Introduction
Alport syndrome (AS) is a hereditary nephritis caused by mutations in COL4A3, COL4A4, and COL4A5, which encode the collagen IV alpha3, alpha4, and alpha5 chains, respectively.1 The collagen IV alpha345 molecule is the major component of the mature glomerular basement membrane in the kidney.2 Disease-causing mutations in these genes may affect the synthesis, assembly, deposition and/or function of the collagen IV alpha345 molecule.1 Phenotypically, the disease is characterized by hematuria and proteinuria, progressing to end-stage renal disease. Some patients experience extrarenal symptoms, such as progressive high-frequency sensorineural deafness and ocular abnormalities (anterior lenticonus or myopia).3
The 3 inheritance types of AS are X-linked, autosomal dominant, and autosomal recessive.1 COL4A3 and COL4A4, located on chromosome 2, are associated with an autosomal pattern. COL4A5, residing at chrXq22.3, is X-linked.1 Rarely a combination of certain mutations in these genes will cause digenic inheritance.1 X-linked Alport syndrome (XLAS) is the most common form of disease.4 X-linked female patients are heterozygous, whereas X-linked male patients are hemizygous (having only 1 X chromosome). Loss of function mutations, such as large deletions, frameshift mutations, and nonsense mutations, generally cause severe phenotypes in affected XLAS hemizygous male patients, with essentially 100% risk factor for progression to end-stage renal disease and complete loss of the alpha5 chain in the glomerular basement membrane.1 Conversely, heterozygous female patients, due to random X-inactivation, have variable mild phenotypes, a lower risk of progressing to end-stage renal disease, and frequently show a mosaic pattern with segmental loss of alpha5 chain in the glomerular basement membrane.1 Splicing and missense mutations demonstrate greater phenotypic variability in the expression of alpha3, alpha4, and alpha5 in the glomerular basement membrane.
There are very few reported cases of XLAS with somatic mosaic mutations. We herein describe the pathology and molecular findings in a 10-year-old male patient with somatic mosaicism in XLAS and review previously reported cases of somatic mosaicism in XLAS male and female patients from the literature.
Case Presentation
Clinical History and Initial Laboratory Data
A 10-year-old boy was referred to pediatric nephrology for consultation regarding gross hematuria, persistent microscopic hematuria, and nephrotic range proteinuria. He was delivered at full term with normal development. He has 3 healthy older siblings. There was no family history of renal disease or hearing defects. His physical examination was normal and the pertinent laboratory results were as follows: serum creatinine, 0.53 mg/dl; albumin, 2.7 g/dl; and urinary total protein-to-creatinine ratio, 4.18 g/g. Urinalysis showed a moderate amount of blood and 45 red blood cells per high power field. Renal ultrasound showed a duplicated collecting system on the right but no other anatomic abnormalities. Audiometry and an ocular examination were normal. Two weeks after the initial visit, a diagnostic renal biopsy was performed.
Renal Biopsy Findings
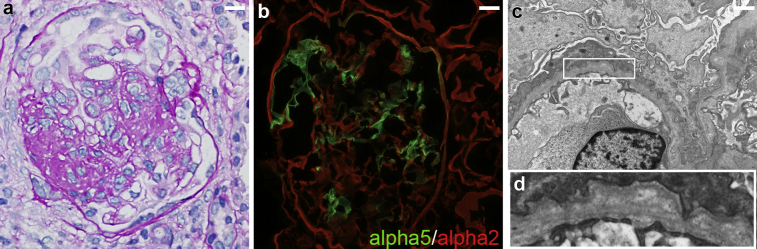
Renal biopsy tissue of the left kidney was submitted for light, immunofluorescence, and electron microscopy evaluation. The light microscopy specimen consisted of 2 needle cores of renal corticomedullary tissue containing 19 glomeruli, 3 of which were globally sclerotic, and the remaining were normocellular. Four glomeruli showed ischemic capillary wall wrinkling and periglomerular fibrosis. One glomerulus showed segmental sclerosis, with a morphologic appearance consistent with focal segmental glomerulosclerosis (FSGS), not otherwise specified (Figure 1a). There was approximately 10% tubular atrophy and interstitial fibrosis with very mild lymphocytic infiltrate. Scattered foam cells were noted in the interstitium. Three arteries and arterioles present were largely unremarkable.
Figure 1.
Findings of renal biopsy. (a) A glomerulus with segmental sclerosis on periodic acid–Schiff stain. (b) Immunofluorescence staining for alpha 5 (green) and alpha 2 (red) type IV collagen shows segmental absence of glomerular basement membrane staining for alpha 5 type IV collagen. (c, d) Electron microscopy shows glomerular basement membrane (d for high magnification) with irregular thickening, thinning, and lamination of lamina densa, with severe foot process effacement. Bar = 30 μm in (a) and (b); bar = 1 μm in (c).
The immunofluorescence microscopy showed segmental, granular mesangial staining, with possible capillary wall extension, for IgG (1+), IgA (trace), IgM (0 to trace), C1q (1+), C3 (0 to trace), and kappa (0 to trace) and lambda (trace) light chains. There was no tubular basement membrane staining and the remaining immune reactants were negative.
Ultrastructurally, there was segmental irregularity of glomerular basement membrane with thickening, thinning, and lamination of lamina densa, along with associated severe podocyte foot process effacement (Figure 1c). Electron moderate to dense deposits were seen in the mesangial regions and rare subendothelial areas (not shown). There were no tubuloreticular inclusions noted.
Additional immunofluorescence staining for alpha2 and alpha5 type IV collagen (fluorescein isothiocyanate–conjugated monoclonal rat anti-human collagen IV alpha 5 chain and Texas-Red–conjugated monoclonal rat anti-human collagen IV alpha2 chain; SGE-CFT45325; Cosmo Bio Co., Ltd., Tokyo, Japan) was performed with appropriate controls. There was segmental absence of the capillary basement membrane staining for alpha5 type IV collagen (Figure 1b). There was diffuse glomerular mesangial and capillary wall staining for alpha2 chain with segmental absence of alpha5 staining in glomerular capillaries. A positive control section displayed expected staining patterns for alpha5 and alpha2 type IV collagens.
Additional Investigations
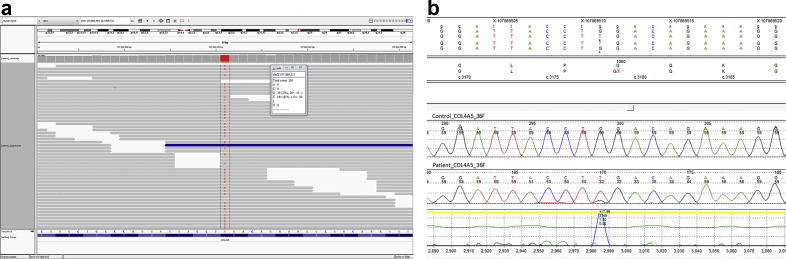
Cytogenetic analysis of cultured, synchronized peripheral blood cells demonstrated a normal 46,XY male karyotype in all 50 Giemsa-stained metaphase cells examined. An AS next generation sequencing panel was also performed on peripheral blood,5, 6, 7, 8 which identified a nonsense mutation within exon 36 of COL4A5 (NM_033380.2; c.3178G>T; p.Gly1060*). This G>T nucleotide substitution was observed in approximately 80% of the unique individual reads covering this genomic region, at a total sequencing depth of 200X (Figure 2a); the remaining 20% of reads demonstrated the wild-type G nucleotide. To confirm this finding, Sanger sequencing of the patient's peripheral blood DNA sample was performed and showed an imbalanced allelic ratio consistent with the presence of approximately 80% mutated alleles (Figure 2b). This combination of an imbalanced allelic ratio in the sequencing data with normal 46,XY karyotype by G-bands is consistent with constitutional somatic mosaicism of this mutation in the patient.
Figure 2.
(a) Integrated Genomics Viewer visualization of COL4A5 nonsense mutation c.3178G>T (p.Gly1060*); 181 reads demonstrated the single nucleotide threonine substitution versus 39 reads containing the wild-type guanine residue. (b) Sanger sequencing confirms the allelic imbalance of G>T at c.3178 in COL4A5. Top trace is a synthetic control; bottom trace is the patient’s forward strand data showing a T:G ratio of approximately 80:20. The reverse strand also confirmed the finding (data not shown for brevity).
Diagnosis
The 10-year-old boy was diagnosed with somatic mosaic X-linked AS.
Clinical Follow-up
The patient was referred for ophthalmologic and audiologic examination to evaluate for abnormalities commonly associated with AS. There were no ocular or audiologic findings of AS. The patient was also referred to genetic counseling. His proteinuria started to decrease after treatment with oral enalapril 2.5 mg twice a day. At the 6-month follow-up, his urinary total protein-to-creatinine ratio had dropped to 1.31 g/g.
Discussion
Mosaicism has been described in many genetic disorders9,S1 and may occur when a mutation arises in the early development of an embryo, causing a group of cells to differ in their genetic component from the other cells of the body. A variety of mechanisms, including chromosome non-disjunction, anaphase lagging, and endoreplication, can cause mosaicism.S2 The mutation can be present in both somatic and germ cells, if the mutation occurs before the differentiation of primordial germ cells.S3 Hashimura et al.S4 reported on 52 XLAS male patients, finding 2 patients with somatic mosaic XLAS (approximately 3.8%). Across previously reported cases, there are a total of 10 families, including 18 individuals with genetically confirmed XLAS with somatic mosaicism (Table 1). Of these 10 families, 4 families have probands of young girls (ranging from 3 to 11 years of age); the same mutation variants are detected in 3 fathers, and 2 of them are asymptomatic. However, 1 of the fathers with COL4A5 mutation, previously asymptomatic, developed end-stage renal disease at the age of 43 years (family 3).S5 Of the 6 families with male probands, 4 inherited the same mutation variant from their mothers; 1 mother is not tested (patient 52)S4and 1 mother is confirmed without mutation (patient 1)S6 (Table 1).
Table 1.
Summary of X-linked Alport syndrome with somatic mosaicism
| Reference | Family or patient no. | Sex-Age | Pedigree | ESRD | Hematuria/Proteinuria | Renal biopsy IF EM | Genetic testing | cDNA | Amino acid | |
|---|---|---|---|---|---|---|---|---|---|---|
| 4 | Family 2 | F-3 | Proband | NA | +/+(0.2g/g Cr) | Mosaic | Typical | Blood | c.2147–2A>G; IVS28–2A>G | Exon 28 skip |
| F-3 | Sister | NA | +/NA | NP | NP | Blood | IVS28–2A>G | Exon 28 skip | ||
| M-38 | Father | NA | −/− | NP | NP | Blood and urine sediment | c.2147–2A>G; r.2147_2164del | Exon 28 skip p.Gly716_Pro721del; | ||
| 4 | Family 4 | F-6 | Proband | NA | +/+ | Mosaic | Typical | Blood and urine sediment | c.4787G>T | p.Gly1596Val |
| M-42 | Father | NA | +/− | NP | NP | Blood, urine sediment, and hair roots | ||||
| S5 | Family 3 | F -4 | Proband | NA | +/− | NA | Typical | Blood | c.2114G>A | p.Gly638Ser |
| M-NA | Father | 43 | −/− | NA | NA | Blood | ||||
| S7 | Patient 2 | F-11 | Proband | NA | +/+(1g/g Cr) | NA | Typical | Blood and urine sediment | c.2732G>Aa | p.Gly911Glu |
| S4 | Patient 52 | M-NA | Proband | NA | NA/NA | Mosaic | NA | Blood | c.1912G>A | p.Gly638Ser |
| S5 | Patient 1 | M-11 | Proband | NA | +/− | NP | NP | Blood | c.2208G>C | p.Gly669Ala |
| F-NA | Mother | NA | +/− | NP | NP | Blood | ||||
| S5 | Patient 2 | M-NA | Proband | 17 | +/− | NA | Typical | Blood | c.849–3C>A | Exon 12 skip |
| F-NA | Mother | NA | −/− | NA | NA | Blood | ||||
| S6 | Patient 1 | M-8 | Proband | NA | +/+(1g/24h) | Mosaic | Typical | Blood, urine sediment, hair roots, and skin | c.3998–2A>T | Exon 44 skip |
| S7 | Patient 1 | M-NA | Proband | NA | NA/NA | NA | NA | Blood | c.2405G>T | p.Gly802Val |
| F-NA | Mother | NA | +/+ | NP | NP | Blood | ||||
| S9 | Patient 1 | M-15 | Proband | NA | +/+ | NP | NP | Blood, urine sediment, hair roots, oral mucosa | c.2393–1G>A | Loss or exchange of Gly799 |
| F-46 | Mother | NA | +/NA | NP | NP | Blood, urine sediment, hair roots, oral mucosa | ||||
EM, electron microscopy of kidney biopsy; F, female; IF, immunofluorescence microscopy of kidney biopsy; M, male; NA, not available; NP, not performed; mosaic: mosaic alpha5 staining in glomerular basement membrane.
Modifier gene variant: COL4A3 exon 42 c.3691G>A (p.Gly1231Ser).
The phenotypic features for patients with XLAS include a spectrum of nephropathy, from microscopic hematuria to progressive renal failure. Somatic mosaic XLAS male patients were thought to have mild phenotypes, especially when such findings were observed in the earlier reported cases.S4–S6 Unlike previous cases, the 10-year-old boy we present here has a severe phenotype with gross hematuria, persistent microscopic hematuria, and nephrotic range proteinuria. This suggests a spectrum of phenotypic severity in XLAS male patients with somatic mosaicism, potentially dependent on the allelic balance of mutant and wild-type cells or on the tissue distribution of affected cells. Similarly, this spectrum exists in somatic mosaic XLAS female patients (Table 1). Possibly, a severe phenotype might be related to the type of mutation, such as large deletions, frameshift mutations, and nonsense mutations.1 In addition, it is reported that AS phenotype is related to coexisting mutations in COL4A3 and/or COL4A4. It is reasonable to assume similar relationship is present between the severity of somatic mosaic XLAS and presence of coexisting mutations. For instance, a modifier gene variant on COL4A3 exon 42 c.3691G>A (p.Gly1231Ser) is detected in an 11-year-old girl with a severe phenotype, although no clear mechanism of the severe phenotype was elucidated.S6
Patients with XLAS may show extrarenal involvement, such as sensorineural deafness, ocular anomalies, and leimyomatosis.S8 No patients or families with somatic mosaic XLAS experienced sensorineural hearing loss or ocular abnormalities except 1 family reported by Beicht et al.S9 In this family, the 15-year-old boy received hearing aid devices because of sensorineural hearing loss of 50 to 60 dz and a reduced midrange, whereas the mother, a 46-year-old woman with the same mutation, was described to have experienced sensorineural hearing loss of 30 dz in the left ear and 40 dz in the right ear with a reduced midrange and myopia of unknown degree.S9 Leiomyomatosis was not discussed in any previous cases of somatic mosaic XLAS.
In contrast to unremarkable light microscopy findings in all previous cases of AS with somatic mosaicism, FSGS was seen in our patient, consistent with the patient's clinical characteristics with nephrotic range proteinuria. Although FSGS may represent a disease entity, FSGS may be seen as secondary lesion in a variety of renal disorders, such as AS. Gast et al.S10 studied the distribution of gene mutations in 81 adult patients with FSGS from 76 families and found the most frequent underlying definitely pathogenic mutations were COL4A3/COL4A4/COL4A5 mutations. Other studies have shown that type 4 collagen variants may be mistaken for FSGS,S11 especially when clinical and biopsy features are not classic for AS. Abnormal electron microscopy findings raise suspicion for AS; however, electron microscopy findings with normal glomerular basement membrane thickness and appearance do not completely exclude the possibility of AS, because abnormal findings may be seen on repeat biopsies.S11
Mosaicism in this case was suspected on the basis of immunostaining for the alpha5 collagen IV chain. Mosaic staining for the alpha5 collagen IV chain is frequently observed in female patients who are heterozygous for COL4A5 mutations3 but is atypical for hemizygous male patients. Mosaic immunostaining for the alpha5 collagen IV has been described in previous reports of somatic mosaicism in male patients with COL4A5 mutationsS4,S6 (Table 1). In addition, karyotype analysis is helpful during diagnostic workup even though the concomitant occurrence of AS and Klinefelter syndromeS12 is extremely rare, with only 3 reported casesS13–S15 and an estimated occurrence of 1 in 5 million births.S12 Furthermore, next generation sequencing is critical for the confirmation of diagnosis. This is especially true for the 4 families/patients with female somatic mosaic XLAS (family 2,4 family 4,4 family 3,S6 and patient 2S7 in Table 1), because the mosaic staining pattern of alpha5 collagen IV is expected in female patients with XLAS due to random X-inactivation. Because disease-modifying treatment is available for AS,S16,S17 an early diagnosis is extremely important. Many patients with asymptomatic or mild forms of AS will not seek evaluation until much later in life when it might be too late. For example, the mothersS6,S7,S9 and the asymptomatic fathers4,S6 only received genetic testing when their children were diagnosed with AS. With a kidney biopsy and additional workup, including immunofluorescence study of alpha5 chain staining pattern, karyotype analysis, and next generation sequencing, we can ensure an early diagnosis, genetic counseling, appropriate monitoring, and management of patients with genetic abnormality.
In summary, XLAS is the most common form of AS, caused by mutations in COL4A5, residing at chrXq22.3. Somatic mosaic XLAS, although rare, has been reported in both male and female patients with XLAS. Somatic mosaicism is suspected based on mosaic type IV collagen alpha5 chain immunostaining pattern, which is frequently seen in heterozygous female patients but is atypical for hemizygous male patients. Abnormal electron microscopy findings with glomerular basement membrane thinning, thickening, and lamellation would raise suspicion and guide genetic testing. A karyotype analysis and next generation sequencing are helpful for accurate diagnosis and confirmation of somatic mosaicism (Table 2).
Table 2.
Teaching points
| 1. X-linked Alport syndrome (XLAS) is the most common form of Alport syndrome, caused by mutations in COL4A5, residing at chrXq22.3. |
| 2. Mosaic staining of type IV collagen alpha5 chain is frequently observed in renal biopsies of female patients with XLAS, but is atypical for male patients. |
| 3. A karyotype analysis is performed to evaluate for concurrent Klinefelter syndrome in male patients with mosaic type IV collagen alpha5 chain staining. |
| 4. Next generation sequencing of COL4A5 is performed to diagnose and confirm somatic mosaicism in male patients. |
Disclosure
All the authors declared no competing interests.
Footnotes
Supplementary References.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
References
- 1.Kashtan C.E., Ding J., Garosi G. Alport syndrome: a unified classification of genetic disorders of collagen IV α345: a position paper of the Alport Syndrome Classification Working Group. Kidney Int. 2018;93:1045–1051. doi: 10.1016/j.kint.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 2.Massella L., Gangemi C., Giannakakis K. Prognostic value of glomerular collagen IV immunofluorescence studies in male patients with X-linked Alport syndrome. Clin J Am Soc Nephrol. 2013;8:749–755. doi: 10.2215/CJN.07510712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kashtan C.E., Michael A.F. Alport syndrome. Kidney Int. 1996;50:1445–1463. doi: 10.1038/ki.1996.459. [DOI] [PubMed] [Google Scholar]
- 4.Fu X.J., Nozu K., Kaito H. Somatic mosaicism and variant frequency detected by next-generation sequencing in X-linked Alport syndrome. Eur J Hum Genet. 2016;24:387–391. doi: 10.1038/ejhg.2015.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson A.C., Bower M., Baughn L.B. Criteria for clinical reporting of variants from a broad target capture NGS assay without Sanger verification. JSM Biomar. 2015;2:1005. [Google Scholar]
- 6.Yohe S., Hauge A., Bunjer K. Clinical validation of targeted next-generation sequencing for inherited disorders. Arch Pathol Lab Med. 2015;139:204–210. doi: 10.5858/arpa.2013-0625-OA. [DOI] [PubMed] [Google Scholar]
- 7.Onsongo G., Erdmann J., Spears M.D. Implementation of Cloud based next generation sequencing data analysis in a clinical laboratory. BMC Res Notes. 2014;7:314. doi: 10.1186/1756-0500-7-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richards C.S., Bale S., Bellissimo D.B. ACMG recommendations for standards for interpretation and reporting of sequence variations: Revisions 2007. Genet Med. 2008;10(4):294–300. doi: 10.1097/GIM.0b013e31816b5cae. [DOI] [PubMed] [Google Scholar]
- 9.Papavassiliou P., Charalsawadi C., Rafferty K., Jackson-Cook C. Mosaicism for trisomy 21: a review. Am J Med Genet A. 2015;167A:26–39. doi: 10.1002/ajmg.a.36861. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.