epithelial–mesenchymal transition (EMT) in corneal epithelial cells (CECs). (a) Phase contrast images of CECs with or without TGF-β1 treatment. Scale bar, 100 μm. (b) Schematic of experimental method. (c) Gene expression analysis of EMT-related markers in CECs with or without co-culture with AdMSC (10,000 or 20,000 cells/insert). Data are expressed as the means ± SEM; n = 4 cell samples; *p < 0.05, **p < 0.01, and ***p < 0.001. (d) Immunostaining for VIM (red) and CLDN1 (green) in CECs. Nuclei, blue; scale bars, 100 μm.
3.2. AdMSC-CM treatment attenuates TGF-β1-induced EMT in CECs
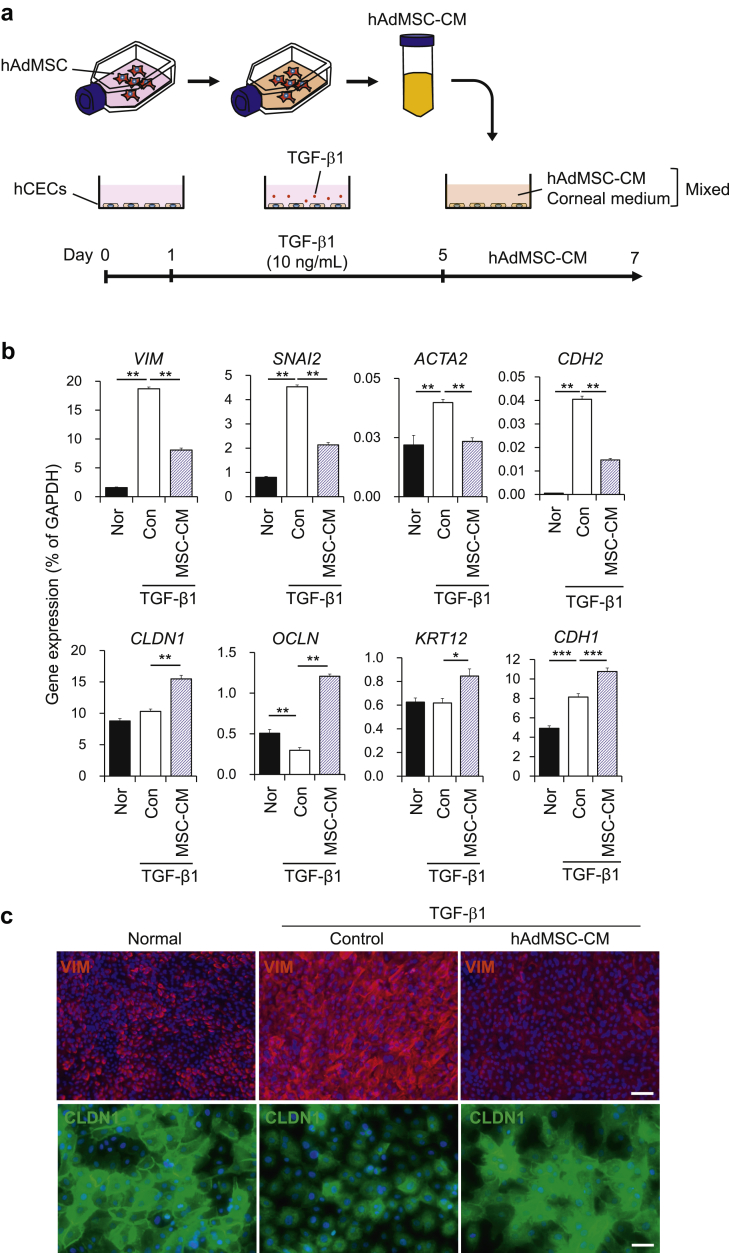
We next examined whether AdMSC-CM could also have an anti-EMT effect on CECs. After treating these cells with TGF-β1, as described previously herein, the medium containing TGF-β1 was removed and AdMSC-CM was added at a 1:1 ratio with medium for CECs (Fig. 2a). For the non-TGFβ1-treated Nor and TGFβ1-treated Con groups, MSC maintenance medium, without cultured AdMSC, was added. Similar to the results of the co-culture experiment, TGF-β1-induced up-regulation of mesenchymal-related genes such as VIM, SNAI2, ACTA2, and CDH2 was attenuated by the addition of AdMSC-CM. This treatment also increased the expression levels of epithelial-related genes such as CLDN1, OCLN, KRT12, and CDH1 (Fig. 2b). Immunostaining results also showed that the increased expression of VIM and mislocalization of CLDN1 in CECs were mitigated by AdMSC-CM treatment (Fig. 2c). We further confirmed that these changes in expression induced by TGF-β were alleviated by AdMSC-CM treatment at the protein level (Supplemental Fig. 2). These results clearly showed that AdMSC-CM could suppress EMT in CECs.
Fig. 2.
Conditioned medium from Adipose-derived mesenchymal stem cells (AdMSC-CM) attenuates TGF-β1-induced epithelial–mesenchymal transition (EMT) in corneal epithelial cells (CECs). (a) Schematic of experimental method. (b) Gene expression analysis of EMT-related markers in CECs. Data are expressed as the means ± SEM; n = 6 cell samples; *p < 0.05, **p < 0.01, and ***p < 0.001. (c) Immunostaining for VIM (red) and CLDN1 (green) in CECs. Nuclei, blue; scale bars, 100 μm (upper panels) and 50 μm (lower panels).
3.3. The apical application of AdMSC-CM attenuates TGF-β1-induced EMT in CEC sheets
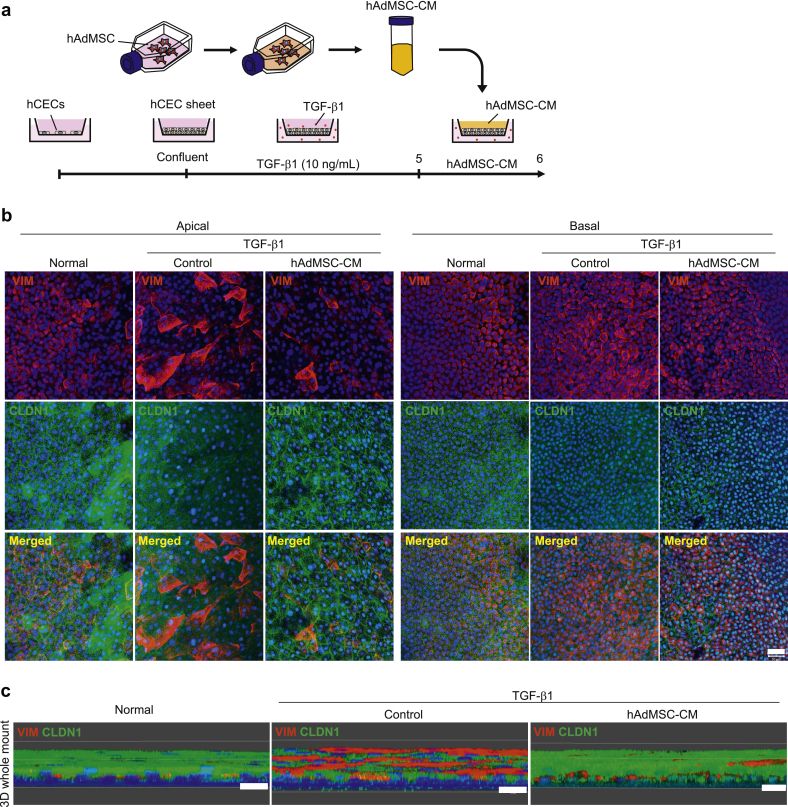
Next, in addition to the influence of the AdMSC secretome on TGF-β1-induced EMT in CEC monolayers, we also examined these effects on confluent and stratified CEC sheets, which more closely resemble the physiological corneal epithelium, and analyzed EMT-related molecules three-dimensionally. We cultured CECs using cell culture inserts until they were confluent and then added TGF-β1 on both the apical and basal sides of the culture insert to induce EMT. Next, AdMSC-CM was added from the apical side (Fig. 3a). Whole-mount immunostaining results revealed that AdMSC-CM could attenuate the TGF-β1-induced up-regulation of VIM and down-regulation of CLDN1, especially on the apical side of the CEC sheet (Fig. 3b). Images of CEC sheets constructed three-dimensionally using Z-stacks are shown in Fig. 3c. From these results, it was confirmed that, even when CEC sheets were structured three-dimensionally, TGF-β1-induced increase in the expression of VIM and decrease in the expression of CLDN1 could be alleviated by the addition of AdMSC-CM.
Fig. 3.
Conditioned medium from Adipose-derived mesenchymal stem cells (AdMSC-CM) attenuates TGF-β1-induced epithelial–mesenchymal transition (EMT) in corneal epithelial cell (CEC) sheets. (a) Schematic of experimental method. (b) Immunostaining for VIM (red) and CLDN1 (green) in CEC sheets at the apical and basal region. Nuclei, blue. Scale bar, 50 μm. (c) Immunostaining for VIM (red) and CLDN1 (green) in whole-mounts of CEC sheets constructed as a three-dimensional image. Nuclei, blue. Scale bars, 20 μm.
3.4. AdMSC-CM restores the TGF-β-induced disruption of cell–cell interactions and barrier function in CEC sheets
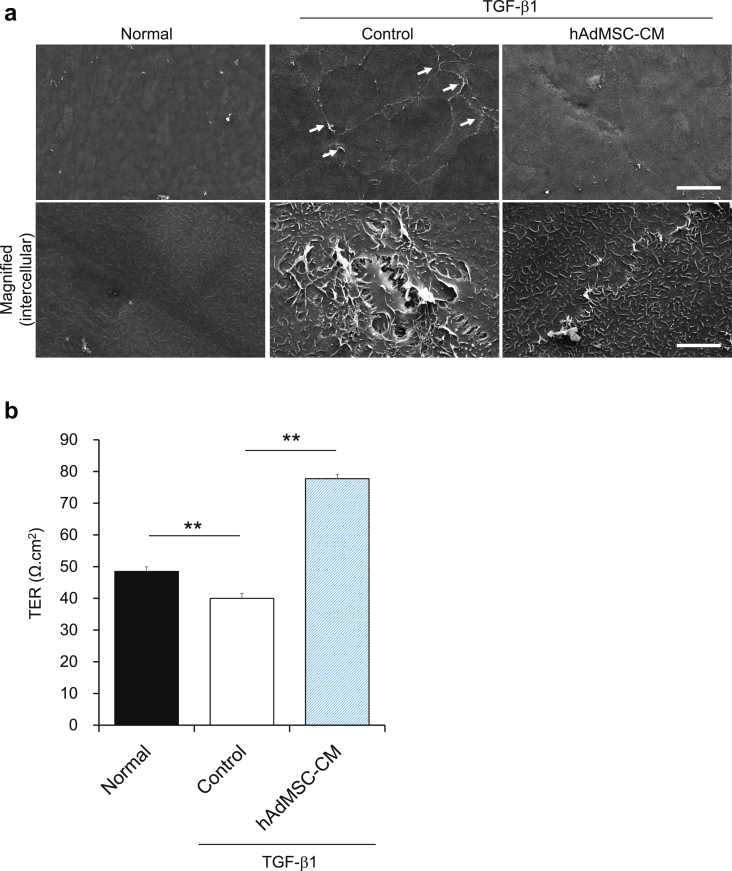
To further investigate the apical surface of the cornea sheet in detail, we observed the ultrastructure of CEC sheets using SEM. We found that TGF-β1 treatment abolished cell–cell interactions and that AdMSC-CM treatment could attenuate this phenotype (Fig. 4a). TGF-β is also known to decrease TER in corneal epithelial cells [27]. We therefore examined whether the decrease in TER induced by TGF-β1 could be recovered by the AdMSC secretome. We found that this treatment could also mitigate the decrease in TER observed in TGF-β1-treated CEC sheets (Fig. 4b). These results suggested that the AdMSC secretome could rescue the TGF-β1-induced disruption of cell–cell interactions, which could contribute to the recovery of barrier functions in stratified CEC sheets.
Fig. 4.
Adipose-derived mesenchymal stem cell (AdMSC) secretome abrogates TGF-β-induced dissociation of cell–cell interactions and decreases in transepithelial electrical resistance (TER) in corneal epithelial cell (CEC) sheets. (a) Ultrastructure of CEC sheet surfaces taken by scanning electron microscopy. The arrows indicate the areas where cell–cell interactions are disrupted. Scale bars, 50 μm (upper panels) and 5 μm (lower magnified images). (b) Quantification of TER in CEC sheets at day 1 after the addition of hAdMSC-CM. The results are presented as the means ± SEM, n = 4 cell samples. **p < 0.01.
4. Discussion
The purpose of the present study was to examine the effects of the human AdMSC secretome on EMT in CECs. We demonstrated that TGF-β1-induced EMT phenotypes in CECs were attenuated by this treatment.
TGF-β is one of the main inducers of EMT. Previous studies have reported the effects of TGF-β on CECs using an SV40-immortalized CEC line [28]. In this study, we investigated the effect of TGF-β1 on primary CECs derived from human donors under serum-free conditions. Consistent with this previous report, the addition of TGF-β1 induced the dissociation of cell–cell contacts and the up-regulation of mesenchymal-related molecules in primary CECs. We also showed that co-culture with AdMSCs and the addition of AdMSC-CM could attenuate these changes in expression in CECs (Fig. 1, Fig. 2). In co-culture experiments, there was a tendency for the effect to increase in a cell number-dependent manner. Epithelial molecules such as CLDN1 were not decreased by TGF-β1 treatment at the mRNA level but were decreased at the protein level at the timepoint of analysis, and this was also attenuated by the AdMSC secretome (Supplemental Fig. 2). CLDN1 also showed aberrant localization with TGF-β1 treatment. However, with AdMSC secretome administration, the localization of CLDN1 at the cell–cell boundary was restored (Fig. 1, Fig. 2). Similarly, the addition of TGF-β1 to CECs increased rather than decreased E-cadherin mRNA level (Fig. 1, Fig. 2). We showed that E-cadherin was reduced at the protein level by treatment with TGF-β1 (Supplementary Fig. 1.). In the assay shown in Fig. 1, Fig. 2, TGF-β1 removal time after TGF-β1 treatment was more than 24 h. During this period, there may have been changes in expression status such as restoration of mRNA expression. To understand the detailed expression mechanism of such epithelial genes showing complex regulation in response to TGF-β1, detailed analysis of the time course and effect of the addition of components to MSC maintenance medium is required. At the same time, the expression at the protein level should be investigated in future studies on regenerative therapy.
We also showed that the AdMSC secretome was effective in attenuating EMT in stratified CEC sheets that recapitulate physiological conditions (Fig. 3). TGF-β1 also caused phenotypic changes in addition to the expression changes in EMT-related molecules in CECs. TGF-β1 induced the dissociation of cell–cell interactions. Moreover, as reported using an immortalized CEC line [27], TGF-β1 decreased the TER in CEC sheets. However, administration of the AdMSC secretome rescued the dissociation of cell–cell interactions and the decrease in TER (Fig. 4). Further, the AdMSC secretome alleviated the expression of mesenchymal factors, which were elevated by TGF-β1, and increased the expression of epithelial factors that were not altered by TGF-β1. This caused the TER to be higher with AdMSC secretome treatment compared to that observed in the untreated controls. This improvement in phenotype when compared with the TGF-β1-untreated group is consistent with the results in Fig. 1, Fig. 2 that show that AdMSC secretome increased expression of epithelial genes when compared with the TGF-β1-untreated Nor group. These results suggest that the AdMSC secretome increases the expression of epithelial genes and molecules responsible for barrier function of CECs, with or without TGF-β1 treatment, in addition to suppressing EMT. The barrier of the corneal epithelium has an important function and protects against invasion by pathogens. The pathways of substance permeation are mainly paracellular and transcellular. Tight junction protein complexes and the mucin layer are involved in the former and latter pathway, respectively [29]. The AdMSC secretome was effective in strengthening tight junctions and repairing abnormalities in cell–cell interactions, which are critical for the barrier function of the corneal epithelium.
As EMT is known to be involved in fibrosis, it could be that some of the effects of MSCs are meditated by suppressing EMT. Actually, MSCs were found to exert an EMT-inhibitory effect on peritoneal mesothelial cells and the human liver [19]. Regarding the application of MSCs to the ocular surface, its efficacy has been proven using in vivo models such as alkali injury [30], [31], dry eye [32], and limbal stem cell deficiency [33], and also dry eye in patients with chronic graft-versus-host disease via intravenous injection [34]. The MSC secretome has anti-inflammatory and anti-angiogenic effects on the cornea [35]. A previous report also showed that CEC damage induced by ethanol can be alleviated by the MSC secretome, and that these therapeutic effects are enhanced by pre-treating MSCs with inflammatory cytokines such as TNF-α [36]. TSG-6 has been reported to be an active component in the MSC secretome [37], [38]. The effect of suppressing EMT in CECs, revealed in this study, is expected to lead to therapeutics based on the MSC secretome. Further studies are needed to verify the efficacy of the MSC secretome using in vivo disease models and to elucidate the underlying mechanism. The MSC secretome is expected to have multiple effects, as previously reported. This cell-free therapy might be associated with fewer safety concerns and could be simply administered as eye drops. Thus, the MSC secretome has great potential for the treatment of ocular surface diseases. However, there are also some obstacles. One is difficulties associated with standardizing ingredients. If we can identify the active component or surrogate markers responsible for the therapeutic effects of the secretome, such as TSG-6, HGF, or BMP7, this will accelerate the development of such therapeutics [39], [40].
5. Conclusions
This is the first study to report that the AdMSC secretome can suppress EMT in CECs. This finding demonstrates usefulness for the administration of the AdMSC secretome as a cell-free treatment and suggests a novel mechanism through which MSCs could act on EMT-related corneal diseases induced by elevated TGF-β1.
Declaration of interest
S.S., T.O., Y.K., and Y.H. are employees of ROHTO Pharmaceutical Co., Ltd. R.H. is affiliated with the endowed chair of ROHTO Pharmaceutical Co., Ltd.
Acknowledgements
We would like to thank S. Hara, Y. Ishikawa, and J. Harrington of the Osaka University for their technical and writing assistance.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.reth.2019.06.005.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Kalluri R., Neilson E.G. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Investig. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeisberg M., Yang C., Martino M., Duncan M.B., Rieder F., Tanjore H. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem. 2007;282:23337–23347. doi: 10.1074/jbc.M700194200. [DOI] [PubMed] [Google Scholar]
- 3.Kim K.K., Kugler M.C., Wolters P.J., Robillard L., Galvez M.G., Brumwell A.N. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A. 2006;103:13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian L., Lu Z.-P., Cai B.-B., Zhao L.-T., Qian D., Xu Q.-C. Activation of pancreatic stellate cells involves an EMT-like process. Int J Oncol. 2016;48:783–792. doi: 10.3892/ijo.2015.3282. [DOI] [PubMed] [Google Scholar]
- 5.Shu D.Y., Lovicu F.J. Myofibroblast transdifferentiation: the dark force in ocular wound healing and fibrosis. Prog Retin Eye Res. 2017;60:44–65. doi: 10.1016/j.preteyeres.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamiya S., Kaplan H.J. Role of epithelial–mesenchymal transition in proliferative vitreoretinopathy. Exp Eye Res. 2016;142:26–31. doi: 10.1016/j.exer.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 7.de Iongh R.U., Wederell E., Lovicu F.J., McAvoy J.W. Transforming growth factor-beta-induced epithelial-mesenchymal transition in the lens: a model for cataract formation. Cells Tissues Organs. 2005;179:43–55. doi: 10.1159/000084508. [DOI] [PubMed] [Google Scholar]
- 8.Wormstone I.M. Posterior capsule opacification: a cell biological perspective. Exp Eye Res. 2002;74:337–347. doi: 10.1006/exer.2001.1153. [DOI] [PubMed] [Google Scholar]
- 9.Kato N., Shimmura S., Kawakita T., Miyashita H., Ogawa Y., Yoshida S. Beta-catenin activation and epithelial-mesenchymal transition in the pathogenesis of pterygium. Investig Ophthalmol Vis Sci. 2007;48:1511–1517. doi: 10.1167/iovs.06-1060. [DOI] [PubMed] [Google Scholar]
- 10.Kawashima M., Kawakita T., Higa K., Satake Y., Omoto M., Tsubota K. Subepithelial corneal fibrosis partially due to epithelial-mesenchymal transition of ocular surface epithelium. Mol Vis. 2010;16:2727–2732. [PMC free article] [PubMed] [Google Scholar]
- 11.Park G Bin, Kim D., Kim Y.S., Kim S., Lee H.-K., Yang J.W. The Epstein-Barr virus causes epithelial-mesenchymal transition in human corneal epithelial cells via Syk/src and Akt/Erk signaling pathways. Investig Ophthalmol Vis Sci. 2014;55:1770–1779. doi: 10.1167/iovs.13-12988. [DOI] [PubMed] [Google Scholar]
- 12.Tiwari A., Loughner C.L., Swamynathan S., Swamynathan S.K. KLF4 plays an essential role in corneal epithelial homeostasis by promoting epithelial cell fate and suppressing epithelial–mesenchymal transition. Investig Opthalmology Vis Sci. 2017;58:2785. doi: 10.1167/iovs.17-21826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawakita T., Espana E.M., Higa K., Kato N., Li W., Tseng S.C.G. Activation of Smad-mediated TGF-β signaling triggers epithelial-mesenchymal transitions in murine cloned corneal progenitor cells. J Cell Physiol. 2013;228:225–234. doi: 10.1002/jcp.24126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ullah I., Subbarao R.B., Rho G.J. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep. 2015;35:1–18. doi: 10.1042/BSR20150025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang X., Ding Y., Zhang Y., Tse H.-F., Lian Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant. 2014;23:1045–1059. doi: 10.3727/096368913X667709. [DOI] [PubMed] [Google Scholar]
- 16.Glenn J.D., Whartenby K.A. Mesenchymal stem cells: emerging mechanisms of immunomodulation and therapy. World J Stem Cell. 2014;6:526. doi: 10.4252/wjsc.v6.i5.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Squillaro T., Peluso G., Galderisi U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant. 2016;25:829–848. doi: 10.3727/096368915X689622. [DOI] [PubMed] [Google Scholar]
- 18.Vizoso F.J., Eiro N., Cid S., Schneider J., Perez-Fernandez R. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci. 2017;18:1852. doi: 10.3390/ijms18091852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ueno T., Nakashima A., Doi S., Kawamoto T., Honda K., Yokoyama Y. Mesenchymal stem cells ameliorate experimental peritoneal fibrosis by suppressing inflammation and inhibiting TGF-β1 signaling. Kidney Int. 2013;84:297–307. doi: 10.1038/ki.2013.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu B., Ding F.-X., Liu Y., Xiong G., Lin T., He D.-W. Human umbilical cord-derived mesenchymal stem cells conditioned medium attenuate interstitial fibrosis and stimulate the repair of tubular epithelial cells in an irreversible model of unilateral ureteral obstruction. Nephrology (Carlton) 2017;23:728–736. doi: 10.1111/nep.13099. [DOI] [PubMed] [Google Scholar]
- 21.Huang S., Wu Y., Gao D., Fu X. Paracrine action of mesenchymal stromal cells delivered by microspheres contributes to cutaneous wound healing and prevents scar formation in mice. Cytotherapy. 2015;17:922–931. doi: 10.1016/j.jcyt.2015.03.690. [DOI] [PubMed] [Google Scholar]
- 22.Wu Y., Huang S., Enhe J., Ma K., Yang S., Sun T. Bone marrow-derived mesenchymal stem cell attenuates skin fibrosis development in mice. Int Wound J. 2014;11:701–710. doi: 10.1111/iwj.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qi Y., Jiang D., Sindrilaru A., Stegemann A., Schatz S., Treiber N. TSG-6 released from intradermally injected mesenchymal stem cells accelerates wound healing and reduces tissue fibrosis in murine full-thickness skin wounds. J Investig Dermatol. 2014;134:526–537. doi: 10.1038/jid.2013.328. [DOI] [PubMed] [Google Scholar]
- 24.Strioga M., Viswanathan S., Darinskas A., Slaby O., Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cell Dev. 2012;21:2724–2752. doi: 10.1089/scd.2011.0722. [DOI] [PubMed] [Google Scholar]
- 25.Yoshihara M., Sasamoto Y., Hayashi R., Ishikawa Y., Tsujikawa M., Hayashizaki Y. High-resolution promoter map of human limbal epithelial cells cultured with keratinocyte growth factor and rho kinase inhibitor. Sci Rep. 2017;7:2845. doi: 10.1038/s41598-017-02824-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyashita H., Yokoo S., Yoshida S., Kawakita T., Yamagami S., Tsubota K. Long-term maintenance of limbal epithelial progenitor cells using rho kinase inhibitor and keratinocyte growth factor. Stem Cells Transl Med. 2013;2:758–765. doi: 10.5966/sctm.2012-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Contreras-Ruiz L., Schulze U., García-Posadas L., Arranz-Valsero I., López-García A., Paulsen F. Structural and functional alteration of corneal epithelial barrier under inflammatory conditions. Curr Eye Res. 2012;37:971–981. doi: 10.3109/02713683.2012.700756. [DOI] [PubMed] [Google Scholar]
- 28.Aomatsu K., Arao T., Sugioka K., Matsumoto K., Tamura D., Kudo K. TGF-β induces sustained upregulation of SNAI1 and SNAI2 through Smad and non-Smad pathways in a human corneal epithelial cell line. Investig Ophthalmol Vis Sci. 2011;52:2437–2443. doi: 10.1167/iovs.10-5635. [DOI] [PubMed] [Google Scholar]
- 29.Leong Y.-Y., Tong L. Barrier function in the ocular surface: from conventional paradigms to new opportunities. Ocul Surf. 2015;13:103–109. doi: 10.1016/j.jtos.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Almaliotis D., Koliakos G., Papakonstantinou E., Komnenou A., Thomas A., Petrakis S. Mesenchymal stem cells improve healing of the cornea after alkali injury. Graefes Arch Clin Exp Ophthalmol. 2015;253:1121–1135. doi: 10.1007/s00417-015-3042-y. [DOI] [PubMed] [Google Scholar]
- 31.Yao L., Li Z., Su W., Li Y., Lin M., Zhang W. Role of mesenchymal stem cells on cornea wound healing induced by acute alkali burn. PLoS One. 2012;7 doi: 10.1371/journal.pone.0030842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee M.J., Ko A.Y., Ko J.H., Lee H.J., Kim M.K., Wee W.R. Mesenchymal stem/stromal cells protect the ocular surface by suppressing inflammation in an experimental dry eye. Mol Ther. 2015;23:139–146. doi: 10.1038/mt.2014.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galindo S., Herreras J.M., López-Paniagua M., Rey E., de la Mata A., Plata-Cordero M. Therapeutic effect of human adipose tissue-derived mesenchymal stem cells in experimental corneal failure due to limbal stem cell niche damage. Stem Cell. 2017;35:2160–2174. doi: 10.1002/stem.2672. [DOI] [PubMed] [Google Scholar]
- 34.Weng J., He C., Lai P., Luo C., Guo R., Wu S. Mesenchymal stromal cells treatment attenuates dry eye in patients with chronic graft-versus-host disease. Mol Ther. 2012;20:2347–2354. doi: 10.1038/mt.2012.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oh J.Y., Kim M.K., Shin M.S., Lee H.J., Ko J.H., Wee W.R. The anti-inflammatory and anti-angiogenic role of mesenchymal stem cells in corneal wound healing following chemical injury. Stem Cell. 2008;26:1047–1055. doi: 10.1634/stemcells.2007-0737. [DOI] [PubMed] [Google Scholar]
- 36.Oh J.Y., Ko J.H., Kim M.K., Wee W.R. Effects of mesenchymal stem/stromal cells on cultures of corneal epithelial progenitor cells with ethanol injury. Investig Ophthalmol Vis Sci. 2014;55:7628–7635. doi: 10.1167/iovs.14-15424. [DOI] [PubMed] [Google Scholar]
- 37.Roddy G.W., Oh J.Y., Lee R.H., Bartosh T.J., Ylostalo J., Coble K. Action at a distance: systemically administered adult stem/progenitor cells (MSCs) reduce inflammatory damage to the cornea without engraftment and primarily by secretion of TNF-α stimulated gene/protein 6. Stem Cell. 2011;29:1572–1579. doi: 10.1002/stem.708. [DOI] [PubMed] [Google Scholar]
- 38.Oh J.Y., Roddy G.W., Choi H., Lee R.H., Ylöstalo J.H., Rosa R.H. Anti-inflammatory protein TSG-6 reduces inflammatory damage to the cornea following chemical and mechanical injury. Proc Natl Acad Sci U S A. 2010;107:16875–16880. doi: 10.1073/pnas.1012451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mittal S.K., Omoto M., Amouzegar A., Sahu A., Rezazadeh A., Katikireddy K.R. Restoration of corneal transparency by mesenchymal stem cells. Stem Cell Reports. 2016;7:583–590. doi: 10.1016/j.stemcr.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung J.K., Park S.A., Hwang H.S., Kim K.S., Cho Y.J., You Y.S. Effects of exogenous recombinant human bone morphogenic protein-7 on the corneal epithelial mesenchymal transition and fibrosis. Int J Ophthalmol. 2017;10:329–335. doi: 10.18240/ijo.2017.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.