Abstract
Expression of programmed cell death ligand 1 (PD‐L1) on tumor cells contributes to cancer immune evasion by interacting with programmed cell death 1 on immune cells. γ‐Interferon (IFN‐γ) has been reported as a key extrinsic stimulator of PD‐L1 expression, yet its mechanism of expression is poorly understood. This study analyzed the role of CD74 and its ligand macrophage migration inhibitory factor (MIF) on PD‐L1 expression, by immunohistochemical analysis of melanoma tissue samples and in vitro analyses of melanoma cell lines treated with IFN‐γ and inhibitors of the MIF‐CD74 interaction. Immunohistochemical analyses of 97 melanoma tissue samples showed significant correlations between CD74 and the expression status of PD‐L1 (P < .01). In vitro analysis of 2 melanoma cell lines, which are known to secrete MIF constitutively and express cell surface CD74 following IFN‐γ stimulation, showed upregulation of PD‐L1 levels by IFN‐γ stimulation. This was suppressed by further treatment with the MIF‐CD74 interaction inhibitor, 4‐iodo‐6‐phenylpyrimidine. In the analysis of melanoma cell line WM1361A, which constitutively expresses PD‐L1, CD74, and MIF in its non‐treated state, treatment with 4‐iodo‐6‐phenylpyrimidine and transfection of siRNAs targeting MIF and CD74 significantly suppressed the expression of PD‐L1. Together, the results indicated that MIF‐CD74 interaction directly regulated the expression of PD‐L1 and helps tumor cells escape from antitumorigenic immune responses. In conclusion, the MIF‐CD74 interaction could be a therapeutic target in the treatment of melanoma patients.
Keywords: CD74, interferon gamma, macrophage migration inhibitory factor, melanoma, PD‐L1
1. INTRODUCTION
Programmed cell death ligand 1 (PD‐L1) is a type I transmembrane protein that plays a major role in suppressing the immune response.1 High expression of PD‐L1 is frequently observed in tumor cells, and its interaction with programmed cell death 1 (PD‐1) on immune cells subsequently leads to immune cell dysfunction and prevents cytotoxic T cells from targeting tumor cells.2 Programmed cell death 1 and PD‐L1 constitute a key pathway in local tumor immunosuppression, and inhibition of the PD‐1‐PD‐L1 axis has been reported to be an effective therapy for various malignancies.3, 4, 5, 6
Expression of PD‐L1 in tumor cells is regulated by several extrinsic and intrinsic factors.7, 8 In particular, γ‐interferon (IFN‐γ) in the tumor microenvironment has been reported to be a key extrinsic regulator of PD‐L1 expression. IFN‐γ principally activates JAK and STAT signaling pathways, which induce the direct binding of its transcription factor, IFN regulatory factor 1, to the promoter region of PD‐L1.3, 4, 5, 9 However, expression of PD‐L1 by IFN‐γ stimulation is mediated by multiple mechanisms. In medulloblastomas, IFN‐γ induces PD‐L1 upregulation, which is required for the supportive effect of cyclin‐dependent kinase 5.10
CD74, also known as MHC class II‐associated invariant chain, is a type II transmembrane glycoprotein.11 Part of this glycoprotein is exposed on the cell surface and functions as a receptor for macrophage migration inhibitory factor (MIF).12, 13, 14 The MIF‐CD74 interactions induce activation of RAS‐RAF‐MEK‐ERK MAPK signaling and the PI3K‐AKT signaling pathway.14, 15 Stimulation of IFN‐γ has also been reported to upregulate the expression of CD74.16 In a previous analysis of melanoma patient samples, serum IFN‐γ levels and CD74 expression levels in tumor cells showed a significant correlation. In addition, treating melanoma cell lines with recombinant IFN‐γ protein resulted in upregulation of cell surface CD74 protein levels.17
Based on these findings that IFN‐γ stimulation upregulates expression levels of both CD74 and PD‐L1, we hypothesized that expression of PD‐L1 is mediated by MIF‐CD74 interactions. Here we showed a significant association between the expression levels of PD‐L1 and MIF‐CD74 interactions.
2. MATERIALS AND METHODS
2.1. Patients and tumor samples
The Surveillance Committee for Human Subjects Research at the School of Medicine of Keio University (Tokyo, Japan) approved this research project. This study involved 97 histological samples (64 primary and 33 metastatic samples) obtained from 70 patients treated at the Department of Dermatology of Keio University Hospital from 1993 to 2014. All patients gave written informed consent prior to participating in the study. Diagnoses of malignant melanoma were histologically confirmed for all samples by 2 pathologists (YM and MS).
2.2. Immunohistochemical and immunocytochemical analyses
Formalin‐fixed, paraffin‐embedded sections of melanoma tumor samples and formalin‐fixed cytospin sections of melanoma cell line samples were examined for CD74 and PD‐L1 expression. Mouse monoclonal anti‐human CD74 Ab (LN2; Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit monoclonal anti‐human PD‐L1 Ab (E1L3N; Cell Signaling Technology, Danvers, MA, USA), mouse monoclonal anti‐human Ki‐67 Ab (MIB‐1; Santa Cruz Biotechnology), and rabbit polyclonal anti‐human MIF Ab (Santa Cruz Biotechnology) were used as primary Abs. Immunostaining was carried out using a Leica Bond‐Max Automation and Leica Red Refine detection kit (Leica Biosystems, Richmond, VA, USA) following the manufacturer's protocols. Slides were incubated with primary Abs for 15 min (CD74 and MIF) or 45 min (PD‐L1), followed by treatment with hydrogen peroxide and alkaline phosphatase reagent (Leica Biosystems). For the double staining of PD‐L1 and Ki‐67, immunostaining of PD‐L1 was undertaken using the abovementioned method followed by incubating mouse monoclonal anti‐human Ki67 Ab (MIB‐1) as the primary Ab and HRP‐conjugated goat monoclonal anti‐mouse Ab (Nichirei, Tokyo, Japan) as the secondary Ab. Positive staining was visualized using diaminobenzidine as a chromogen (Muto Chemicals, Tokyo, Japan). Immunolabeling was scored by the percentage of melanoma cells with positive staining after treatment with each Ab (CD74, >1%; PD‐L1, >1%). All specimens were manually and independently evaluated by 2 investigators (MI and KT) without prior knowledge of any other clinical information, and any discrepancies in the findings were subsequently reconciled by a third investigator (YM).
2.3. Cell lines and reagents
The human melanoma A375 cell line was obtained from ATCC (Manassas, VA, USA), SB2 was provided by Dr. M. Davies (University of Texas MD Anderson Cancer Center, Houston, TX, USA), and WM1361A was provided by Dr. M. Herlyn (Wistar Institute, Philadelphia, PA, USA). All cell lines were grown in RPMI‐1640 medium supplemented with 10% (for A375 and SB2) or 20% (for WM1361A) heat‐inactivated bovine serum, 100 μg/mL glutamine, 100 U/mL penicillin, and 100 U/mL streptomycin (all from Invitrogen, Carlsbad, CA, USA). All cells were grown at 37°C in a 5% CO2 atmosphere and cultured to 30%‐40% confluence a day before the start of the experiment. The cell lines were validated by STR DNA fingerprinting using the AmpF/STR identifier kit according to the manufacturer's instructions (Applied Biosystems, Foster city, CA, USA).
Cells were treated with the MIF inhibitor, 4‐iodo‐6‐phenylpyrimidine (4‐IPP; Sigma‐Aldrich, St. Louis, MO, USA) and human recombinant IFN‐γ protein (Invitrogen). For siRNA transfection, cells were transfected with 5 μmol/L nontargeting siRNA (OriGene‐SR30004; OriGene Technologies, Rockville, MD, USA) or CD74‐ and MIF‐targeting siRNA (OriGene‐SR300694 and SR302900, respectively) using Lipofectamine RNAiMAX Transfection Reagent (Invitrogen) according to the manufacturer's protocol. The sequences used were as follows: siCD74‐1, rArGrUrCrGrGrArArCrArGrCrArGrArUrArArCrArArUrGCA; siCD74‐2, rArGrUrArUrGrGrCrArArCrArUrGrArCrArGrArGrGrArCCA; siMIF‐1, rCrCrCrGrGrArCrArGrGrGrUrCrUrArCrArUrCrArArCrUAT; and siMIF‐2, rCrGrArCrArUrGrArArCrGrCrGrGrCrCrArArUrGrUrGrGGC.
2.4. Cell counting and cell viability assay
Cells were seeded in 6‐well plates at 1 × 105 cells/well and incubated as described above. After treatment, cell numbers were counted every 24 hours up to 72 hours with a Bürker‐Türk counting chamber (Hausser Scientific, Horsham, PA, USA) by staining with 0.2% Trypan blue.
2.5. Extraction of RNA and quantitative real‐time PCR
Total cellular RNA was isolated using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA). RNA samples were treated with TURBO DNase (Ambion, Carlsbad, CA, USA) to remove genomic DNA and converted to first‐strand cDNA using the PrimeScript RT reagent kit (Perfect Real Time; Takara Bio, Kusatsu, Japan). Quantitative real‐time PCR analysis was undertaken using a Thermal Cycler Dice Real Time System with SYBR Premix Ex Taq (Perfect Real Time; Takara Bio). The primer sequences used were as follows: GAPDH forward (5′–3′), ATCATCCCTGCCTCTACTGG; GAPDH reverse (5′–3′), TTTCTAGACGGCAGGTCAGGT; CD74 forward (5′–3′), GAGCTGTCGGGAAGATCAGA; CD74 reverse (5′–3′), AGGAAGTAGGCGGTGGTG; PD‐L1 forward (5′–3′), TGCCGACTACAAGCGAATTACTG; PD‐L1 reverse (5′–3′), CTGCTTGTCCAGATGACTTCGG; MIF forward (5′–3′), CGGACAGGGTCTACATCAA; and MIF reverse (5′–3′), CTTAGGCGAAGGTGGAGTT. GAPDH was used as an internal reference. Fold‐induction values were calculated using the 2−ΔΔCt method.
2.6. Antibodies and western blot analysis
Mouse monoclonal anti‐human CD74 Ab (LN2; Santa Cruz Biotechnology), rabbit monoclonal anti‐human PD‐L1 Ab (SP142; Spring Bioscience, Fremont, CA, USA), rabbit polyclonal anti‐human MIF Ab (Santa Cruz Biotechnology), and mouse monoclonal anti‐human Hsp70 Ab (l1409; Santa Cruz Biotechnology) were used as primary Abs at a dilution of 1:1000. Horseradish peroxidase‐labeled anti‐mouse or anti‐rabbit Abs (1:1000; Dako, Carpinteria, CA, USA) were used as secondary Abs. The Amersham ECL western blot detection reagent (GE Healthcare, Piscataway, NJ, USA) was used to detect protein expression. Whole cell extracts (40 μg) were subjected to SDS‐PAGE (NuPAGE Bis‐Tris Gel; Life Technologies, Gaithersburg, MD, USA) and were transferred to a PVDF membrane (GE Healthcare). The membranes were incubated with ECL immunoblotting detection reagent followed by exposure to hyperfilm (GE Healthcare).
2.7. Flow cytometry analysis
Melanoma cells (1 × 106) were allowed to grow in medium with or without IFN‐γ (100 IU/mL) or 4‐IPP for 48 hours. Cells were washed with ice‐cold PBS and detached by gentle pipetting, subsequently fixed with acetone. Cells were incubated with mouse monoclonal anti‐human CD74 Ab (LN2), rabbit anti‐human PD‐L1 Ab (E1L3N) or its isotype control Abs; normal mouse IgG1 (sc‐3877; Santa Cruz Biotechnology) and rabbit (DA1E) mAb IgG XP Isotype Control (Cell Signaling Technology) as primary Abs followed by FITC‐conjugated rabbit anti‐mouse immunoglobulins and swine anti‐rabbit immunoglobulins (Dako Japan) as secondary Abs. Ten thousand events per sample were collected with a Coulter Epics XL‐MCL flow cytometer (Beckman Coulter, Miami, FL, USA). Data analysis was performed using FlowJo FACS analysis software (Tree Star, Ashland, OR, USA).
2.8. Focused protein array
The expression status of proteins in each lysate was measured using a human phospho‐kinase array kit (Proteome Profiler; R&D Systems, Minneapolis, MN, USA). The lysates of WM1361A cells were collected 48 hours after the treatment with or without 4‐IPP 200 μmol/L. Lysates were applied to a human phosphor‐kinase array kit according to the manufacturer's protocol. Protein expression dots were scanned using a computer scanner, and dot pixel density was quantified with Photoshop CS5.1 software (Adobe, San Jose, CA, USA).
2.9. Statistical analysis
To assess the correlation between the expression levels of CD74 and PD‐L1 by immunohistochemistry, Fisher's exact tests were applied. For all in vitro experiments, statistical analyses were carried out using Student's t test. All P values were 2‐sided, and a value of P < .05 was considered statistically significant. All statistical analyses were performed using Excel software (Microsoft, Redmond, WA, USA).
3. RESULTS
3.1. Significant correlation between CD74 and PD‐L1 expression in melanoma tissue samples
To examine the CD74 and PD‐L1 expression levels in melanoma tissue samples, we undertook immunohistochemical staining in 97 melanoma samples obtained from 70 patients (patient characteristics are summarized in Table 1). Anti‐CD74 stained positively for both the cytoplasm and membrane of melanoma cells, and anti‐PD‐L1 stained positively only for the membrane (cytoplasmic localization was considered to be negative). Representative positive staining results of CD74 and PD‐L1 are shown in Figure 1A. The PD‐L1 expression in tumor cells was confirmed by the double staining of PD‐L1 and Ki‐67, as PD‐L1 can be expressed not only in tumor cells but also in myeloid cells infiltrated around tumor cells (Figure S1A). Expression of each sample was evaluated as positive by the percentage of melanoma cells with positive staining (CD74 >1%, PD‐L1 >1%). We then analyzed the correlation between the expression levels of CD74 and PD‐L1, which showed significant correlations in all 97 analyzed samples (χ2, 12.44; P < .01), and in 64 primary tumor samples (χ2, 13.97; P < .01) and 33 metastatic tumor samples (χ2, 9.03; P < .01) (Figure 1B). In the further analysis evaluating expression of each sample as positive by the percentage of melanoma cells with positive staining (CD74 >50%, PD‐L1 >50%), increased percentage of PD‐L1 expression was significantly correlated with that in CD74 expression (P < .01; Figure S1B). In the correlation analysis between those expression levels and tumor progression, PD‐L1 positivity significantly correlated with tumor progression (P < .05; Figure S1C).
Table 1.
Characteristics of melanoma patients analyzed in this study
| N (%) | |
|---|---|
| Sex | |
| Male | 24 (34.3) |
| Female | 46 (65.7) |
| Age (y) | |
| Mean ± SD (range) | 60.31 ± 14.96 (15‐86) |
| Median | 61 |
| Primary lesion, n | 64 |
| Tumor subtype | |
| ALM | 25 (39.1) |
| SSM | 18 (28.1) |
| NM | 23 (35.9) |
| LMM | 1 (1.6) |
| Mucosal | 2 (3.1) |
| In situ | 1 (1.6) |
| Tumor site | |
| Extremity | 39 (61.0) |
| Trunk | 13 (20.3) |
| Head and neck | 8 (12.5) |
| Mucosal | 4 (6.3) |
| Genital | 2 (3.1) |
| Metastatic lesion, n | 33 |
| Lymph node, n | 21 |
| Skin metastasis, n | 12 |
Data are presented as n (%) or mean ± SD (range) unless otherwise indicated.
ALM, acral lentiginous melanoma; LMM, lentigo maligna melanoma; NM, nodular melanoma; SSM, superficial spreading melanoma.
Figure 1.
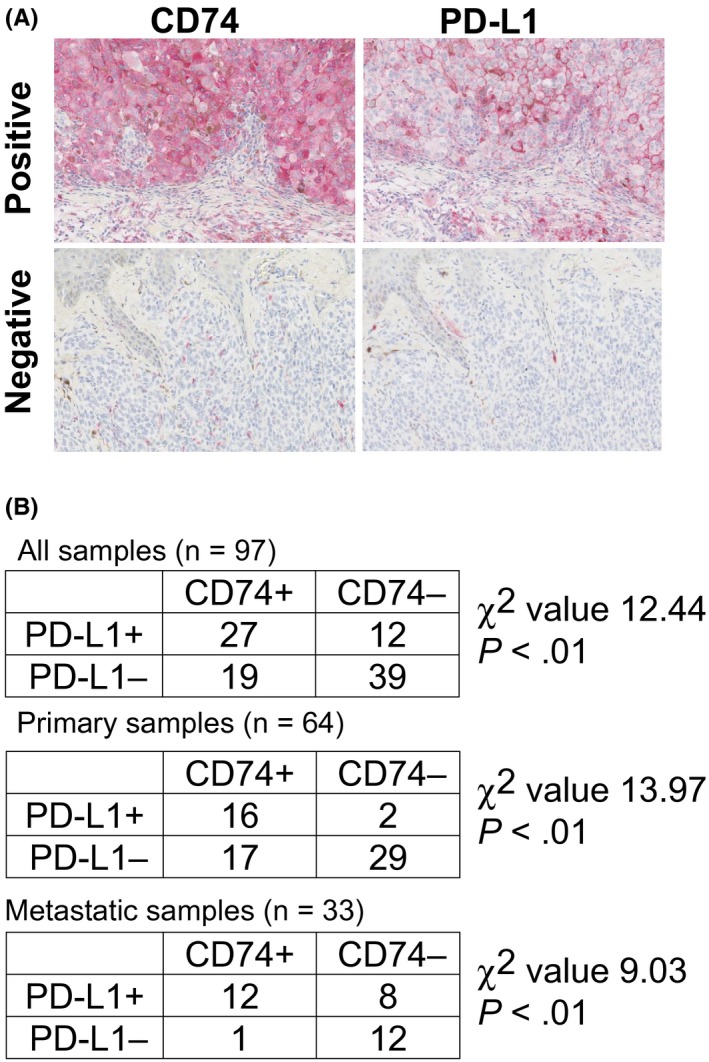
Expressions of CD74 and programmed cell death ligand 1 (PD‐L1) in melanoma tissue samples. A, Representative immunohistochemical staining of CD74 (left panels) and (PD‐L1; right panels) in melanoma tissue samples. Magnification, 200×. Positive staining is visualized by red staining. Upper left panel: metastatic melanoma sample stained positively for CD74 in both the cytoplasm and cell membrane. Upper right panel: the same metastatic melanoma sample in the upper left panel stained positively for PD‐L1 in the cell membrane. Positive staining in the cytoplasm was considered to be negative in the present study. Lower left panel: primary melanoma sample stained negatively for CD74. Lower right panel: same primary melanoma sample in the lower left panel stained negatively for PD‐L1. B, Correlation analysis of CD74 and PD‐L1 expression status in all 97 samples (upper table), 64 primary samples (middle table), and 33 metastatic samples (lower table). The χ2 values were 12.44 (P < .01), 13.97 (P < .01), and 9.03 (P < .01) in all samples, primary lesions, and metastatic lesions, respectively, suggesting a high correlation between the expression levels of CD74 and PD‐L1
3.2. Upregulated expression of CD74 by IFN‐γ stimulation
On the basis of previous findings that IFN‐γ upregulates both PD‐L1 and cell surface CD74 expression,17 we hypothesized that the MIF‐CD74 interaction mediated the expression of PD‐L1 after stimulation by IFN‐γ. To test this hypothesis, we treated 2 melanoma cell lines, A375 and SB2, with IFN‐γ and an antagonist of the MIF‐CD4 interaction, 4‐IPP. Although A375 and SB2 harbor different mutational backgrounds (A375 has a BRAF mutation and SB2 has an NRAS mutation), both cell lines expressed MIF and secreted it into the culture supernatant and also upregulated cell surface CD74 levels in response to IFN‐γ treatment, to augment the MIF‐CD74 interaction.17 Initially, viability of both cell lines was evaluated under the IFN‐γ and/or 4‐IPP treated condition. The IFN‐γ 100 IU/mL treatment alone did not affect the cell proliferation in either cell line, and 4‐IPP 100 μmol/L treatment or combined treatment of IFN‐γ 100 IU/mL and 4‐IPP 100 μmol/L suppressed the cell proliferation, yet viable cells continued to proliferate (Figure 2A). Under such conditions, expression of CD74 was upregulated when stimulated with IFN‐γ, in terms of mRNA (Figure 2B), protein (Figure 2C), and cell surface expression levels (Figure 2D). Further treatment with 4‐IPP did not suppress the CD74 expression level (Figure 2B‐D). In addition, neither IFN‐γ nor 4‐IPP affected the expression level of MIF (Figures 2C and S2A).
Figure 2.
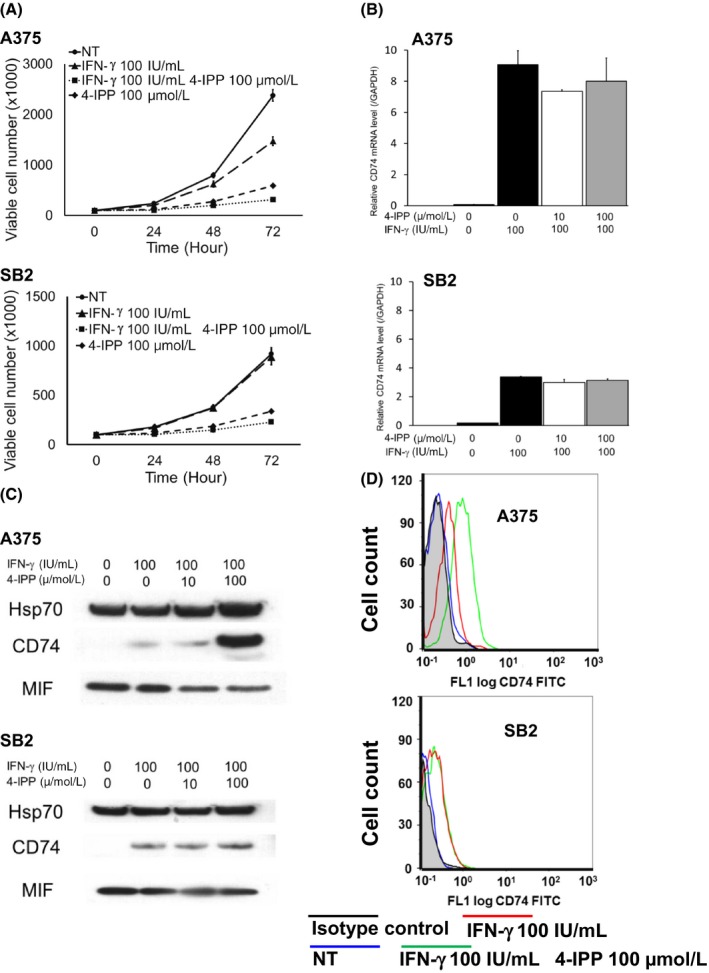
γ‐Interferon (IFN‐γ) stimulation upregulates the expression of CD74 in melanoma cells. A375 and SB2 cells were treated with/without IFN‐γ and/or 4‐iodo‐6‐phenylpyrimidine (4‐IPP). A, Cell viability analysis. A375 (upper panel) and SB2 (lower panel). Treatment with IFN‐γ 100 IU/mL alone did not affect the cell proliferation in either cell line. However, 4‐IPP 100 μmol/L treatment alone or combined with IFN‐γ 100 IU/mL suppressed cell proliferation. B, Quantitative real‐time PCR analysis to measure mRNA levels of CD74 in A375 cells (upper panel) and SB2 cells (lower panel). Stimulation with IFN‐γ‐ upregulated the expression of CD74, which was not affected by further treatment with 4‐IPP. Shown are representative data from 1 of 3 experiments. C, Western blot analysis of CD74 protein expression in A375 cells (upper panel) and SB2 cells (lower panel). Stimulation with IFN‐γ upregulated the expression of CD74. Further treatment of A375 cells with 4‐IPP showed further upregulation of CD74 expression, possibly due to a compensatory mechanism. MIF, macrophage migration inhibitory factor. D, Flow cytometry analysis of cell surface CD74 protein in A375 cells (upper panel) and SB2 cells (lower panel). IFN‐γ stimulation upregulated the expression of cell surface CD74 protein level in both cell lines. Further treatment of A375 cells with 4‐IPP showed further upregulation of CD74 expression. Mean fluorescence intensity (MFI) of each condition was as follows. A375 cells: isotype control, 0.26, nontreated (NT), 0.30; IFN‐γ 100 IU/mL, 0.48; IFN‐γ 100 IU/mL and 4‐IPP 100 μmol/L, 0.69. SB2 cells: isotype control, 0.16; NT, 0.19; IFN‐γ 100 IU/mL, 0.34; IFN‐γ 100 IU/mL and 4‐IPP 100 μmol/L, 0.25
3.3. Upregulated expression of PD‐L1 by IFN‐γ stimulation suppressed by inhibition of MIF‐CD74 interaction
Next we evaluated the expression levels of PD‐L1 under IFN‐γ and/or 4‐IPP treated conditions. Expression of PD‐L1 was negative in both cell lines under normal culture conditions, but was dramatically upregulated when stimulated with IFN‐γ, in terms of mRNA (Figure 3A), protein (Figure 3B), and cell surface expression levels (Figure 3C,D). After addition of 4‐IPP, the expression of PD‐L1 was suppressed in a dose‐dependent manner, in terms of both mRNA (Figure 3A) and protein levels (Figure 3B). Suppression of PD‐L1 expression by 4‐IPP was also confirmed using flow cytometry analysis and immunocytochemistry (Figure 3C,D).
Figure 3.
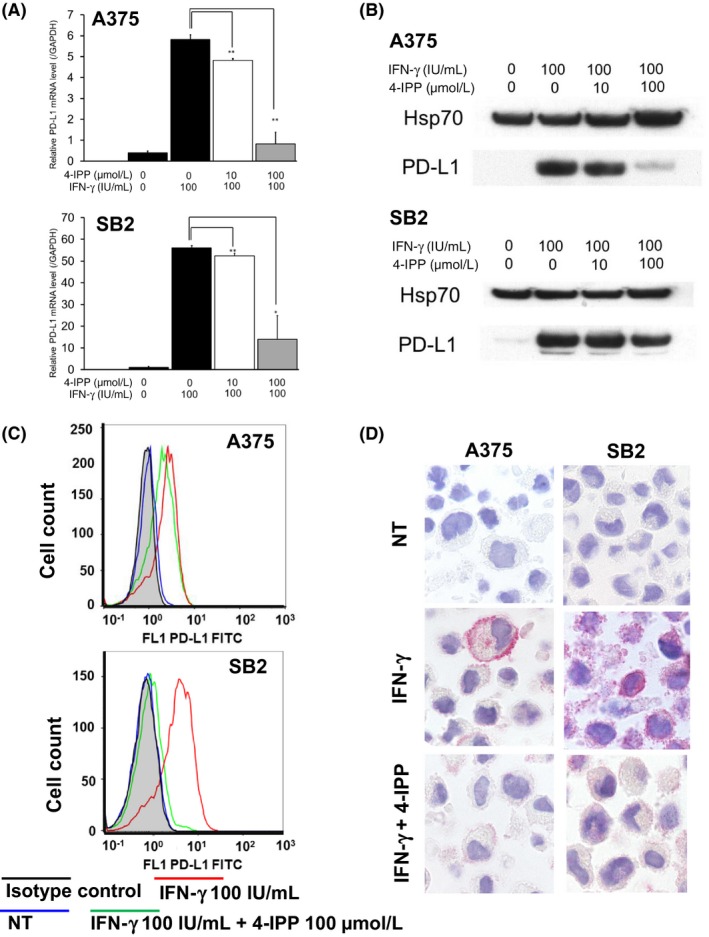
γ‐Interferon (IFN‐γ) stimulation upregulates the expression of programmed cell death ligand 1 (PD‐L1) in melanoma cells through macrophage migration inhibitory factor (MIF)‐CD74 interaction. A375 and SB2 cells were treated with 4‐iodo‐6‐phenylpyrimidine (4‐IPP) for 48 h under IFN‐γ stimulatory conditions. A, Quantitative real‐time PCR analysis to measure mRNA levels of PD‐L1 in A375 cells (upper panel) and SB2 cells (lower panel). IFN‐γ stimulation upregulated expression of PD‐L1, which was suppressed by further treatment with 4‐IPP. *P < .05; **P < .01. Shown are representative data from 1 of 3 experiments. B, Western blot analysis of PD‐L1 protein expression in A375 cells (upper panel) and SB2 cells (lower panel). IFN‐γ stimulation upregulated the expression of PD‐L1, which was suppressed by further treatment with 4‐IPP. C, Flow cytometry analysis of cell surface PD‐L1 protein in A375 cells (upper panel) and SB2 cells (lower panel). IFN‐γ stimulation upregulated the expression of cell surface PD‐L1 protein level in both cell lines. Further treatment with 4‐IPP showed downregulation of CD74 expression. Mean fluorescence intensity of each condition was as follows. A375 cells: isotype control, 0.86; nontreated (NT), 0.87; IFN‐γ 100 IU/mL, 2.57; IFN‐γ 100 IU/mL; and 4‐IPP 100uM, 1.77. SB2 cells: isotype control, 0.90; nontreated (NT), 0.91; IFN‐γ 100 IU/mL, 3.90; IFN‐γ 100 IU/mL and 4‐IPP 100 μmol/L, 1.16. D, Immunocytochemistry of A375 cells (left panels) and SB2 cells (right panels) PD‐L1 was negative under normal culture conditions (upper panels). IFN‐γ stimulation upregulated the membrane staining of PD‐L1 (middle panels), and cell staining was suppressed by further treatment with 4‐IPP (lower panels) in both cell lines. Shown are representative data from 1 of 3 experiments
3.4. Inhibition of MIF‐CD74 interaction suppressed expression of PD‐L1, phosphorylation of STAT2 [Y689], and phosphorylated c‐JUN [S63] in WM1361A cells, which constitutively express PD‐L1
To confirm that autocrine MIF‐CD74 interaction directly regulated the expression of PD‐L1, we initially attempted to establish CD74‐overexpressing A375 cells and SB2 cells by transfecting CD74 expression vectors in these cell lines. However, although transfection showed overexpression of CD74 mRNA and proteins, it did not show upregulation of cell surface CD74 levels (data not shown). Therefore, we studied the WM1361A melanoma cell line, which constitutively expressed PD‐L1, CD74, and MIF under untreated conditions (Figure 4A). Cell surface expression of CD74 was also confirmed by flow cytometry analysis (Figure 4B). In the evaluation of WM1361A cell viability under the 4‐IPP treated condition, 4‐IPP 50 or 200 μmol/L treatment suppressed cell proliferation, yet viable cells continued to proliferate (Figure 4C). Under this treatment condition, phosphorylation or expression of the several signaling molecules, as well as the expression level of PD‐L1, was evaluated. In the analysis of protein array, treatment with 4‐IPP 200 μmol/L suppressed expression level of several signaling molecules, including STAT2 (Y689) and c‐JUN (S63), and upregulated STAT3 (S727) and p53 (S392) (Figure 4D). Treatment with 4‐IPP decreased the expression of PD‐L1, in terms of both mRNA (Figure 5A) and protein levels (Figure 5B), in a dose‐dependent manner. Downregulation of cell surface expression of PD‐L1 by 4‐IPP treatment was also confirmed by flow cytometry analysis and immunocytochemistry (Figure 5C,D). However, 4‐IPP treatment did not affect the expression levels of CD74 or MIF (Figures 4C and 5B). We also undertook siRNA targeting of CD74 and MIF. Transfection of 2 siRNA sequences targeting CD74 decreased the expression levels of CD74 by 40.7% and 3.8%. Under this CD74 knockdown condition, the expression levels of PD‐L1 were suppressed by 55.2% and 34.9%, respectively (Figure 5E). Cell surface expressions of CD74 and PD‐L1 were also suppressed by 2 siRNA sequences targeting CD74 (Figure 5F). In a similar manner, transfection of 2 sequences of siRNA targeting MIF decreased the expression levels of MIF by 45.7% and 7.1%, and the expression levels of PD‐L1 were suppressed by 50.0% and 26.4%, respectively (Figure S2B). Together, these results suggested that the MIF‐CD74 interaction directly regulated the expression of PD‐L1 in melanoma cells.
Figure 4.
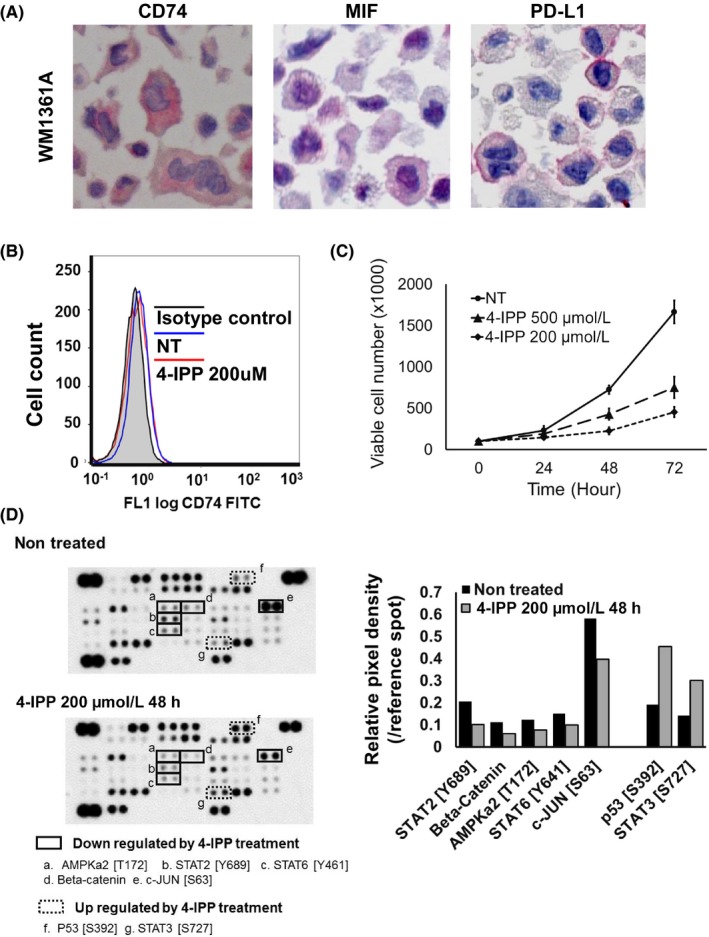
Macrophage migration inhibitory factor (MIF)‐CD74 interaction regulates activity of several signaling molecules in the WM1361A melanoma cell line. A, Immunocytochemical analysis. WM1361A cells were confirmed to express programmed cell death ligand 1 (PD‐L1) (left panel), MIF (middle panel), and CD74 (right panel) in untreated cultures. B, Flow cytometry analysis. WM1361A cells showed cell surface expression of CD74. Mean fluorescence intensity of each condition was as follows. Isotype control, 0.47; nontreated (NT), 0.71; 4‐iodo‐6‐phenylpyrimidine (4‐IPP) 200 μmol/L, 0.65. C, Cell viability analysis. 4‐IPP treatment suppressed the proliferation of WM1361A cells in a dose‐dependent manner. D, Protein array. 4‐IPP treatment for 48 h decreased expression of phosphorylated (phospho‐)AMPKa2 (T172) (a), phospho‐STAT2 (Y689) (b), phospho‐STAT6 (Y461) (c), β‐catenin (d), and phospho‐c‐JUN (S63) (e), and increased expression of phospho‐P53 (S392) (f) and phospho‐STAT3 (S727) (g). Right panel, relative dot density of protein array analysis
Figure 5.
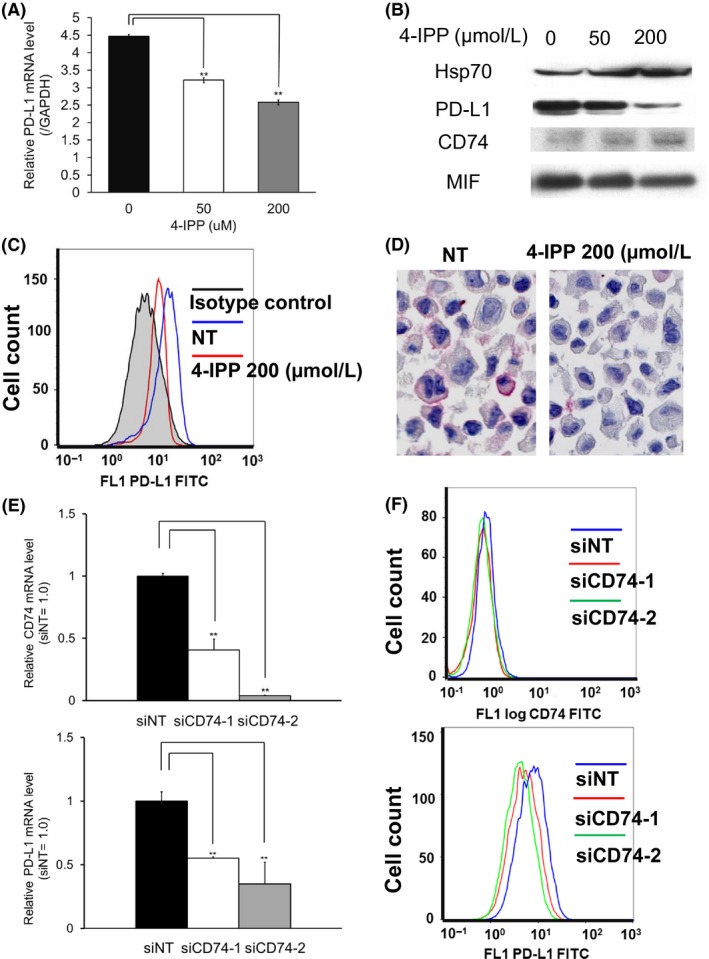
Macrophage migration inhibitory factor (MIF)‐CD74 interaction regulates expression of programmed cell death ligand 1 (PD‐L1) in the WM1361A melanoma cell line. WM1361A cells were treated with 4‐iodo‐6‐phenylpyrimidine (4‐IPP) for 72 h. A, Quantitative real‐time PCR analysis. The mRNA level of PD‐L1 was suppressed in a dose‐dependent manner by 74.4% when treated with 50 μmol/L 4‐IPP, and suppressed by 60.7% with 200 μmol/L 4‐IPP. **P < .01. B, Western blot analysis. The expression level of PD‐L1 protein was suppressed in a dose‐dependent manner. C, Flow cytometry analysis. Cell surface PD‐L1 expression was positive in untreated condition. 4‐IPP treatment decreased its expression level. Mean fluorescence intensity of each condition was: isotype control, 5.74; nontreated (NT), 17.9; 4‐IPP 200 μmol/L, 12.5. D, Membrane staining of PD‐L1 was suppressed when treated with 200 μmol/L 4‐IPP. Number of PD‐L1 positive cells from 5 different randomly selected areas were counted using a high‐powered field (400× magnification), and the average of the 5 sums was calculated. The mean number of PD‐L1‐positive cells were 21.8 in the untreated condition and 4.6 in the 4‐IPP 200 μmol/L treated condition. E, WM1361A cells were transfected with siRNA targeting CD74, and the expression levels of CD74 (upper panel) and PD‐L1 (lower panel) were analyzed by quantitative real‐time PCR 48 h after transfection. Transfections of 2 sequences of siRNA targeting CD74 decreased the expression levels of CD74 by 40.7% (siCD74‐1) and 3.8% (siCD74‐2). E, Flow cytometry analysis. Expression of cell surface CD74 (upper panel) and PD‐L1 (lower panel) were analyzed 72 h after the transfections of 2 sequences of siRNA targeting CD74. Both sequences decreased expression level of CD74 and PD‐L1. Mean fluorescence intensity of each condition was follows. CD74: siNT, 0.51; siCD74‐1, 0.43; siCD74‐2, 0.40. PD‐L1: siNT, 7.20; siCD74‐1, 4.78; siCD74‐2, 4.12. Shown are representative data from 1 of 3 experiments
4. DISCUSSION
Melanoma is the most aggressive form of skin cancer and is believed to have a highly immunogenic potential. Recent advances in therapy have provided immune checkpoint inhibitors that show impressive clinical benefits in patients with metastatic melanoma.18, 19, 20, 21 However, blockade of the PD‐1‐PD‐L1 interaction does not induce tumor regression in all patients.20, 21 Further understanding of the molecular mechanisms underlying immune evasion of cancers, as well as the mechanisms for controlling PD‐L1 expression, will therefore be important in improving the efficacy of antitumor immunotherapy.
In the present study, we showed that the MIF‐CD74 interaction regulated the expression of PD‐L1, especially under the stimulatory conditions of IFN‐γ treatment (Figure 6). Immunohistochemistry of melanoma tissue samples showed a significant correlation between the expression levels of CD74 and PD‐L1. A375 and SB2 cells showed upregulation of PD‐L1 and CD74 levels by IFN‐γ stimulation, and the PD‐L1 level was suppressed by further treatment with 4‐IPP. In further analyses of WM1361A cells, treatment with 4‐IPP and transfection of siRNAs targeting MIF and CD74 significantly suppressed the expression levels of PD‐L1.
Figure 6.
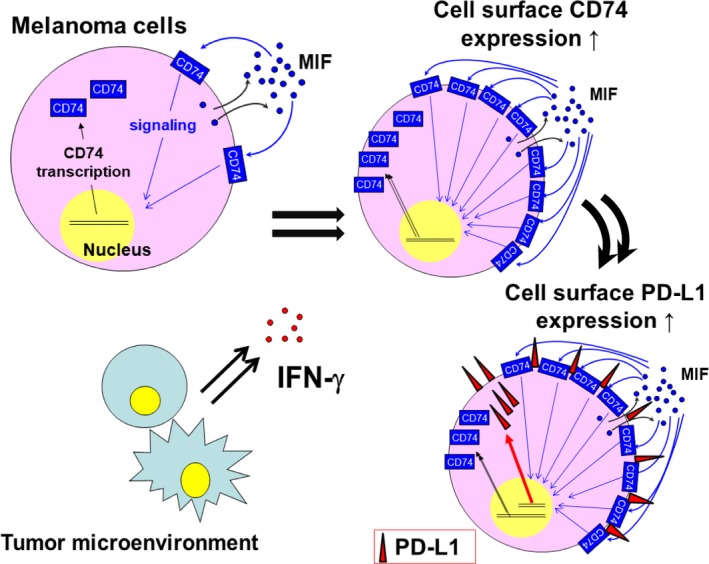
Schematic image of this study. γ‐Interferon (IFN‐γ) stimulation upregulates expression levels of programmed cell death ligand 1 (PD‐L1) mediated by the activation of macrophage migration inhibitory factor (MIF)‐CD74 interactions, which is induced by the upregulation of cell surface CD74 expression by IFN‐γ stimulation
Expression of PD‐L1 is regulated by several genomic, epigenetic, and transcriptional mechanisms.7, 8 Genomic rearrangements, including gene amplification and translocation in chromosomes, have been reported to upregulate PD‐L1 expression in several malignancies.22, 23, 24 Epigenetically, bromodomain‐containing protein 425, 26 and histone methyltransferase MLL1 have been associated with the expression of PD‐L1.27 Regarding its regulation at the transcriptional level, various signaling pathways, including the JAK‐STAT,5 NF‐κB,28 MAPK, and PI3K‐AKT signaling pathways29, 30 have been reported to be upstream regulators of PD‐L1 expression. In this study, protein array analysis of signaling molecules in WM1361A cell treated with/without 4‐IPP identified that inhibition of MIF‐CD74 interaction suppressed phosphorylation of STAT2 (Y689) and c‐JUN (S63), and upregulated phosphorylation of STAT3 (S727). Reportedly, STAT2 is one of the mediators to promote PD‐L1 transcription.5 Additionally, STAT3 plays an important transcriptional role of PD‐L1,31 and c‐JUN has been reported to promote its transcription by acting together with STAT3.8 However, S‐727 phosphorylation of STAT3 has been reported to have an intrinsic mechanism for shortening the duration of STAT3 activity,32 hence S‐727 phosphorylation is expected to regulate PD‐L1 expression negatively. These results suggest that MIF‐CD74 interaction regulates expression of PD‐L1 in tumor cells by augmenting activation of the IFN‐γ‐JAK‐STAT signaling pathway.
CD74 has dual functional roles. It has usually been identified as a chaperone of MHC class II Dα and Dβ chains,33 but a small proportion of CD74 is found on the cell surface independent of the MHC class II complex,12 where it functions as a receptor for MIF.12, 13, 14 CD74 has been reported to be expressed in various malignancies and serves as a prognostic factor, disease progression marker, and promoter of invasion and metastasis.17, 34, 35, 36, 37, 38 Interaction between MIF and CD74 has been reported to regulate the expression of various genes, including IL‐6, IL‐8, and BCL‐2.17, 39, 40 Expression of BCL‐2 contributes to apoptosis and chemotherapy resistance.41 IL‐6 and IL‐8 are associated with the invasiveness and metastatic potential of tumor cells.42, 43 Additionally, secretion of both IL‐6 and IL‐8 from tumor cells has been reported to enrich the Foxp3+ CD4‐regulatory T‐cell subset among T cells migrating toward melanoma, thereby creating an immunosuppressive microenvironment.44 Together with its regulatory role in PD‐L1 expression in tumor cells, activation of the MIF‐CD74 interaction plays a critical role in melanoma cells by causing immune evasion and promoting survival in the microenvironment of the antitumorigenic immune reaction.
In conclusion, MIF‐CD74 interaction is a regulator of PD‐L1 expression and plays a key role in maintaining an advantageous tumor microenvironment for tumor cells. The MIF‐CD74 interaction is therefore a possible target for effective treatment of melanoma patients.
DISCLOSURE
The authors declare no potential conflicts of interest.
Supporting information
Imaoka M, Tanese K, Masugi Y, Hayashi M, Sakamoto M. Macrophage migration inhibitory factor‐CD74 interaction regulates the expression of programmed cell death ligand 1 in melanoma cells. Cancer Sci. 2019;110:2273‐2283. 10.1111/cas.14038
Masako Imaoka and Keiji Tanese equally contributed to this work.
REFERENCES
- 1. Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD‐1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu P, Wu D, Li L, Chai Y, Huang J. PD‐L1 and survival in solid tumors: a meta‐Analysis. PLoS ONE. 2015;10:e0131403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mandai M, Hamanishi J, Abiko K, Matsumura N, Baba T, Konishi I. Dual faces of IFNγ in cancer progression: a role of PD‐L1 induction in the determination of pro‐ and antitumor immunity. Clin Cancer Res. 2016;22:2329‐2334. [DOI] [PubMed] [Google Scholar]
- 4. Abiko K, Matsumura N, Hamanishi J, et al. IFN‐γ from lymphocytes induces PD‐L1 expression and promotes progression of ovarian cancer. Br J Cancer. 2015;112:1501‐1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garcia‐Diaz A, Shin DS, Moreno BH, et al. Interferon receptor signaling pathways regulating PD‐L1 and PD‐L2 expression. Cell Rep. 2017;19:1189‐1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pasquali S, Hadjinicolaou AV, Chiarion Sileni V, Rossi CR, Mocellin S. Systemic treatments for metastatic cutaneous melanoma. Cochrane Database Syst Rev. 2018;2:CD011123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD‐L1 checkpoint. Immunity. 2018;48:434‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen J, Jiang CC, Jin L, Zhang XD. Regulation of PD‐L1: a novel role of pro‐survival signalling in cancer. Ann Oncol. 2016;27:409‐416. [DOI] [PubMed] [Google Scholar]
- 9. Murray PJ. The JAK‐STAT signaling pathway: input and output integration. J Immunol. 2007;178:2623‐2629. [DOI] [PubMed] [Google Scholar]
- 10. Dorand RD, Nthale J, Myers JT, et al. Cdk5 disruption attenuates tumor PD‐L1 expression and promotes antitumor immunity. Science. 2016;353:399‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koch N, Moldenhauer G, Hofmann WJ, Möller P. Rapid intracellular pathway gives rise to cell surface expression of the MHC class II‐associated invariant chain (CD74). J Immunol. 1991;147:2643‐2651. [PubMed] [Google Scholar]
- 12. Wraight CJ, van Endert P, Möller P, et al. Human major histocompatibility complex class II invariant chain is expressed on the cell surface. J Biol Chem. 1990;265:5787‐5792. [PubMed] [Google Scholar]
- 13. Leng L, Metz CN, Fang Y, et al. MIF signal transduction initiated by binding to CD74. J Exp Med. 2003;197:1467‐1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shi X, Leng L, Wang T, et al. CD44 is the signaling component of the macrophage migration inhibitory factor‐CD74 receptor complex. Immunity. 2006;25:595‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Starlets D, Gore Y, Binsky I, et al. Cell‐surface CD74 initiates a signaling cascade leading to cell proliferation and survival. Blood. 2006;107:4807‐4816. [DOI] [PubMed] [Google Scholar]
- 16. Cao ZA, Moore BB, Quezada D, Chang CH, Jones PP. Identification of an IFN‐gamma responsive region in an intron of the invariant chain gene. Eur J Immunol 2000;30:2604‐2611. [DOI] [PubMed] [Google Scholar]
- 17. Tanese K, Hashimoto Y, Berkova Z, et al. Cell surface CD74‐MIF interactions drive melanoma survival in response to interferon‐γ. J Invest Dermatol. 2015;135:2775‐2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wolchok JD, Hodi FS, Weber JS, et al. Development of ipilimumab: A novel immunotherapeutic approach for the treatment of advanced melanoma. Ann N Y Acad Sci. 2013;1291:2273‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320‐330. [DOI] [PubMed] [Google Scholar]
- 22. Roemer MG, Advani RH, Ligon AH, et al. PD‐L1 and PD‐L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J Clin Oncol. 2016;34:2690‐2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Twa DD, Chan FC, Ben‐Neriah S, et al. Genomic rearrangements involving programmed death ligands are recurrent in primary mediastinal large B‐cell lymphoma. Blood. 2014;123:2062‐2065. [DOI] [PubMed] [Google Scholar]
- 24. Cancer Genome Atlas Research Network . Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhu H, Bengsch F, Svoronos N, et al. BET bromodomain inhibition promotes anti‐tumor immunity by suppressing PD‐L1 expression. Cell Rep. 2016;16:2829‐2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hogg SJ, Vervoort SJ, Deswal S, et al. BET‐bromodomain inhibitors engage the host immune system and regulate expression of the immune checkpoint ligand PD‐L1. Cell Rep. 2017;18:2162‐2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lu C, Paschall AV, Shi H, et al. The MLL1‐H3K4me3 axis‐mediated PD‐L1 expression and pancreatic cancer immune evasion. J Natl Cancer Inst. 2017;109:djw283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peng J, Hamanishi J, Matsumura N, et al. Chemotherapy induces programmed cell death‐ligand 1 overexpression via the nuclear factor‐κB to foster an immunosuppressive tumor microenvironment in ovarian cancer. Cancer Res. 2015;75:5034‐5045. [DOI] [PubMed] [Google Scholar]
- 29. Jiang X, Zhou J, Giobbie‐Hurder A, Wargo J, Hodi FS. The activation of MAPK in melanoma cells resistant to BRAF inhibition promotes PD‐L1 expression that is reversible by MEK and PI3K inhibition. Clin Cancer Res. 2013;19:598‐609. [DOI] [PubMed] [Google Scholar]
- 30. Atefi M, Avramis E, Lassen A, et al. Effects of MAPK and PI3K pathways on PD‐L1 expression in melanoma. Clin Cancer Res. 2014;20:3446‐3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marzec M, Zhang Q, Goradia A, et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD‐L1, B7‐H1). Proc Natl Acad Sci USA. 2008;105:20852‐20857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wakahara R, Kunimoto H, Tanino K, et al. Phospho‐Ser727 of STAT3 regulates STAT3 activity by enhancing dephosphorylation of phospho‐Tyr705 largely through TC45. Genes Cells. 2012;17:132‐145. [DOI] [PubMed] [Google Scholar]
- 33. Roche PA, Marks MS, Cresswell P. Formation of a nine‐subunit complex by HLA class II glycoproteins and the invariant chain. Nature. 1991;354:392‐394. [DOI] [PubMed] [Google Scholar]
- 34. Burton JD, Ely S, Reddy PK, et al. CD74 is expressed by multiple myeloma and is a promising target for therapy. Clin Cancer Res. 2004;10:6606‐6611. [DOI] [PubMed] [Google Scholar]
- 35. Datta MW, Shahsafaei A, Nadler LM, Freeman GJ, Dorfman DM. Expression of MHC class II‐associated invariant chain (Ii;CD74) in thymic epithelial neoplasms. Appl Immunohistochem Mol Morphol. 2000;8:210‐215. [DOI] [PubMed] [Google Scholar]
- 36. Ishigami S, Natsugoe S, Tokuda K, et al. Invariant chain expression in gastric cancer. Cancer Lett. 2001;168:87‐91. [DOI] [PubMed] [Google Scholar]
- 37. Koide N, Yamada T, Shibata R, et al. Establishment of perineural invasion models and analysis of gene expression revealed an invariant chain (CD74) as a possible molecule involved in perineural invasion in pancreatic cancer. Clin Cancer Res. 2006;12:2419‐2426. [DOI] [PubMed] [Google Scholar]
- 38. Ekmekcioglu S, Davies MA, Tanese K, et al. Inflammatory marker testing identifies CD74 expression in melanoma tumor cells, and its expression associates with favorable survival for stage III melanoma. Clin Cancer Res. 2016;22:3016‐3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Binsky I, Haran M, Starlets D, et al. IL‐8 secreted in a macrophage migration‐inhibitory factor‐ and CD74‐dependent manner regulates B cell chronic lymphocytic leukemia survival. Proc Natl Acad Sci USA. 2007;104:13408‐13413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gore Y, Starlets D, Maharshak N, et al. Macrophage migration inhibitory factor induces B cell survival by activation of a CD74‐CD44 receptor complex. J Biol Chem. 2008;283:2784‐2792. [DOI] [PubMed] [Google Scholar]
- 41. Bedikian AY, Millward M, Pehamberger H, et al. Bcl‐2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study Group. J Clin Oncol. 2006;24:4738‐4745. [DOI] [PubMed] [Google Scholar]
- 42. Bar‐Eli M. Role of interleukin‐8 in tumor growth and metastasis of human melanoma. Pathobiology. 1999;67:12‐18. [DOI] [PubMed] [Google Scholar]
- 43. Kushiro K, Chu RA, Verma A, Núñez NP. Adipocytes promote B16BL6 melanoma cell invasion and the epithelial‐to‐mesenchymal transition. Cancer Microenviron. 2012;5:73‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Eikawa S, Ohue Y, Kitaoka K, et al. Enrichment of Foxp3 + CD4 regulatory T cells in migrated T cells to IL‐6‐ and IL‐8‐expressing tumors through predominant induction of CXCR50 by IL‐6. J Immunol. 2010;185:6734‐6740. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


