Abstract
The tumor microenvironment is associated with various tumor progressions, including cancer metastasis, immunosuppression, and tumor sustained growth. Tumor‐associated macrophages (TAMs) are considered an indispensable component of the tumor microenvironment, participating in the progression of tumor microenvironment remodeling and creating various compounds to regulate tumor activities. This study aims to observe enriched TAMs in tumor tissues during bladder cancer development, which markedly facilitated the proliferation of bladder cancer cells and promoted tumor growth in vivo. We determined that TAMs regulate tumor sustained growth by secreting type I collagen, which can activate the prosurvival integrin α2β1/PI3K/AKT signaling pathway. Furthermore, traditional chemotherapeutic drugs combined with integrin α2β1 inhibitor showed intensive anticancer effects, revealing an innovative approach in clinical bladder cancer treatment.
Keywords: bladder cancer, collagen, integrin α2β1, PI3K/AKT, tumor‐associated macrophages
Abbreviations
- CCL17
chemokine (C‐C motif) ligand 17
- CCL22
chemokine (C‐C motif) ligand 22
- DOX
doxorubicin
- HCPT
hydroxycamptothecine
- IL‐10
interleukin‐10
- MMC
mitomycin C
- NMIBC
nonmuscle‐invasive bladder cancer
- TAM
tumor‐associated macrophage
- TGF‐β
transforming growth factor‐b
- VEGF
vascular endothelial growth factor
1. INTRODUCTION
Bladder cancer is one of the leading urogenital malignancies worldwide. Approximately 70% of diagnoses of bladder cancer are initially detected as a nonmuscle‐invasive superficial disease, classified as stages Ta, T1, or carcinoma in situ (CIS).1 In the clinical setting, NMIBC is treated with a combination of transurethral resection of the bladder tumor and intravesical chemotherapy. However, approximately half of the patients experience tumor recurrence because of remnant tumor cells and limited chemotherapy effects.2 In addition, the mechanism underlying the bladder cancer cells’ proliferation remains unclear, thereby highlighting the necessity of exploring innovative approaches to suppress cancer cell proliferation and enhance curative effects in bladder cancer treatment.
Tumor progression does not only depend on cancer cells’ autonomous features and is related to several factors, including the activation of prosurvival signaling pathways in tumor cells,3 intercellular cross‐talk between cancer and stroma cells,4, 5 and the immune microenvironment.6, 7 Accumulating evidence suggests that TAMs in the tumor microenvironment play a vital role in tumor growth.8, 9, 10, 11 As one of the most crucial immune cells residing within the tumor microenvironment, TAMs constitute a subpopulation of immunosuppressive cells to avert the tumor cell attack by natural killer and T cells during tumor progression.11, 12 Such TAMs are recruited by tumor cells and present at all stages of tumor progression,13, 14 including tumor cell invasion, extravasation, survival, and persistent growth.15, 16 However, the mechanism underlying TAM‐induced tumor progression remains unclear, and the innovative approach to target those protumoral macrophages has been an attractive strategy as part of combination therapy in cancer therapy.17, 18
Previously, we demonstrated that TAMs 17 could facilitate tumor growth in bladder cancer and that those TAMs isolated from tumor tissues markedly promoted bladder cancer cell proliferation in vitro and in vivo. In addition, we illustrated that TAMs produced type I collagen to regulate tumor growth of integrin α2β1 in bladder cancer cells. More importantly, the combination of integrin α2β1 inhibitor and chemotherapeutic agents showed dramatic tumor suppression in orthotopic bladder cancer treatment, indicating a potential strategy in clinical bladder cancer therapy. This study aims to observe enriched TAMs in tumor tissues during bladder cancer development, which markedly facilitated the proliferation of bladder cancer cells and promoted tumor growth in vivo.
2. MATERIALS AND METHODS
2.1. Cell lines and reagents
We purchased DOX, MMC, and HCPT from Sangon Biotech (Shanghai, China). E7820, LY294002, and MK‐2206 were purchased from Selleck Chemicals (Houston, TX, USA). In addition, type I collagen and type I collagenase were purchased from Sigma (St. Louis, MO, USA). We purchased murine bladder cancer cell line MB49 and human bladder cancer cell line T24 from the ATCC (Manassas, VA, USA). All cells were maintained in RPMI‐1640 complete medium (Gibco, Waltham, MA, USA) supplemented with 10% FCS (Gibco), at 37°C in 5% CO2 atmosphere. Then human and mice TAMs were isolated from patients or mouse bladder tumor tissues. Briefly, the tumor tissues were cut into pieces as small as possible after washing with PBS. Next, tissues were digested with RPMI‐1640 complete culture medium (Gibco) containing Accumax (Sigma) medium at 37°C, 5% CO2 in an incubator for 2 hours. After washing with PBS, cell precipitation was collected and seeded into a 6‐well plate containing 2 mL RPMI‐1640 medium supplemented with 10% FBS overnight at 37°C. Next day, we replaced the medium with fresh medium to remove the nonadherent cells and collected the remaining cells to sort CD68+ or F4/80+ cells with a BD FACSAria III (BD Biosciences, Franklin Lakes, NJ, USA) to collect TAMs and culture them for further analysis. In addition, we cultured 105 TAMs in 2 mL RPMI‐1640 complete medium containing 10% FCS for 12 hours. The TAM cultured medium was collected for further analysis (Figure S1A). In this study, all samples were reviewed by a pathologist and graded according to the World Health Organization classification. The collection and processing of all samples were carried out in accordance with the Declaration of Helsinki. The study protocol received ethical approval from the Committee of the West China Hospital, Sichuan University (Chengdu, China).
2.2. Cell proliferation analysis and colony formation experiments
We detected cell proliferation using the MTT Analysis Kit (Solarbio, Beijing, China). Briefly, MB49 or T24 cells were pretreated as described previously. Then 2000 sorted MB49 (F4/80 negative) or T24 (CD68 negative) cells were seeded into 96‐well culture plates. After 72 hours, we measured cell growth after the addition of 10 μL of 0.5 mg/mL MTT solution. After 4 hours of incubation at 37°C, the medium was replaced with 100 μL DMSO and vortexed for 10 minutes. We measured the absorbance at 570 nm by a microplate reader (Bio‐Rad, Hercules, CA, USA). Of note, each experiment was carried out at least 3 times independently.
Conversely, the pretreated MB49 or T24 cells were seeded into 6‐well culture plates (1000 cells/well) in RPMI‐1640 complete culture medium with 10% FCS. After 8 days, we stained the cells with crystal violet solution (Solarbio) and calculated the number. Each experiment was carried out independently at least 3 times.
2.3. Flow cytometry
We added anti‐CD68 (Abcam, Cambridge, UK), anti‐F4/80 (Abcam), or CD206 (Abcam) Abs to the cell suspension to isolate TAMs in tumor tissues. After incubating for 30 minutes at room temperature, the samples were sorted by BD FACSAria III (BD Biosciences). The isotype was stained as a negative control.
2.4. Real‐time PCR
We used 1 μg cDNA as the template for the quantitative real‐time PCR to detect the target genes (SYBR Green Real‐Time PCR master mixes; Thermo Fisher Scientific, Waltham, MA, USA). Actin was used as the internal control, and 3 independent experiments were carried out in each sample. The relative expression was quantified by normalizing the target gene level to the actin by the ΔΔC t method. The primer pairs used are listed in Table S1.
2.5. Western blot analysis
Samples were solubilized with an equal volume of loading buffer (125 mmol/L Tris‐HCl, pH 6.8, 4% SDS, 20% glycerol, 0.05% bromophenol blue, and 5% β‐mercaptoethanol) and boiled for 10 minutes. Samples were separated by SDS‐PAGE then transferred to PVDF membranes and detecting by immunoblotting with primary Abs against integrin α2β1 (1:1000; Abcam) and actin (1:1000; Abcam) at 4°C overnight. After that, HRP‐conjugated secondary Ab (1:1000; Abcam) was incubated for 1 hour at room temperature and visualized by the ECL Detection Kit (Cell Signaling Technology, Danvers, MA, USA).
2.6. Immunofluorescence staining
We fixed and permeabilized MB49 cells treated with collagen or E7820 to examine the PI3K/AKT signaling pathway in bladder cancer cells. Then the cells were labeled with anti‐integrin β1 (1:200; Abcam), anti‐p‐PI3K (1:200; Abcam), or p‐AKT (1:200; Abcam) followed by Alexa 594 secondary Abs (1:800; Abcam). In addition, nuclei were labeled with DAPI. All immunofluorescence images were captured from FV1000 laser scanning confocal microscope (Leica, Wetzlar, Germany).
2.7. Immunohistochemistry
All tumor tissues were kept in 4% paraformaldehyde overnight, then processed, embedded in paraffin, and sectioned at 4 μm. The tumor sections were incubated with F4/80 (1:200; Abcam) or type I collagen (1:500; Abcam) at 4°C overnight, followed by signal amplification using the ABC HRP Kit (Thermo Fisher Scientific) and counterstaining with hematoxylin, dehydration with series of graded ethanol, and cleaned with xylene. We used a microscope (Leica) to visualize the sections.
2.8. Enzyme‐linked immunosorbent assay
The quantification of type I collagen in macrophage cultured medium was carried out with a type I collagen ELISA analysis kit (Kramer) as guided. One hundred microliters (10×) of macrophage cultured medium was used for the ELISA analysis. The assays used the quantitative sandwich enzyme immunoassay technique using murine collagen I with Abs raised against the recombinant proteins. Optical density was read with a microtiter plate reader by dual wavelength at 450 nm. All samples were assayed in duplicate.
2.9. Animal protocol
We purchased female C57BL mice (6‐8 weeks old) from Huafukang Company (Beijing, China) and maintained them in specific pathogen‐free conditions. The orthotopic bladder cancer model was established as described previously. Briefly, 106 MB49 bladder cancer cells in 100 μL PBS were intravesically instilled into the bladders of mice after 20 minutes of pretreatment with poly‐l‐lysine (0.1 mg/mL; Sigma). In addition, tumor cell suspension was kept in the bladder for 60 minutes with a venous indwelling catheter. On days 4 and 8, mice were treated with PBS, DOX (0.1 mg), DOX (0.1 mg) combined with E7820 (50 μg), HCPT (0.05 mg), HCPT (0.05 mg) combined with E7820 (50 μg), MMC (0.1 mg), or MMC (0.1 mg) combined with E7820 (50 μg) by bladder irrigation. On day 12, 8 mice were killed for the tumor weight analysis. The survival of tumor‐bearing mice (5/group) was observed every day from day 12. Animal experiments undertaken in this study were approved and monitored by the Animal Care and Use Committee of West China Hospital, Sichuan University.
2.10. Statistical analysis
All data are presented as mean ± SEM. GraphPad Prism 6.0 (San Diego, CA) was used for statistical analysis (P < .05). We used Student's t test to analyze between‐group differences. In addition, the survival analysis was carried out by the Kaplan‐Meier method and evaluated using the log‐rank test. We considered P < .05 as considered significant.
3. RESULTS
3.1. Tumor‐associated macrophages facilitate tumor growth in bladder cancer
To investigate the role of TAMs in bladder cancer, we collected bladder tumors from patients in stage T0 and T3. Remarkably, we observed an increased number of macrophages in tumor sites in highly malignant tumor tissues (stage T3) compared to tumor tissues with low malignancy (stage T0) (Figure 1A,B). Those TAMs isolated from patients bladder tumors revealed higher expression of CD206 (Figure S1B) and M2 macrophage‐associated genes (Arg‐1, IL‐10, and TGF‐β) (Figure S1C), indicating that those TAMs are M2 macrophages. We hypothesized that TAMs might play a vital role in bladder tumor growth. Accordingly, we isolated TAMs from mouse tumor tissues and then cocultured MB49 cells with those TAMs to investigate the impact of TAMs on bladder cancer cells (Figure S1A). Consistently, TAMs could markedly facilitate the proliferation of MB49 cells (Figure 1C), whereas the mouse peritoneal macrophages suppressed the MB49 proliferation. In addition, we observed similar results in human bladder cancer cells T24 cocultured with TAMs from patients’ tissues (Figure 1D). Moreover, TAM‐treated MB49 and T24 cells revealed enhanced tumor formation ability compared with the PBS groups, and normal macrophages from paracarcinoma tissues or mice enterocoelia inhibited the colony formation (Figure 1E,F). Next, to further validate our hypothesis, we instilled TAMs into the bladders of mice with orthotopic bladder tumors. As anticipated, TAM‐treated mice markedly promoted tumor growth (Figure 1G,H), suggesting that TAMs in tumor sites play a vital role in bladder cancer progression.
Figure 1.
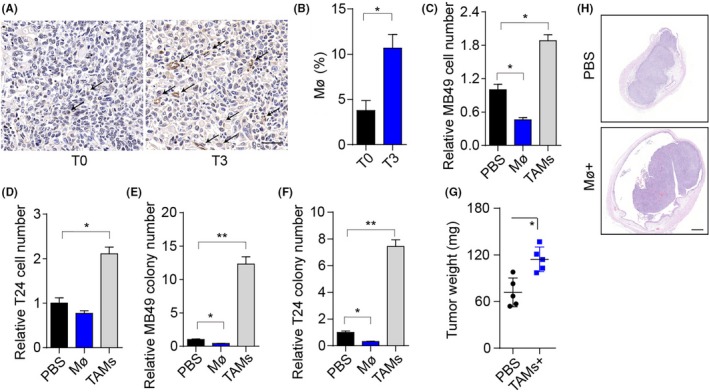
Tumor‐associated macrophages (TAMs) promote bladder cancer cell growth and tumor progression. A, Immunohistochemistry of CD68 in bladder tumor tissues from patients in stage T0 and T3. Scale bar, 50 μm. The arrows indicate the macrophages in tumor sites. B, Percentage of macrophages in the immune cell subpopulation of patients (stage T0 and T3) bladder tumor tissues. C, Relative cell number of MB49 cells cocultured with mouse peritoneal macrophages, TAMs, or PBS for 72 h. D, Relative numbers of T24 cells cocultured with macrophages from patients’ paracarcinoma tissues, TAMs, or PBS for 72 h. E, Relative colony numbers of MB49 cells pretreated with mouse peritoneal macrophages, TAMs, or PBS for 72 h. F, Relative colony numbers of T24 cells pretreated with macrophages from patients’ paracarcinoma tissues, TAMs, or PBS for 72 h. G, Bladder tumor weights of mice treated with PBS or TAMs. Of note, 106 MB49 cells were intravesically instilled into C57 mice bladders. On days 4 and 8, mice were instilled with 2 × 105 TAMs by bladder irrigation. H, Histological H&E staining of the bladders of C57 mice in PBS or TAM. Scale bar, 500 μm. Error bars, mean ± SEM; *P < .05; **P < .01
3.2. Tumor‐associated macrophages facilitate bladder cancer cell growth through secretion of collagen
We wondered how TAMs participated in tumor development in bladder cancer. Current knowledge suggests that TAMs can remodel the surrounding cells by secreting soluble factors.19, 20 Thus, we added the cultured medium of TAMs to tumor cells and detected the cell proliferation and colony‐formation ability of cancer cells. Intriguingly, we observed enhanced cell proliferation and colony‐formation ability in MB49 and T24 cells treated with TAM cultured medium (Figure 2A,B), suggesting that TAMs facilitate bladder cancer cell growth by secreting soluble factors. Next, we detected the major compound or cytokine expression of type I collagen, IL‐10, TGF‐β, VEGF, consensus clustering 2 (CC2), CCL17, and CCL22 in peritoneal macrophages and TAMs isolated from bladder tumors of mice to elucidate the specific cytokine inducing the bladder cancer proliferation (Figure 2C). We found that TAMs have an increasing expression of type I collagen (Figure 2C,D). Accordingly, we screened receptors of those cytokines in tumor cells, and the type I collagen receptor integrin β1 revealed markedly increasing expression in MB49 cells treated with TAMs (Figure 2E,F). The same results were observed in human‐derived macrophages (Figure S1D) and T24 cells (Figure S1E), suggesting that TAMs might produce type I collagen to induce bladder cancer proliferation.
Figure 2.
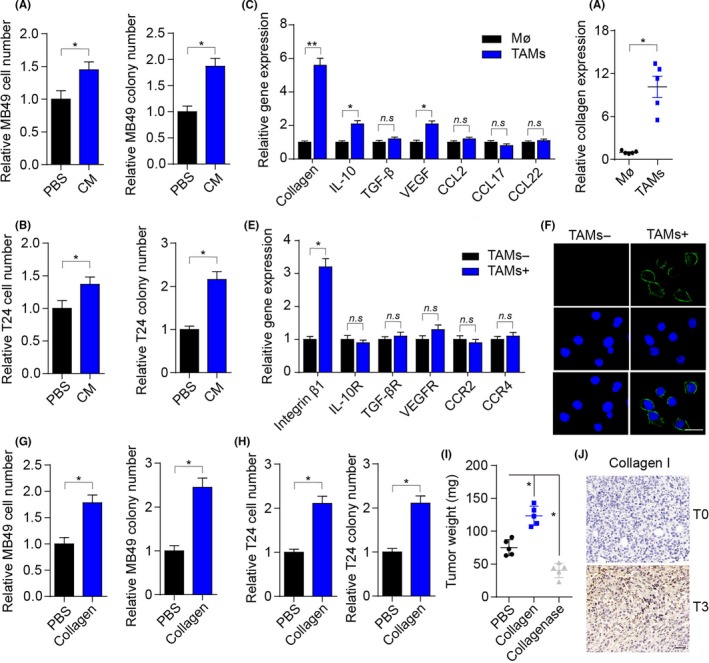
Tumor‐associated macrophages (TAMs) facilitate bladder cancer cell growth by secreting collagen. A, Relative cell numbers and colony numbers of MB49 cells treated with TAM cultured medium (CM) or not (PBS) for 72 h. B, Relative cell numbers and colony numbers of T24 cells treated with CM or PBS for 72 h. C, Relative gene expression of collagen, interleukin (IL)‐10, transforming growth factor (TGF)‐β, vascular endothelial growth factor (VEGF), chemokine (C‐C motif) ligand (CCL)2, CCL17, and CCL22 in mice peritoneal macrophages and TAMs. D, Relative type I collagen quantity in peritoneal macrophage cultured medium or TAM cultured medium detected by ELISA. E, Relative gene expression of integrin β1, IL‐10 receptor (R), TGF‐βR, VEGFR, CCR2, and CCR4 in MB49 cells treated with TAMs or not. F, Immunofluorescence of integrin β1 of MB49 cells treated with TAM cultured medium or not for 72 h. Scale bar, 10 μm. G, Relative cell numbers and colony numbers of MB49 cells treated with collagen (0.1 μg/mL) or PBS for 72 h. H, Relative cell numbers and colony numbers of T24 cells treated with collagen (0.1 μg/mL) or PBS for 72 h. I, Bladder tumor weights of mice treated with PBS, collagen, or collagenase. Of note, 106 MB49 cells were intravesically instilled into C57 mice bladders. On days 4 and 8, mice were instilled with 1 μg collagen or 0.1 μg collagenase by bladder irrigation. J, Immunohistochemistry of type I collagen in bladder cancer tissues from patients in stage T0 or stage T3. Scale bar, 50 μm. Error bars, mean ± SEM. *P < .05; **P < .01. n.s, not statistically significant
We treated MB49 and T24 cells with type I collagen to further verify the proliferative effects induced by collagen. We observed enhanced cell proliferation and colony‐formation ability in collagen‐treated bladder cancer cells compared with the PBS‐treated group (Figure 2G,H). In addition, instillation of collagen into the bladders remarkably facilitated tumor growth, whereas type I collagenase suppressed tumor progression (Figure 2I). Moreover, elevated expression of type I collagen was observed in highly malignant (stage T3) bladder tumors compared to bladder tumors with low malignancy (stage T0) (Figure 2J). Overall, these findings showed that collagen produced by TAMs promotes bladder cancer growth.
3.3. Collagen promotes tumor growth through activation of the integrin α2β1/PI3K/AKT signaling pathway
Previously, we revealed that bladder cancer cells show a dramatic increase in expression of collagen receptor integrin β1 after TAMs coculture. Growing evidence suggests that integrin α1, α2, α10, and α11 could combine with integrin β1 to form integrin heterodimers to regulate the downstream signaling pathway induced by collagen.21 In addition, we determined the upregulation of integrin α2 in collagen‐treated cancer cells (Figure 3A). Furthermore, we observed the upregulation of integrin α2β1 in the protein level in collagen‐treated cancer cells, suggesting that integrin α2β1 participates in collagen‐induced tumor cell proliferation (Figure 3B). We used E7820, an integrin α2β1 inhibitor, which efficiently suppressed the expression of integrin α2 induced by collagen (Figure S1F) without influencing cell proliferation or colony formation (Figure S1G), to further verify our hypothesis and treat cancer cells and, subsequently, to detect collagen‐induced proliferative effects. As anticipated, E7820 efficiently reversed the collagen‐induced proliferative effects in bladder cancer cells (Figure 3C,D). A similar result was observed in mice with orthotopic bladder tumors (Figure 3E). Overall, these findings suggested that collagen promotes bladder cancer growth through integrin α2β1.
Figure 3.
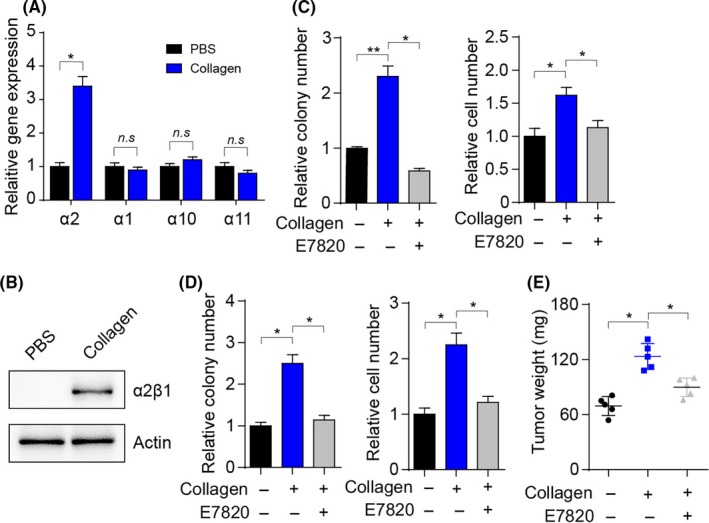
Collagen induces tumor progression through integrin α2β1. A, Relative gene expression of integrin α2, α1, α10, and α10 in collagen‐treated MB49 cells (0.1 μg/mL) for 72 h or not (PBS). B, Western blot analysis of integrin α2β1 and actin in MB49 cells treated with collagen (0.1 μg/mL) for 72 h. C, Relative colony numbers and cell numbers of MB49 cells treated with collagen (0.1 μg/mL) or collagen (0.1 μg/mL) combined with E7820 (0.05 μg/mL) for 72 h. D, Relative colony numbers and cell numbers of T24 cells treated with collagen (0.1 μg/mL) or collagen (0.1 μg/mL) combined with E7820 (0.05 μg/mL) for 72 h. E, Bladder tumor weights of mice treated with collagen (1 μg) or collagen (1 μg) combined with E7820 (50 μg) on days 4 and 8. Error bars, mean ± SEM. *P < .05; **P < .01. n.s, not statistically significant
As a classic downstream molecular pathway of the integrin β1 signaling axis, PI3K/AKT participates in various physiological activities, including cell cycle, metabolism, and sustained growth.22, 23, 24 Previous studies reported that the PI3K/AKT signaling pathway is correlative in the sustained growth of various solid tumors.25 Intriguingly, we observed enhanced phosphorylated PI3K and AKT in collagen‐treated MB49 cells, suggesting the activation of the PI3K/AKT signaling pathway (Figure 4A,B). In addition, we used LY294002 and MK2206, PI3K and AKT inhibitors, respectively, without effects on cell proliferation or colony formation (Figure S1H), to treat bladder cancer cells. We found that the blockade of PI3K/AKT signals effectively reversed the enhanced proliferative and colony‐formation abilities induced by collagen (Figure 4C‐F). Notably, similar results were observed in mice with orthotopic bladder tumors (Figure 4G). These findings implied that collagen could activate the integrin α2β1/PI3K/AKT signaling pathway to facilitate bladder cancer cell proliferation.
Figure 4.
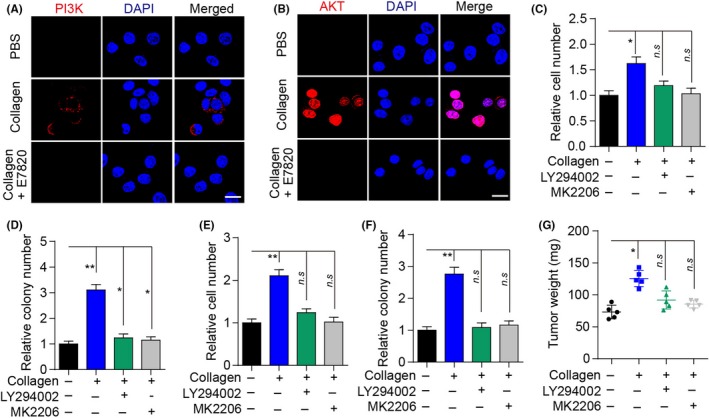
Collagen‐induced tumor progression by activation of the PI3K/AKT signaling pathway. A, Immunofluorescence of p‐PI3K in MB49 cells treated with collagen (0.1 μg/mL), collagen (0.1 μg/mL) combined with E7820 (0.05 μg/mL), or PBS for 72 h. Scale bar, 10 μm. B, Immunofluorescence of p‐AKT in MB49 cells treated with collagen (0.1 μg/mL), collagen (0.1 μg/mL) combined with E7820 (0.05 μg/mL), or PBS for 72 h. Scale bar, 10 μm. C, Relative numbers of MB49 cells treated with collagen (0.1 μg/mL), or collagen (0.1 μg/mL) combined with LY294002 (0.5 μmol/L) or MK‐2206 (10 nmol/L) for 72 h. D, Relative colony numbers of MB49 cells treated with collagen (0.1 μg/mL), or collagen (0.1 μg/mL) combined with LY294002 (0.5 μmol/L) or MK‐2206 (10 nmol/L) for 72 h. E, Relative numbers of T24 cells treated with collagen (0.1 μg/mL), or collagen (0.1 μg/mL) combined with LY294002 (0.5 μmol/L) or MK‐2206 (10 nmol/L) for 72 h. F, Relative colony numbers of T24 cells treated with collagen (0.1 μg/mL), or collagen (0.1 μg/mL) combined with LY294002 (0.5 μmol/L) or MK‐2206 (10 nmol/L) for 72 h. G, Bladder tumor weights of mice treated with collagen (1 μg), or collagen (1 μg) combined with LY294002 (50 μg) or MK‐2206 (2 μg) on days 4 and 8. Error bars, mean ± SEM. *P < .05; **P < .01. n.s, not statistically significant
3.4. Combination of integrin α2β1 and chemotherapeutic agents revealed enhanced anticancer effects in vivo
Considering the proliferative effects induced by the integrin α2β1/PI3K/AKT signaling pathway, we hypothesized that the combination of integrin α2β1 inhibitor and chemotherapeutic agents might serve as a potential strategy in clinical bladder therapy. In this study, we established the NMIBC mouse model by intravesically instilling 2 × 106 MB49 cells into the mouse bladder. On days 4 and 8, we treated mice with PBS, DOX, E7820, or DOX combined with E7820 by intravesical instillation. On day 12, >90% PBS control mice showed macroscopic hematuria, and we observed declined hematuria (~60%) in mice treated with DOX alone. In addition, fewer mice (<30%) presented with hematuria following pre‐instillation of E780 combined with DOX (Figure 5A). Accordingly, we observed a striking inhibition of tumor growth in the group treated with E7820 and DOX (Figure 5B). Additionally, mice treated with E7820 and DOX revealed markedly prolonged survival time compared with the PBS or DOX groups (Figure 5C). Notably, E7820 combined with other chemotherapeutic agents, such as MMC and HCPT, showed enhanced curative effects against bladder cancer (Figure 5D,E). Overall, these findings suggested that the combination of integrin α2β1 and chemotherapeutic agents revealed enhanced tumor suppression and might serve as a potential strategy in the treatment of bladder cancer.
Figure 5.
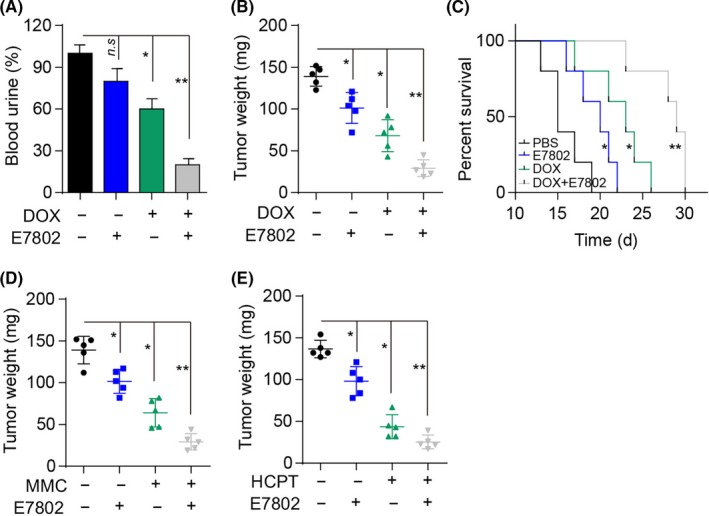
Combination of integrin α2β1 and chemotherapeutic agents enhanced the anticancer effects in bladder cancer treatment. A, Incidence of blood urine on day 12 in mice treated with PBS, doxorubicin (DOX; 0.1 mg), or DOX (0.1 mg) combined with E7820 (50 μg) on days 4 and 8. B, Bladder tumor weights in mice on day 12 treated with PBS, DOX (0.1 mg), or DOX (0.1 mg) combined with E7820 (50 μg) on days 4 and 8. C, Survival time of mice with bladder tumors treated with PBS, DOX (0.1 mg), or DOX (0.1 mg) combined with E7820 (50 μg) on days 4 and 8. D, Bladder tumor weight in mice on day 12 treated with PBS, mitomycin C (MMC; 0.1 mg), or MMC (0.1 mg) combined with E7820 (50 μg) on days 4 and 8. E, Bladder tumor weights in mice on day 12 treated with PBS, hydroxycamptothecine (HCPT; 0.05 mg), or HCPT (0.05 mg) combined with E7820 (50 μg) on days 4 and 8. Error bars, mean ± SEM. *P < .05; **P < .01. n.s, not statistically significant
4. DISCUSSION
This study established that TAMs isolated from tumor tissues exert a proliferative effect on bladder cancer cells. Previous studies have reported the involvement of TAMs in various tumor progressions, including tumor metastasis and sustained growth; however, the underlying mechanism remains unclear. In this study, we found that TAMs could produce type I collagen to stimulate tumor growth. Collagen secreted from TAMs could activate the PI3K/AKT signaling pathway through integrin α2β1. Furthermore, the combination of integrin α2β1 inhibitor and chemotherapeutic agents revealed enhanced anticancer effects, which provided a new strategy for bladder cancer treatment.
Recent findings have provided new insights into the role of TAMs biology in the tumor microenvironment. Previous studies have reported that the presence of TAMs in tumor sites closely correlated with the tumor development.26 However, the specific cytokines secreted by TAMs to induce tumor cell proliferation remain unidentified, and little is known about the subsequent signaling pathways activated in tumor cells. In this study, we identified type I collagen as a critical factor to regulate the biological activities of bladder cancer cells. In fact, tumor cells revealed enhanced proliferation and colony‐formation ability because of the collagen‐activated PI3K/AKT signaling pathway. Furthermore, we clarified the role of TAMs in the tumor microenvironment and elucidated the underlying mechanisms of TAM‐induced bladder cancer development.
Critically, we also determined that type I collagen participates in bladder tumor growth and the potential role of integrin α2β1 in bladder cancer cell proliferation.27 Reportedly, collagen serves as a vital component of the ECM, which facilitates the maintenance of cancer stem cells. In addition, integrin expression is associated with tumor cell adhesion and stemness.27, 28, 29, 30 Furthermore, we determined the functions of collagen and integrin in tumor progression and provided new insight into the tumor environment. The findings provided a feasible approach for therapeutically targeting the tumor microenvironment, especially TAMs. Based on the tumor growth suppression experiments, targeting integrin α2β1, the collagen receptor to induce the PI3K/AKT prosurvival signaling pathway, is one possibility in clinical bladder cancer therapy. Furthermore, several integrin inhibitors have been developed for the clinical evaluation of tumor inhibition, such as integrin αvβ3 inhibitor cilengitide in melanoma treatment.30, 31 More importantly, the irrigation of integrin α2β1 inhibitor and chemotherapeutic into bladders, instead of i.v. injection, ensures safety while markedly enhancing the anticancer effects.
In conclusion, this study found that TAMs in tumor sites could facilitate bladder cancer proliferation by secreting collagen. Collagen activates the PI3K/AKT prosurvival signaling pathway. Furthermore, the combination of integrin α2β1 inhibitor and chemotherapeutic agents could be explored as a potential therapeutic strategy in bladder cancer.
DISCLOSURE
Authors declare no conflicts of interest for this article.
Supporting information
ACKNOWLEDGMENTS
The authors acknowledge Ian Charles Tobias for reviewing the manuscript. We would also like to acknowledge all of the staff members for their participation with study recruitment. This study was supported by The National Key Research and Development Program of China (Grant No. 2017YFC0908003), the Post‐Doctoral Research Project, West China Hospital, Sichuan University (Grant No.2018HXBH085), the National Natural Science Foundation of China (Grant Nos. 81300627, 81200551, 81270841, 81460148, and 81370855), funding from the Science and Technology Department of Sichuan Province (Grant Nos. 2014JY0219 and 2017HH0063), and the Prostate Cancer Foundation Young Investigator Award 2013.
Qiu S, Deng L, Liao X, et al. Tumor‐associated macrophages promote bladder tumor growth through PI3K/AKT signal induced by collagen. Cancer Sci. 2019;110:2110–2118. 10.1111/cas.14078
Qiu, Deng, and Liao contributed equally to this work.
Funding information
Science and Technology Department of Sichuan Province, Grant/Award Numbers 2014JY0219, 2017HH0063;Post‐Doctoral Research Project, West China Hospital, Sichuan University, Grant/Award Number 2018HXBH085; National Key Research and Development Program of China, Grant/Award Number 2017YFC0908003; National Natural Science Foundation of China, Grant/Award Numbers 81300627, 81200551, 81270841, 81460148a; Prostate Cancer Foundation Young Investigator Award 2013
Contributor Information
Lu Yang, Email: wycleflue@163.com.
Qiang Wei, Email: weiqiang163163@163.com.
REFERENCES
- 1. Kamat AM, Hahn NM, Efstathiou JA, et al. Bladder cancer. Lancet. 2016;388(10061):2796‐2810. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7‐30. [DOI] [PubMed] [Google Scholar]
- 3. Schnepp PM, Lee DD, Guldner IH, et al. GAD1 upregulation programs aggressive features of cancer cell metabolism in the brain metastatic microenvironment. Cancer Res. 2017;77(11):2844‐2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11(10):889‐896. [DOI] [PubMed] [Google Scholar]
- 5. Pietras K, Ostman A. Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res. 2010;316(8):1324‐1331. [DOI] [PubMed] [Google Scholar]
- 6. Holzel M, Bovier A, Tuting T. Plasticity of tumour and immune cells: a source of heterogeneity and a cause for therapy resistance? Nat Rev Cancer. 2013;13(5):365‐376. [DOI] [PubMed] [Google Scholar]
- 7. Davalos AR, Coppe JP, Campisi J, Desprez PY. Senescent cells as a source of inflammatory factors for tumor progression. Cancer Metastasis Rev. 2010;29(2):273‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ostman A. The tumor microenvironment controls drug sensitivity. Nat Med. 2012;18(9):1332‐1334. [DOI] [PubMed] [Google Scholar]
- 9. Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309‐322. [DOI] [PubMed] [Google Scholar]
- 10. Wu D. Innate and adaptive immune cell metabolism in tumor microenvironment. Adv Exp Med Biol. 2017;1011:211‐223. [DOI] [PubMed] [Google Scholar]
- 11. Zhang Y, Choksi S, Chen K, et al. ROS play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor‐associated macrophages. Cell Res. 2013;23(7):898‐914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423‐1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Colegio OR, Chu NQ, Szabo AL, et al. Functional polarization of tumour‐associated macrophages by tumour‐derived lactic acid. Nature. 2014;513(7519):559‐563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gordon SR, Maute RL, Dulken BW, et al. PD‐1 expression by tumour‐associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545(7655):495‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gocheva V, Wang HW, Gadea BB, et al. IL‐4 induces cathepsin protease activity in tumor‐associated macrophages to promote cancer growth and invasion. Genes Dev. 2010;24(3):241‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shi Y, Ping YF, Zhou W, et al. Tumour‐associated macrophages secrete pleiotrophin to promote PTPRZ1 signalling in glioblastoma stem cells for tumour growth. Nat Commun. 2017;8:15080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Monteiro LN, Rodrigues MA, Gomes DA, Salgado BS, Cassali GD. Tumour‐associated macrophages: relation with progression and invasiveness, and assessment of M1/M2 macrophages in canine mammary tumours. Vet J. 2018;234:119‐125. [DOI] [PubMed] [Google Scholar]
- 19. Su S, Liu Q, Chen J, et al. A positive feedback loop between mesenchymal‐like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell. 2014;25(5):605‐620. [DOI] [PubMed] [Google Scholar]
- 20. Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22(2):231‐237. [DOI] [PubMed] [Google Scholar]
- 21. McAtee CO, Booth C, Elowsky C, et al. Prostate tumor cell exosomes containing hyaluronidase Hyal1 stimulate prostate stromal cell motility by engagement of FAK‐mediated integrin signaling. Matrix Biol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fresno VJ, Casado E, de Castro J, et al. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30(2):193‐204. [DOI] [PubMed] [Google Scholar]
- 23. Dong P, Konno Y, Watari H, et al. The impact of microRNA‐mediated PI3K/AKT signaling on epithelial‐mesenchymal transition and cancer stemness in endometrial cancer. J Transl Med. 2014;12:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martini M, De Santis MC, Braccini L, Gulluni F, Hirsch E. PI3K/AKT signaling pathway and cancer: an updated review. Ann Med. 2014;46(6):372‐383. [DOI] [PubMed] [Google Scholar]
- 25. Watabe H, Furuhama T, Tani‐Ishii N, Mikuni‐Takagaki Y. Mechanotransduction activates alpha(5)beta(1) integrin and PI3K/Akt signaling pathways in mandibular osteoblasts. Exp Cell Res. 2011;317(18):2642‐2649. [DOI] [PubMed] [Google Scholar]
- 26. Bailey C, Negus R, Morris A, et al. Chemokine expression is associated with the accumulation of tumour associated macrophages (TAMs) and progression in human colorectal cancer. Clin Exp Metastasis. 2007;24(2):121‐130. [DOI] [PubMed] [Google Scholar]
- 27. Chen L, Xiao Z, Meng Y, et al. The enhancement of cancer stem cell properties of MCF‐7 cells in 3D collagen scaffolds for modeling of cancer and anti‐cancer drugs. Biomaterials. 2012;33(5):1437‐1444. [DOI] [PubMed] [Google Scholar]
- 28. Seguin L, Desgrosellier JS, Weis SM, Cheresh DA. Integrins and cancer: regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol. 2015;25(4):234‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Seguin L, Kato S, Franovic A, et al. An integrin beta(3)‐KRAS‐RalB complex drives tumour stemness and resistance to EGFR inhibition. Nat Cell Biol. 2014;16(5):457‐468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McDonald PC, Oloumi A, Mills J, et al. Rictor and integrin‐linked kinase interact and regulate Akt phosphorylation and cancer cell survival. Cancer Res. 2008;68(6):1618‐1624. [DOI] [PubMed] [Google Scholar]
- 31. Tentori L, Dorio AS, Muzi A, et al. The integrin antagonist cilengitide increases the antitumor activity of temozolomide against malignant melanoma. Oncol Rep. 2008;19(4):1039‐1043. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


