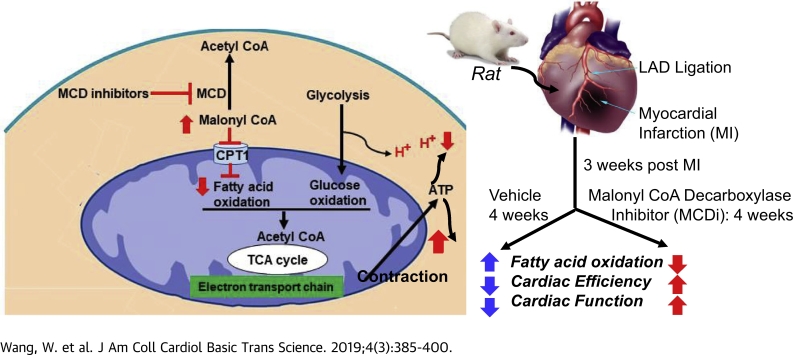
Visual Abstract
Key Words: fatty acid oxidation, heart failure, glucose oxidation, uncoupling of glycolysis
Abbreviations and Acronyms: ATGL, adipose triglyceride lipase; CPT1, carnitine palmitoyltransferase 1; EF, ejection fraction; FOXO3, forkhead box O3; MCD, malonyl coenzyme A decarboxylase; MI, myocardial infarction; SERCA2, sarco(endo)plasmic reticulum Ca2+-ATPase 2; SOD, superoxide dismutase; SPT, serine palmitoyltransferase; TAG, triacylglycerol; Trx, thioredoxin
Highlights
-
•
MCD inhibition decreases fatty acid oxidation via increasing malonyl coenzyme A levels to prevent fatty acid uptake into mitochondria in the failing hearts induced by coronary artery ligation.
-
•
Downregulating fatty acid oxidation by MCD inhibition occurrs in conjuction with a decrease in glycolysis and in proton production while an increase in triacylglycerol biosynthesis.
-
•
MCD inhibition enhances antioxidative capacity through increasing mitochondrial superoxide dismutase activity via reducing its acetylation.
Summary
Alterations in cardiac energy metabolism after a myocardial infarction contribute to the severity of heart failure (HF). Although fatty acid oxidation can be impaired in HF, it is unclear if stimulating fatty acid oxidation is a desirable approach to treat HF. Both immediate and chronic malonyl coenzyme A decarboxylase inhibition, which decreases fatty acid oxidation, improved cardiac function through enhancing cardiac efficiency in a post–myocardial infarction rat that underwent permanent left anterior descending coronary artery ligation. The beneficial effects of MCD inhibition were attributed to a decrease in proton production due to an improved coupling between glycolysis and glucose oxidation.
Ischemic heart disease and heart failure (HF) are the major causes of death worldwide. As such, there is an urgent need for novel therapeutic approaches that focus on different mechanisms to improve outcomes of patients suffering from ischemic heart disease and HF. Adverse remodeling of the myocardium after a myocardial infarction (MI) contributes to the severity of HF (1), which includes alterations in myocardial energy metabolism 1, 2. Whereas it is generally accepted that mitochondrial oxidative metabolism is compromised in the failing heart (3), there is less agreement as to whether the heart switches energy substrate preference in the failing heart (3). Impaired mitochondrial oxidative metabolism in the failing heart can decrease not only fatty acid oxidation, but also glucose oxidation 2, 4, 5. This is accompanied by an increase in glycolysis as the remodeled heart attempts to compensate for the decrease in mitochondrial energy production (2). This can result in an uncoupling between glycolysis and glucose oxidation, which accelerates proton production. As a result, energy has to be used for correcting acidosis-induced dysregulation of ion homeostasis (including Na+ and Ca2+ overload) rather than supporting mechanical function, which ultimately decreases cardiac mechanical efficiency 2, 6, 7.
Although fatty acid oxidation can be impaired in HF (due to a decrease in mitochondrial oxidative capacity) it is unclear if stimulating fatty acid oxidation is a desirable approach to treat HF. Increasing fatty acid oxidation by cardiac-specific overexpression of peroxisome proliferator-activated receptor α results in the development of cardiomyopathy (8). Administration of peroxisome proliferator-activated receptor α agonists to isolated perfused hearts also decreases cardiac efficiency (9). In contrast, cardiac-specific deletion of acetyl CoA carboxylase 2, which lowers malonyl CoA, accelerates fatty acid oxidation, and improves cardiac function in mice subjected to transverse aortic constriction (10). However, this approach also markedly improves the coupling of glycolysis to glucose oxidation, resulting in a decreased lactate production. Improved coupling of glycolysis to glucose oxidation has been shown to be beneficial in reducing the symptoms of myocardial ischemia 2, 6, 7, 11, 12. This can be achieved by inhibiting fatty acid oxidation, which increases glucose oxidation, thereby improving the coupling of glycolysis to glucose oxidation (13). This approach benefits cardiac function 6, 11, 14 and reduces infarct size (6) in the immediate post-ischemic period.
Malonyl CoA is a key regulator of fatty acid oxidation, which inhibits carnitine palmitoyltransferase 1 (CPT1) and prevents the mitochondrial uptake of fatty acids. Myocardial malonyl CoA levels can be increased by inhibiting malonyl CoA decarboxylase (MCD), the enzyme responsible for malonyl CoA degradation 11, 12. Deletion of MCD (MCD−/−) protects the hearts from ischemia/reperfusion injury and decreases infarct size (6), which is associated with an increase in malonyl CoA levels, a decrease in fatty acid oxidation, and an increase in glucose oxidation 6, 12. Similar findings have been recapitulated following immediate pharmacological inhibition of MCD in an experimental model of ex vivo ischemia/reperfusion injury in the heart 11, 15.
The objective of this study was to determine whether immediate or chronic inhibition of cardiac fatty acid oxidation by pharmacological inhibiting MCD in MI rats, induced by a permanent left anterior descending coronary artery ligation, could decrease the severity of HF post-MI.
Methods
Animal care
All protocols performed on male Sprague-Dawley rats (200 to 300 g, Charles River, Wilmington, Massachusetts) were reviewed and approved by the Animal Care and Use Committee at University of Alberta, or by Amgen’s Internal Animal Care and Use Committee. All animal experiments were performed in accordance with the guidelines of Canadian Council of Animal Care.
Immediate effect of MCD inhibition on hemodynamic analysis, cardiac malonyl CoA levels, and exercise capacity testing
A series of male Sprague-Dawley rats (n = 25) underwent a sham (n = 8) or an MI (n = 17) 6, 16 procedure. At 9 weeks post-MI, rats were subjected to transthoracic echocardiogram for invasive hemodynamic analysis with a 120-min short-term intravenous stepped-dose infusion of the MCD inhibitor (CBM-3001106, obtained from Metabolic Modulators Research Ltd., Edmonton, AB, Canada) to assess the cardiac function. Then the hearts were isolated for assessing the levels of malonyl CoA. To measure the exercise capacity, rats were randomized (sham, n = 6; MI, n = 7) to a graded exercise test that was performed at a 5% incline, beginning with 10 m/min for 1 min, 11 m/min for 1 min, 12 m/min for 1 min, 13 m/min for 2 min, 15 m/min for 5 min, 17 m/min for 5 min, and 20 m/min until exhaustion (17). A rat was deemed to be fatigued when it was no longer able to run on the treadmill, as judged by rat spending >50% of time or >30 consecutive seconds on the platform and resistant to prodding by the air puff.
Immediate effect of malonyl CoA on inhibiting fatty acid oxidation in liver and heart mitochondria
The dose-dependent effect of malonyl CoA on inhibiting fatty acid oxidation in liver (n = 12) and heart (n = 6) was discovered in vitro by using freshly isolated mitochondria from the tissue homogenate centrifuged at 27,000 × g for 10 min. The pellet was re-suspended in a A buffer containing 70 mmol/l sucrose, 210 mmol/l mannitol, 5 mmol/l 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid, N-(2-Hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid) (HEPES), 1 mmol/l ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), and 0.5% (w/v) fatty acid-free bovine serum albumin (BSA) (pH 7.2) and centrifuged at 10,000 × g. The resulting pellets were re-suspended in the ice-cold A buffer without BSA, from which, 1.5 μg of protein were used for recording fatty acid oxidation as a rate of oxygen consumption by using a Seahorse XF96 Extracellular Flux Analyzer (Seahorse Bioscience, North Billerica, Massachusetts). The substrates consist of either 10 mmol/l pyruvate/2 mmol/l malate or 160 μmol/l palmitoyl CoA/0.5 mmol/l carnitine/0.2 mmol/l malate. Malonyl CoA was added to a final concentration of either 100 μmol/l, 10 μmol/l, 1 μmol/l, 100 nmol/l, or 0 nmol/l. Following this, adenosine diphosphate was added to a final concentration of 6 mmol/l, followed by oligomycin (2 μmol/l final) and carbonyl cyanide 4-(trifluoromethoxy)-phenylhydrazone (4 μmol/l final).
Malonyl CoA content assessment
This was performed via high-performance liquid chromatography 6, 18. To optimize the dosage of MCD inhibitor on altering malonyl CoA levels, the male rats (n = 32) were administrated with different oral doses of MCD inhibitor (0, 1, 3, 10, 20, 50, 100 mg/kg BW (body weight), n = 4 to 5/group). The hearts and gastrocnemius muscle were isolated at 12 h post-treatment for assessing the levels of malonyl CoA.
Chronic interventions of MI model and infarct size assessment
Sham (n = 16) and post-MI rats (n = 31) were randomized into 5 groups: 1) sham + vehicle (ethanol + DMSO + Cremophor + H2O); 2) sham + high-dose MCD inhibitor (100 mg/kg/day); 3) MI + vehicle; 4) MI + low-dose MCD inhibitor (50 mg/kg/day); and 5) MI + high-dose MCD inhibitor (100 mg/kg/day), for daily oral gavage for a 4-week period. The infarct size was assessed by cutting the frozen hearts (n = 7 to 8/group) into ∼10 μm slices followed by incubation in 1% triphenyltetrazolium chloride for 10 min at 37°C as described previously (6).
Assessment of in vivo cardiac function
At 3 weeks post-MI and 4 weeks post-treatment (7 weeks post-MI), rats were subjected to an ultrasound echocardiography using a VisualSonics Vevo 2100 machine (VisualSonics Inc, Canada) to assess in vivo left ventricular ejection fraction (%EF). Parasternal long axis and short axis views were performed in both systole and diastole from the base to apex. The Simpson method was used for the data analysis (19).
Assessment of ex vivo cardiac function and energy metabolism by heart perfusion
At the end of the 7-week study period, heart perfusions were performed with oxygenated Krebs-Henseleit solution, consisting of either 0.8 mmol/l palmitate bound to 3% fatty acid-free BSA, 5 mmol/l [5-3H/U-14C]glucose for glycolysis and glucose oxidation measurements, or with 0.8 mmol/l [9,10-3H] palmitate bound to 3% fatty acid-free bovine serum albumin, and 5 mmol/l glucose for fatty acid oxidation measurements (16). Hearts were perfused aerobically for 30 min without insulin and an additional 30 min with insulin (100 μU/ml), then were snap frozen for later biochemical analysis.
Calculations of proton production rates, adenosine triphosphate production rate, cardiac work
Proton production rates were calculated following the equation: 2 × (glycolysis rates − glucose oxidation rates) (16). Adenosine triphosphate (ATP) production rates were calculated based on ATP production from each substrate (104 for palmitate oxidation, 29 for glucose oxidation, and 2 for glycolysis) (18). Cardiac work (unit as Joules/min/g dry weight) was calculated following the equation: cardiac output × (peak systolic pressure − preload pressure) × 0.1332 × 10−3/3,600/heart dry mass. The preload pressure is determined by the flow to the atria, which is controlled by the height of the oxygenator above the perfused heart with a value of 11.5 mm Hg. The cardiac efficiency was calculated as: cardiac work/total ATP production rates (18).
Measurement of triacylglycerol and the incorporation of 3H-palmitate into triacylglycerol
Triacylglycerol (TAG) content was assessed in the set of hearts perfused with [9,10-3H]palmitate in the perfusate (n = 6 to 7/group) as described previously (16) by colorimetric assay kit (Wako Pure Chemical Industries, Richmond, Virginia), while another potion of the lipid extraction was counted with scintillation fluid to measure the incorporation of [9,10-3H]palmitate into TAG based on the specific activity of [9,10-3H]palmitate from the perfusate.
Measurement of glycogen and the incorporation of glycogen from 3H-glucose
Glycogen was assessed in the set of hearts perfused with [5-3H]glucose (n = 6 to 9/group) via measurement of glucose content by using glucose assay kit (Sigma), as described previously (16).
Detection of lactate dehydrogenase isoenzymes
Heart proteins (20 μg/well) were electrophoresed on 6% native gel at 4°C as described previously (20), and followed by western blot to detect isoforms of lactate dehydrogenase (LDH), of which the densitometry were analyzed using Image J-win64 software.
Measurement of enzyme activities
Citrate synthase activity (n = 7 to 8/group) was measured based on continuous kinetic changes of absorbance at 412 nm over 2 to 5 min by recording the reduction of dithiobis-nitrobenzoic acid. Complex I activity (n = 7 to 8/group) was measured by recording the reduction of 2,6-dichloroindophenol at 600 nm (21). Cytosolic glycerol-3-phosphate acyltransferase activity (n = 6/group) was measure by using cytosolic fractions of heart samples in the assay buffer containing 1 mmol/l glycerol-3-phosphate, 0.1 mmol/l palmitoyl CoA, and 0.1 mmol/l 5,5′-dithio-bis-2-nitrobenzoic acid at 412 nm (22). Superoxide dismutase 2 (SOD2) activity (n = 7 to 8/group) was measured in the presence of 1 mmol/l KCN potassium cyanide (to inhibit Cu/ZnSOD activity) at 450 nm by using water soluble tetrazolium salt-1 (WST-1) that produces a water-soluble formazan dye upon reduction with superoxide anion (23).
Fractionation of nuclear or cytosolic proteins
Frozen heart tissues were homogenized in a buffer containing 10 mmol/l HEPES (pH 7.9), 1.5 mmol/l MgCl2, 10 mmol/l KCl, and 0.05% NP40 with cocktail of protease inhibitor and 2 mmol/l DTT. The resulting pellets, after the centrifugation at 1,000 × g for 15 min at 4oC, were resuspended for nuclear protein assays. The resulted supernatant was subjected to centrifugation at 10,000 × g for 60 min at 4oC to obtain the supernatant as cytosolic protein fraction.
Western blotting and immunoprecipitation
The following antibodies were used: adipose triglyceride lipase (ATGL), lamin A and thioredoxin-1 (Cell Signaling Technology); sarco(endo)plasmic reticulum Ca2+-ATPase 2 (SERCA2, Pierce); CD36 and forkhead box O3 (FOXO3), SIRT3, and LDH (Abcam). Serine palmitoyltransferase (SPT1) (Santa Cruz Biotechnology). To detect acetylation of mitochondrial SOD2, 500 μg of the total heart lysates were used for incubation with 10 μl acetyl-lysine antibodies (Millipore) and protein A/G-agarose beads (Santa Cruz Biotechnology) overnight at 4°C followed by incubation with the antibody against SOD2. To determine the interaction of MCD with SOD2 (Millipore), the heart lysates were immunoprecipitated with MCD antibody or with mitochondrial SOD2 antibody, then western blots were performed to detect SOD2 or MCD from the respective membranes. Positive control was the same sample(s) used for immunocapture without incubation of antibody. The negative control was the antibody (immunoglobulin G) used for immune-capture without incubation of the respective samples. Bound antibody was visualized by incubation with enhanced chemiluminescent substrate. ImageJ-win64 (by Johannes Schindelin, Albert Cardona, Mark Longair, Benjamin Schmid, and others) was used for evaluation of each band (n = 4 to 5/group, except n = 3/group for immunoprecipitation).
Statistical analysis
Values represent the mean ± SD. Samples sizes are indicated in the table and figure legends. The significance of differences for multiple comparisons in Figures 1D, 2B, 2F to 2K, and 3, 4, and 5 was analyzed by 1-way analysis of variance (ANOVA). If ANOVA revealed differences, selected datasets were compared by Bonferroni's multiple comparison test. The significance of differences for the effect of MCD inhibition on time-dependent changes of cardiac function in Figures 1F to 1H, 1J to 1L, and 6 was analyzed by 2-way ANOVA. The significance of differences between 2 groups was estimated by unpaired, 2-tailed Student's t test where appropriate. Differences were considered significant at p < 0.05.
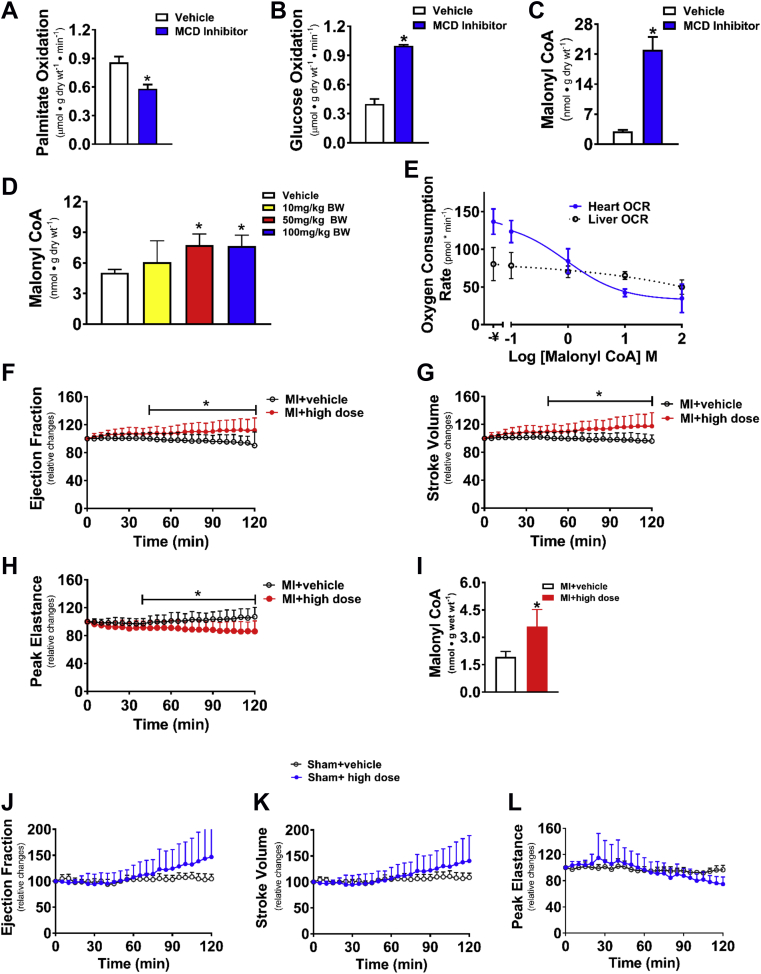
Figure 1.
Acute Effects of CoA Decarboxylase Inhibition on Cardiac Energy Metabolism and Malonyl CoA Levels in Normal Rat Hearts and on Cardiac Function in Post-Myocardial Infarction Failing Rat Hearts
Effects of 1 μmol/l CBM-300116 on rates of palmitate oxidation (A), glucose oxidation (B), and levels of malonyl CoA (C) in perfused hearts (n = 5). Dose-dependent changes in cardiac malonyl CoA content (n = 4 to 5/group) 12-h post-malonyl CoA decarboxylase (MCD) inhibition (D). Differential effect of malonyl CoA on inhibiting fatty acid oxidation in heart (n = 6/group) and liver mitochondria (n = 12/group) (E). Invasive hemodynamic analysis in failing and sham rat hearts with a 120-min acute intravenous stepped-dose infusion of the MCD inhibitor, including relative changes from the baseline of systolic function in percent ejection fraction (EF) (F and J), of stroke volume (G and K), and of peak elastance (H and L) as well as the malonyl CoA content in myocardial infarction (MI) hearts (I) (n = 7 to 8/group). Values represent the mean ± SD. *p < 0.05, significantly different from the vehicle group. BW = body weight; CoA = Malonyl Coenzyme A; OCR = oxygen consumption rate.
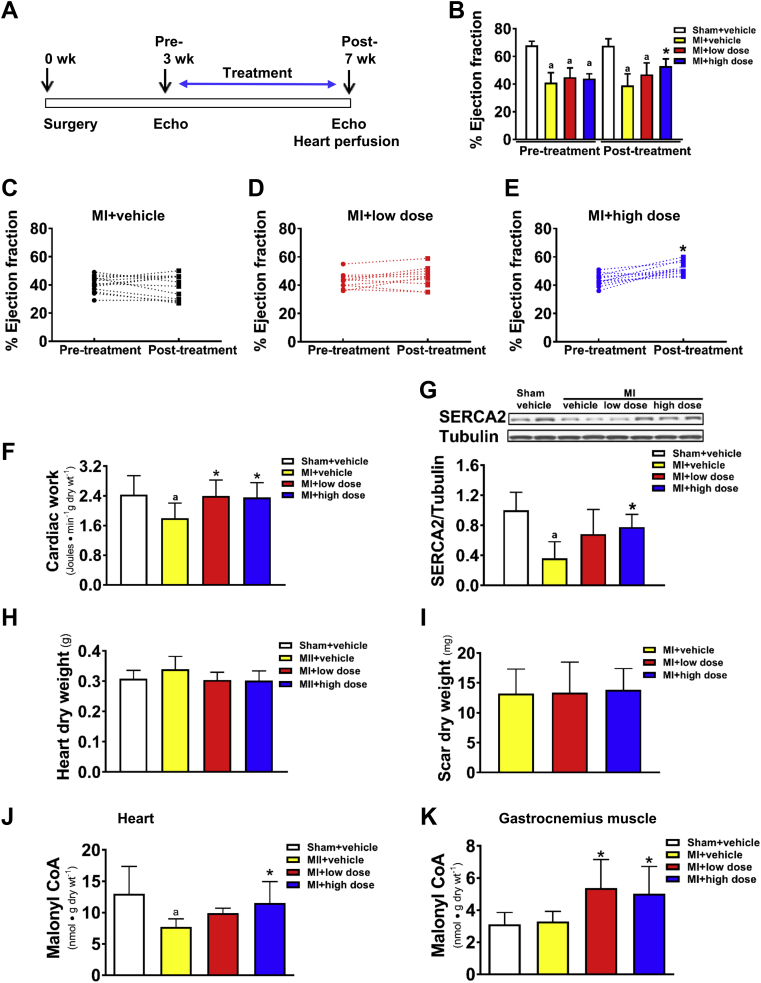
Figure 2.
Effect of Chronic MCD Inhibition on In Vivo and Ex Vivo Cardiac Function in Failing Rat Hearts
Schematic drawing of the experimental timeline (A). Rats were subjected to a permanent left anterior descending artery ligation to product an MI. Three-weeks post-MI, rats were treated with 50 mg/kg/day (low dose) or 100 mg/kg/day (high dose) of CBM-3001106 for an additional 4 weeks. Alterations of %EF pre- and post-MCD inhibition (B). Alterations of %EF in the individual MI rats pre- and post-MCD inhibition (C to E). Cardiac work in perfused working hearts (F). Representative blots of SERCA2 and densitometric analyses (G). Heart dry weight (H). Scar dry weight (I). Malonyl CoA content in perfused hearts (J) and in gastrocnemius muscle (K). Values represent the mean ± SD (n = 5 to 11/group). ap < 0.05 or *p < 0.05, significantly different from sham + vehicle or MI + vehicle group, respectively. Abbreviations as in Figure 1.
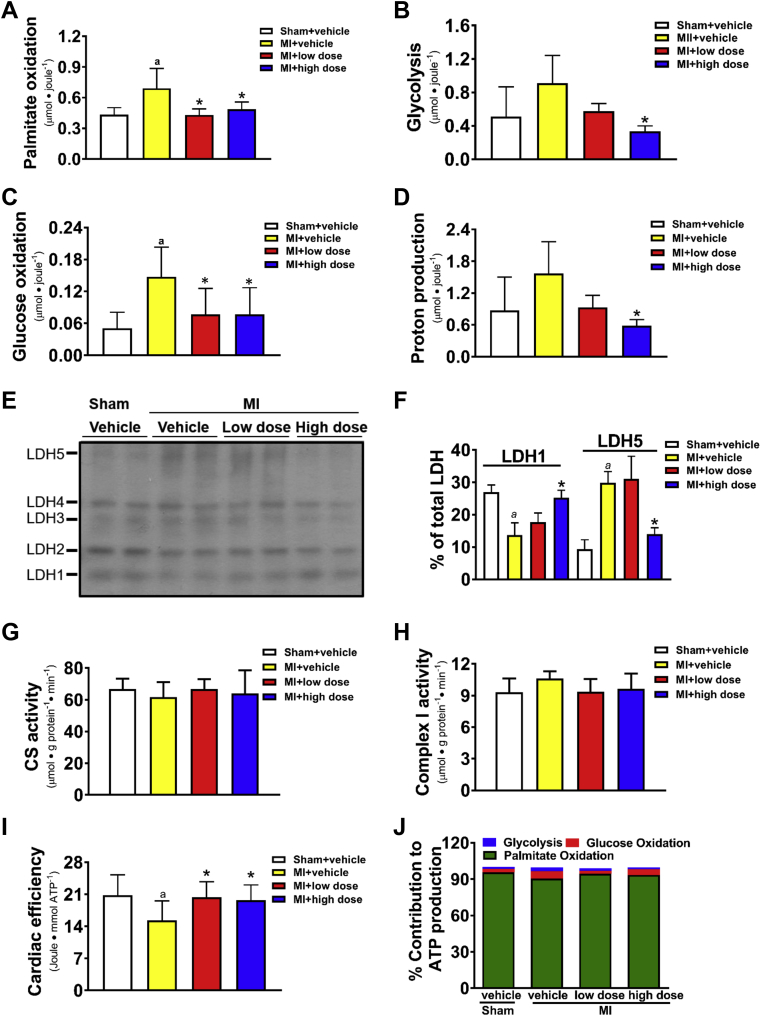
Figure 3.
Effect of Chronic MCD Inhibition on Cardiac Energy Metabolism in Failing Rat Hearts
Post-MI rats were treated with 50 mg/kg/day (low dose) or 100 mg/kg/day (high dose) of CBM-3001106 for 4 weeks, followed by isolated working heart perfusions. Rates of palmitate oxidation per cardiac work (A). Rates of glycolysis per cardiac work (B). Rates of glucose oxidation per cardiac work (C). Rates of proton production (D). Expression of lactic acid dehydrogenase (LDH) isoenzymes (E) and % of LDH1 and LDH5 in total LDH (F). Citrate synthase activity (G). Mitochondrial complex I activity (H). Cardiac efficiency (I). Percent contribution of adenosine triphosphate (ATP) production from individual substrate (J). Values represent the mean ± SD (n = 3 to 10). ap < 0.05 or *p < 0.05, significantly different from sham + vehicle or MI + vehicle group, respectively. CS = citrate synthase; other abbreviations as in Figure 1.
Figure 4.
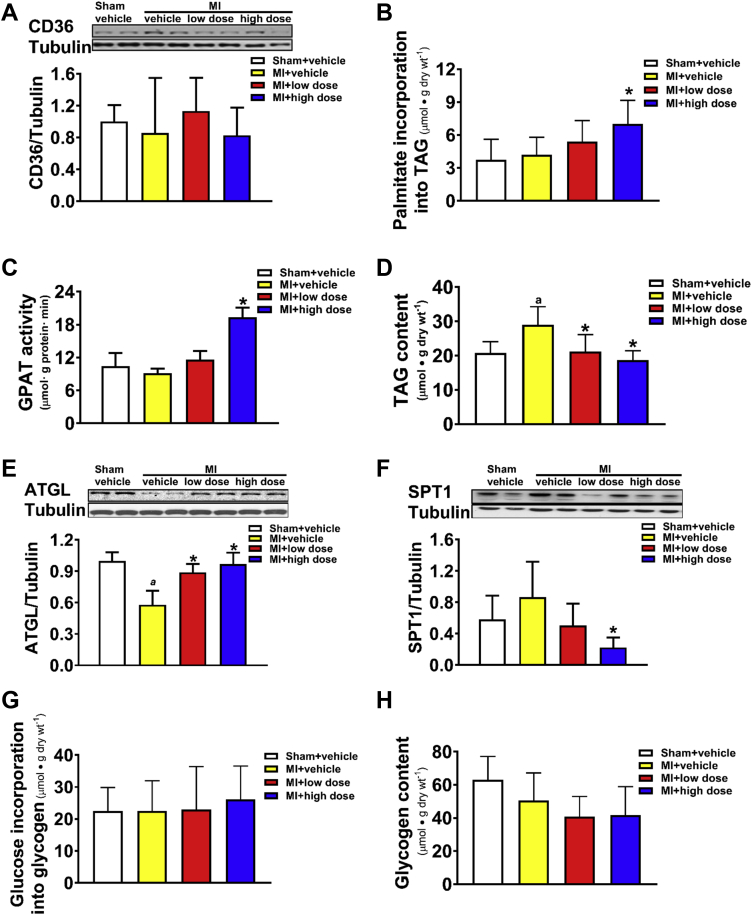
Effect of Chronic MCD Inhibition on Status of Myocardial Triacylglycerol, on Serine Palmitoyltransferase 1 Protein Expression, and Alterations of Glycogen
Post-MI rats were treated with 50 mg/kg/day (low dose) or 100 mg/kg/day (high dose) of CBM-3001106 for 4 weeks. Protein expression of CD36 (A). Incorporation of [3H]-palmitate into triacylglycerol (TAG) during the 1-h perfusion (B). Cytosolic GPAT activity (C). Myocardial TAG content (D). Protein expression of ATGL (E), and of SPT1 (F). Glucose incorporation into glycogen (G). Glycogen content (H). Values represent the mean ± SD (n = 4 to 7). ap < 0.05 or *p < 0.05, significantly different from sham + vehicle or MI + vehicle group, respectively. ATGL = adipose triglyceride lipase; GPAT = Glycerol-3-phosphate acyltransferase; SPT1 = serine palmitoyltransferase 1; other abbreviations as in Figure 1.
Figure 5.
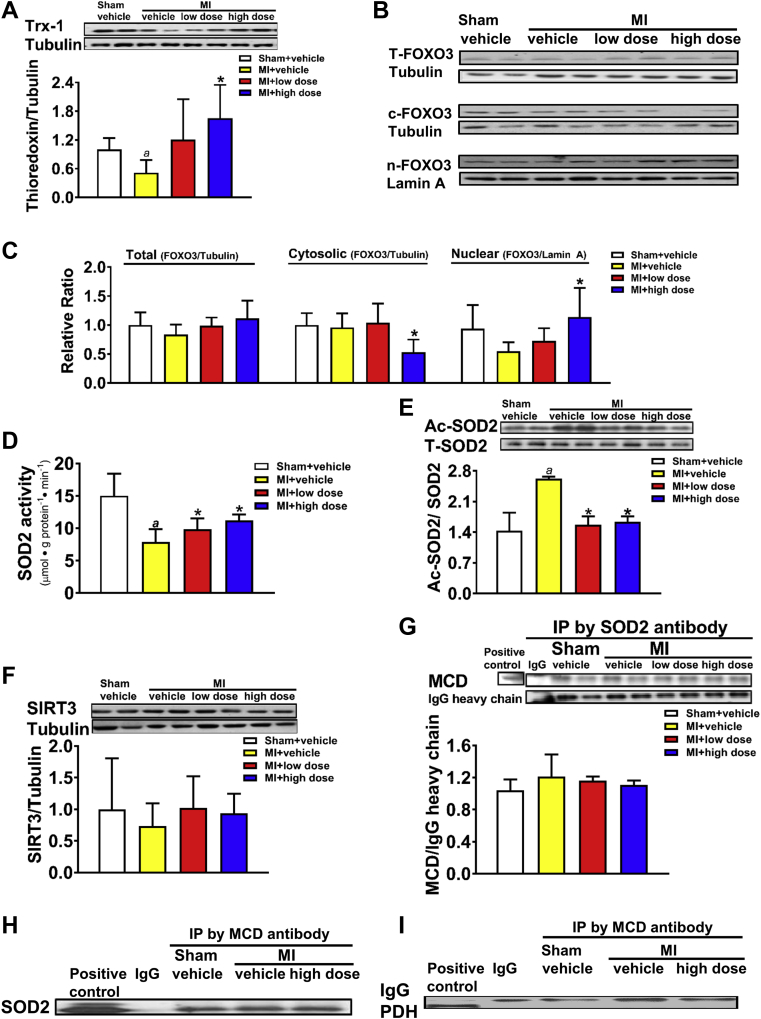
Effect of Chronic MCD Inhibition on Anti-Oxidants Expression and Interaction of MCD With Superoxide Dismutase 2
Post-MI rats were treated with 50 mg/kg/day (low dose) or 100 mg/kg/day (high dose) of CBM-3001106 for 4 weeks. Protein expression of thioredoxin-1 (Trx-1) (A). Protein expression of total forkhead box O3 (T-FOXO3), cytosolic FOXO3 (c-FOXO3) and nuclear FOXO3 (n-FOXO3) (B), as well as the densitometric analyses (C). Superoxide dismutase 2 (SOD2) activity (D). Acetylated SOD2 (Ac-SOD2) (E). Protein expression of SIRT3 (F). MCD-SOD2 interaction (G,H). Negative interaction of MCD-PDH (I). Values represent the mean ± SD (n = 3 to 7). ap < 0.05 or *p < 0.05, significantly different from sham + vehicle or MI + vehicle group, respectively. IgG = immunoglobulin G; Ip = immunoprecipitation; PDH = pyruvate dehydrogenase; SIRT3 = Sirtuin-3; other abbreviations as in Figure 1.
Figure 6.
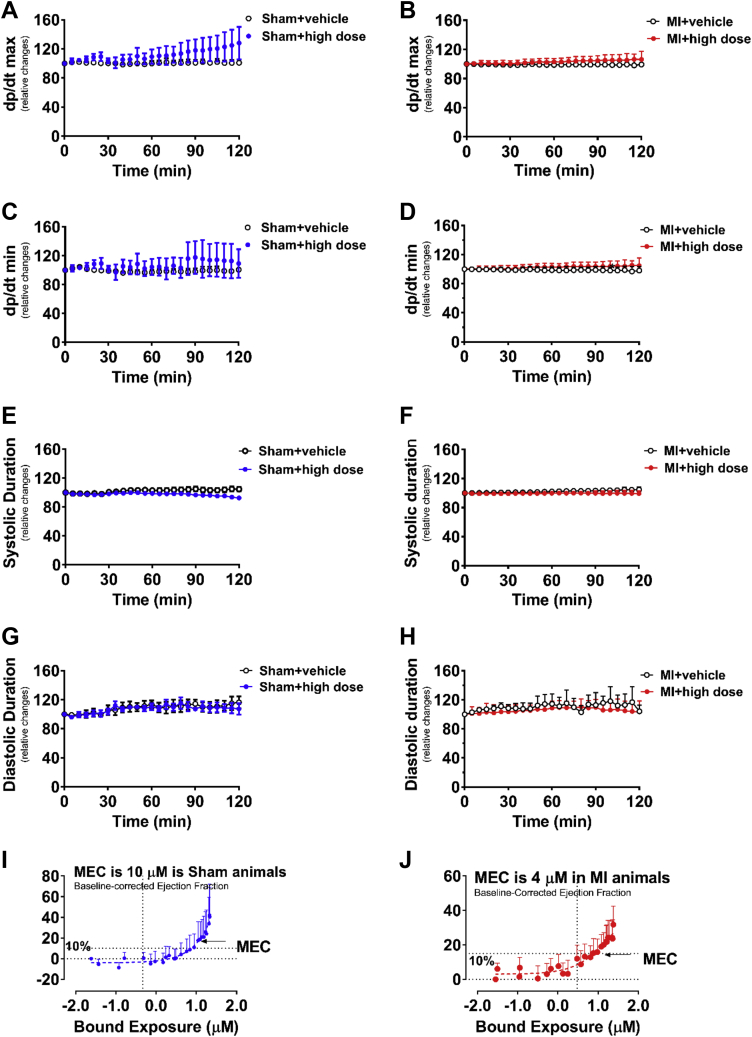
Effect of Acute MCD Inhibition on Invasive Hemodynamic Analysis in Failing and Sham Rat Hearts and the Minimally Effective Concentration to Improve Cardiac Function
Invasive hemodynamic analysis in failing and sham rat hearts with a 120-min acute intravenous stepped-dose infusion of the MCD inhibitor, including relative changes of dp/dt max (A,B) and dp/dt min (C,D), relative changes of systolic duration (E,F), diastolic duration (G,H), as well as the minimal effective concentration (MEC) to improve cardiac function (I,J). Values represent the mean ± SD (n = 8 to 17). dp/dt max/min = maximal/minimal rate of rise of left ventricular pressure; other abbreviations as in Figure 1.
Results
Immediate MCD inhibition increases malonyl CoA levels and inhibits fatty acid oxidation and stimulates glucose oxidation in normal rat hearts and improves cardiac function in failing hearts
The direct effects of the MCD inhibitor CBM-3001106 on fatty acid and glucose oxidation were initially examined in isolated working rat hearts. A dose of 1 μmol/l CBM-3001106 added to the hearts resulted in a decrease in fatty acid oxidation rates (Figure 1A) and a significant increase in glucose oxidation rates (Figure 1B) without affecting ex vivo cardiac function (data not shown). This was accompanied by a significant increase in cardiac malonyl CoA levels (Figure 1C). Twelve hours after oral administration a single dose of either 50 or 100 mg/kg of CBM-3001106 to rats, a significant increase in cardiac malonyl CoA levels was also evident (Figure 1D). Malonyl CoA showed a dose-dependent inhibition of fatty acid oxidation as reflected by a decrease in oxygen consumption rates (Figure 1E). However, the concentration that inhibits 50% was 900 nmol/l in heart mitochondria and greater than 100 μmol/l in liver mitochondria (Figure 1E), supporting the concept that malonyl CoA has a higher binding affinity to CPT1 in heart than in liver (24).
To investigate if Immediate inhibition of MCD could improve cardiac function in failing hearts, invasive hemodynamics was used to assess cardiac function following a 120 min, immediate intravenous stepped-dose infusion of CBM-3001106 to 9-week post-MI rats. MI-rats exhibited a significant decrease in %EF, stroke volume, and exercise capacity, whereas an increase in left atria diameter was seen (Table 1). Immediate exposures of CBM-3001106 to the post-MI hearts dose-dependently increased %EF (Figure 1F) and stroke volume (Figure 1G), whereas there was decreased peak elastance (Figure 1H), which was accompanied by an increase in malonyl CoA levels (Figure 1I). In contrast, Immediate exposure to CBM-3001106 had no effects on these factors in sham hearts (Figures 1J to 1L), neither had effect on maximal/minimal rate of rise of left ventricular pressure (dp/dt max/min), and systolic/diastolic duration in either sham (Figures 6A, 6C, 6E, and 6G) or post-MI hearts (Figures 6B, 6D, 6F, and 6H). The minimally effective concentration to improve cardiac function was 10 μmol/l in the sham group (Figure 6I), but required a lower concentration (4 μmol/l) in the MI group (Figure 6J).
Table 1.
Alterations in Cardiac Function and Exercise Capacity in Sprague Dawley Rats That Underwent Either a Sham or a Coronary Artery Ligation Procedure (Myocardial Infarction) for 9 Weeks
| Sham | MI | |
|---|---|---|
| Cardiac function | 8 | 17 |
| Heart rate (beats/min) | 357.3 ± 13.8 | 364.7 ± 22.5 |
| Ejection fraction % | 65.9 ± 5.7 | 41.6 ± 8.9∗ |
| Stroke volume (μl) | 300.5 ± 47.4 | 216.2 ± 44.3∗ |
| Left atria diameter (mm) | 4.4 ± 0.5 | 5.7 ± 0.7∗ |
| Exercise capacity | 6 | 7 |
| Body weight (g) | 479.8 ± 63.5 | 507.1 ± 56.9 |
| Running speed (m · min-1) | 18.3 ± 2.0 | 12.2 ± 5.2∗ |
| Running distance (m) | 271.2 ± 145.2 | 169.2 ± 67.5∗ |
Values are n or mean ± SD.
p < 0.05, significantly different from sham + vehicle.
Chronic MCD inhibition improves cardiac function in failing hearts
To determine if chronic MCD inhibition was beneficial in failing hearts, rats were treated orally for a 4-week period with a high or low dose of CBM-3001106, starting at 3 weeks post-MI surgery (Figure 2A). Before treatment, the decrease in %EF was similar among all post-MI groups compared to sham (Figure 2B). However, over the subsequent 4-week treatment period a significant increase in %EF was evident in the MI + high-dose group (Figures 2B to 2E), which was further supported by comparing individual %EF value in the post-MI rats between the pre-treatment and post-treatment periods (Figure 2E). In contrast, %EF continued to decline in the majority of the vehicle-treated post-MI rats (Figure 2C).
To further illustrate the chronic effect of MCD inhibition on cardiac function, ex vivo cardiac function was assessed in isolated working hearts at the end of the 4-week treatment period (Figure 3F). Cardiac work was similar between the sham groups (Table 2), but was reduced in the MI + vehicle group (Figure 2F). The reduction in cardiac work was significantly restored by both the low- and high-dose MCD inhibition (Figure 2F), regardless of the presence or absence of insulin (Table 3). The restoration of function was accompanied by an increase in the protein expression of SERCA2 (Figure 2G) without affecting heart weight (Figure 2H), and the size of the left ventricular infarct scar tissues (Figure 2I), confirming that the beneficial effect of MCD inhibition on cardiac function was not due to the differences in the severity of the infarct among the groups.
Table 2.
Effect of Chronic Malonyl Coenzyme A Decarboxylase Inhibition on Ex Vivo Cardiac Function
| Sham + ve (n = 10) | Sham + High (n = 6) | MI + ve (n = 11) | MI + Low (n = 10) | MI + High (n = 10) | |
|---|---|---|---|---|---|
| Heart rate (beats/min) | |||||
| − Insulin | 264 ± 25 | 269 ± 15 | 255 ± 51 | 265 ± 15 | 264 ± 26 |
| + Insulin | 288 ± 19 | 281 ± 13 | 266 ± 50 | 284 ± 26 | 289 ± 31 |
| Peak systolic pressure (mm Hg) | |||||
| − Insulin | 108 ± 7 | 112 ± 6 | 102 ± 9 | 106 ± 5 | 107 ± 4 |
| + Insulin | 104 ± 7 | 108 ± 5 | 101 ± 8 | 103 ± 5 | 103 ± 5 |
| Developed pressure (mm Hg) | |||||
| − Insulin | 42 ± 8 | 50 ± 10 | 37 ± 9 | 40 ± 8 | 38 ± 6 |
| + Insulin | 37 ± 3 | 44 ± 8 | 35 ± 10 | 35 ± 8 | 33 ± 6 |
| Cardiac output (ml · min−1) | |||||
| − Insulin | 47 ± 11 | 50 ± 6 | 41 ± 9 | 44 ± 5 | 41 ± 9 |
| + Insulin | 49 ± 10 | 52 ± 7 | 39 ± 10 | 46 ± 6 | 43 ± 10 |
| Aortic outflow (ml · min−1) | |||||
| − Insulin | 22 ± 8 | 24 ± 6 | 15 ± 7 | 21 ± 6 | 19 ± 7 |
| + Insulin | 24 ± 6 | 25 ± 7 | 15 ± 6 | 20 ± 6 | 18 ± 7 |
| Coronary flow (ml · min−1) | |||||
| − Insulin | 26 ± 8 | 26 ± 6 | 22 ± 6 | 24 ± 6 | 23 ± 8 |
| + Insulin | 25 ± 7 | 27 ± 7 | 23 ± 6 | 26 ± 6 | 25 ± 9 |
| Cardiac work (J· min−1· g dry wt−1) | |||||
| − Insulin | 2.4 ± 0.5 | 3.1 ± 0.9 | 1.7 ± 0.4∗ | 2.4 ± 0.4† | 2.4 ± 0.4† |
| + Insulin | 2.5 ± 0.1 | 3.1 ± 0.8 | 1.8 ± 0.4∗ | 2.5 ± 0.5† | 2.3 ± 0.4† |
Values are mean ± SD. Sham or post-MI rats were treated with either vehicle (ve), 50 mg/kg/day (low = low dose) or 100 mg/kg/day (high = high dose) of CBM-3001106 for 4 weeks, following which isolated working heart perfusions were performed for 30 min in the absence of insulin, and then 30 min in the presence of 100 μU/ml insulin.
MI = myocardial infarction.
p < 0.05 or †p < 0.05, significantly different from sham + vehicle or MI + vehicle group, respectively.
Table 3.
Effect of Chronic Malonyl Coenzyme A Decarboxylase Inhibition on Cardiac Energy Metabolism
| Sham + ve | Sham + High | MI + ve | MI + Low | MI + High | |
|---|---|---|---|---|---|
| Glucose oxidation: μmol · g dry wt−1 · min−1 | |||||
| − Insulin | 0.1 ± 0.05 | 0.1 ± 0.02 | 0.2 ± 0.06 | 0.2 ±0.1 | 0.2 ± 0.1 |
| + Insulin n | 0.3 ± 0.2 10 |
0.1 ± 0.04 6 |
0.5 ± 0.2 11 |
0.4 ± 0.2 10 |
0.4 ± 0.2 10 |
| Glycolysis: μmol · g dry wt−1 · min−1 | |||||
| − Insulin | 1.0 ± 0.5 | 1.0 ± 0.8 | 1.9 ± 0.7 | 1.3 ± 0.1 | 0.9 ± 0.3 |
| + Insulin n | 1.3 ± 0.7 3 |
0.9 ± 0.5 3 |
2.0 ± 0.5 5 |
2.0 ± 0.8 4 |
1.6 ± 0.4 3 |
| Palmitate oxidation: μmol · g dry wt−1 · min−1 | |||||
| − Insulin | 1.1 ± 0.2 | 1.0 ± 0.1 | 1.0 ± 0.2 | 1.1 ± 0.2 | 1.1 ± 0.2 |
| + Insulin n | 1.0 ± 0.2 7 |
0.9 ± 0.1 3 |
1.0 ± 0.1 6 |
1.1 ± 0.2 6 |
1.1 ± 0.4 7 |
| Proton production: μmol · g dry wt−1 · min−1 | |||||
| − Insulin | 1.7 ± 0.8 | 1.9 ± 1.5 | 3.1 ± 1.3 | 2.0 ± 0.2 | 1.5 ± 0.4 |
| + Insulin n | 2.0 ± 1.1 3 |
1.4 ± 1.0 3 |
3.2 ± 1.1 5 |
3.1 ± 1.2 4 |
2.5 ± 0.6 3 |
| ATP production: μmol · g dry wt−1 · min−1 | |||||
| − Insulin | 118 ± 16 | 104 ± 6 | 119 ± 18 | 118 ± 12 | 120 ± 14 |
| + Insulin n | 113 ± 20 10 |
102 ± 9 6 |
127 ± 11 11 |
129 ± 15 10 |
133 ± 35 10 |
Values are n or mean ± SD. Sham or post-MI rats were treated with either vehicle (ve), 50 mg/kg/day (low = low dose) or 100 mg/kg/day (high = high dose) of CBM-3001106 for 4 weeks, following which isolated working heart perfusions were performed for 30 min in the absence of insulin, and then 30 min in the presence of 100 μU/ml insulin.
ATp = adenosine triphosphate; MI = myocardial infarction.
The efficacy of in vivo MCD inhibitor in increasing tissue malonyl CoA levels was evident not only in ex vivo perfused hearts (Figure 2J), but also in gastrocnemius muscle (Figure 2K) from post-MI rats after the high- and low-dose MCD inhibitor treatment.
Chronic MCD inhibition enhances cardiac efficiency by altering energy metabolism in failing hearts
To understand the mechanisms underlying chronic MCD inhibition-mediated improvement of cardiac function, the energy metabolism rates in the perfused hearts were assessed. Since cardiac insulin resistance has been suggested to be a contributor to HF 5, 18, the heart perfusions were performed with and without insulin to check if cardiac insulin sensitivity was altered in post-MI hearts. Absolute fatty acid oxidation rates were similar among the groups regardless of the absence or the presence of insulin (Table 3). Because cardiac work is a major determinant of cardiac mitochondrial oxidative metabolism 2, 25, we normalized the energy metabolic rates by cardiac work. The increases in fatty acid oxidation rates (Figure 3A), in glycolysis rates (Figure 3B) and in glucose oxidation rates (Figure 3C) were prevented in post-MI hearts by MCD inhibition. Absolute rates of glycolysis and glucose oxidation tended to increase in the MI + vehicle group when compared to sham (Table 3), which resulted in an increased proton production in MI + vehicle group (Figure 3D), whereas the increase was prevented in MI + high-dose group (Figure 3D).
An increase in the expression of M subunit of LDH has been shown to be an indication of an increased glycolytic capacity (26). Five isoenzymes of LDH were observed in hearts (Figure 3E). An increase in the expression of LDH5, the M4 isoenzyme, was evident in the post-MI hearts relative to sham (Figure 3F), whereas MCD inhibition reduced LDH5 but increased LDH1 (Figure 3F), the H4 isoenzyme, in post-MI hearts.
ATP production rates (Table 3) were unchanged among all the groups, as were citrate synthase activity (Figure 3G) and mitochondrial complex I activity (Figure 3H). However, a reduced cardiac efficiency was observed in the MI + vehicle group relative to the sham group (Figure 3I), whereas the reduction was restored by MCD inhibition (Figure 3I). Despite the concept that the failing heart switches from fatty acid to glucose metabolism (2), overall, fatty acid oxidation remained the predominant source of ATP production in the post-MI hearts (Figure 3J).
Chronic MCD inhibition enhances incorporation of [3H]-palmitate into TAG while reducing myocardial TAG content and biosynthesis of ceramide in post-MI hearts
Inhibiting MCD could divert palmitate into TAG synthesis as an effect of decreasing fatty acid oxidation. Although protein expression of CD36, a major enzyme involved in plasma fatty acid uptake, was unaltered (Figure 4A), the incorporation of 3H-palmitate into TAG (Figure 4B) was increased in the MCD inhibition groups relative to the MI + vehicle group. This increase was mirrored by the activity of cytosolic glycerol phosphate acyltransferase (Figure 4C), the rate-limiting enzyme for de novo TAG synthesis. However, despite this, myocardial TAG content was decreased by MCD inhibition (Figure 4D), accompanied by an upregulation of ATGL, a major enzyme involved in TAG lipolysis (Figure 4E), while downregulating SPT1 (Figure 4F), a key enzyme for ceramide biosynthesis that has been suggested to be a contributor of cardiac dysfunction (27). Unlike myocardial lipids, neither the incorporation of radiolabeled glucose into glycogen (Figure 4G), nor total glycogen content was altered among any of the groups (Figure 4H).
Chronic MCD inhibition increases antioxidative capacity in post-MI hearts
As MCD inhibition-mediated improvement of cardiac dysfunction was accompanied by the alterations in lipid biosynthesis, we determined if any alteration in myocardial antioxidative capacity, which is associated with pathophysiology of HF in humans (28), also occurred concurrently. The protein expression of cardiac thioredoxin-1 (Trx-1), 1 of the molecules with a high antioxidative action in cytosol, was significantly reduced in the MI + vehicle group relative to sham, whereas the reduction was restored in the MI + MCD inhibitor high-dose group (Figure 5A). FOXO3 is known to bind to the Trx-1 promoter, thereby upregulating expression of Trx-1 (29). The total protein expression of cardiac FOXO3 was unaltered among the groups (Figures 5B to 5C). However, the cytosolic expression of FOXO3 was significantly reduced, concomitant with an increased nuclear expression of FOXO3, in the MI + high-dose group relative to the MI + vehicle group (Figures 5B to 5C), implicating an association between upregulation of myocardial Trx-1 and nuclear translocation of FOXO3 in the MI + high-dose group.
In addition, SOD2, a mitochondrial antioxidant, was less active in the post-MI hearts, but the activity was enhanced by MCD inhibition (Figure 5D). Alterations in SOD2 activity were inversely reflected by the acetylation of SOD2 (Figure 5E). Neither protein expression of SIRT3 (Figure 5F), a mitochondrial deacetylase (30), nor GCN5L1, a mitochondrial acetylase, was altered among the groups (data not shown). MCD has been suggested to exhibit acetyltransferase activity (31); therefore, we investigated if acetylation of SOD2 was associated with MCD through a direct interaction. Immunoprecipitation of the heart lysates revealed that SOD2 was captured by the MCD antibody (Figure 5G), and vice versa (Figure 5H), suggestive of an association between MCD and SOD2. In contrast, pyruvate dehydrogenase, a key mitochondrial enzyme modulating glucose oxidation (6), was unable to be captured by MCD antibody in the same heart lysates (Figure 5I).
Discussion
This study shows several important novel findings. 1) Inhibition of MCD is able to reverse pre-existing HF as the MCD inhibitor was added after HF had already occurred in the post-MI rat (Figures 1F to 1H and 2B). Pharmacological inhibiting MCD in post-MI rats decreases the severity of pre-existing HF post-MI, which is relevant and practical to clinic treatment for HF patients. In the previous studies with MCD-deficient mice it was not possible to separate possible beneficial effects of MCD inhibition during the infarct development from preventing adverse remodeling of the post-MI heart. 2) MCD inhibition is able to immediately improve cardiac function in the failing heart (Figures 1F to 1H), suggesting that optimizing cardiac energy metabolism can acutely improve heart function in the failing heart. 3) Both immediate and chronic inhibition of MCD improves cardiac contractility in the failing hearts, which is associated with an increase in malonyl CoA content in the post-MI hearts, resulting in a decreased cardiac fatty acid oxidation rate. 4) MCD inhibition improves cardiac efficiency that is associated with a reduced proton production through increasing LDH1 expression while decreasing LDH5 expression, thereby ameliorating coupling of glycolysis with glucose oxidation. 5) MCD inhibition can enhance cardiac antioxidant capacity through increasing SOD2 activity by reducing its acetylation. As a result, our data suggest a mechanism that failing heart is metabolically inefficient at using energy, while inhibiting MCD reprograms fuel use prevents proton production that improves cardiac efficiency in the post-MI heart. Thus, inhibiting fatty acid oxidation may be a promising therapeutic approach to treat HF.
It is generally assumed that the heart switches from fatty acid to glucose metabolism in the failing heart (2). If this was the case, it would not appear logical to inhibit fatty acid oxidation as an approach for improving cardiac energetics in the failing heart. It has been proposed by Kolowicz et al. (10) that stimulating fatty acid oxidation may be beneficial in the failing heart. However, it is probably more accurate to suggest that mitochondrial oxidative metabolism decreases in the HF, with a switch in energy metabolism towards glycolysis in HF (2).
Glycolysis is a source of lactate and protons especially when glucose oxidation is impaired 2, 22. The clearance of protons is rapid. However, this still decreases the cardiac efficiency because of the association with the influx of Na+, which can subsequently lead to accumulation of intracellular Ca2+. As a result, a significant amount of cardiac ATP is shunted away from providing energy for contraction to re-establishing normal Na+ and Ca2+ ionic homeostasis 7, 32. The assessment of proton production rates in the current study is a technical limitation, which is not a direct measurement but a calculation from the rates of glycolysis and glucose oxidation. Our previous studies have shown that the calculated proton production from glycolysis and glucose oxidation is inversely correlated to intracellular pH, measured by using 31-P-NMR spectroscopy, and to cardiac efficiency in post-ischemic hearts (7), whereas cardiac efficiency is improved in post-ischemic hearts by altering both the source and fate of proton 7, 32. Kolowicz et al. (10) also observed an improved coupling of glucose metabolism with a decreased lactate production in mice with cardiac-specific deletion of acetyl CoA carboxylase 2. We further extended our understanding towards the mechanisms underlying MCD inhibition-mediated coupling of glucose metabolism and found an increased expression of LDH1 with a decreased expression of LDH5 in MI hearts post-MCD inhibition. An increase in M subunit of LDH is an indicator of increased glycolytic capacity (26). An increase in LDH5 and a decrease in LDH1 isoenzyme expression was seen in myocardium from patients with chronic HF, in which a shift of LDH pattern towards an increase in LDH1 paralleled by a decrease in LDH5 was observed after treatment with the angiotensin-converting enzyme inhibitor (33). Therefore, we propose that the alteration of LDH isoenzymes in MI hearts post-MCD inhibition may increase to the conversion of lactate to pyruvate, thereby reducing glycolysis to improve the coupling of glucose metabolism. Thus, monitoring of cardiac LDH enzymatic adaptation may be of clinical interest (34).
Decreased mitochondrial fatty acid oxidation with MCD inhibition has the potential to shunt fatty acids towards TAG. However, instead of increasing TAG levels, the decrease in TAG level and an increased ATGL expression were observed concomitant with a reduction of SPT1 in MI hearts post-MCD inhibition, suggesting a reduction of ceramide biosynthesis occurred in conjunction with an increased TAG turnover by MCD inhibition. Tian et al. (35) have recently shown that elevated rates of TAG turnover due to cardiac-specific overexpression of DGAT1 Diacylglycerol O-Acyltransferase 1 are associated with improved functional recovery from ischemic stress, in part, by sequestering fatty acids into the TAG pool and reducing the accumulation of ceramides (35). In addition, among the lipids, ceramide, as a potent massager molecule to induce oxidative stress, is associated with pathophysiology of HF in humans (28). Our data also suggest that MCD inhibition enhances antioxidative capacity through at least 2 pathways via FOXO3/Trx-1 axis and acetylation/SOD2 axis. The mechanisms for downregulating Trx-1 in the post-MI heart are unclear, but degradation of Trx-1, 1 of the mechanisms induced by oxidative stress (36), needs further studies. Deacetylation of SOD2 was also observed in gastrocnemius muscle from aged MCD−/− mice, in which reduction of oxidative stress was evident (37). MCD has been suggested to function as an acetyltransferase (31). At this point it remains unclear whether the deacetylation of SOD2 following MCD inhibition is due to a reduced acetyltransferase activity of MCD as our findings do not confirm whether MCD acts as an acetylase or simply controls protein acetylation via controlling acetyl CoA content. Thus, the dynamic nature of endogenous cardiac ceramide and TAG may indicate a novel role of enhanced intracellular lipids metabolism as a result of MCD inhibition in preventing cardiac oxidative stress in post-MI hearts.
The use of MCD inhibitors in vivo has to consider issues such as the potential for liver steatosis due to redirecting lipids towards TAG synthesis. However, we observed that hepatic TAG content was similar among the MI groups (data not shown), which may be due to a fact that malonyl CoA is more sensitive in inhibiting fatty acid oxidation in heart versus liver mitochondria (Figure 1E). In addition, aged MCD−/− mice, despite elevated hepatic TAG levels, displayed a significant increase in life span of ∼30% than the wild-type littermates (37).
Infarct size is a key determinant of the outcome of an acute MI (38). It was therefore important to rule out that any of the beneficial effects of MCD inhibition post-MI were occurring secondary to a reduction in infarct size. This possibility is unlikely because rats were subjected to a permanent MI and MCD inhibition was not initiated until 3 weeks post-MI, and the scar sizes were similar among the post-MI groups. This is of potential important clinical importance, considering many patients who suffer from chronic HF have permanent myocardial infarcts/scars that cannot be revascularized or repaired. Thus, the cardioprotective effects of MCD inhibition may not only enhance coronary revascularization therapy in patients with acute MI, but may also be useful as long-term therapy to prevent the transition to HF.
Study limitations
In this study, MCD inhibition of fatty acid oxidation was primarily observed when fatty acid oxidation rates were normalized for cardiac work, with absolute fatty acid oxidation rates being similar among the groups. This normalization of fatty acid oxidation rates was performed to account for the dramatic changes in cardiac work, which is a major determinant of cardiac mitochondrial oxidative metabolism 2, 25. However, it must be recognized that the possibility exists that the absence of change in absolute metabolic fatty acid oxidation rates following chronic MCD inhibitor treatment could suggest that the underlying mechanisms may not be dependent on changes in metabolic pathways. A second limitation concerns the mechanisms underlying the effect of MCD inhibition on cardiac function. Emerging evidence has shown that downregulating malic enzyme 1 expression in failing hearts induces favorable shifts in not only improving coupling between glycolysis and glucose oxidation, but also improving redox state and contractile function (39). As MCD inhibition enhances the antioxidative capacity in MI hearts in conjunction with the improved uncoupling of glycolysis and glucose oxidation, whether this is achieved by preserving nicotinamide adenine dinucleotide phosphate through reducing malic enzyme 1 thereby mediating coupling of glycolysis and glucose oxidation, needs further investigation. We are unable to discern whether the MCD inhibition-mediated functional benefits are a direct result of either restoration of cellular redox state due to a better coupling between glycolysis and glucose oxidation for preserving nicotinamide adenine dinucleotide phosphate and enhancing antioxidative capacity, or induction of the more efficient oxidation of glucose to reduce protons thereby altering calcium dynamics, or the consequence of multiple metabolic responses.
In conclusion, chronic MCD inhibition reverses cardiac dysfunction in rats with established HF by improving cardiac energy inefficiency due to optimizing cardiac energy metabolism. MCD inhibition might be a novel potential therapy for treating post-MI heart disease. Certainly, establishing efficacy and safety in large animal models would be essential before the MCD inhibitor would be introduced into clinical studies.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Cardiac energetics is compromised in the human failing heart, due both to a decrease in energy production and a decrease in cardiac efficiency. Inhibition of fatty acid oxidation with MCD inhibition improves cardiac efficiency in the failing heart, which is associated with an improvement of cardiac function. As a result, MCD inhibition is a potentially promising approach to decrease the severity of HF.
TRANSLATIONAL OUTLOOK: Therapeutic strategies that involve the inhibition of cardiac fatty acid oxidation have been shown to be clinically beneficial in the setting of ischemic heart disease and heart failure. This includes the use of CPT1 inhibitors and direct fatty acid ß-oxidation inhibitors. The strategy of increasing malonyl CoA levels, which inhibits CPT1, by inhibiting MCD is another novel approach to inhibiting fatty acid oxidation in the heart. From a clinical viewpoint, oral delivery of MCD inhibitors for the treatment of ischemic heart disease would be practical. We have shown previously that this did not affect peripheral tissue metabolism because there was no insulin resistance observed in skeletal muscle, and no TAG accumulation in liver. In addition, MCD inhibition-mediated improvement of cardiac efficiency was independent of the scar size, suggesting that the cardioprotective effects of MCD inhibition may occur by directly optimizing energy metabolism in the remodeled post-MI myocardium.
Footnotes
Drs. Nguyen, Fu, Motani, and Reagan are employees/shareholders of Amgen, Inc. Dr. Lupaschuk has received grants from the Canadian Institutes of Health Research; is an Alberta Heritage Foundation for Medical Research Medical Scientist; and is a shareholder in Metabolic Modulators Research Ltd. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and U.S. Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Heusch G., Libby P., Gersh B. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet. 2014;383:1933–1943. doi: 10.1016/S0140-6736(14)60107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopaschuk G.D., Ussher J.R., Folmes C.D., Jaswal J.S., Stanley W.C. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–258. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 3.Huss J.M., Kelly D.P. Mitochondrial energy metabolism in heart failure: a question of balance. J Clin Invest. 2005;115:547–555. doi: 10.1172/JCI200524405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mori J., Basu R., McLean B.A. Agonist-induced hypertrophy and diastolic dysfunction are associated with selective reduction in glucose oxidation: a metabolic contribution to heart failure with normal ejection fraction. Circ Heart Fail. 2012;5:493–503. doi: 10.1161/CIRCHEARTFAILURE.112.966705. [DOI] [PubMed] [Google Scholar]
- 5.Zhabyeyev P., Gandhi M., Mori J. Pressure-overload-induced heart failure induces a selective reduction in glucose oxidation at physiological afterload. Cardiovasc Res. 2013;97:676–685. doi: 10.1093/cvr/cvs424. [DOI] [PubMed] [Google Scholar]
- 6.Ussher J.R., Wang W., Gandhi M. Stimulation of glucose oxidation protects against acute myocardial infarction and reperfusion injury. Cardiovasc Res. 2012;94:359–369. doi: 10.1093/cvr/cvs129. [DOI] [PubMed] [Google Scholar]
- 7.Liu Q., Docherty J.C., Rendell J.C., Clanachan A.S., Lopaschuk G.D. High levels of fatty acids delay the recovery of intracellular pH and cardiac efficiency in post-ischemic hearts by inhibiting glucose oxidation. J Am Coll Cardiol. 2002;39:718–725. doi: 10.1016/s0735-1097(01)01803-4. [DOI] [PubMed] [Google Scholar]
- 8.Finck B.N., Lehman J.J., Leone T.C. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest. 2002;109:121–130. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hafstad A.D., Khalid A.M., Hagve M. Cardiac peroxisome proliferator-activated receptor-alpha activation causes increased fatty acid oxidation, reducing efficiency and post-ischaemic functional loss. Cardiovasc Res. 2009;83:519–526. doi: 10.1093/cvr/cvp132. [DOI] [PubMed] [Google Scholar]
- 10.Kolwicz S.C., Jr., Olson D.P., Marney L.C., Garcia-Menendez L., Synovec R.E., Tian R. Cardiac-specific deletion of acetyl CoA carboxylase 2 prevents metabolic remodeling during pressure-overload hypertrophy. Circ Res. 2012;111:728–738. doi: 10.1161/CIRCRESAHA.112.268128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dyck J.R., Cheng J.F., Stanley W.C. Malonyl coenzyme a decarboxylase inhibition protects the ischemic heart by inhibiting fatty acid oxidation and stimulating glucose oxidation. Circ Res. 2004;94:e78–e84. doi: 10.1161/01.RES.0000129255.19569.8f. [DOI] [PubMed] [Google Scholar]
- 12.Dyck J.R., Hopkins T.A., Bonnet S. Absence of malonyl coenzyme A decarboxylase in mice increases cardiac glucose oxidation and protects the heart from ischemic injury. Circulation. 2006;114:1721–1728. doi: 10.1161/CIRCULATIONAHA.106.642009. [DOI] [PubMed] [Google Scholar]
- 13.Randle P.J., Sugden P.H., Kerbey A.L., Radcliffe P.M., Hutson N.J. Regulation of pyruvate oxidation and the conservation of glucose. Biochem Soc Symp. 1978:47–67. [PubMed] [Google Scholar]
- 14.Stanley W.C., Morgan E.E., Huang H. Malonyl-CoA decarboxylase inhibition suppresses fatty acid oxidation and reduces lactate production during demand-induced ischemia. Am J Physiol Heart Circ Physiol. 2005;289:H2304–H2309. doi: 10.1152/ajpheart.00599.2005. [DOI] [PubMed] [Google Scholar]
- 15.Wu H., Zhu Q., Cai M. Effect of inhibiting malonyl-CoA decarboxylase on cardiac remodeling after myocardial infarction in rats. Cardiology. 2014;127:236–244. doi: 10.1159/000356471. [DOI] [PubMed] [Google Scholar]
- 16.Masoud W.G., Ussher J.R., Wang W. Failing mouse hearts utilize energy inefficiently and benefit from improved coupling of glycolysis and glucose oxidation. Cardiovasc Res. 2014;101:30–38. doi: 10.1093/cvr/cvt216. [DOI] [PubMed] [Google Scholar]
- 17.Dolinsky V.W., Jones K.E., Sidhu R.S. Improvements in skeletal muscle strength and cardiac function induced by resveratrol during exercise training contribute to enhanced exercise performance in rats. J Physiol. 2012;590:2783–2799. doi: 10.1113/jphysiol.2012.230490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L., Jaswal J.S., Ussher J.R. Cardiac insulin-resistance and decreased mitochondrial energy production precede the development of systolic heart failure after pressure-overload hypertrophy. Circ Heart Fail. 2013;6:1039–1048. doi: 10.1161/CIRCHEARTFAILURE.112.000228. [DOI] [PubMed] [Google Scholar]
- 19.Picard M.H., Popp R.L., Weyman A.E. Assessment of left ventricular function by echocardiography: a technique in evolution. J Am Soc Echocardiogr. 2008;21:14–21. doi: 10.1016/j.echo.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Singh T.D., Barbhuiya M.A., Gupta S. Quantitative assessment of expression of lactate dehydrogenase and its isoforms 3 and 4 may serve as useful indicators of progression of gallbladder cancer: a pilot study. Indian J Clin Biochem: IJCB. 2011;26:146–153. doi: 10.1007/s12291-011-0117-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janssen A.J., Trijbels F.J., Sengers R.C. Spectrophotometric assay for complex I of the respiratory chain in tissue samples and cultured fibroblasts. Clin Chem. 2007;53:729–734. doi: 10.1373/clinchem.2006.078873. [DOI] [PubMed] [Google Scholar]
- 22.Krishnan J., Suter M., Windak R. Activation of a HIF1alpha-PPARgamma axis underlies the integration of glycolytic and lipid anabolic pathways in pathologic cardiac hypertrophy. Cell Metab. 2009;9:512–524. doi: 10.1016/j.cmet.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Peskin A.V., Winterbourn C.C. A microtiter plate assay for superoxide dismutase using a water-soluble tetrazolium salt (WST-1) Clinica Chimica Acta. 2000;293:157–166. doi: 10.1016/s0009-8981(99)00246-6. [DOI] [PubMed] [Google Scholar]
- 24.Bird M.I., Saggerson E.D. Binding of malonyl-CoA to isolated mitochondria. Evidence for high- and low-affinity sites in liver and heart and relationship to inhibition of carnitine palmitoyltransferase activity. Biochem J. 1984;222:639–647. doi: 10.1042/bj2220639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heineman F.W., Balaban R.S. Control of mitochondrial respiration in the heart in vivo. Annu Rev Physiol. 1990;52:523–542. doi: 10.1146/annurev.ph.52.030190.002515. [DOI] [PubMed] [Google Scholar]
- 26.Cahn R.D.Z.E., Kaplan N.O., Levine L. Nature and development of lactic dehydrogenases: the 2 major types of this enzyme form molecular hybrids which change in makeup during development. Science. 1962;136:962–969. doi: 10.1126/science.136.3520.962. [DOI] [PubMed] [Google Scholar]
- 27.Perman J.C., Bostrom P., Lindbom M. The VLDL receptor promotes lipotoxicity and increases mortality in mice following an acute myocardial infarction. J Clin Invest. 2011;121:2625–2640. doi: 10.1172/JCI43068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Myoishi M., Hao H., Minamino T. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation. 2007;116:1226–1233. doi: 10.1161/CIRCULATIONAHA.106.682054. [DOI] [PubMed] [Google Scholar]
- 29.Li X.N., Song J., Zhang L. Activation of the AMPK-FOXO3 pathway reduces fatty acid-induced increase in intracellular reactive oxygen species by upregulating thioredoxin. Diabetes. 2009;58:2246–2257. doi: 10.2337/db08-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiu X., Brown K., Hirschey M.D., Verdin E., Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12:662–667. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 31.Aparicio D., Perez-Luque R., Carpena X. Structural asymmetry and disulfide bridges among subunits modulate the activity of human malonyl-CoA decarboxylase. J Biol Chem. 2013;288:11907–11919. doi: 10.1074/jbc.M112.443846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu B., Clanachan A.S., Schulz R., Lopaschuk G.D. Cardiac efficiency is improved after ischemia by altering both the source and fate of protons. Circ Res. 1996;79:940–948. doi: 10.1161/01.res.79.5.940. [DOI] [PubMed] [Google Scholar]
- 33.Schultheiss H.P. Effect on the myocardial energy metabolism of angiotensin-converting enzyme inhibition in chronic heart failure. Am J Cardiol. 1990;65:74–81. doi: 10.1016/0002-9149(90)90965-4. [DOI] [PubMed] [Google Scholar]
- 34.Lin L., Sylven C., Astrom H., Liska J., Ljungquist A., Jansson E. Myocardial lactate dehydrogenase and its isoenzyme activities in transplanted human hearts. Scand J Thorac and Cardiovasc Surg. 1991;25:51–55. doi: 10.3109/14017439109098083. [DOI] [PubMed] [Google Scholar]
- 35.Kolwicz S.C., Jr., Liu L., Goldberg I.J., Tian R. Enhancing cardiac triacylglycerol metabolism improves recovery from ischemic stress. Diabetes. 2015;64:2817–2827. doi: 10.2337/db14-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kato T., Niizuma S., Inuzuka Y. Analysis of metabolic remodeling in compensated left ventricular hypertrophy and heart failure. Circ Heart Fail. 2010;3:420–430. doi: 10.1161/CIRCHEARTFAILURE.109.888479. [DOI] [PubMed] [Google Scholar]
- 37.Ussher J.R., Fillmore N., Keung W. Genetic and pharmacological inhibition of malonyl CoA decarboxylase does not exacerbate age-related insulin resistance in mice. Diabetes. 2016;65:1–9. doi: 10.2337/db15-1145. [DOI] [PubMed] [Google Scholar]
- 38.Bosch X., Theroux P. Left ventricular ejection fraction to predict early mortality in patients with non-ST-segment elevation acute coronary syndromes. Am Heart J. 2005;150:215–220. doi: 10.1016/j.ahj.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 39.Lahey R., Carley A.N., Wang X. Enhanced redox state and efficiency of glucose oxidation with miR based suppression of maladaptive NADPH-dependent malic enzyme 1 expression in hypertrophied hearts. Circ Res. 2018;122:836–845. doi: 10.1161/CIRCRESAHA.118.312660. [DOI] [PMC free article] [PubMed] [Google Scholar]