Abstract
In the context of tumorigenesis, telomere shortening is associated with apparent antagonistic outcomes: On one side, it favors cancer initiation through mechanisms involving genome instability, while on the other side, it prevents cancer progression, due to the activation of the DNA damage response (DDR) checkpoint behaving as a cell‐intrinsic proliferation barrier. Consequently, telomerase, which can compensate for replicative erosion by adding telomeric DNA repeats at the chromosomal DNA extremities, is crucial for cancer progression and is upregulated in nearly 90% of human cancers. Therefore, telomeres are considered potential anti‐cancer targets and, to date, most of the studies have focused on telomerase inhibition. However, the development of clinically efficient telomerase targeting therapies is still in its infancy. In this context, the findings reported in this issue of EMBO Molecular Medicine by Bejarano et al (2019) open new avenues for alternative telomere therapies.
Subject Categories: Cancer, Pharmacology & Drug Discovery
In human cells, telomeric chromatin is organized into a terminal loop (t‐loop), nucleosomes, the non‐coding RNA TERRA, the protein complex shelterin, and a network of nuclear factors. The shelterin complex is essential for telomere protection and comprises six subunits: Three subunits bind telomeric DNA (TRF1, TRF2, and POT1), while the three others establish protein–protein contacts: RAP1 with TRF2, TIN2 with TRF1, TRF2, and TPP1 with TIN2 and POT1. Each shelterin subunit has a specific role in telomere protection, i.e., TRF1 prevents replication stress, TRF2 blocks ataxia telangiectasia‐mutated (ATM) signaling and non‐homologous end joining (NHEJ), while POT1 blocks ataxia telangiectasia and Rad3‐related (ATR) signaling.
A wealth of recent findings points toward shelterin as a valuable alternative to telomerase to fight cancer. POT1 mutations, as well as increased dosages of TRF1 and TRF2, are observed in several types of human malignancies (Nakanishi et al, 2003; Ramsay et al, 2013; Bejarano et al, 2017; Cherfils Vicini et al, 2019). Consistently, the TRF1 and TRF2 expression is regulated by key cancer signaling pathways such as canonical Wnt (Diala et al, 2013), WT1 (El Maï et al, 2014), and PI3K/AKT (Bejarano et al, 2017). An overexpression of TRF1 and TRF2 has been implicated in various pro‐tumorigenic mechanisms including initiation and progression, migration, metastasis, angiogenesis (El Maï et al, 2014; Picco et al, 2016; Zizza et al, 2019), cancer stemness (Bejarano et al, 2017), telomere maintenance (García‐Beccaria et al, 2015; Bejarano et al, 2017), or immunosurveillance bypass (Biroccio et al, 2013; Cherfils Vicini et al, 2019), making these shelterin subunits interesting multi‐hit targets for cancer treatment.
In this issue of EMBO Molecular Medicine, Bejarano et al (2019) identified small compounds targeting TRF1 using an FDA‐approved library to screen for TRF1 expression and localization. Several of the drugs downregulating TRF1 expression interfere with common cancer signaling pathways including ERK and MEK, Aurora, CDK, PLK1, HSP90, mTOR, RTK, or chemotherapy drugs like gemcitabine and docetaxel. Treatment of lung cancer and glioblastoma cells with these compounds triggered DDR activation at telomeres and telomere replication defects. In patient‐derived glioblastoma stem cells (GSC), these TRF1 inhibitors reduced stemness in vitro. Importantly, the authors showed that TRF1 is a direct substrate for ERK2 and bRAF, two kinases of the Ras pathway, and mTOR. Among the 13 residues they identified for TRF1 phosphorylation by ERK2 and the 4 for bRAF, T330 appears critical for both bRAF and ERK2 phosphorylation as well as for TRF1‐telomere complex formation. Inhibiting T330 phosphorylation is sufficient to increase telomere uncapping, to reduce proliferation and stemness in vitro, and to reduce tumorigenesis in vivo. Finally, combined treatment with PI3K inhibitors and ERK2 or bRAF inhibitors showed synergistic effects in vitro and in vivo on patient‐derived glioblastoma mouse model, showing that TRF1 targeting via phosphorylation inhibition could be a promising strategy.
Interestingly, data from Bejarano et al (2019) make a direct connection between TRF1 phosphorylation by common cancer signaling pathways, telomere protection, and cancer treatment. This link is certainly not limited to TRF1, since TRF2 can also be phosphorylated by the ERK1/2 kinases and interacts with Ras signaling to bypass DDR in cancer cells (Biroccio et al, 2013; Picco et al, 2016). This is in agreement with the partial rescue of telomere protection reported in Bejarano et al, through TRF1 overexpression in cells treated with ERK inhibitor, which suggests that other telomere components are involved. In this context, testing known molecules inhibiting Ras farnesylation could be an interesting anti‐telomere strategy.
Taken together, these findings indicate that known drugs targeting common cancer signaling pathways can act through shelterin downregulation, suggesting valuable drug combinations in future chemotherapies and precise medicine against cancer (Fig 1).
Figure 1. Telomere uncapping and anti‐cancer effect induced by Ras signaling inhibition.
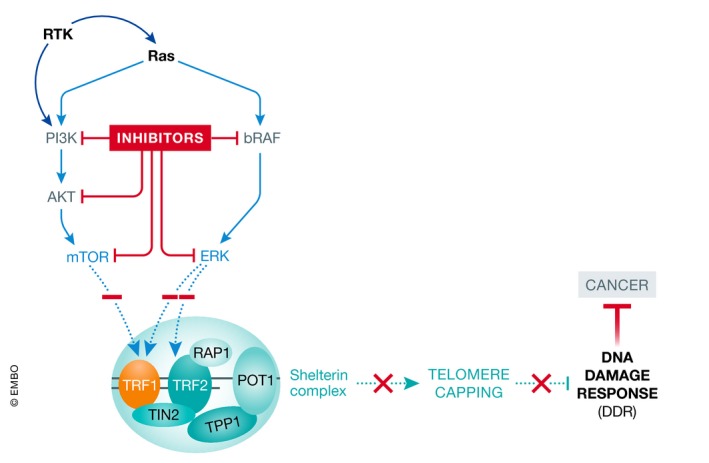
The inhibition of FDA‐approved inhibitors targeting key molecules downstream of Ras inhibits the phosphorylation of the shelterin TRF1 and TRF2. This inhibition of phosphorylation triggers telomere uncapping, DDR activation, and anti‐cancer effect in vitro and in vivo.
EMBO Mol Med (2019) 11: e10845
See also: L Bejarano et al (July 2019)
References
- Bejarano L, Schuhmacher AJ, Méndez M, Megías D, Blanco‐Aparicio C, Martínez S, Pastor J, Squatrito M, Blasco MA (2017) Inhibition of TRF1 telomere protein impairs tumor initiation and progression in glioblastoma mouse models and patient‐derived xenografts. Cancer Cell 32: 590–607.e4 [DOI] [PubMed] [Google Scholar]
- Bejarano L, Bosso G, Louzame J, Serrano R, Gómez‐Casero E, Martínez‐Torrecuadrada J, Martínez S, Blanco‐Aparicio C, Pastor J, Blasco MA (2019) Multiple cancer pathways regulate telomere protection. EMBO Mol Med 11: e10292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biroccio A, Cherfils Vicini J, Augereau A, Pinte S, Bauwens S, Ye J, Simonet T, Horard B, Jamet K, Cervera L et al (2013) TRF2 inhibits a cell‐extrinsic pathway through which natural killer cells eliminate cancer cells. Nat Cell Biol 15: 818–828 [DOI] [PubMed] [Google Scholar]
- Cherfils Vicini J, Iltis C, Cervera L, Pisano S, Croce O, Sadouni N, Győrffy B, Collet R, Renault VM, Rey Millet M et al (2019) Cancer cells induce immune escape via glycocalyx changes controlled by the telomeric protein TRF2. EMBO J 38: e100012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diala I, Wagner N, Magdinier F, Shkreli M, Sirakov M, Bauwens S, Schluth‐Bolard C, Simonet T, Renault VM, Ye J et al (2013) Telomere protection and TRF2 expression are enhanced by the canonical Wnt signalling pathway. EMBO Rep 14: 356–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Maï M, Wagner K‐D, Michiels J‐F, Ambrosetti D, Borderie A, Destree S, Renault V, Djerbi N, Giraud‐Panis M‐J, Gilson E et al (2014) The telomeric protein TRF2 regulates angiogenesis by binding and activating the PDGFRβ promoter. Cell Rep 9: 1047–1060 [DOI] [PubMed] [Google Scholar]
- García‐Beccaria M, Martínez P, Méndez‐Pertuz M, Martínez S, Blanco‐Aparicio C, Cañamero M, Mulero F, Ambrogio C, Flores JM, Megías D et al (2015) Therapeutic inhibition of TRF1 impairs the growth of p53‐deficient K‐RasG12V‐induced lung cancer by induction of telomeric DNA damage. EMBO Mol Med 7: 930–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi K, Kawai T, Kumaki F, Hiroi S, Mukai M, Ikeda E, Koering C‐E, Gilson E (2003) Expression of mRNAs for telomeric repeat binding factor (TRF)‐1 and TRF2 in atypical adenomatous hyperplasia and adenocarcinoma of the lung. Clin Cancer Res 9: 1105–1111 [PubMed] [Google Scholar]
- Picco V, Coste I, Giraud‐Panis M‐J, Renno T, Gilson E, Pages G (2016) ERK1/2/MAPK pathway‐dependent regulation of the telomeric factor TRF2. Oncotarget 7: 46615–46627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay AJ, Quesada V, Foronda M, Conde L, Martínez‐Trillos A, Villamor N, Rodríguez D, Kwarciak A, Garabaya C, Gallardo M et al (2013) POT1 mutations cause telomere dysfunction in chronic lymphocytic leukemia. Nat Genet 45: 526–530 [DOI] [PubMed] [Google Scholar]
- Zizza P, Dinami R, Porru M, Cingolani C, Salvati E, Rizzo A, D'Angelo C, Petti E, Amoreo CA, Mottolese M et al (2019) TRF2 positively regulates SULF2 expression increasing VEGF‐A release and activity in tumor microenvironment. Nucleic Acids Res 3: 48 [DOI] [PMC free article] [PubMed] [Google Scholar]


