Abstract
Inflammation plays an essential role in the development and progression of most cancers. Chemokine C‐C motif chemokine 2 (CCL2) and its receptor C‐C chemokine receptor type 2 (CCR2) constitute a key signaling axis in inflammation that has recently attracted much interest on the basis of evidence showing its association with cancer progression. Propagermanium (3‐oxygermylpropionic acid polymer) is an organogermanium compound that is given for the treatment of hepatitis B in Japan and which inhibits the CCL2‐CCR2 signaling pathway. Herein, we review the importance of the CCL2‐CCR2 axis as a target in cancer treatment as shown by studies in mice and humans with pharmacological agents including propagermanium.
Keywords: CCL2, CCR2, inflammation, metastasis, propagermanium
Abbreviations
- ALT
alanine aminotransferase
- CCL2
C‐C motif chemokine 2
- CCR2
C‐C chemokine receptor type 2
- GeSP
germanium straight‐chain polymer
- HBV
hepatitis B virus
- MDSC
myeloid‐derived suppressor cell
- Mo‐MDSC
monocytic myeloid‐derived suppressor cell
- PDAC
pancreatic ductal adenocarcinoma
- PGe
propagermanium
- RGe
repagermanium
- TAM
tumor‐associated macrophage
1. INTRODUCTION
Inflammation is associated with the development of many diseases including cancer.1, 2 Under normal physiological conditions, the immune system eliminates pathogens and unwanted cells such as those that are damaged, senescent, or immature. However, cancer hijacks this system to ensure tumor survival and long‐term growth. Cancer cells recruit immune cells such as macrophages, neutrophils, and MDSC to form a microenvironment known as a “niche.”3 Cancer cells and their niche cells produce various cytokines, chemokines, growth factors, ECM proteins, and proteases that promote tumor growth and metastasis.
C‐C motif chemokine 2 (CCL2, also known as monocyte chemoattractant protein 1) and its receptor CCR2 have attracted much interest in recent years because of their relation to cancer progression.4, 5 Although CCL2 was first described as a cytokine with a physiological role in the regulation of inflammation,6, 7 more recent studies have shown a protumorigenic function of CCL2 in the promotion of cancer development and metastasis (Figure 1). Binding of CCL2 to CCR2, a G protein‐coupled receptor, triggers intracellular signaling in cancer and other cell types. CCL2‐CCR2 signaling promotes cancer progression by supporting cancer cell proliferation and survival, inducing cancer cell migration and invasion, and stimulating inflammation and angiogenesis.8 CCL2 is secreted by many cell types including endothelial cells, fibroblasts, monocytes, and cancer cells, whereas CCR2 is expressed at a high level in inflammatory monocytes, dendritic cells, and natural killer cells as well as at a low level in neutrophils and T and B lymphocytes. In the early stages of metastasis, CCL2 guides tumor cell migration by interacting with CCR2 expressed on the surface of cancer cells.9, 10 CCL2 also induces expression of MMP2 and MMP9 in cancer cells, both of which facilitate cancer cell invasion,11, 12 and it promotes the intra‐ and extravasation of cancer cells by attracting TAM.13 TAM and MDSC recruited by CCL2 trigger an angiogenic switch14, 15 and suppress immune‐mediated attack of cancer cells.16 In addition, CCL2 attracts cancer cells to future sites of metastasis and supports their proliferation and survival at such premetastatic niches.17
Figure 1.
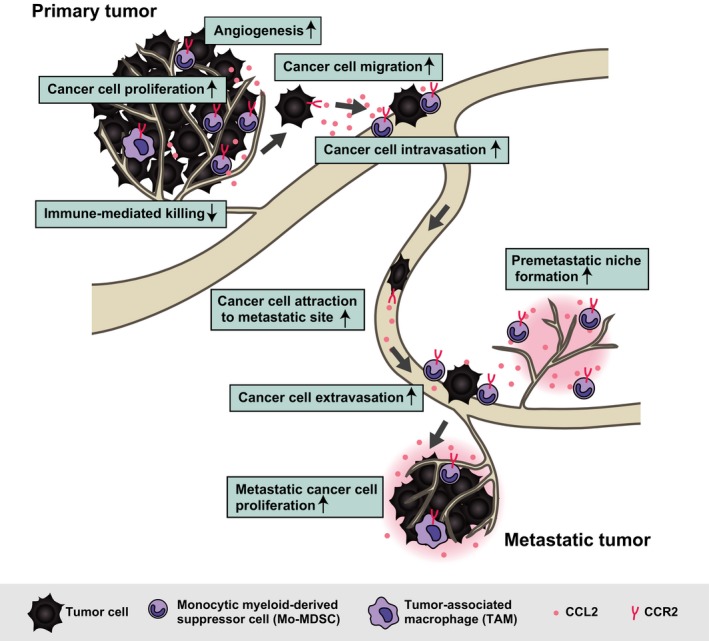
Role of chemokine C‐C motif chemokine 2–C‐C chemokine receptor type 2 (CCL2‐CCR2) signaling in cancer progression. CCL2 is secreted by cancer cells and surrounding stromal cells. It induces tumor cell proliferation at the primary tumor site, and CCR2+ myeloid cells attracted by CCL2 suppress immune‐mediated killing of tumor cells. CCL2 also promotes tumor cell migration and invasion into the surrounding ECM followed by tumor cell intravasation into the circulation. The subsequent dissemination of cancer cells is directed by a chemotactic gradient of CCL2 toward potential sites of metastasis. CCL2 and CCR2+ cells then promote tumor cell extravasation and colonization and growth at such metastatic sites
Increased levels of CCL2 in tumor cells or serum have been detected in individuals with melanoma or with breast, prostate, colorectal, gastric, or ovarian cancer, and they are frequently associated with disease progression, tumor grade, or metastasis, suggestive of the clinical importance of the CCL2‐CCR2 signaling pathway.18, 19, 20, 21, 22, 23, 24, 25, 26, 27 These observations also suggest that targeting of the CCL2‐CCR2 axis might be an effective strategy for cancer treatment.
Herein, we review currently available agents that target the CCL2‐CCR2 axis and studies of their efficacy for cancer treatment. Among such agents, we focus on an organogermanium compound, PGe (3‐oxygermylpropionic acid polymer), that has been shown to act as a blocker of CCR2 signaling.
2. INHIBITORS OF THE CCL2‐CCR2 AXIS
2.1. Antibodies to CCL2 and CCR2
Experimental studies and clinical information have implicated the CCL2‐CCR2 axis in tumor promotion and suggested that its inhibition might be of therapeutic value. One approach to inhibition of this axis is the administration of neutralizing antibodies to CCL2, which has been shown to suppress the growth of tumors formed by transplanted human prostate,28, 29 breast,30 or pancreatic31 cancer cells in mice as well as to attenuate macrophage infiltration in breast cancer. This strategy was also found to inhibit metastatic seeding in the lung and to prolong survival in mice with tumors formed by transplanted human breast cancer cells32or mouse melanoma cells.33 Administration effects of antibodies to CCL2 were associated with inhibition of the recruitment to the tumors of CCR2+ inflammatory monocytes.
Antibodies to human CCL2 have been evaluated for safety, pharmacokinetic‐pharmacodynamic profile, and antitumor activity in cancer patients. Phase 1 clinical trials (NCT00537368 and NCT01204996) and a phase 2 study (NCT00992186) for a mAb to human CCL2 (CNTO888, carlumab)34, 35, 36 showed that it induced a transient decrease in the concentration of CCL2 in serum, but that this decrease was followed by an increase to levels higher than pretreatment baseline values. Pharmacodynamics analysis indicated that, in contrast to its high affinity for CCL2 (dissociation constant of 15 pmol/L) in vitro, the affinity of CNTO888 for CCL2 was much higher (dissociation constant of 2.4 nmol/L) in patients, suggestive of a reduced capacity to inhibit CCL2 in vivo.34
Another phase 2 clinical trial (NCT01015560) with a humanized mAb to CCR2 (MLN1202, plozalizumab) was conducted with regard to treatment of bone metastasis of unspecified tumors.37 MLN1202 was given to 44 patients with bone metastasis to evaluate its effects on tumor cell proliferation, monocyte‐macrophage trafficking, and osteoclast maturation. Urinary concentration of N‐telopeptide, a biomarker of bone turnover, was decreased in 14% of the treated patients, and serious adverse events developed in 7% of the patients. A phase 1 clinical trial (NCT02723006) of MLN1202 in combination with the immune‐checkpoint inhibitor nivolumab for patients with advanced melanoma was terminated as a result of the emergence of serious adverse events. Together, these results suggest that antibodies to CCL2 and CCR2 have limited potential for cancer therapy.
2.2. Small‐molecule compounds
As an alternative to neutralizing antibodies, chemical agents have been shown to restrain cancer progression by inhibiting the CCL2‐CCR2 axis. Treatment with one such small‐molecule antagonist of CCR2 (PF‐04136309) alone or in combination with gemcitabine slowed tumor progression and reduced the number of infiltrating TAM in mice subjected to orthotopic transplantation of established PDAC tumors.38 Similar results were obtained when PF‐04136309 was combined with FOLFIRINOX chemotherapy in mice.39 PF‐04136309 in combination with gemcitabine also completely inhibited metastasis of pancreatic cancer cells injected into mice through the tail vein.40 In addition, PF‐04136309 treatment significantly attenuated lung metastasis of lung squamous carcinomas cells injected i.v. in mice.41 Giving CCX872‐B, another small‐molecule antagonist of CCR2, prolonged overall survival in a mouse model of breast cancer, although it neither extended tumor‐free survival nor suppressed tumor growth.42
On the basis of the preclinical findings for the potential of PF‐04136309 for treatment of pancreatic cancer, a phase 1 clinical trial (NCT01413022) was conducted for this agent in combination with FOLFIRINOX in patients with nonmetastatic PDAC.43 Incidence of adverse events of grade 3 or higher in patients treated with FOLFIRINOX plus PF‐04136309 was similar to that in those who received FOLFIRINOX alone. Treatment with PF‐04136309 prevented the PDAC‐mediated mobilization of bone marrow‐derived CCR2+ monocytes into the peripheral circulation, resulting in a decrease in the number of TAM. Approximately half of the patients treated with FOLFIRINOX plus PF‐04136309 achieved an objective tumor response, suggesting that such therapy is both effective and tolerable. A phase 1b/2 clinical study of PF‐04136309 in combination with nab‐paclitaxel and gemcitabine in patients with metastatic PDAC (NCT02732938) was terminated as a business‐related decision by Pfizer. Clinical trials of small‐molecule CCR2 blockers for patients with other inflammatory diseases such as insulin resistance, multiple sclerosis, nonalcoholic steatohepatitis, and rheumatoid arthritis have also been conducted (Table 1).
Table 1.
Structures, IC50, and clinical trials for C‐C chemokine receptor type 2 blockers
| Reagent | Structure | Originator | IC50 (in vitro binding) | IC50 (chemotaxis) | Clinical trial | ClinicalTrials.gov | Status |
|---|---|---|---|---|---|---|---|
| 15a |
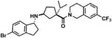
|
50 nmol/L | – | – | |||
| AZD2423 |
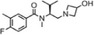
|
AstraZeneca | 2.6 nmol/L | 4.4 nmol/L | Chronic obstructive pulmonary disease (COPD) | NCT01153321 | Completed (inhibition of) |
| Chronic obstructive pulmonary disease (COPD) | NCT01215279 | Completed (no effect) | |||||
| Post‐traumatic neuralgia | NCT01200524 | Completed (modest improvement) | |||||
| Painful diabetic polyneuropathy | NCT01201317 | Completed (no effect) | |||||
| BMS741672 |
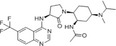
|
Bristol‐Myers Squibb | 1.4 nmol/L | – | Type 2 diabetes mellitus | NCT00699790 | Completed |
| Neuropathic pain | NCT00683423 | Completed | |||||
| BMS813160 |
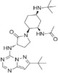
|
Bristol‐Myers Squibb | 6.2 nmol/L | 0.8 nmol/L | Pancreatic ductal adenocarcinoma (PDAC) | NCT03496662 | Recruiting |
| Pancreatic ductal adenocarcinoma (PDAC) | NCT03767582 | Ongoing | |||||
| Colorectal cancer and pancreatic cancer | NCT03184870 | Recruiting | |||||
| Diabetic kidney disease | NCT01752985 | Completed (no result published) | |||||
| Accelerated intimal hyperplasia | NCT01049165 | Completed (no result published) | |||||
| CAS 445479‐97‐0 |
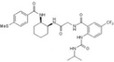
|
Bristol‐Myers Squibb | 5.1 nmol/L | 1 nmol/L | – | ||
| CCX140‐B |
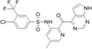
|
ChemoCentryx | 17 nmol/L | 8 nmol/L | Diabetic nephropathy | NCT01447147 | Completed (modest improvement) |
| Type 2 diabetes mellitus | NCT01440257 | Completed (no result published) | |||||
| Type 2 diabetes mellitus | NCT01028963 | Completed (no result published) | |||||
| Focal segmental glomerulosclerosis | NCT03536754 | Recruiting | |||||
| CCX872‐B | Undisclosed | ChemoCentryx | 3 nmol/L | 32 nmol/L | Pancreatic adenocarcinoma | NCT02345408 | Ongoing |
| Cenicriviroc |
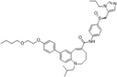
|
National Institute for Health Research; Takeda | 5.9 nmol/L | – | Nonalcoholic steatohepatitis (NASH) | NCT02217475 | Completed (no result published) |
| HIV‐1 infection | NCT01092104 | Completed (no result published) | |||||
| Nonalcoholic steatohepatitis (NASH) | NCT03028740 | Recruiting | |||||
| Nonalcoholic fatty liver disease (NAFLD) | NCT02330549 | Completed (no result published) | |||||
| Primary sclerosing cholangitis | NCT02653625 | Completed (modest improvement) | |||||
| INCB3284 |
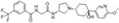
|
Incyte Corporation | 3.7 nmol/L | 4.7 nmol/L | – | ||
| INCB3344 |
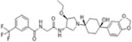
|
Incyte Corporation | 5.1 nmol/L | 3.8 nmol/L | – | ||
| JNJ‐17166864 |
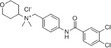
|
Johnson & Johnson | – | – | Allergic rhinitis | NCT00604123 | Completed (no result published) |
| JNJ‐41443532 |
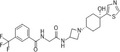
|
Johnson & Johnson | – | – | Type 2 diabetes mellitus | NCT01230749 | Completed (modest improvement) |
| MK0812 |
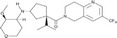
|
Merck & Co. | 3.2 nmol/L | 8 nmol/L | Rheumatoid arthritis | NCT00542022 | Completed (no result published) |
| Multiple sclerosis | NCT00239655 | Terminated | |||||
| PF04136309 |
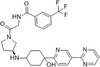
|
Pfizer | 5.2 nmol/L | 3.9 nmol/L | Metastatic pancreatic ductal adenocarcinoma | NCT02732938 | Terminated |
| Pancreatic adenocarcinoma | NCT01413022 | Completed (no result published) | |||||
| Osteoarthritic pain | NCT00689273 | Completed (no result published) | |||||
| Chronic hepatitis C infection | NCT01226797 | Terminated | |||||
| PF04634817 |
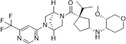
|
Pfizer | 3.68 nmol/L | – | Type 2 diabetes and overt nephropathy | NCT01712061 | Completed (modest improvement) |
| Renal insufficiency | NCT01791855 | Completed (no result published) | |||||
| Diabetic macular edema | NCT01994291 | Terminated | |||||
| RO5234444 |
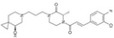
|
Roche | 22 nmol/L | 50.2 nmol/L | – | ||
| RS504393 |
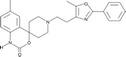
|
Roche | 89 nmol/L | 330 nmol/L | – | ||
| RS102895 |
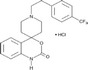
|
Roche | 360 nmol/L | 1700 nmol/L | – | ||
| TLK19705 |
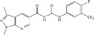
|
Telik, Inc. | – | 700 nmol/L | – |
'‐' indicates that there is no data available.
3. CHEMICAL AND PHARMACOLOGICAL PROPERTIES OF PROPAGERMANIUM
Organogermanium compounds manifest various biological actions including antibacterial and antioxidant effects, effects on the blood circulation system, and anticancer activities. PGe, with the formula (C3H5GeO3.5)n, is the only approved medicine among such compounds. It was discovered in 1979 together with other organic germanium polymers, and it was approved for the treatment of chronic hepatitis B in Japan in 1994, having now been given to such patients for 25 years. PGe has the same essential formula as RGe (or Ge‐132), poly‐trans‐[(2‐carboxyethyl) germasesquioxane] or (C18H30Ge6O21)n, although the physicochemical properties of the two compounds are distinct.44 PGe is thus more susceptible to hydrolysis by water than is RGe. At low concentrations, PGe and RGe are hydrolyzed to the same monomer species. At high concentrations, however, whereas RGe is hydrolyzed to the same monomer, PGe is hydrolyzed to GeSP (Figure 2).
Figure 2.
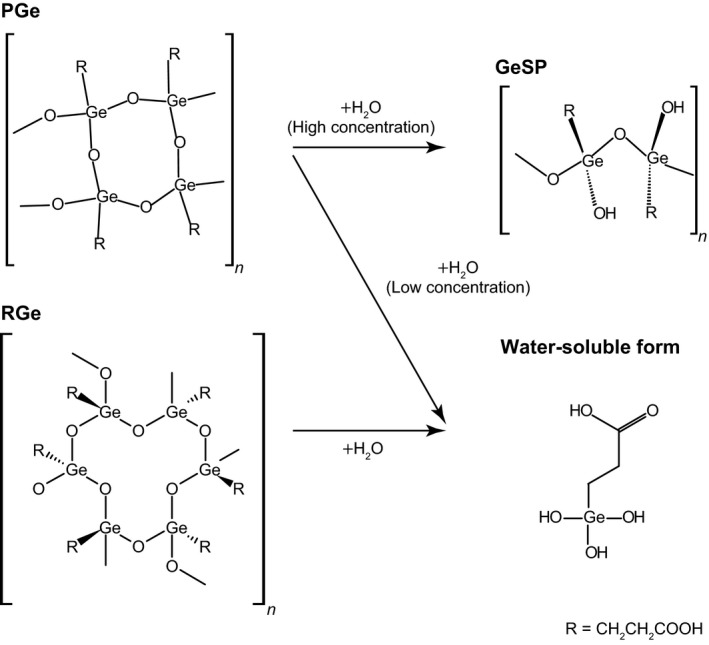
Solid and water‐soluble forms of propagermanium (PGe) and repagermanium (RGe). GeSP, germanium straight‐chain polymer
PGe exerts immunomodulatory effects through interaction with glycosylphosphatidylinositol‐anchored proteins associated with CCR2. It thus interrupts the CCL2‐CCR2 signaling pathway and thereby suppresses the chemotaxis of monocytes‐macrophages without disrupting the receptor‐ligand interaction.45 Inhibition of such signaling by treatment with PGe promotes retention of all leukocyte subsets—in particular, inflammatory monocytes—in bone marrow, resulting in a reciprocal reduction in the number of these cells in peripheral blood and consistent with the suppressive effect of PGe on a range of chronic inflammatory conditions mediated primarily by inflammatory monocytes and macrophages.
A double‐blind controlled trial of PGe was conducted with HBV antigen‐positive chronic hepatitis in the late 1980s.46 Titers of antibodies to HBV were significantly increased after treatment in the PGe group, whereas HBV antigen titers were significantly lower in the PGe group than in the placebo group. Serum HBV antigen levels and serum (ALT levels were significantly lower in the PGe group than in the control group at 12 and 16 weeks after treatment onset. PGe is also effective for the treatment of other types of liver injury in mice. Giving oral PGe thus inhibited the development of liver injury induced by injection of concanavalin A or lipopolysaccharide injection, Corynebacterium parvum infection or genetic deletion of fumarylacetoacetate hydrolase.47, 48, 49 PGe also has therapeutic effects in other inflammatory diseases such as atherosclerosis,50, 51 fibrosis,52, 53, 54 and obesity induced by a high‐fat diet.55, 56, 57
4. PROPAGERMANIUM FOR TREATMENT OF CANCER
Recent studies have shown that interference with key mediators of metastatic development is a promising alternative strategy for cancer treatment. The CCL2‐CCR2 signaling pathway is an attractive therapeutic target in such an approach, given its key functions in metastasis described above. We previously showed that Fbxw7 (also known as Fbw7, Sel‐10, hCdc4, or hAgo), a receptor protein of SCF (Skp1‐Cul1‐F‐box protein)‐type ubiquitin ligases, suppresses cancer metastasis by inhibition of CCL2‐dependent inflammation.58 A low level of Fbxw7 in tumor cells and peripheral blood is associated with poor prognosis in cancer patients. Moreover, mice in which Fbxw7 is specifically ablated in bone marrow (Fbxw7 bmΔ/Δ mice) manifest enhanced metastasis of melanoma, lung carcinoma, and breast adenocarcinoma. Serum level of CCL2 was also increased in Fbxw7 bmΔ/Δ mice compared with control mice both before and after orthotopic transplantation of breast cancer cells. In addition, the numbers of Ly6C+ Mo‐MDSC and F4/80+ macrophages were increased in peripheral blood and at sites of metastasis in Fbxw7 bmΔ/Δ mice (Figure 3).
Figure 3.
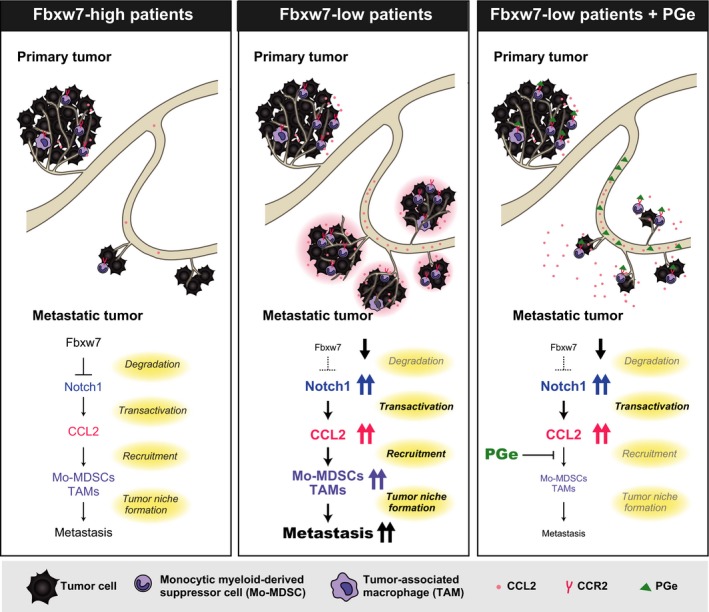
Model for the promotion of cancer metastasis by loss of Fbxw7 in the host environment and its suppression by treatment with propagermanium (PGe). Excessive signaling by Notch1 due to the impairment of its degradation as a result of Fbxw7 ablation gives rise to increased production of chemokine C‐C motif chemokine 2 (CCL2). Consequent recruitment of monocytic myeloid‐derived suppressor cells (Mo‐MDSC) and tumor‐associated macrophages (TAM) facilitates metastatic tumor growth. PGe suppresses CCL2‐dependent recruitment of Mo‐MDSC and TAM and thereby attenuates cancer metastasis. CCR2, C‐C chemokine receptor type 2
Giving PGe significantly attenuated metastasis of melanoma cells and breast cancer cells in Fbxw7 bmΔ/Δ mice, with the size of metastatic nodules of breast adenocarcinoma in the lungs being reduced.58 Such treatment also reduced the number of Ly6C+ Mo‐MDSC found in the lungs of Fbxw7 bmΔ/Δ mice after transplantation of breast cancer cells (Figure 3).
Treatment with PGe in a mouse model of colon carcinogenesis reduced the number and size of tumors as well as the number of TAM, and it attenuated adenocarcinomatous changes in the colon tumors.59 A phase 2 clinical trial (UMIN000017123) is underway to assess the efficacy of PGe in 15 patients with untreatable advanced or metastatic gastric cancer. Another study found that PGe treatment in 10 multiple myeloma patients resulted in complete remission in two patients, partial remission in two patients, stable disease in four patients, and progressive disease in two patients.60 After discontinuation of PGe, the multiple myeloma progressed in two patients who had achieved stable disease and in the two patients who had achieved partial remission. Phase 1 studies are also ongoing to evaluate the safety and effectiveness of PGe for patients with breast cancer (UMIN000022494), pancreatic cancer (UMIN000017715), and colorectal cancer (UMIN000022129).
5. CONCLUSIONS
The CCL2‐CCR2 signaling pathway plays a central role in inflammatory diseases including cancer metastasis. Treatment targeted to CCR2 such as that with PGe alleviates pathological phenotypes associated with these diseases. PGe has been approved for the treatment of hepatitis B in Japan, and its bioavailability and safety have been established. Drug repositioning, which aims to identify new indications for existing drugs, has been gaining popularity as an approach to drug discovery. PGe is thus a candidate for drug repositioning with regard to its suppressive effect on cancer metastasis. Further studies will determine whether this agent is truly effective for the treatment of cancer.
DISCLOSURE
Authors declare no conflicts of interest for this article.
ACKNOWLEDGMENTS
This work was supported in part by Japan Society for the Promotion of Science KAKENHI grants 17H05795, 17K07171, and 18H05037, Japan Agency for Medical Research and Development (AMED) P‐CREATE grant 19cm0106105h0004, and the NAITO foundation.
Yumimoto K, Sugiyama S, Mimori K, Nakayama KI. Potentials of C‐C motif chemokine 2–C‐C chemokine receptor type 2 blockers including propagermanium as anticancer agents. Cancer Sci. 2019;110:2090‐2099. 10.1111/cas.14075
REFERENCES
- 1. Lazennec G, Richmond A. Chemokines and chemokine receptors: new insights into cancer‐related inflammation. Trends Mol Med. 2010;16:133‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer‐related inflammation and treatment effectiveness. Lancet Oncol. 2014;15:e493‐e503. [DOI] [PubMed] [Google Scholar]
- 3. Barcellos‐Hoff MH, Lyden D, Wang TC. The evolution of the cancer niche during multistage carcinogenesis. Nat Rev Cancer. 2013;13:511‐518. [DOI] [PubMed] [Google Scholar]
- 4. Svensson S, Abrahamsson A, Rodriguez GV, et al. CCL2 and CCL5 are novel therapeutic targets for estrogen‐dependent breast cancer. Clin Cancer Res. 2015;21:3794‐3805. [DOI] [PubMed] [Google Scholar]
- 5. Lim SY, Yuzhalin AE, Gordon‐Weeks AN, Muschel RJ. Targeting the CCL2‐CCR5 signaling axis in cancer metastasis. Oncotarget. 2016;7:28697‐28710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Matsushima K, Larsen CG, Dubois GC, Oppenheim JJ. Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell‐line. J Exp Med. 1989;169:1485‐1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yoshimura T, Robinson EA, Tanaka S, Appella E, Kuratsu J, Leonard EJ. Purification and amino acid analysis of two human glioma‐derived monocyte chemoattractants. J Exp Med. 1989;169:1449‐1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein‐1 (MCP‐1): an overview. J Interferon Cytokine Res. 2009;29:313‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chiu HY, Sun KH, Chen SY, et al. Autocrine CCL2 promotes cell migration and invasion via PKC activation and tyrosine phosphorylation of paxillin in bladder cancer cells. Cytokine. 2012;59:423‐432. [DOI] [PubMed] [Google Scholar]
- 10. Monti P, Leone BE, Marchesi F, et al. The CC chemokine MCP‐1/CCL2 in pancreatic cancer progression: regulation of expression and potential mechanisms of antimalignant activity. Cancer Res. 2003;63:7451‐7461. [PubMed] [Google Scholar]
- 11. Tang CH, Tsai CC. CCL2 increases MMP‐9 expression and cell motility in human chondrosarcoma cells via the Ras/Raf/MEK/ERK/NF‐κB signaling pathway. Biochem Pharmacol. 2012;83:335‐344. [DOI] [PubMed] [Google Scholar]
- 12. Dagouassat M, Suffee N, Hlawaty H, et al. Monocyte chemoattractant protein‐1 (MCP‐1)/CCL2 secreted by hepatic myofibroblasts promotes migration and invasion of human hepatoma cells. Int J Cancer. 2010;126:1095‐1108. [DOI] [PubMed] [Google Scholar]
- 13. Wyckoff JB, Wang Y, Lin EY, et al. Direct visualization of macrophage‐assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67:2649‐2656. [DOI] [PubMed] [Google Scholar]
- 14. Lin EY, Pollard JW. Tumor‐associated macrophages press the angiogenic switch in breast cancer. Cancer Res. 2007;67:5064‐5066. [DOI] [PubMed] [Google Scholar]
- 15. Low‐Marchelli JM, Ardi VC, Vizcarra EA, van Rooijen N, Quigley JP, Yang J. Twist1 induces CCL2 and recruits macrophages to promote angiogenesis. Cancer Res. 2013;73:662‐671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang B, Lei Z, Zhao J, et al. CCL2/CCR16 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer Lett. 2007;252:86‐92. [DOI] [PubMed] [Google Scholar]
- 17. Roca H, Varsos Z, Pienta KJ. CCL2 protects prostate cancer PC3 cells from autophagic death via phosphatidylinositol 3‐kinase/AKT‐dependent survivin up‐regulation. J Biol Chem. 2008;283:25057‐25073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chun E, Lavoie S, Michaud M, et al. CCL2 promotes colorectal carcinogenesis by enhancing polymorphonuclear myeloid‐derived suppressor cell population and function. Cell Rep. 2015;12:244‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhao L, Lim SY, Gordon‐Weeks AN, et al. Recruitment of a myeloid cell subset (CD11b/Gr1mid) via CCL2/CCR19 promotes the development of colorectal cancer liver metastasis. Hepatology. 2013;57:829‐839. [DOI] [PubMed] [Google Scholar]
- 20. Gorlov IP, Sircar K, Zhao H, et al. Prioritizing genes associated with prostate cancer development. BMC Cancer. 2010;10:599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hefler L, Tempfer C, Heinze G, et al. Monocyte chemoattractant protein‐1 serum levels in ovarian cancer patients. Br J Cancer. 1999;81:855‐859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tonouchi H, Miki C, Tanaka K, Kusunoki M. Profile of monocyte chemoattractant protein‐1 circulating levels in gastric cancer patients. Scand J Gastroenterol. 2002;37:830‐833. [PubMed] [Google Scholar]
- 23. Lu Y, Cai Z, Galson DL, et al. Monocyte chemotactic protein‐1 (MCP‐1) acts as a paracrine and autocrine factor for prostate cancer growth and invasion. Prostate. 2006;66:1311‐1318. [DOI] [PubMed] [Google Scholar]
- 24. Futagami S, Tatsuguchi A, Hiratsuka T, et al. Monocyte chemoattractant protein 1 and CD40 ligation have a synergistic effect on vascular endothelial growth factor production through cyclooxygenase 2 upregulation in gastric cancer. J Gastroenterol. 2008;43:216‐224. [DOI] [PubMed] [Google Scholar]
- 25. Hu H, Sun L, Guo C, et al. Tumor cell‐microenvironment interaction models coupled with clinical validation reveal CCL2 and SNCG as two predictors of colorectal cancer hepatic metastasis. Clin Cancer Res. 2009;15:5485‐5493. [DOI] [PubMed] [Google Scholar]
- 26. Ueno T, Toi M, Saji H, et al. Significance of macrophage chemoattractant protein‐1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res. 2000;6:3282‐3289. [PubMed] [Google Scholar]
- 27. Tao LL, Shi SJ, Chen LB, Huang GC. Expression of monocyte chemotactic protein‐1/CCL2 in gastric cancer and its relationship with tumor hypoxia. World J Gastroenterol. 2014;20:4421‐4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Loberg RD, Ying C, Craig M, et al. Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Cancer Res. 2007;67:9417‐9424. [DOI] [PubMed] [Google Scholar]
- 29. Kirk PS, Koreckij T, Nguyen HM, et al. Inhibition of CCL2 signaling in combination with docetaxel treatment has profound inhibitory effects on prostate cancer growth in bone. Int J Mol Sci. 2013;14:10483‐10496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fujimoto H, Sangai T, Ishii G, et al. Stromal MCP‐1 in mammary tumors induces tumor‐associated macrophage infiltration and contributes to tumor progression. Int J Cancer. 2009;125:1276‐1284. [DOI] [PubMed] [Google Scholar]
- 31. Snyder LA, Kesavan P, Kaiser E, et al. Neutralization of CCL2 inhibits tumor angiogenesis and pancreatic tumor growth. Mol Cancer Ther. 2007;6:3357s. [Google Scholar]
- 32. Qian BZ, Li JF, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast tumor metastasis. Nature. 2011;475:222–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Knight DA, Ngiow SF, Li M, et al. Host immunity contributes to the anti‐melanoma activity of BRAF inhibitors. J Clin Invest. 2013;123:1371‐1381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34. Sandhu SK, Papadopoulos K, Fong PC, et al. A first‐in‐human, first‐in‐class, phase I study of carlumab (CNTO 888), a human monoclonal antibody against CC‐chemokine ligand 2 in patients with solid tumors. Cancer Chemother Pharmacol. 2013;71:1041‐1050. [DOI] [PubMed] [Google Scholar]
- 35. Brana I, Calles A, LoRusso PM, et al. Carlumab, an anti‐C‐C chemokine ligand 2 monoclonal antibody, in combination with four chemotherapy regimens for the treatment of patients with solid tumors: an open‐label, multicenter phase 1b study. Target Oncol. 2015;10:111‐123. [DOI] [PubMed] [Google Scholar]
- 36. Pienta KJ, Machiels JP, Schrijvers D, et al. Phase 2 study of carlumab (CNTO 888), a human monoclonal antibody against CC‐chemokine ligand 2 (CCL2), in metastatic castration‐resistant prostate cancer. Invest New Drugs. 2013;31:760‐768. [DOI] [PubMed] [Google Scholar]
- 37. Vela M, Aris M, Llorente M, Garcia‐Sanz JA, Kremer L. Chemokine receptor‐specific antibodies in cancer immunotherapy: achievements and challenges. Front Immunol. 2015;6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mitchem JB, Brennan DJ, Knolhoff BL, et al. Targeting tumor‐infiltrating macrophages decreases tumor‐initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73:1128‐1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nywening TM, Belt BA, Cullinan DR, et al. Targeting both tumour‐associated CXCR39+ neutrophils and CCR39+ macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma. Gut. 2018;67:1112‐1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sanford DE, Belt BA, Panni RZ, et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR40 axis. Clin Cancer Res. 2013;19:3404‐3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Porrello A, Leslie PL, Harrison EB, et al. Factor XIIIA‐expressing inflammatory monocytes promote lung squamous cancer through fibrin cross‐linking. Nat Commun. 2018;9:1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen X, Wang Y, Nelson D, et al. CCL2/CCR42 regulates the tumor microenvironment in HER‐2/neu‐driven mammary carcinomas in mice. PLoS ONE. 2016;11:e0165595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nywening TM, Wang‐Gillam A, Sanford DE, et al. Targeting tumour‐associated macrophages with CCR43 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single‐centre, open‐label, dose‐finding, non‐randomised, phase 1b trial. Lancet Oncol. 2016;17:651‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mizuno N, Nishibori E, Oka M, Jomori T, Takata M, Kumasaka T. Structural basis for polymer packing and solvation properties of the organogermanium crystalline polymer propagermanium and its derivatives. J Pharm Sci. 2015;104:2482‐2488. [DOI] [PubMed] [Google Scholar]
- 45. Yokochi S, Hashimoto H, Ishiwata Y, et al. An anti‐inflammatory drug, propagermanium, may target GPI‐anchored proteins associated with an MCP‐1 receptor, CCR45. J Interferon Cytokine Res. 2001;21:389‐398. [DOI] [PubMed] [Google Scholar]
- 46. Hirayama C, Suzuki H, Ito M, Okumura M, Oda T. Propagermanium: a nonspecific immune modulator for chronic hepatitis B. J Gastroenterol. 2003;38:525‐532. [DOI] [PubMed] [Google Scholar]
- 47. Ishiwata Y, Yokochi S, Hashimoto H, Ninomiya F, Suzuki T. Protection against concanavalin A‐induced murine liver injury by the organic germanium compound, propagermanium. Scand J Immunol. 1998;48:605‐614. [DOI] [PubMed] [Google Scholar]
- 48. Yokochi S, Ishiwata Y, Hashimoto H, Ninomiya F, Suzuki T. Hepatoprotective effect of propagermanium on Corynebacterium parvum and lipopolysaccharide‐induced liver injury in mice. Scand J Immunol. 1998;48:183‐191. [DOI] [PubMed] [Google Scholar]
- 49. Qi ZP, Wang X, Wei HM, Sun R, Tian ZG. Infiltrating neutrophils aggravate metabolic liver failure in fah‐deficient mice. Liver Int. 2015;35:774‐785. [DOI] [PubMed] [Google Scholar]
- 50. Yamashita T, Kawashima S, Ozaki M, et al. Propagermanium reduces atherosclerosis in apolipoprotein E knockout mice via inhibition of macrophage infiltration. Arterioscler Thromb Vasc Biol. 2002;22:969‐974. [DOI] [PubMed] [Google Scholar]
- 51. Shimokawa H, Eto Y, Miyata K, et al. Propagermanium suppresses macrophage‐mediated formation of coronary arteriosclerotic lesions in pigs in vivo. J Cardiovasc Pharmacol. 2003;41:372‐380. [DOI] [PubMed] [Google Scholar]
- 52. Kitagawa K, Wada T, Furuichi K, et al. Blockade of CCR52 ameliorates progressive fibrosis in kidney. Am J Pathol. 2004;165:237‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hara A, Sakai N, Furuichi K, et al. CCL2/CCR53 augments the production of transforming growth factor‐beta1, type 1 collagen and CCL2 by human CD45‐/collagen 1‐positive cells under high glucose concentrations. Clin Exp Nephrol. 2013;17:793‐804. [DOI] [PubMed] [Google Scholar]
- 54. Rao VH, Meehan DT, Delimont D, et al. Role for macrophage metalloelastase in glomerular basement membrane damage associated with alport syndrome. Am J Pathol. 2006;169:32‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ito A, Suganami T, Yamauchi A, et al. Role of CC chemokine receptor 2 in bone marrow cells in the recruitment of macrophages into obese adipose tissue. J Biol Chem. 2008;283:35715‐35723. [DOI] [PubMed] [Google Scholar]
- 56. Tamura Y, Sugimoto M, Murayama T, et al. Inhibition of CCR56 ameliorates insulin resistance and hepatic steatosis in db/db mice. Arterioscler Thromb Vasc Biol. 2008;28:2195‐2201. [DOI] [PubMed] [Google Scholar]
- 57. Mulder P, van den Hoek AM, Kleemann R. The CCR57 inhibitor propagermanium attenuates diet‐induced insulin resistance, adipose tissue inflammation and non‐alcoholic steatohepatitis. PLoS ONE. 2017;12:e0169740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yumimoto K, Akiyoshi S, Ueo H, et al. F‐box protein FBXW7 inhibits‐cancer metastasis in a non‐cell‐autonomous manner. J Clin Invest. 2015;125:621‐635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Popivanova BK, Kostadinova FI, Furuichi K, et al. Blockade of a chemokine, CCL2, reduces chronic colitis‐associated carcinogenesis in mice. Cancer Res. 2009;69:7884‐7892. [DOI] [PubMed] [Google Scholar]
- 60. Tsutsumi Y, Tanaka J, Kanamori H, et al. Effectiveness of propagermanium treatment in multiple myeloma patients. Eur J Haematol. 2004;73:397‐401. [DOI] [PubMed] [Google Scholar]


