Abstract
Purpose
To describe the outcome of adjuvant high fluence photoactivated chromophore for infectious keratitis cross-linking (PACK-CXL) used to treat an advanced form of refractory Acanthamoeba keratitis (AK) diagnosed several months after initial presentation.
Observations
An otherwise healthy 24-year old female presented with a severe unilateral keratitis. The diagnosis eluded clinicians for several months and when finally confirmed as AK, anti-amoebic therapy was instated and only appeared to be effective after addition of high fluence PACK-CXL.
Conclusion and importance
In this case of advanced AK, high fluence PACK-CXL treatment given adjuvant to pharmacologic anti-amoebic therapy resulted in lasting pain relief, re-epithelization and eradication of the Acanthamoeba parasite. Given adjuvant to anti-amoebic pharmacotherapy, high fluence PACK-CXL might be a useful method for treating typically refractory advanced AK.
Keywords: Acanthamoeba keratitis, Phototherapy
1. Introduction
Acanthamoeba keratitis (AK) is a rare but severe ocular parasitic infection caused by the ubiquitously present protozoans A. Castellanii and A. Polyphagia.1, 2, 3, 4, 5 Reckoned to be on the rise due to increased use of soft contact lenses and multi-purpose solutions6, AK is characterized by an agonizing pain generally not corresponding to the clinical findings upon initial presentation. AK is often misdiagnosed as having a fungal or viral etiology with which it shares common clinical features early in the course of the infection.7, 8, 9, 10, 11 The delay in initiating appropriate treatment can have sight-threatening consequences, due to the invasiveness and persistence of the Acanthamoeba parasite. Typically a late diagnosed advanced-stage AK is resistant to standard treatments, may require keratoplasty and in some cases may even lead to permanent blindness.8,11, 12, 13, 14, 15, 16 Here we report a case of AK diagnosed several months after presentation, where high fluency photoactivated chromophore for infectious keratitis cross-linking (PACK-CXL) was used as an adjuvant to standard anti-amoebic therapy to treat the advanced, refractory AK. The result was a complete eradication of the Acanthamoeba infection.
2. Case report
A previously healthy 24-year woman presented with progressively worsening photophobia and pain in the right eye. The symptoms had started 5–6 days prior to admission and the patient, who wore contact lenses for myopia, had ceased wearing them a shortly after the symptoms started. An initial slit lamp examination showed conjunctival hyperemia; diffuse punctate staining of the cornea, subepithelial infiltrates, presence of anterior chamber cells and a decreased visual acuity of the right eye (BSCVA 0.3 decimal, 20/67 Snellen). A tentative diagnosis of adenoviral keratitis was made, and the patient was prescribed topical hydrocortisone and oxytetracycline (Terracortril with Polymyxin B®, Pfizer Inc, New York, USA) tid. The following six weeks the treatment was changed to topical acyclovir 3% (Zovirax®, GSK, Brendtford, UK) due to suspicion of herpes simplex keratitis and later adjusted to valacyclovir (Valtrex®, GSK, Brendtford, UK) 500 mg bid and topical dexamethasone (Dexafree®, Laboratories Théa, Clermont-Ferrand, France) instilled bid due to suspicion of discoid herpes keratitis. No signs of clinical improvement were observed during this time with persistent pain and deteriorating visual acuity (BSCVA 0.05 decimal, 20/400 Snellen). A conjunctival culture was taken and sent for analysis due to the refractory nature of the keratitis without signs of bacterial growth. Based on the rapidly deteriorating status of the cornea, a PACK-CXL according to the Dresden Protocol was carried out (3mW/cm2 for 30 minutes, total fluence 5.4 J/cm2) in a circular treatment area of 9 mm diameter of the corneal surface. Two days after the PACK-CXL, the pain had subsided and the patient was able to open her eye but photophobia remained. A few days later the cornea was completely re-epithelized and a few weeks later a maculae cornea had formed. An improved visual acuity was noted (BSCVA 0.25 decimal, 20/80 Snellen) and the cornea was deemed stable.
Two months after the first PACK-CXL treatment the patient was again admitted for severe pain and decreased visual acuity in the right eye. The cornea showed signs of diffuse infiltrates and central thinning (Fig. 1A). The clinical picture of ineffective pharmacotherapy combined with constant and intensive pain led to the suspicion of Acanthamoeba infection and the patient was referred to our partner university hospital in Linköping, where the diagnosis of AK was confirmed microbiologically and in vivo through the use of in vivo confocal microscopy (IVCM; Heidelberg Retinal Tomograph 3 with Rockstock Corneal Module) (Fig. 2A). Pharmacotherapy was initiated with topical propamidine isethionate 0.1% (Brolene®, Sanofi, Paris, France) and chlorhexidine 0.02% (available from APL manufacturing pharmacy, Stockholm, Sweden) hourly, chloramphenicol (Chloramphenicol®, Santen Pharmaceutical Co. Ltd, Osaka, Japan) tid and Cyclopentholate 1% (Cyclogyl®, Novartis, Basel, Switzerland) bid. A few days later patient could cope with the prescribed regimen at home and was discharged. By this time a small central de-epithelized lesion with an indistinct surrounding ring-formed opacity was noted (Fig. 1B and C). Before the next planned follow-up, however, the patient's condition worsened leading to re-hospitalization. The therapy was adjusted from chlorhexidine 0.02% to topical voriconazole 1% (available from APL manufacturing pharmacy, Stockholm, Sweden) given hourly together with propamidine isethionate 0.1% eight times a day. During the week-long stay the patient was agonized by severe pain and photophobia necessitating morphine. Prior to discharge the treatment resulted in a small improvement, was tapered and topical tobramycin (Tobrex Depot®, Novartis, Basel, Switzerland) bid and topical hydrocortisone and oxytetracycline once daily was added to the regimen. Clinically a large central de-epithelized lesion area with an increasingly evident opaque edge was noted (Fig. 1D and E).
Fig. 1.
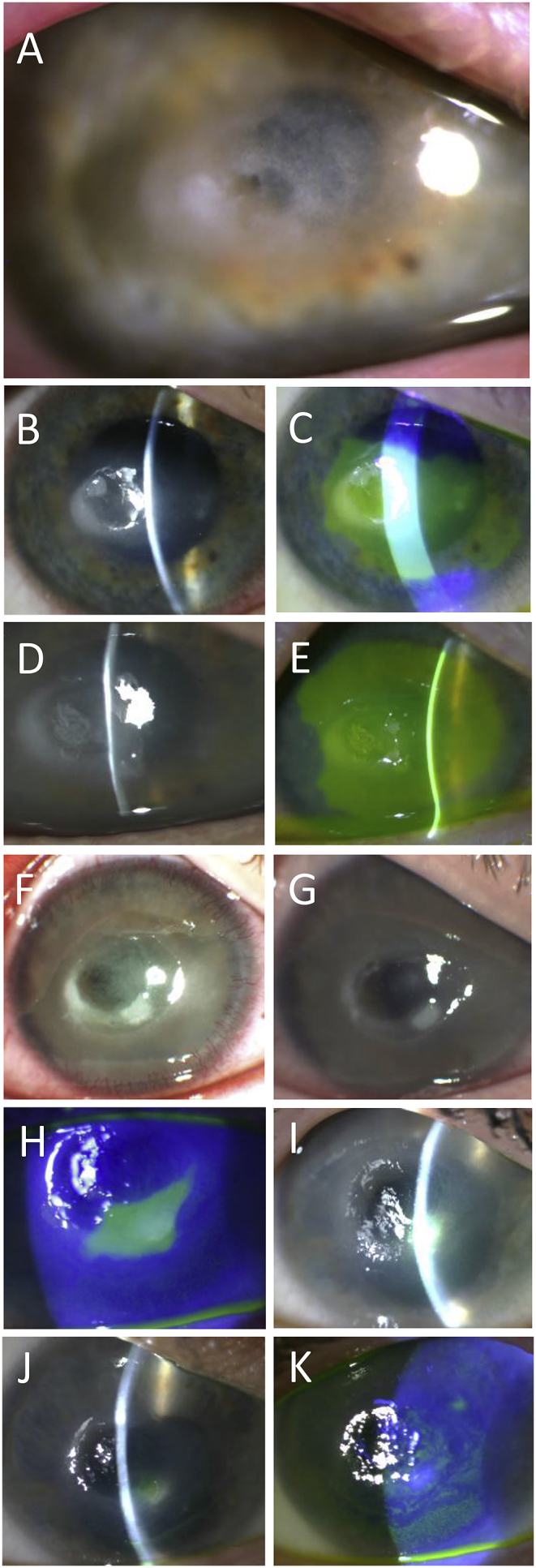
Time series slit lamp biomicroscopy images documenting the clinical course. (A) 7 weeks following the first PACK-CXL, Acanthamoeba infection is suspected and a central thinning is observed. (B, C) Lesion area with surrounding ring-shaped opacity observed after 3 weeks of anti-amoebic therapy. The lesion and surrounding opacity is stained with fluorescein. (D, E) Lesion area 2 weeks later, with distinct ring-shaped opacity evident and large central de-epithelized area. (F) Three days following the second PACK-CXL procedure. (G) Two weeks after the second PACK-CXL procedure. (H) Nine weeks after the second PACK-CXL procedure, only a small inferior de-epithelized area remains. (I) At 11 weeks, the clinical picture remained relatively unchanged. (J) At four months, healing has continued and the lesion size has further diminished. (K) Five months after the second PACK-CXL treatment, only intermittent punctate staining of the epithelium is found and the eye is stable and pain-free.
Fig. 2.
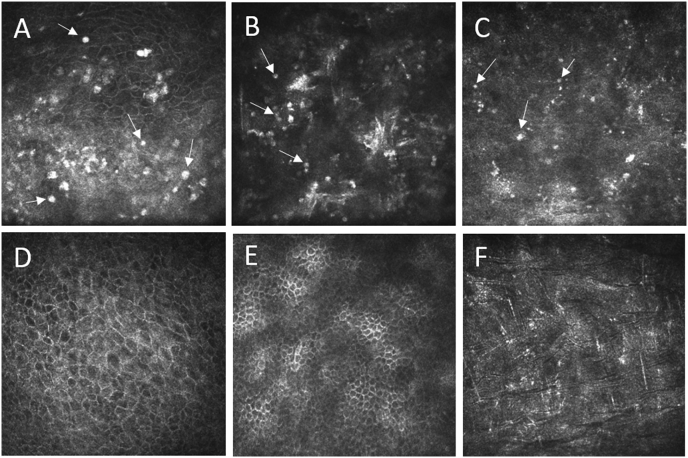
In vivo confocal microscopy images taken during the course of treatment. (A) Acanthamoeba infection is detected 7 weeks after first PACK-CXL by the presence of hyper-reflective round structures in the epithelium (arrows) indicative of cysts. (B) Two weeks after second PACK-CXL, round structures in the epithelium (arrows) with typical appearance and distribution indicating Acanthamoeba cysts, but with diffuse reflectivity. (C) 5 weeks after the second PACK-CXL, small hyper-reflective round structures (arrows) are observed in the anterior stroma, possibly indicative of inactive cysts or apoptotic/necrotic debris. Note the absence of stromal keratocytes which take a longer time to repopulate following CXL. (D–F) 5 months after second PACK-CXL. No cysts were found in the regenerated superficial epithelium (D), in regenerated epithelial wing cell layers (E), or in the anterior stroma (F).
One week after discharge, increasingly disabling pain, photophobia, mixed bulbar injection and a large central corneal ulcer prompted a second PACK-CXL treatment adjuvant to the topical treatment regimen. A higher fluence, in total 7.2 J/cm2 (4 mW/cm2 for 30 minutes) was administered within a circular treatment area of 9 mm diameter. The following day the patient reported relief of pain, topical levofloxacin (Oftaquix®, Santen Pharmaceutical Co. Ltd, Osaka, Japan) was instated qid prophylactically against secondary bacterial infections and the anti-amoebic drops were discontinued due to potential negative side effects on epithelial wound healing and inadequate therapeutic effect. Three days later the patient experienced further improvement with reduced pain, and initial re-epithelization was noted (Fig. 1F). By the fifth day the patient was pain-free, partial corneal transparency was restored and re-epithelization continued. The second week following the higher-dose PACK-CXL, a small central epithelial defect was still present, a haze covered the cornea (attributed to the PACK-CXL) and the bulbar injection had receded (Fig. 1G). Less obvious pathologic activity was now observed in IVCM (Fig. 2B). By the fifth week, a smaller central crater representing de-epithelization was noted while IVCM showed signs of features presumed to be inactive cyst-like remnants (Fig. 2C). Nine weeks after the high fluence PACK-CXL, a central de-epithelized crater remained visible although this was smaller in size (Fig. 1H) and the patient was free of pain, but still photophobic. This status remained relatively unchanged during subsequent weekly visits (Fig. 1I). A few months later a re-epithelized lesion area covered most of the corneal surface (Fig. 1J and K), visual acuity was hand motions at 1-meter distance, and the patient was pain-free and intermittently photophobic. IVCM at this time revealed a normalized histological appearance of the corneal superficial layers (Fig. 2D–F). The cornea is considered stable and the patient is a candidate for potential future keratoplasty.
3. Discussion
CXL has in recent years showed promising results when used to treat severe forms of infectious keratitis17, 18, 19, 20, 21, 22, 23, 24, where in such cases the treatment is termed PACK-CXL. The microbicidal effect of PACK-CXL is thought to be mediated through the combined effects of UVA-induced DNA damage and release of reactive oxygen species (ROS) by riboflavin25,26, while the following post-procedure relief in pain is thought to be mediated by the combined effects of suppression of inflammation27 and a transient reduction of nociceptive subepithelial nerves.28
To our understanding, both in terms of long lasting clinical improvement and histological eradication of the infection, the first PACK-CXL treatment did not seem to have an effect, nor was the drug regimen successful alone. The second more intense PACK-CXL however, had a clear effect on the pain and as no further anti-amoebic treatment was given, also had an effect on the infection.
Despite these observations, there are reports of both in vivo and in vitro failure to eradicate Acanthamoeba with PACK-CXL given both as monotherapy5,29, and adjuvant to anti-amoebic treatment.30,31 The former report, however, concerned an animal model, did not use PACK-CXL as adjuvant to medical therapy, and used the standard 5.4 J/cm2 dose. In our case, deterioration seemingly first came to a halt when 7.2 J/cm2 was utilized adjuvant to the topical anti-amoebic therapy. An earlier study30, which came to the conclusion that the anti-amoebic properties solely relied on the concentrations of different anti-amoebic agents without any effect attributed to PACK-CXL, was performed in an in vitro setting. Although current anti-amoebic drugs show low minimal cysticidal concentrations (MCC) in vitro, the clinical correlation between MCC in vitro and those required in a clinical setting seems poor. Clinical failures have been reported despite the MCC of these drugs being exceeded by 66 times for PHMB and 16 times for chlorohexidine, rendering the correlation between the in vitro sensitivities and the clinical outcomes less evident.32,33 Considering the contradictory outcomes between the results achieved in vitro versus those achieved in vivo, the possibility of a confounding factor not accounted for in vitro seems plausible. Whether this factor is an immunologic response upheld by the Acanthamoeba or by the host tissue microenvironment which impedes pharmacotherapy or promotes the photoreactive elements of PACK-CXL should be further investigated.
Finally, it should be mentioned that the adjuvant PACK-CXL given with a higher fluence setting (7.2 J/cm2), seemingly had potent cysticidal properties in an advanced, late stage of the infection. High fluence PACK-CXL may therefore be preferred over standard fluence protocol, as an adjuvant to standard treatment in persistent cases of Acanthamoeba infection. Whilst most anti-amoebic drops are effective in eradicating the Acanthamoeba parasite in its active trophozitic form, only biguanides (PHMB and chlorhexidine) and diamides (Brolene) possess cysticidal effects.12 These drugs are, however, laden with adverse effects especially when used in higher concentrations, to potentially achieve a clinical effect late in the course of the infection. In our case, whether the combination treatment, repeated PACK-CXL, or the higher dose of energy was responsible for successful eradication, is not known, but it remains a distinct possibility that for some patients an optimal combination of drugs and high fluence cross-linking may exist, and that this combination may even be effective against long-standing, advanced AK.
4. Conclusion
Our observations indicate that PACK-CXL, possibly given repeatedly and possibly with the use of an increased fluence adjuvant to traditional pharmacotherapy, may be an effective means of eradicating the Acanthamoeba infection in late-stage, refractory patients.
Funding
Financial support was granted by Futurum – academy for Health and Care Region Jönköping County, Jönköping, Sweden.
Conflicts of interest
The authors have no financial disclosures.
Patient consent
Informed consent was obtained from this patient in writing for publication of her case details.
Authorship
All the authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgments
Karim Makdoumi. MD, PhD. Örebro University Hospital | USÖ · Department of Ophthalmology For his valuable support in the writing of this article.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajoc.2019.100499.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Marciano-Cabral F., Cabral G. Acanthamoeba spp. as agents of disease in humans. Clin Microbiol Rev. 2003;16:273–307. doi: 10.1128/CMR.16.2.273-307.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niederkorn J.Y., Alizadeh H., Leher H., McCulley J.P. The pathogenesis of Acanthamoeba keratitis. Microb Infect. 1999;1:437–443. doi: 10.1016/s1286-4579(99)80047-1. [DOI] [PubMed] [Google Scholar]
- 3.Panjwani N. Pathogenesis of acanthamoeba keratitis. Ocul Surf. 2010;8:70–79. doi: 10.1016/s1542-0124(12)70071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siddiqui R., Khan N.A. Biology and pathogenesis of acanthamoeba. Parasit Vectors. 2012;5:6. doi: 10.1186/1756-3305-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berra M., Galperin G., Boscaro G. Treatment of Acanthamoeba keratitis by corneal cross-linking. Cornea. 2013;32:174–178. doi: 10.1097/ICO.0b013e31825cea99. [DOI] [PubMed] [Google Scholar]
- 6.Carnt N., Hoffman J.J., Verma S. Acanthamoeba keratitis: confirmation of the UK outbreak and a prospective case-control study identifying contributing risk factors. Br J Ophthalmol. 2018;102(12) doi: 10.1136/bjophthalmol-2018-312544. https://bjo.bmj.com/content/102/12/1621.long [DOI] [PubMed] [Google Scholar]
- 7.Jiang C., Sun X., Wang Z., Zhang Y. Acanthamoeba keratitis: clinical characteristics and management. Ocul Surf. 2015;13:164–168. doi: 10.1016/j.jtos.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Bacon A.S., Frazer D.G., Dart J.K., Matheson M., Ficker L.A., Wright P. A review of 72 consecutive cases of Acanthamoeba keratitis, 1984-1992. Eye. 1993;7(Pt 6):719–725. doi: 10.1038/eye.1993.168. [DOI] [PubMed] [Google Scholar]
- 9.Thebpatiphat N., Hammersmith K.M., Rocha F.N. Acanthamoeba keratitis: a parasite on the rise. Cornea. 2007;26:701–706. doi: 10.1097/ICO.0b013e31805b7e63. [DOI] [PubMed] [Google Scholar]
- 10.Patel D.V., McGhee C.N. Acanthamoeba keratitis: a comprehensive photographic reference of common and uncommon signs. Clin Exp Ophthalmol. 2009;37:232–238. doi: 10.1111/j.1442-9071.2008.01913.x. [DOI] [PubMed] [Google Scholar]
- 11.Illingworth C.D., Cook S.D., Karabatsas C.H., Easty D.L. Acanthamoeba keratitis: risk factors and outcome. Br J Ophthalmol. 1995;79:1078–1082. doi: 10.1136/bjo.79.12.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dart J.K., Saw V.P., Kilvington S. Acanthamoeba keratitis: diagnosis and treatment update 2009. Am J Ophthalmol. 2009;148:487–499 e2. doi: 10.1016/j.ajo.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Ficker L.A., Kirkness C., Wright P. Prognosis for keratoplasty in Acanthamoeba keratitis. Ophthalmology. 1993;100:105–110. doi: 10.1016/s0161-6420(93)31707-0. [DOI] [PubMed] [Google Scholar]
- 14.Berger S.T., Mondino B.J., Hoft R.H. Successful medical management of Acanthamoeba keratitis. Am J Ophthalmol. 1990;110:395–403. doi: 10.1016/s0002-9394(14)77020-5. [DOI] [PubMed] [Google Scholar]
- 15.Lorenzo-Morales J., Khan N.A., Walochnik J. An update on Acanthamoeba keratitis: diagnosis, pathogenesis and treatment. Parasite. 2015;22:10. doi: 10.1051/parasite/2015010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis M.J., Packo K.H., Epstein R.J., Grostern R.J., Cohen J.A. Acanthamoeba endophthalmitis following penetrating keratoplasty for Acanthamoeba keratitis. Arch Ophthalmol. 2010;128:505–506. doi: 10.1001/archophthalmol.2010.33. [DOI] [PubMed] [Google Scholar]
- 17.Tabibian D., Mazzotta C., Hafezi F., PACK -C.X.L. Corneal cross-linking in infectious keratitis. Eye Vis (Lond) 2016;3:11. doi: 10.1186/s40662-016-0042-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alio J.L., Abbouda A., Valle D.D., Del Castillo J.M., Fernandez J.A. Corneal cross linking and infectious keratitis: a systematic review with a meta-analysis of reported cases. J Ophthalmic Inflamm Infect. 2013;3:47. doi: 10.1186/1869-5760-3-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makdoumi K., Mortensen J., Crafoord S. Infectious keratitis treated with corneal crosslinking. Cornea. 2010;29:1353–1358. doi: 10.1097/ICO.0b013e3181d2de91. [DOI] [PubMed] [Google Scholar]
- 20.Price M.O., Tenkman L.R., Schrier A., Fairchild K.M., Trokel S.L., Price F.W., Jr. Photoactivated riboflavin treatment of infectious keratitis using collagen cross-linking technology. J Refract Surg. 2012;28:706–713. doi: 10.3928/1081597X-20120921-06. [DOI] [PubMed] [Google Scholar]
- 21.Said D.G., Elalfy M.S., Gatzioufas Z. Collagen cross-linking with photoactivated riboflavin (PACK-CXL) for the treatment of advanced infectious keratitis with corneal melting. Ophthalmology. 2014;121:1377–1382. doi: 10.1016/j.ophtha.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Tabibian D., Richoz O., Hafezi F. PACK-CXL: corneal cross-linking for treatment of infectious keratitis. J Ophthalmic Vis Res. 2015;10:77–80. doi: 10.4103/2008-322X.156122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papaioannou L., Miligkos M., Papathanassiou M. Corneal collagen cross-linking for infectious keratitis: a systematic review and meta-analysis. Cornea. 2016;35:62–71. doi: 10.1097/ICO.0000000000000644. [DOI] [PubMed] [Google Scholar]
- 24.Moren H., Malmsjo M., Mortensen J., Ohrstrom A. Riboflavin and ultraviolet a collagen crosslinking of the cornea for the treatment of keratitis. Cornea. 2010;29:102–104. doi: 10.1097/ICO.0b013e31819c4e43. [DOI] [PubMed] [Google Scholar]
- 25.Martins S.A., Combs J.C., Noguera G. Antimicrobial efficacy of riboflavin/UVA combination (365 nm) in vitro for bacterial and fungal isolates: a potential new treatment for infectious keratitis. Invest Ophthalmol Vis Sci. 2008;49:3402–3408. doi: 10.1167/iovs.07-1592. [DOI] [PubMed] [Google Scholar]
- 26.Schrier A., Greebel G., Attia H., Trokel S., Smith E.F. In vitro antimicrobial efficacy of riboflavin and ultraviolet light on Staphylococcus aureus, methicillin-resistant Staphylococcus aureus, and Pseudomonas aeruginosa. J Refract Surg. 2009;25:S799–S802. doi: 10.3928/1081597X-20090813-07. [DOI] [PubMed] [Google Scholar]
- 27.Corbin F., 3rd Pathogen inactivation of blood components: current status and introduction of an approach using riboflavin as a photosensitizer. Int J Hematol. 2002;76(Suppl 2):253–257. doi: 10.1007/BF03165125. [DOI] [PubMed] [Google Scholar]
- 28.Myung D., Manche E.E., Tabibian D., Hafezi F. The future of corneal cross-linking. In: Sinjab M.M., Cummings A.B., editors. Corneal Collagen Cross Linking. Springer International Publishing; Cham: 2017. pp. 269–292. [Google Scholar]
- 29.del Buey M.A., Cristobal J.A., Casas P. Evaluation of in vitro efficacy of combined riboflavin and ultraviolet a for Acanthamoeba isolates. Am J Ophthalmol. 2012;153:399–404. doi: 10.1016/j.ajo.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 30.Lamy R., Chan E., Good S.D., Cevallos V., Porco T.C., Stewart J.M. Riboflavin and ultraviolet A as adjuvant treatment against Acanthamoeba cysts. Clin Exp Ophthalmol. 2016;44:181–187. doi: 10.1111/ceo.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kashiwabuchi R.T., Carvalho F.R., Khan Y.A. Assessing efficacy of combined riboflavin and UV-A light (365 nm) treatment of Acanthamoeba trophozoites. Investig Ophthalmol Vis Sci. 2011;52:9333–9338. doi: 10.1167/iovs.11-8382. [DOI] [PubMed] [Google Scholar]
- 32.Elder M.J., Kilvington S., Dart J.K. A clinicopathologic study of in vitro sensitivity testing and Acanthamoeba keratitis. Invest Ophthalmol Vis Sci. 1994;35:1059–1064. [PubMed] [Google Scholar]
- 33.Perez-Santonja J.J., Kilvington S., Hughes R., Tufail A., Matheson M., Dart J.K. Persistently culture positive acanthamoeba keratitis: in vivo resistance and in vitro sensitivity. Ophthalmology. 2003;110:1593–1600. doi: 10.1016/S0161-6420(03)00481-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.