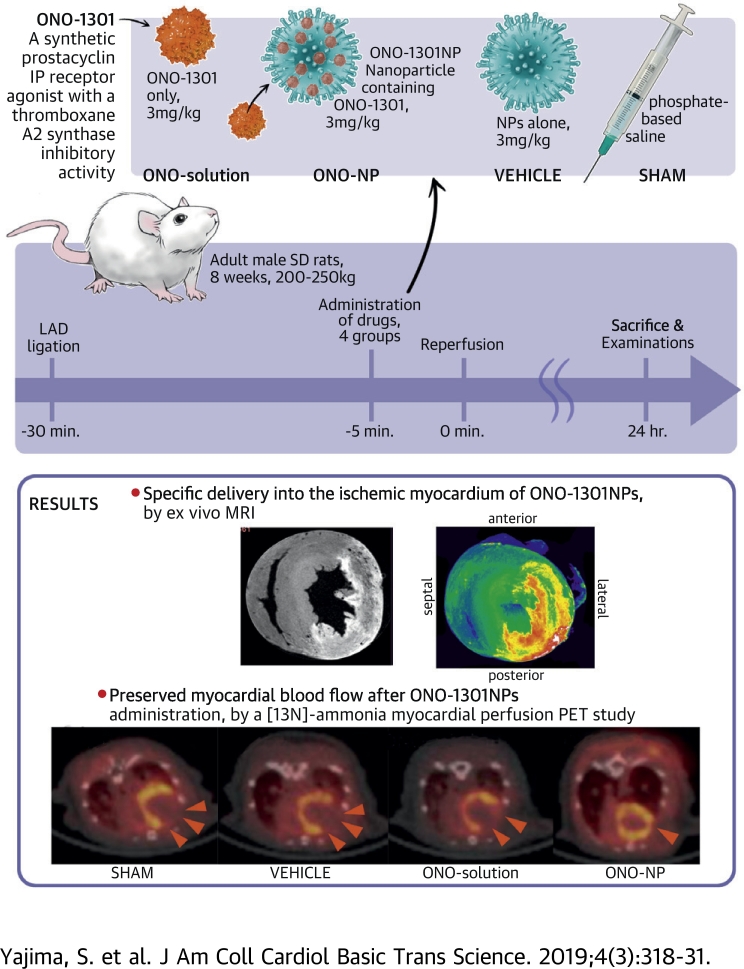
Visual Abstract
Key Words: ischemia/reperfusion injury, nanoparticles, ONO-1301, prostacyclin
Abbreviations and Acronyms: ANG, angiopoietin; EPR, enhanced permeability and retention; IL, interleukin; I/R, ischemia/reperfusion; MBF, myocardial blood flow; MRI, magnetic resonance imaging; NP, nanoparticle; PET, positron emission tomography; PMNL, polymorphonuclear leukocyte; VEGF, vascular endothelial growth factor
Highlights
-
•
Intravenously injected ONO-1301–containing nanoparticles selectively accumulated in the ischemic border area of the myocardium.
-
•
Prominent up-regulation occurred of proangiogenic cytokines such as vascular endothelial growth factor and angiopoietin-1 in the ischemic myocardium, which may have contributed to the preservation of the native vascular and capillary networks, thus preserving regional myocardial blood flow.
-
•
Down-regulation of the proinflammatory cytokines interleukin-1β, interleukin-6, and tumor necrosis factor-α in the ischemic myocardium might have led to the attenuation of myocyte swelling and the suppression of the endothelial bleb formation, also contributing to the preservation of myocardial blood flow or the reduced infarct size.
Summary
Intravenously injected ONO-1301–containing nanoparticles (ONO-1301NPs), unlike an ONO-1301 solution, selectively accumulated in the ischemia/reperfusion (I/R)-injured myocardium of rats and contributed to the prolonged retention of ONO-1301 in the targeted myocardial tissue. In the ischemic area, proangiogenic cytokines were up-regulated and inflammatory cytokines were down-regulated upon ONO-1301NP administration. Consequently, ONO-1301NP–injected rats exhibited a smaller infarct size, better-preserved capillary networks, and a better-preserved myocardial blood flow at 24 h after I/R injury, compared with those in vehicle-injected or ONO-1301 solution–injected rats. ONO-1301NPs attenuate the myocardial I/R injury via proangiogenic and anti-inflammatory effects of the drug.
Myocardial ischemia/reperfusion (I/R) injury is a pivotal therapeutic target to optimize revascularization therapy for acute coronary syndrome (1). Although an array of basic studies have reported efficacious treatments targeting myocardial I/R injury 2, 3, 4, 5, the therapeutic efficacy has not been established for any treatment in clinical settings 6, 7, 8, 9. This critical gap between the basic and clinical findings may be explained by the narrow pharmacological therapeutic window of each treatment (10). Because myocardial I/R injury involves dynamic biological and physiological events regulated by serially invoked complex pathways, any simple treatments, targeting single cellular and/or molecular processes, would not modify this event in the therapeutic direction to a clinically beneficial extent.
A new drug, ONO-1301, is a synthetic prostacyclin IP receptor agonist lacking the typical prostanoid structures, which contributes to the biological and chemical stability of this compound, resulting in long-lasting prostacyclin activity in vivo 11, 12. In addition, ONO-1301 has a 3-pyridine radical, which exerts thromboxane A2 synthase inhibitory activity, inducing an intrinsic prostaglandin I2 synthesis-promoting effect to augment the IP receptor agonistic activity (13). Our laboratory and others have reported beneficial effects of a slow-releasing form of ONO-1301 for acute and chronic ischemic cardiac failure via its role as an inducer of protective cytokines 14, 15, 16. Indeed, it has been shown that ONO-1301 binds to IP receptors on endothelial cells, vascular smooth muscle cells, or fibroblasts to induce the release of protective cytokines such as vascular endothelial growth factor (VEGF), hepatocyte growth factor, or stromal cell–derived factor 1, contributing to the repair of an experimentally induced ischemic myocardium.
Drug targeting is more critical in myocardial I/R injury than in acute or chronic ischemic cardiac failure because multiple complex events are serially, dynamically, and multidirectionally provoked in the myocardium after reperfusion. In this regard, systemically injected lipid nanoparticles (NPs) reportedly accumulate in acutely damaged tissue, represented by I/R injury, via enhanced permeability and retention (EPR) effects 17, 18. Importantly, the drug incorporated in NPs is selectively released into the target tissue 2, 19. We herein hypothesized that NPs containing ONO-1301 (ONO-1301NPs) would accumulate in I/R-injured cardiac tissue and induce multiple protective cytokines, thereby contributing to the attenuation of myocardial I/R injury. Thus, the aim of the present study was to evaluate whether ONO-1301NPs attenuate myocardial I/R injury.
Methods
Preparation of ONO-1301NPS
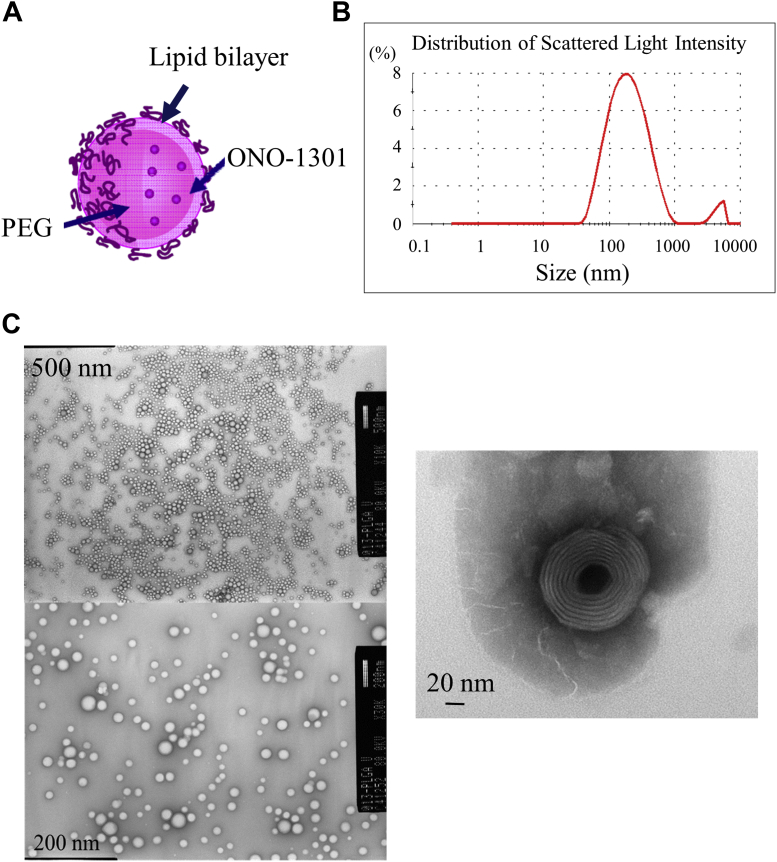
NPs containing ONO-1301 were prepared as follows. Two types of lipids, 1,2-dierucoyl-sn-glycerol-3-phosphorylcholine (1.88 g, CAS No. 5177-95-4, Nippon Fine Chemical, Osaka, Japan) and N-(carbonyl-methoxypolyethyleneglycol 2000)-1,2 distearoyl-sn-glycero-3-phosphoethanolamine sodium salt (0.12 g, CAS No. 147867-65-0, Nippon Fine Chemical), were dissolved, together with ONO-1301 (100 mg, Ono Pharmaceuticals, Osaka, Japan), in 20 g of t-butanol at 70°C. The obtained solution was immediately frozen in dry ice/acetone, followed by freeze-drying for 17 h. The obtained powder was dispersed in phosphate-buffered saline in a warm bath (50°C) and sonicated until lumps disappeared. The solution was passed through a polycarbonate filter with pores of 400 nm in diameter and then through another filter, with pores of 200 nm in diameter, to obtain a translucent liposome fluid. The obtained solution was purified by ultrafiltration to prepare consistently nanosized and round-shaped ONO-1301NPs (Figure 1). ONO-1301NPs were prepared by mixing the drug and the lipid (1,2-dierucoyl-sn-glycerol-3-phosphorylcholine:N-(carbonyl-methoxypolyethyleneglycol 2000)-1,2 distearoyl-sn-glycero-3-phosphoethanolamine sodium salt = 94:6) in a drug-to-lipid ratio of 0.05. After encapsulation and ultrafiltration, the drug-to-lipid ratio determined by using high-performance liquid chromatography was 0.043, indicating that loading efficiency was 86%.
Figure 1.
Characteristics of ONO-1301NPs
(A) Scheme of ONO-1301–containing nanoparticles (ONO-1301NPs). ONO-1301NPs are composed of a lipid bilayer with a polyethylene glycol (PEG) side chain on the surface and contain the ONO-1301 compound. (B) Details of ONO-1301NPs. Average particle diameter was obtained by the dynamic light scattering method. (C) Representative electron micrographic images of ONO-1301NPs. Upper: 10,000 × magnification; Lower: 30,000 magnification; Right: 50,000 × magnification.
Surgery and grouping of animals
The institutional ethics committee approved all experimental procedures. Animal care was conducted humanely in compliance with the principles of laboratory animal care.
Male Sprague-Dawley rats (200 to 250 g, Charles River Laboratories, Margate, United Kingdom) underwent left thoracotomy under general anesthesia with inhalation of isoflurane (2%, 0.2 ml/min); they were then intubated and placed on a respirator during surgery to maintain ventilation. The myocardial I/R model was prepared as follows. The left anterior descending artery was ligated at approximately 2 mm below the atrioventricular groove for 30 min by using a 7-0 monofilament with a silicon tube (outside diameter: 0.7 mm) placed along the left anterior descending artery and then released for reperfusion (2). Five minutes before reperfusion, 5 ml/kg phosphate-buffered saline (sham group), 3 mg/kg nanoliposomes in 5 ml/kg phosphate-buffered saline (vehicle group), 3 mg/kg ONO-1301 in 5 ml/kg phosphate-buffered saline (ONO-solution group), and 3 mg/kg ONO-1301NPs in 5 ml/kg phosphate-buffered saline (ONO-NP group) were injected into the rats (Supplemental Figure 1A). In addition, nanosized liposomes with a fluorescently labeled reagent (cyanine 5.5, Katayama Chemical Co., Osaka, Japan) encapsulated were prepared in the same manner as ONO-1301NPs and injected intravenously 5 min before reperfusion into I/R model rats (n = 6) for confocal microscopic analysis. Moreover, a nanosized liposomal contrast agent (Gadolisome, 5 ml/kg; DS Pharma Biomedical, Osaka, Japan) or 5 ml/kg saline was intravenously injected 5 min before reperfusion into I/R model rats (n = 3 each) for ex vivo magnetic resonance imaging (MRI) analysis. In addition, the same amount of Gadolisome or saline was injected into normal rats (n = 2 each) for the control of the MRI study. The characteristics of NP preparations (empty, fluorescently labeled, and Gadolisome) are described in Supplemental Figure 2.
Definition of myocardial area
Cardiac segmental analysis was performed according to the American Heart Association 17-segment model (20). The 17 segments were compiled into 3 territories according to the area of the ischemic insult: the infarct (1, 2, 7, 8, 13, and 14), border (3, 6, 9, 12, 15, and 16), and remote (4, 5, 10, and 11) areas (Supplemental Figure 1B).
Other methods
The methods of measurement of ONO-1301 concentrations in the plasma and heart, analysis of fluorescently labeled NPs, ex vivo MRI, [13N]-ammonia positron emission tomography (PET), enzyme-linked immunosorbent assay, triphenyltetrazolium chloride–Evans blue staining, histology and immunohistolabeling, and real-time polymerase chain reaction are detailed in the Supplemental Methods.
Statistical analysis
Continuous variables are presented as the mean ± SD. Statistical analyses were performed by using nonparametric methods because the sample sizes were too small to determine if a normal or a skewed distribution was followed. Intergroup differences were compared by using a Kruskal-Wallis analysis with a Mann-Whitney U test ad hoc analysis. A p value < 0.05 was considered statistically significant. All statistical analyses were performed with JMP version 12 software (SAS Institute, Inc., Cary, North Carolina).
Results
Stability of ONO-1301NPs in artificial plasma
The stability of the ONO-1301NP preparation in artificial plasma (i.e., plasma that does not contain plasma components such as proteins and lipids) and lipase (which breaks down the ONO-1301/ONO-1301NP preparation) was investigated. Approximately 7% of free ONO-1301 was detected in artificial plasma 72 h after culture, whereas approximately 55% of free ONO-1301 was hydrolyzed by the lipoprotein lipase. However, separation of ONO-1301 due to ONO-1301NP breakdown could not be confirmed with phospholipase A2 processing even after 72 h. ONO-1301/ONO-1301NPs were hydrolyzed by the lipase in the plasma (mainly lipoprotein lipase), and then bare ONO-1301 was gradually separated. In addition, >50% of ONO-1301 was separated from ONO-1301NPs through hydrolysis by lipase after 72 h (Supplemental Figure 3).
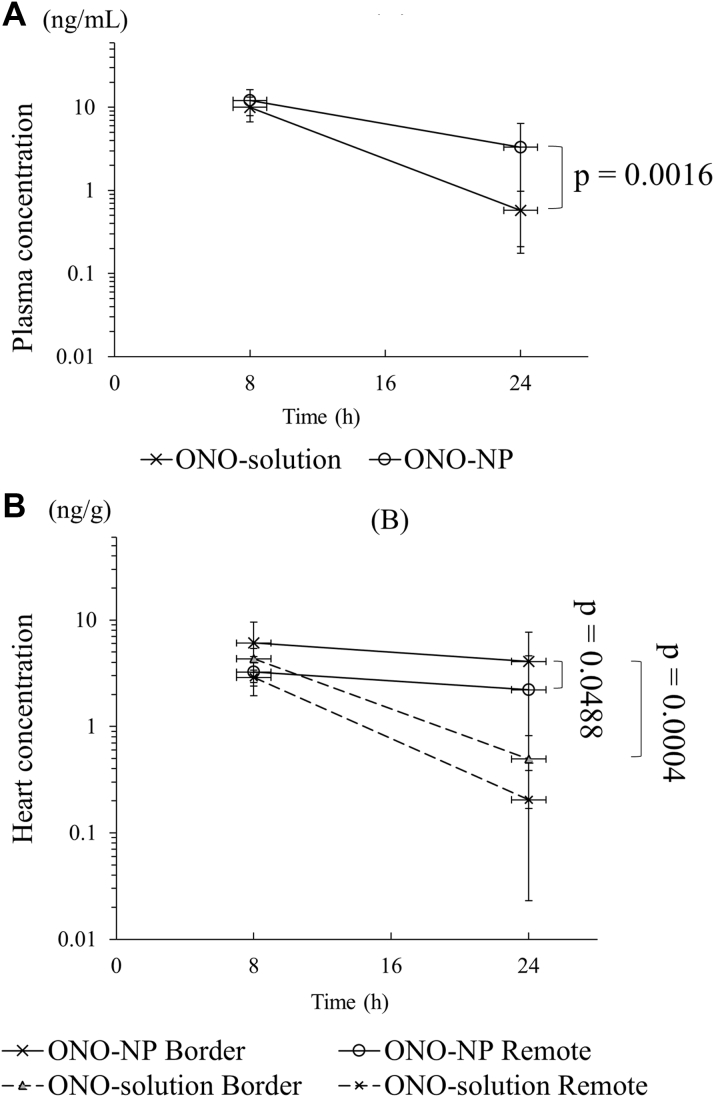
Prolonged retention of ONO-1301 in the plasma and heart after NP injection
ONO-1301 (ONO-solution group) or ONO-1301NPs (ONO-NP group) were injected intravenously into rats with myocardial I/R injury. Concentrations of ONO-1301 were measured in the plasma and heart according to liquid chromatography–tandem mass spectrometry analysis 8 and 24 h after the injection to explore targeted delivery of ONO-1301 incorporated into the liposome-based NPs. The results show that the plasma concentrations were not significantly different between the ONO-solution and ONO-NP groups at 8 h after the injection (ONO-solution: 10.0 ± 3.3 ng/ml; ONO-NP: 12.1 ± 4.2 ng/ml; p = 0.2611), whereas concentrations at 24 h were more prominently decreased in the ONO-solution group than in the ONO-NP group (ONO-solution: 0.6 ± 0.4 ng/ml; ONO-NP: 3.3 ± 3.1 ng/ml; p = 0.0016) (Figure 2A). The cardiac concentrations of ONO-1301 at 8 h were not significantly different between the 2 groups in the border area (ONO-solution: 4.3 ± 1.1 ng/g; ONO-NP: 6.1 ± 3.5 ng/g; p = 0.3703) and the remote area (ONO-solution: 2.9 ± 0.5 ng/g; ONO-NP: 3.2 ± 1.3 ng/g; p = 0.4292). Of note, the cardiac ONO-1301 concentrations were not significantly different at 8 and 24 h in the border and remote areas in the ONO-NP group. In contrast, the cardiac ONO-1301 concentrations significantly decreased at 24 h in the border and remote areas in the ONO-solution group. The cardiac concentrations of ONO-1301 at 24 h were significantly greater in the ONO-NP group than in the ONO-solution group in the border (4.1 ± 3.6 ng/g vs. 0.5 ± 0.3 ng/g, respectively; p = 0.0004) and remote (2.2 ± 2.4 ng/g vs. 0.2 ± 0.2 ng/g; p = 0.0003) areas (Figure 2B). Furthermore, the concentration in the border area was still significantly greater than in the remote area (p = 0.0488). The half-life of ONO-1301NPs in the plasma was 13.2 h, whereas those in the border and remote areas were 23.8 and 20.5 h.
Figure 2.
Plasma and Cardiac Concentrations of ONO-1301 After Intravenous Injection
ONO-1301 concentrations (A) in the plasma and (B) in the heart. Injected ONO-1301NPs showed better migration properties than did an injected ONO-1301 solution, leading to a greater ONO-1301 concentration in the infarct border myocardium. NP = nanoparticle.
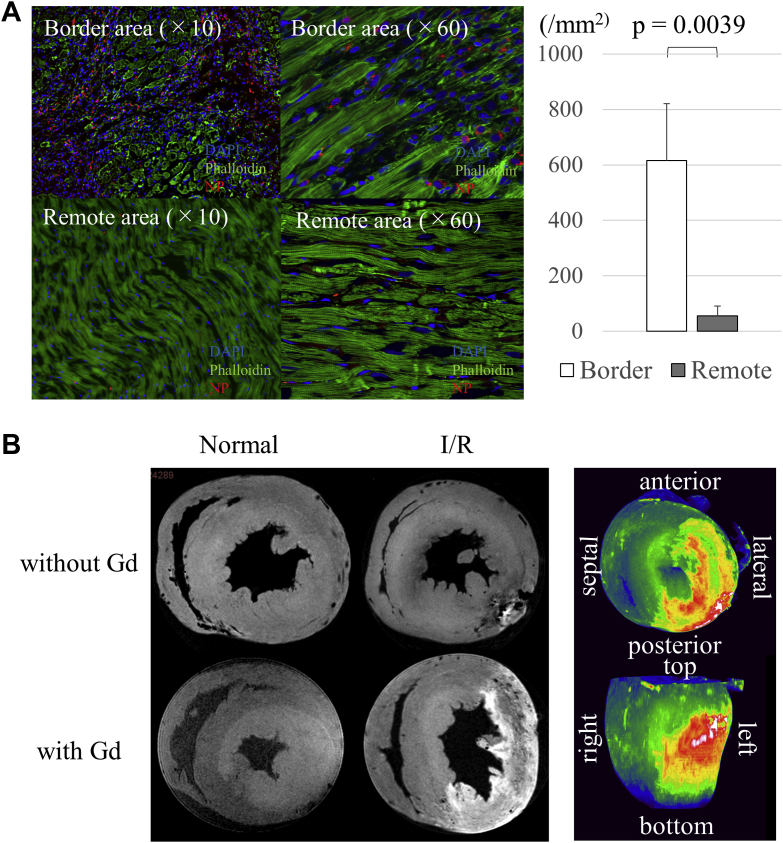
Predominant accumulation of NPs in the border area of the myocardium
Fluorescently labeled NPs and Gadolisome were intravenously injected into myocardial I/R model rats to assess the distribution of NPs in cardiac tissue by using confocal microscopy and by ex vivo MRI, respectively. The results showed that the fluorescently labeled NPs were more abundantly present in the border area of the myocardium than in the remote area at 24 h after reperfusion (border: 615 ± 206 mm2; remote: 56 ± 35 mm2; p = 0.0039) (Figure 3A). The injected fluorescently labeled NPs were predominantly present in the interstitial space of the border and remote areas. Meanwhile, Gadolisome was abundantly detected in the infarct and infarct border myocardium but minimally detected in the remote myocardium and the normal heart (Figure 3B). On a three-dimensional, fast low-angle shot view, specific accumulation of Gadolisome was particularly obvious in the outer and inner layers, rather than in the mid-layers, of the infarct and border myocardium (Figure 3C).
Figure 3.
Distribution of NPs in the Myocardium at 24 h After I/R Injury
(A) Confocal microscopy showed predominant presence of fluorescently labeled liposomes in the border area rather than in the remote area. (B) Magnetic resonance imaging (11.7-T) displayed selective accumulation of gadolinium (Gd)-labeled liposomes in the ischemic area at 24 h after NP injection. I/R = ischemia/reperfusion; NP = nanoparticle.
Up-regulation of therapeutic cytokines in the heart by ONO-1301NP injection
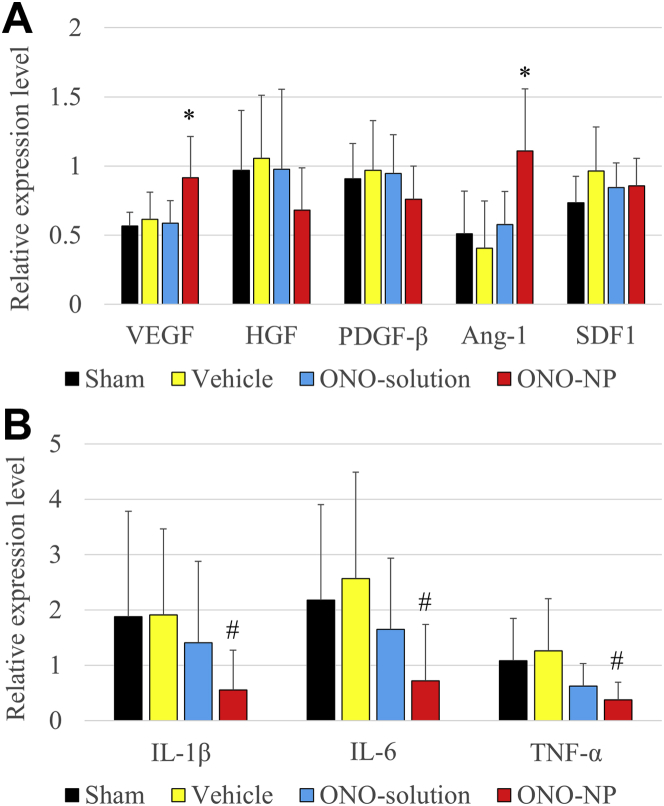
Gene expression of proangiogenic and inflammatory cytokines in the border area of the myocardium was assessed at 24 h after reperfusion by using real-time polymerase chain reaction. The results showed that the Vegf and angiopoietin-1 (Ang-1) genes were significantly up-regulated in the ONO-NP group compared with their expression in the other 3 groups (VEGF: vs. sham; p = 0.0024; vs. vehicle; p = 0.0194; vs. ONO-solution; p = 0.0051; ANG-1: vs. sham; p = 0.0024; vs. vehicle; p = 0.0006; vs. ONO-solution; p = 0.0014) (Figure 4A). In addition, the levels of gene expression for interleukin (IL)-1β, IL-6, and tumor necrosis factor-α were significantly lower in the ONO-NP group than in the other 3 groups (IL-1β: vs. sham; p = 0.0120; vs. vehicle; p = 0.0020; vs. ONO-solution; p = 0.0194; IL-6: vs. sham; p = 0.0226; vs. vehicle; p = 0.0029; vs. ONO-solution; p = 0.0166; tumor necrosis factor-α: vs. sham; p = 0.0020; vs. vehicle; p = 0.0024; vs. ONO-solution; p = 0.0464) (Figure 4B). There were no significant differences in the expression of these genes among the sham, vehicle, and ONO-solution groups.
Figure 4.
Gene Expression in the Heart at 24 h After I/R Injury
(A) Gene expression of the proangiogenic cytokines vascular endothelial growth factor (VEGF) and angiopoietin (ANG)-1 in the border area was significantly greater in the ONO-NP group than in the other 3 groups. (B) Gene expression of the inflammatory cytokines interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α was significantly lower in the ONO-NP group than in the other 3 groups. *Significantly greater than in the sham, vehicle, and ONO-solution groups. #Significantly smaller than in the sham, vehicle, and ONO-solution groups. HGF = hepatocyte growth factor; PDGF = platelet-derived growth factor; SDF1 = stromal cell–derived factor 1; other abbreviations as in Figure 3.
Protection of capillaries in the border area by ONO-1301NP injection
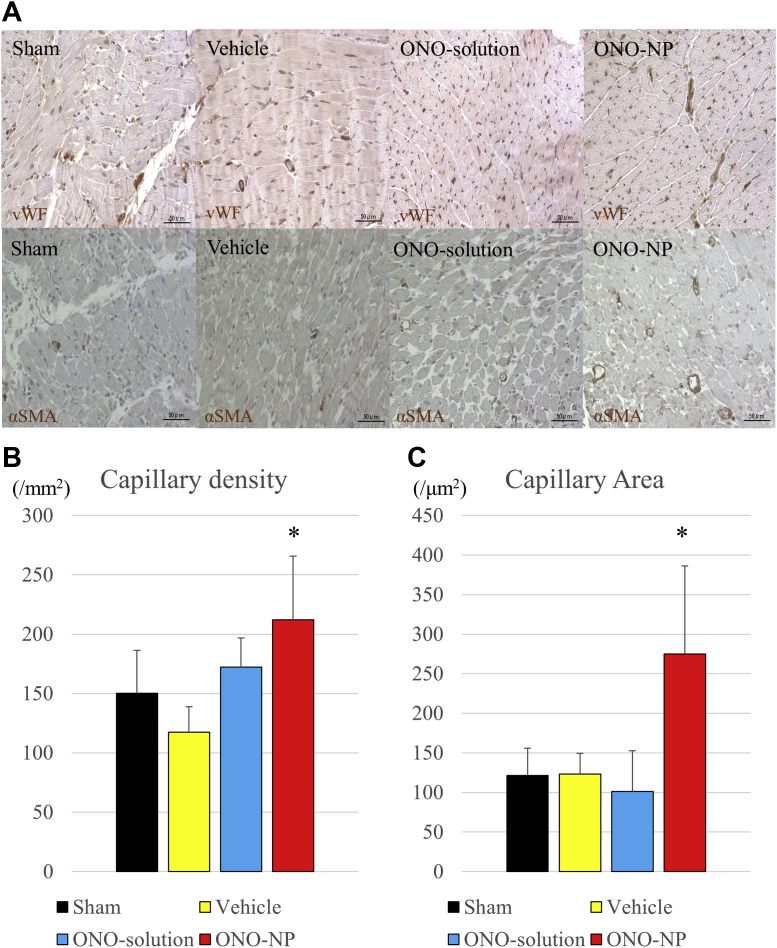
Pathological angiogenesis was assessed at the border area 24 h after the myocardial I/R injury. The residual capillary network in the border area was semi-quantitatively assessed by using immunohistology for the von Willebrand factor. The results showed that the von Willebrand factor–positive capillary network was better preserved in the myocardial interstitium of the ONO-NP group than in the myocardial interstitium of the other groups (Figure 5A). The capillary density was significantly greater in the ONO-NP group (212 ± 54 mm−2) than in the other groups (sham: 150 ± 36 mm−2; p = 0.0124; vehicle: 117 ± 21 mm−2; p = 0.0002; ONO-solution: 172 ± 25 mm−2; p = 0.0397) (Figure 5B). The capillary area was significantly greater in the ONO-NP group (275 ± 111 μm2) than in the other 3 groups (sham: 121 ± 34 μm2; p = 0.0001; vehicle: 123 ± 26 μm2; p = 0.0003; ONO-solution: 101 ± 52 μm; p = 0.0001) (Figure 5C).
Figure 5.
Capillary Density and Area at 24 h After I/R Injury
(A) Pathological angiogenesis at the infarct border. (B) The density of von Willebrand factor–positive capillaries and (C) the capillary area at the border were significantly greater in the ONO-NP group than in the other 3 groups. *Significantly greater than in the sham, vehicle, and ONO-solution groups. SMA = smooth muscle actin; vWF = von Willebrand factor; other abbreviations as in Figure 3.
Attenuation of polymorphonuclear leukocyte infiltration and myocyte swelling by ONO-1301NP injection
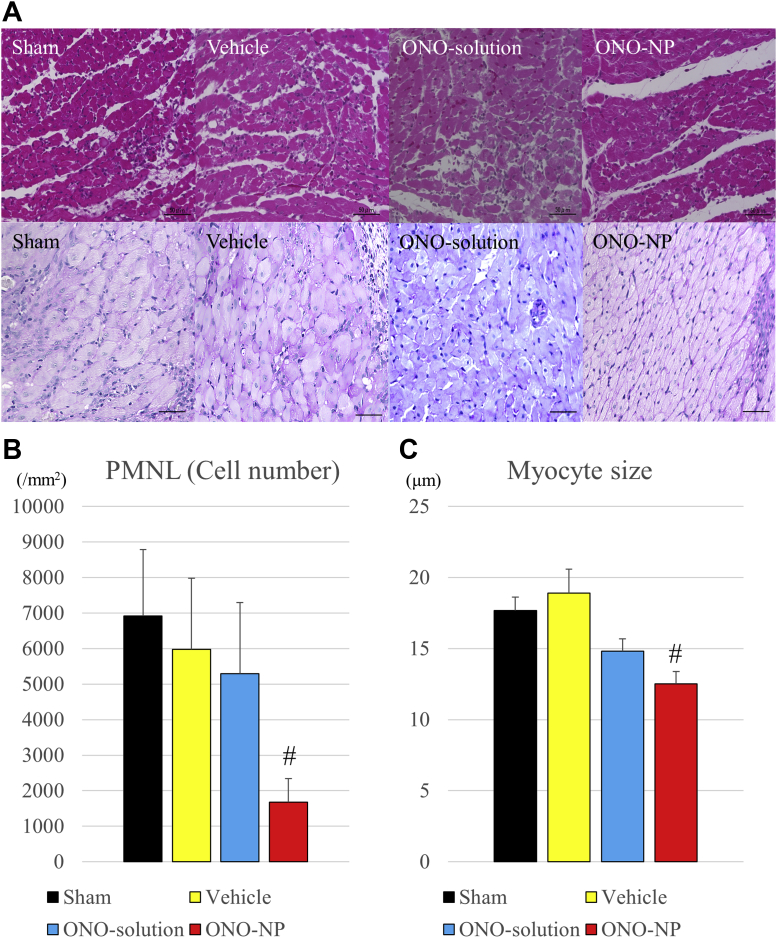
Infiltration of polymorphonuclear leukocytes (PMNLs) into the myocardium and the sizes of the cardiac myocytes were histologically assessed by hematoxylin/eosin and periodic acid-Schiff staining, respectively, 24 h after reperfusion to explore the distribution and degree of PMNL inflammation and myocyte swelling following I/R injury (Figure 6A). The results showed that PMNLs were markedly accumulated in the infarct area in all groups but were rarely observed in the remote area in all groups. In contrast, in the border area, significantly fewer PMNLs were present in the ONO-NP group (1,677 ± 667 mm-2) than in the other groups (sham: 6,923 ± 1,864 mm−2; p = 0.0001; vehicle: 5,979 ± 2,002 mm−2; p = 0.0002; ONO-solution: 5,295 ± 1,499 mm−2; p < 0.0001) (Figure 6B). Meanwhile, the shape and size of myocytes were variable in the infarct area but uniform in the remote area in all groups. In contrast, in the border area, the size of myocytes was significantly smaller in the ONO-NP group (14.8 ± 0.9 μm) than in the other groups (sham: 17.7 ± 0.9 μm; p = 0.0001; vehicle: 18.9 ± 1.7 μm; p = 0.0001; ONO-solution: 14.8 ± 0.9 μm; p = 0.0001) (Figure 6C).
Figure 6.
PNML Accumulation and Myocyte Sizes at 24 h After I/R Injury
(A) Pathological angiogenesis and myocyte swelling at the infarct border. (B) Polymorphonuclear leukocyte (PNML) accumulation was significantly lower and (C) myocyte sizes at the border were significantly smaller in the ONO-NP group than in the other 3 groups. #Significantly smaller than in the sham, vehicle, and ONO-solution groups. Abbreviations as in Figure 3.
Reduction of infarct size by ONO-1301NP injection
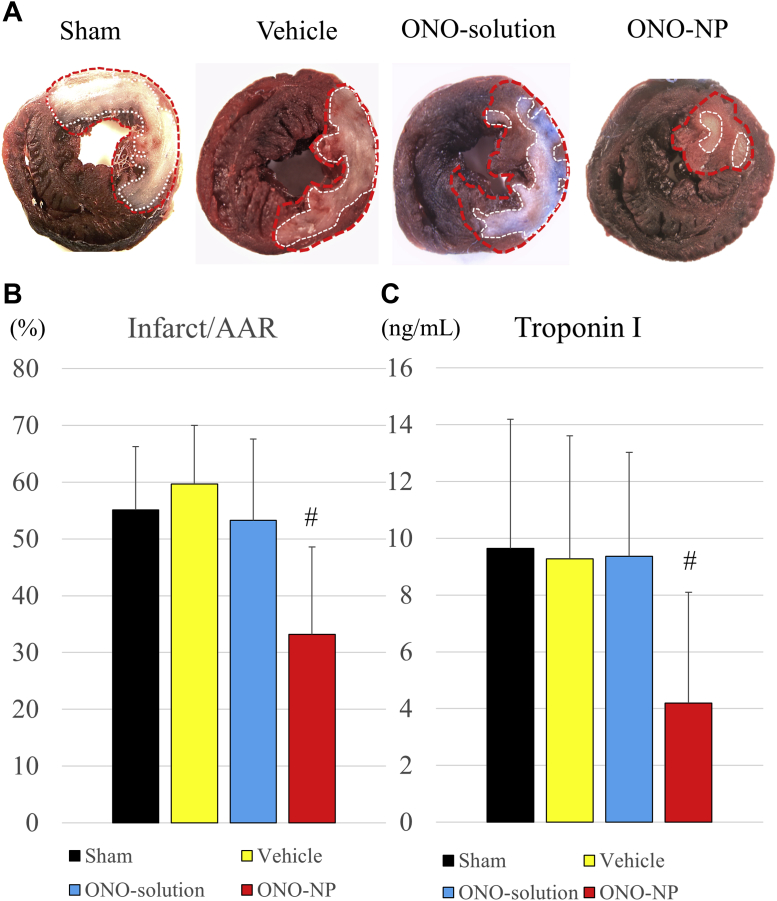
The area at risk of infarction and the infarct area were determined at 24 h after reperfusion by using Evans blue and triphenyltetrazolium chloride staining (Figure 7A). The infarct size per area at risk was significantly smaller (p < 0.01) in the ONO-NP group (33 ± 15%) than in the other groups (sham: 55 ± 11%; p = 0.0149; vehicle: 60 ± 10%; p = 0.0046; ONO-solution: 53 ± 14%; p = 0.0343) (Figure 7B). Consistently, the plasma level of troponin I was significantly lower in the ONO-NP group (4.2 ± 3.9 ng/ml) than in the other groups (sham: 9.6 ± 4.6 ng/ml; p = 0.0074; vehicle: 9.3 ± 4.3%; p = 0.0126; ONO-solution: 9.4 ± 3.7%; p = 0.0054) (Figure 7C), as assessed by using an enzyme-linked immunosorbent assay.
Figure 7.
Infarct Size at 24 h After I/R Injury
(A) Representative images of Evans blue and triphenyltetrazolium chloride staining of the heart in the 4 treatment groups. (B) The size of the area at risk of infarct was significantly smaller in the ONO-NP group than in the other 3 groups, as assessed by triphenyltetrazolium chloride staining. (C) The level of troponin I in the plasma was significantly lower in the ONO-NP group than in the other 3 groups, as assessed by enzyme-linked immunosorbent assay. #Significantly smaller than in the sham, vehicle, and ONO-solution groups. AAR = area at risk; other abbreviations as in Figure 3.
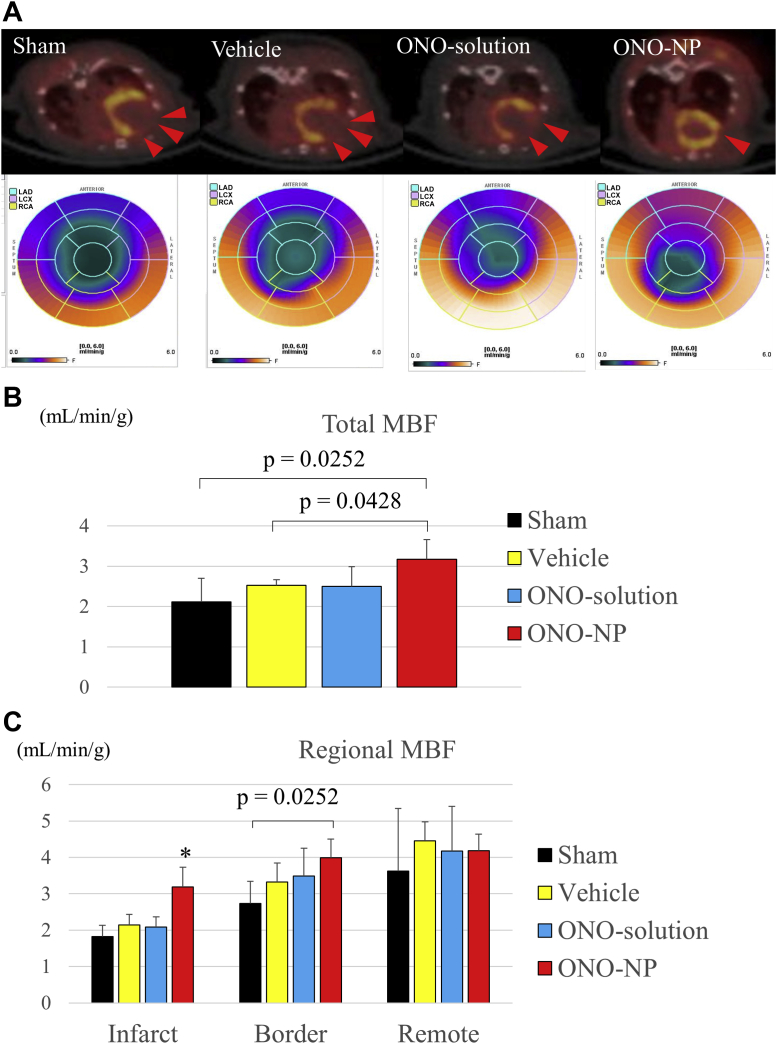
Total and regional myocardial blood flow after myocardial I/R injury
The total and regional myocardial blood flow (MBF) was assessed 24 h after reperfusion according to a [13N]-ammonia myocardial perfusion PET study (Figure 8A). The total MBF was significantly greater in the ONO-NP group (3.2 ± 0.5 ml/min/g) than in the sham and vehicle groups but not in the ONO-solution group (sham: 2.1 ± 0.6 ml/min/g; p = 0.0252; vehicle: 2.5 ± 0.1 ml/min/g; p = 0.0428; ONO-solution: 2.5 ± 0.5 ml/min/g; p = 0.0656) (Figure 8B).
Figure 8.
Total and Regional MBF at 24 h After I/R Injury
(A) Myocardial blood flow (MBF), assessed by a [13N]-ammonia myocardial perfusion positron emission tomography (PET) study, was compared among the 4 treatment groups. (B) Total MBF was significantly greater in the ONO-NP group than in the other 3 groups. (C) MBF in the infarct and peri-infarct regions, but not in the remote regions, was significantly greater in the ONO-NP group than in the sham group. *Significantly greater than in the sham, vehicle, and ONO-solution groups. Abbreviations as in Figure 3.
In the sham, vehicle, and ONO-solution groups, regional MBF in the infarct region was significantly lower than that in the border and remote areas, whereas the former was not significantly different from the latter in the ONO-NP group. Consequently, regional MBF in the infarct area was markedly and significantly greater in the ONO-NP group (3.2 ± 0.5 ml/min/g) than in the other groups (sham: 1.8 ± 0.3 ml/min/g; p = 0.0142; vehicle: 2.1 ± 0.3 ml/min/g; p = 0.0142; ONO-solution: 2.1 ± 0.3 ml/min/g; p = 0.0082). In addition, in the border area, MBF was significantly greater (p = 0.0252) in the ONO-NP group than in the sham group (4.0 ± 0.5 vs. 2.7 ± 0.6 ml/min/g). In the remote area, MBF did not differ significantly among the 4 groups (Figure 8C).
Discussion
Overview of pharmacological effects of ONO-1301NPs
There are 2 potentially different mechanisms of ONO-1301NP: 1) activation of the prostacyclin IP receptor, which is expressed in a variety of cells in the myocardium and in the other organs/tissues; and 2) inhibitory effects on 3-pyridine radical–related thromboxane A2 synthase, which allows bare ONO-1301 to be internalized within cells in the myocardial tissue. We have considered that the IP receptor–related effect is the dominant mechanism of ONO-1301NP therapy, but the 3-pyridine radical–related effect cannot be overlooked. Prostacyclin IP receptor is up-regulated, in response to myocardial I/R, in the endothelial cells, vascular smooth muscle cells, or cardiac fibroblasts. This action causes the release of cytoprotective factors such as VEGF and inhibits proinflammatory factors such as IL-1β, tumor necrosis factor-α, or IL-6 over the 24 h after myocardial I/R.
Intravenously injected ONO-1301NPs selectively accumulated in the ischemic border area of the myocardium where microvasculature was impaired and exhibited enhanced permeability, as assessed by using MRI and confocal microscopy. As shown in the present study, NPs tended to remain in circulation, avoiding a pulmonary trap or renal excretion 17, 21 and, subsequently, effectively accumulated in the ischemic myocardium, particularly in the infarct border area; these findings contrasted with the results obtained when a solution form was administered. This EPR effect resulted in prominent up-regulation of proangiogenic cytokines such as VEGF and ANG-1, which may have contributed to the preservation of the native vascular and capillary networks, thus preserving regional MBF in this group. Furthermore, down-regulation of the proinflammatory cytokines IL-1β, IL-6, and tumor necrosis factor-α in the border area might have led to the attenuation of myocyte swelling and the suppression of the endothelial bleb formation, also contributing to the preservation of MBF. In addition, the antiplatelet and vasodilatory effects of the prostacyclin analogue 22, 23, 24 could have prevented microvascular obstruction, although further studies are warranted to prove this hypothesis. Taken together, these multiple effects of targeted administration of ONO-1301 resulted in prominent cardioprotective effects in this study.
Advantage of ONO-1301NPS over other prostacyclin analogues
Other prostacyclin analogues such as epoprostenol, beraprost, treprostinil, and iloprost have been reported to attenuate myocardial I/R injury via vasodilation, inhibition of platelet aggregation, or anti-inflammation 25, 26. However, clinical applicability of these analogues is limited by adverse effects or drug tolerance, which is due to their short half-lives or the drug delivery systems used (26). It would be more suitable to administer these analogues intravenously immediately after the reperfusion, considering the nature of myocardial I/R injury. ONO-1301NPs have a much longer half-life (13.2 h in vivo) than that of the other prostacyclin analogues; therefore, ONO-1301NPs can be injected once a day, which may lead to the prevention of drug resistance. In addition, NPs containing cardioprotective agents have been shown to effectively operate in the damaged tissue, with minimal adverse effects, because of an EPR effect 2, 19; ONO-1301NPs would thus be an ideal prostacyclin analogue for this pathology.
Advantage of ONO-1301NPs over ONO-1301 solution
In the present study, the therapeutic effects of the ONO-1301 solution were limited, whereas the same dose of ONO-1301NPs revealed proangiogenic and anti-inflammatory effects. In the plasma, the concentration of ONO-1301 was approximately 6-fold higher in the ONO-NP group than in the ONO-solution group at 24 h, even though no difference was observed at 8 h after myocardial I/R injury, demonstrating the superior stealth capability of NPs compared with that of the solution. Because of this characteristic, in addition to the EPR effect, the ONO-1301 concentration in the border area of the I/R myocardium was approximately 8-fold higher in the ONO-NP group than in the ONO-solution group at 24 h after myocardial I/R injury, efficiently contributing to pharmacological effects. Injection of ONO-1301NPs revealed their better histological migration properties compared with those of the ONO-1301 solution, leading to a greater pharmacological efficacy in the reperfused ischemic myocardium. In addition, a protein corona effect 27, 28 might contribute to the peculiar localization of NPs in the ischemic area; this topic was not investigated in the present study. We speculated that some ONO-1301NPs would be surrounded by a corona in circulation, which may interact specifically with the targeted cells and trigger an internalization process, whereas bare ONO-1301 may interact differently.
Angiogenic and arteriogenic effects of ONO-1301NP injection
The Vegf and Ang-1 expression increased at 24 h after ONO-1301NP injection, possibly indicating that the myocardium was still ischemic, thus requiring angiogenesis and arteriogenesis. We consider that angiogenesis and arteriogenesis are still underway at this time point, when further development of the vascular network is needed in the border area. In contrast, in the other 3 groups, which exhibited lower levels of Vegf and Ang-1 expression, angiogenic and arteriogenic activity in the myocardium may have ceased by this time point. Although the expression of hepatocyte growth factor and stromal cell–derived factor-1 did not differ among the 4 groups at 24 h after the injection, these factors might have been elevated early and then normalized by 24 h in the treatment groups. Because expression of proangiogenic factors occurs dynamically in response to an ischemic insult and any angiogenic treatments, the expression pattern may be variable, depending on the nature of the ischemic insult and treatment.
Anti-inflammatory effects of ONO-1301NP injection
Prostacyclin is also known as a regulator of inflammation (26). The prostacyclin analogue iloprost has been reported to attenuate leukocyte adherence in both post-capillary and collecting intestinal venules and to improve the intestinal microvascular blood flow (29). In the present study, ONO-1301NP injection attenuated PMNL accumulation in intramyocardial arterioles of the reperfused myocardium, as assessed by histologic analysis. This and other anti-inflammatory effects, including a decrease in proinflammatory cytokines and suppression of myocardial enzymes, contribute to the attenuation of myocyte swelling, resulting in the relief of microvascular oppression and an increased MBF, particularly in the infarct border myocardium, as confirmed by the [13N]-NH3 PET study. Treatment by ONO-1301NPs attenuated myocardial I/R injury via cytoprotective and angiogenic effects, resulting in a smaller infarct size and better MBF. As a result, swelling of the cardiac myocytes was less severe in the ONO-NP group. Smaller cardiomyocyte size is therefore considered to be due to the indirect effects of ONO-NP. However, there may be a direct effect of the ONO-NP on the cardiac myocytes. Although the fluorescent-NP was not identified inside of the cardiac myocytes in this work as presented in Figure 3, the ONO-1301 molecule might be transferred into the cardiac myocytes, possibly via opsonization, and be ligated with the nuclear receptor of prostacyclin.
MBF increase, particularly in the infarct border area, by ONO-1301NP injection
In the present study, MBF in the infarct area was significantly higher after injection of ONO-1301NPs than after injection of the 3 controls. This finding indicates a reduced myocardial infarct size and augmented MBF in the infarct border area but not an increased amount of viable myocardial tissue, containing abundant arterioles, in the infarct area. Variation in the infarct size would affect MBF in the infarct area because of the narrow border area.
Study limitations
First, the lack of dose-dependent analysis may be a limitation of the study. The rationale to use 3 mg/kg ONO-1301 was based on preliminary experiments, which showed that 3 mg/kg was the maximum dose exhibiting linearity in plasma upon administration of 0.1, 0.3, 1.0, 3.0, and 10 mg/kg ONO-1301 solution to normal rats (Supplemental Table 1, Supplemental Figure 3). We therefore considered that 3 mg/kg was the optimum dose of ONO-1301. As a next step, further investigation is needed to explore the optimal dose and safety and to obtain a maximum benefit with minimum side effects in a large animal model. A second limitation of this study may be its relatively short observation period. Given that the method uses a single injection after myocardial I/R, 24 h was the optimal point to investigate the efficacy of ONO-1301NPs. Further investigation is needed to determine long-term effects, with the manner of drug administration adjusted. Third, evaluation of additional ischemic circulating markers such as the FABP3, which has been shown to provide faster stress-to-recovery response, would be necessary to better understand the mechanism of recovery.
Conclusions
We herein documented that intravenously injected ONO-1301NPs selectively accumulated in the I/R-injured myocardium, resulting in the prolonged retention of ONO-1301 in the targeted myocardial tissue of rats. Compared with the vehicle- or ONO-1301 solution–injected rats, the ONO-1301NP–injected rats showed a smaller infarct size, better-preserved capillary networks, and, importantly, a better-preserved MBF in the border area of the myocardium 24 h after I/R injury. Intravenously injected ONO-1301NPs selectively accumulated in the I/R area, protecting the myocardium from injury via proangiogenic and anti-inflammatory effects. This new drug may have the potential to bridge the gaps between basic and clinical research.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: ONO-1301 is a synthetic prostacyclin IP receptor agonist with inhibitory activity for thromboxane A2 synthase. ONO-1301 lacks the typical prostanoid structures, which contributes to its greater biological and chemical stability. In addition, ONO-1301 has been shown to be a multi-cytokine inducer, acting as a vasodilatory, proangiogenic, anti-inflammatory, and antifibrotic drug. Liposome-based biocompatible nanoparticles containing ONO-1301 may have an advantage over other existing prostacyclin analogues, considering the pathological nature of myocardial I/R injury and long-lasting prostacyclin activity of ONO-1301NPs, as well as their efficient drug delivery, with EPR.
TRANSLATIONAL OUTLOOK: Further investigation is needed to explore the optimal dose and safety of ONO-1301, with maximum pharmacological benefits and minimum adverse effects, and to establish a practical regimen for drug administration to achieve long-term effects.
Acknowledgments
The authors thank Yuri Ide and the staff of the PET Molecular Imaging Center for their excellent technical assistance.
Footnotes
This work was supported by Grants-in-Aid for Scientific Research (KAKENHI), Tokyo, Japan (T16K106280). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors' institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
References
- 1.Yellon D.M., Hausenloy D.J. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 2.Nakano Y., Matoba T., Tokutome M. Nanoparticle-mediated delivery of irbesartan induces cardioprotection from myocardial ischemia-reperfusion injury by antagonizing monocyte-mediated inflammation. Sci Rep. 2016;6:29601. doi: 10.1038/srep29601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukushima S., Coppen S.R., Varela-Carver A. A novel strategy for myocardial protection by combined antibody therapy inhibiting both P-selectin and intercellular adhesion molecule-1 via retrograde intracoronary route. Circulation. 2006;114:I251–I256. doi: 10.1161/CIRCULATIONAHA.105.000794. [DOI] [PubMed] [Google Scholar]
- 4.Hausenloy D.J., Maddock H.L., Baxter G.F., Yellon D.M. Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning? Cardiovasc Res. 2002;55:534–543. doi: 10.1016/s0008-6363(02)00455-8. [DOI] [PubMed] [Google Scholar]
- 5.Gu J., Fan Y., Liu X. SENP1 protects against myocardial ischaemia/reperfusion injury via a HIF1α-dependent pathway. Cardiovasc Res. 2014;104:83–92. doi: 10.1093/cvr/cvu177. [DOI] [PubMed] [Google Scholar]
- 6.Hausenloy D.J., Yellon D.M. Ischaemic conditioning and reperfusion injury. Nat Rev Cardiol. 2016;13:193–209. doi: 10.1038/nrcardio.2016.5. [DOI] [PubMed] [Google Scholar]
- 7.Cung T.T., Morel O., Cayla G. Cyclosporine before PCI in patients with acute myocardial infarction. N Engl J Med. 2015;373:1021–1031. doi: 10.1056/NEJMoa1505489. [DOI] [PubMed] [Google Scholar]
- 8.Ross A.M., Gibbons R.J., Stone G.W., Kloner R.A., Alexander R.W. A randomized, double-blinded, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD-II) J Am Coll Cardiol. 2005;45:1775–1780. doi: 10.1016/j.jacc.2005.02.061. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong P.W., Granger C.B., Adams P.X. Pexelizumab for acute ST-elevation myocardial infarction in patients undergoing primary percutaneous coronary intervention: a randomized controlled trial. JAMA. 2007;297:43–51. doi: 10.1001/jama.297.1.43. [DOI] [PubMed] [Google Scholar]
- 10.Hausenloy D.J., Yellon D.M. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123:92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murakami S., Nagaya N., Itoh T. Prostacyclin agonist with thromboxane synthase inhibitory activity (ONO-1301) attenuates bleomycin-induced pulmonary fibrosis in mice. Am J Physiol Lung Cell Mol Physiol. 2006;290:L59–L65. doi: 10.1152/ajplung.00042.2005. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura K., Sata M., Iwata H. A synthetic small molecule, ONO-1301, enhances endogenous growth factor expression and augments angiogenesis in the ischaemic heart. Clin Sci (Lond) 2007;112:607–616. doi: 10.1042/CS20060301. [DOI] [PubMed] [Google Scholar]
- 13.Fukushima S., Miyagawa S., Sakai Y., Sawa Y. A sustained-release drug-delivery system of synthetic prostacyclin agonist, ONO-1301SR: a new reagent to enhance cardiac tissue salvage and/or regeneration in the damaged heart. Heart Fail Rev. 2015;20:401–413. doi: 10.1007/s10741-015-9477-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishimaru K., Miyagawa S., Fukushima S. Synthetic prostacyclin agonist, ONO1301, enhances endogenous myocardial repair in a hamster model of dilated cardiomyopathy: a promising regenerative therapy for the failing heart. J Thorac Cardiovasc Surg. 2013;146:1516–1525. doi: 10.1016/j.jtcvs.2013.02.045. [DOI] [PubMed] [Google Scholar]
- 15.Imanishi Y., Miyagawa S., Fukushima S. Sustained-release delivery of prostacyclin analogue enhances bone marrow-cell recruitment and yields functional benefits for acute myocardial infarction in mice. PLoS One. 2013;8:e69302. doi: 10.1371/journal.pone.0069302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shirasaka T., Miyagawa S., Fukushima S. A slow-releasing form of prostacyclin agonist (ONO1301SR) enhances endogenous secretion of multiple cardiotherapeutic cytokines and improves cardiac function in a rapid-pacing-induced model of canine heart failure. J Thorac Cardiovasc Surg. 2013;146:413–421. doi: 10.1016/j.jtcvs.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Acharya S., Sahoo S.K. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv Drug Deliv Rev. 2011;63:170–183. doi: 10.1016/j.addr.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Matoba T., Egashira K. Nanoparticle-mediated drug delivery system for cardiovascular disease. Int Heart J. 2014;55:281–286. doi: 10.1536/ihj.14-150. [DOI] [PubMed] [Google Scholar]
- 19.Takahama H., Minamino T., Asanuma H. Prolonged targeting of ischemic/reperfused myocardium by liposomal adenosine augments cardioprotection in rats. J Am Coll Cardiol. 2009;53:709–717. doi: 10.1016/j.jacc.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 20.Cerqueira M.D., Weissman N.J., Dilsizian V. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 21.Markman J.L., Rekechenetskiy A., Holler E., Ljubimova J.Y. Nanomedicine therapeutic approaches to overcome cancer drug resistance. Adv Drug Deliv Rev. 2013;65:1866–1879. doi: 10.1016/j.addr.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camacho M., Rodriguez C., Guadall A. Hypoxia upregulates PGI-synthase and increases PGI2 release in human vascular cells exposed to inflammatory stimuli. J Lipid Res. 2011;52:720–731. doi: 10.1194/jlr.M011007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohite A., Chillar A., So S.P., Cervantes V., Ruan K.H. Novel mechanism of the vascular protector prostacyclin: regulating microRNA expression. Biochemistry. 2011;50:1691–1699. doi: 10.1021/bi101654w. [DOI] [PubMed] [Google Scholar]
- 24.Aherne T., Price D.C., Yee E.S., Hsieh W.R., Ebert P.A. Prevention of ischemia-induced myocardial platelet deposition by exogenous prostacyclin. J Thorac Cardiovasc Surg. 1986;92:99–104. [PubMed] [Google Scholar]
- 25.Chawengsub Y., Gauthier K.M., Campbell W.B. Role of arachidonic acid lipoxygenase metabolites in the regulation of vascular tone. Am J Physiol Heart Circ Physiol. 2009;297:H495–H507. doi: 10.1152/ajpheart.00349.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dorris S.L., Peebles R.S., Jr. PGI2 as a regulator of inflammatory diseases. Mediators Inflamm. 2012;2012:926968. doi: 10.1155/2012/926968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dror Y., Sorkin R., Brand G., Boubriak O., Urban J., Klein J. The effect of the serum corona on interactions between a single nano-object and a living cell. Sci Rep. 2017;7:45758. doi: 10.1038/srep45758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahmoudi M., Lynch I., Ejtehadi M.R., Monopoli M.P., Bombelli F.B., Laurent S. Protein-nanoparticle interactions: opportunities and challenges. Chemical Rev. 2011;111:5610–5637. doi: 10.1021/cr100440g. [DOI] [PubMed] [Google Scholar]
- 29.Lehmann C., Konig J.P., Dettmann J., Birnbaum J., Kox W.J. Effects of iloprost, a stable prostacyclin analog, on intestinal leukocyte adherence and microvascular blood flow in rat experimental endotoxemia. Crit Care Med. 2001;29:1412–1416. doi: 10.1097/00003246-200107000-00019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.