ABSTRACT
Objectives
The aim of the study was to carry out an epidemiological analysis of patients with carbapenemase-producing Enterobacteriaceae (CPE) isolations in our hospital as well as to perform a description of the genotypic temporal evolution of CPE isolated.
Material and methods
. An observational prospective cohort study was performed involving all patients with CPE isolates from clinical samples during November 2014 to November 2016 in a Spanish teaching hospital. Patients were clinically evaluated and classified either as infected or colonized. Information on the consumption of carbapenems in the hospital during the study period was also analyzed. PCR was used for identification of the carbapenemase genes blaKPC, blaVIM, and blaOXA-48.
Results
A total of 301 CPE isolates were obtained (107 in 2014, 89 in 2015 and 105 in 2016). Klebsiella pneumoniae (73.4%) was the most prevalent microorganism. Hundred and seventy (56.7%) of carbapenemases detected were blaOXA-48, 73 (24.3%) were blaKPC and 57 (19%) were blaVIM. In year 2014 KPC was predominant while in 2016 OXA-48 predominated. In 2014 we observed a significant association between the medical wards and the ICU with a higher prevalence of OXA-48 (OR 4.15; P<0.001) and VIM (OR 7.40; P<0.001) in the univariate analysis, in the following years there was no association. Regarding the clinical significance of microbiological results after assessing our patients, 60% of isolates represented infection and 40% behaved as colonizers. One third of hospitalized patients with CPE isolation died within 30 days, regardless of whether they were colonized or infected.
Conclusions
We have observed an epidemiological change in the genotypes of our isolates along the study period. A thorough knowledge of the CPE’s epidemiological distribution in each hospital is fundamental for optimizing antimicrobial chemotherapy.
Keywords: Carbapenem-producing Enterobacteriaceae, Epidemiology, tertiary hospital
RESUMEN
Objetivos
Se realizó un análisis epidemiológico de aquellos pacientes con aislamiento de Enterobacterias portadoras de carbapenemasas (EPC) en un hospital terciario, así como una descripción temporal de los genotipos de dichas EPC.
Material y métodos
Estudio de cohortes prospectivo observacional que incluyó todos los aislamientos de EPC obtenidos de muestras clínicas entre noviembre de 2014 y noviembre de 2016 en un hospital universitario. Los pacientes fueron evaluados clínicamente para determinar si el aislamiento era en el contexto de una infección o de una colonización. También se recopiló la información acerca del consumo de carbapenémicos en el hospital durante el periodo de estudio. Se usó la técnica PCR para la identificación de los genes de carbapenemasas blaKPC, blaVIM, and blaOXA-48.
Resultados
Se obtuvieron un total de 301 aislamientos de EPC (107 en 2014, 89 en 2015 y 105 en 2016). Klebsiella pneumoniae (73,4%) fue el microorganismo más prevalente. De las carbapenemasas aisladas, 170 (56,7%) correspondieron a blaOXA-48, 73 (24,3%) a blaKPC y 57 (19%) a blaVIM. En el año 2014 KPC fue la predominante mientras que en 2016 lo fue OXA-48. En 2014 la prevalencia de OXA-48 (OR 4,15; P<0,001) y de VIM (OR 7,40; P<0,001) fue significativamente mayor en las áreas médicas y en la UCI en el análisis univariante, sin embargo en los siguientes años no hubo ninguna asociación. Respecto a la significación clínica de los resultados microbiológicos, un 60% de los aislamientos correspondían a una infección y un 40% a una colonización. Un tercio de los pacientes hospitalizados con aislamiento para EPC murieron en los 30 días siguientes al mismo, independientemente de si representaba una colonización o una infección.
Conclusiones
. Hemos constatado un cambio en el patrón epidemiológico de los genotipos de nuestros aislamientos a lo largo del período de estudio. Un conocimiento pormenorizado de los patrones de distribución epidemiológica de las EPC dentro de cada hospital es fundamental para optimizar la terapéutica antimicrobiana.
Palabras clave: Enterobacterias portadoras de carbapenemasa, epidemiologia, hospital terciario
INTRODUCTION
The emergence and uncontrolled dissemination of carbapenemase-producing Enterobacteriaceae (CPE) has caused great concern to public healthcare systems worldwide. Genes associated with carbapenemase production are often contained on mobile genetic elements that facilitate transfer among different Enterobacteriaceae species. Until 2009, CPE prevalence in Spain was relatively low and normally related to VIM and KPC enzymes [1, 2]. Recently, CPE prevalence significantly increased owing to the dissemination of successful Klebsiella pneumoniae clones harbouring blaOXA-48, mainly linked to health care exposure and prolonged hospital stay [3, 4]. On the other hand, the emergence of CPE as a cause of community-onset initiated infections, represents a matter of great concern, taking into account the latest detected trends and the increase in carbapenemase reservoirs worldwide, mainly due to the producers of OXA-48 [5, 6].
It is known that precautions based on transmission (contact isolation) for colonized and infected patients is a key factor to reduce their impact [7, 8] but CPE cases go beyond the limits of clinical services and the hospital itself, extending to different types of patients and in clinical settings. That is the reason why not only this measures must been done in general. Epidemiological studies are necessary to better understand the epidemiological trend and what preventive measures and clinical decisions could be implemented to address the challenges related to CPE isolation.
The aim of our study was to carry out an epidemiological analysis of patients with CPE isolations in our hospital from 2014 to 2016. Moreover, a description of the genotypic temporal evolution of CPE isolation in a tertiary hospital was also performed.
MATERIAL AND METHODS
An observational prospective cohort study was performed involving all patients with CPE isolates from clinical samples during November 2014 to November 2016 in a Spanish teaching hospital with over 800 beds and 60/40 medical-surgical activity. Patients were clinically evaluated and classified either as infected or colonized. Information on the consumption of carbapenems in the hospital during the study period was also analyzed. Patients were identified through the Microbiology Department database and only a single episode per patient, the first one, was considered during the study period.
Definition and collection of variables. Data were collected from the microbiology laboratory records of patients with CPE from clinical samples and was evaluated and classified either as infected or colonized. An infection or colonization diagnosis was considered if it was established as a diagnosis during the patient’s care by the responsible physician according to the usual clinical practice. Origin of clinical sample was from hospitalized patients or from primary care. Type of admission ward and the 30-day mortality after the index event also were collected. Global carbapenem use of in the whole Institution, measured as Defined Daily Doses (DDD) per 100 bed-days, was analyzed during the study period.
Microbiological procedures. Analysis included all CPE isolations, but only the first isolation and during the study period. The identification of clinical isolates was performed by MALDI-TOF MS® (Microflex; Bruker Daltonics, Bremen, Germany). Antibiotic susceptibility testing was performed using either the Wider® (Fco. Soria Melguizo, Madrid, Spain) or VITEK® systems (bioMérieux, Marcy L’Étoile, France). Isolates were categorised as either susceptible or resistant to the antibiotics tested according to the interpretative criteria of the Clinical and Laboratory Standards Institute (CLSI) [9].
Enterobacterial strains recovered from any clinical sample with an imipenem or meropenem minimum inhibitory concentration (MIC) of >1 mg/L, or a MIC for ertapenem of > 0.5mg/L were retested using E-test® (bioMérieux, Marcy L’Étoile, France), and screened carbapenamase production by modified Hodge test. All isolates with Hodge test positive were characterised by molecular or immune chromatographic methods. PCR was used for identification of the carbapenemase genes blaKPC, blaVIM, and blaOXA-48 [10, 11] since those are the most frequent types of carbapenemases in Spain.
Statistical analysis. Descriptive statistics were obtained for all variables. Univariate comparison of proportions for categorical variables was performed by either Fisher’s exact test or the Chi-square test. Odds ratio (OR) and 95% confidence interval (CI) were calculated for all valid associations. A significance level of P ≤ 0.05 was considered. CPE population diversity was estimated by using the Simpson’s Dominance Index (SDI) [12]. SDI values ranges from 0 to 1 representing, respectively, the least and the most possible biodiversity possible in the population. All statistical analysis were performed using SPSS® Version 20 (IBM®, Chicago, USA).
RESULTS
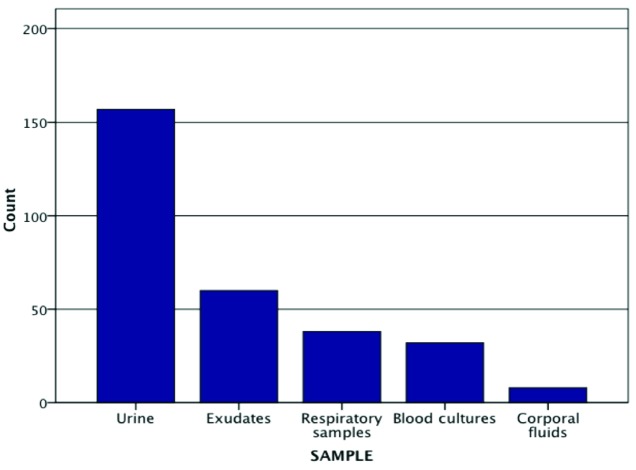
A total of 301 isolates of CPE were obtained (107 in 2014, 89 in 2015, and 105 in 2016). The clinical sample with the highest frequency of isolates was urine (n=157), followed by exudates (n=60), respiratory samples (n=38), blood cultures (n=32) and corporal fluids (n=8) (figure 1). K. pneumoniae (221/301; 73.4%) was the most prevalent microorganism, followed by Enterobacter cloacae (38/301; 12.6%), Serratia marcescens (16/301; 5.3%), Klebsiella oxytoca (10/301; 3.3%), Escherichia coli (6/301; 1.9%), Citrobacter freundii (4/301;1.3%), Enterobacter absuriae (3/301; 0.9%), Enterobacter aerogenes (2/301; 0.6%) and Citobacter koseri (1/301; 0.3%). Analysing the yearly distribution of the variety of species isolated we observed that the Simpson´s Dominance Index (SDI) showed a diversification increase during the study period (SDI year 2014= 0.432, SDI year 2015= 0.427 and SDI year 2016= 0.463).
Figure 1.
Origin of clinical samples
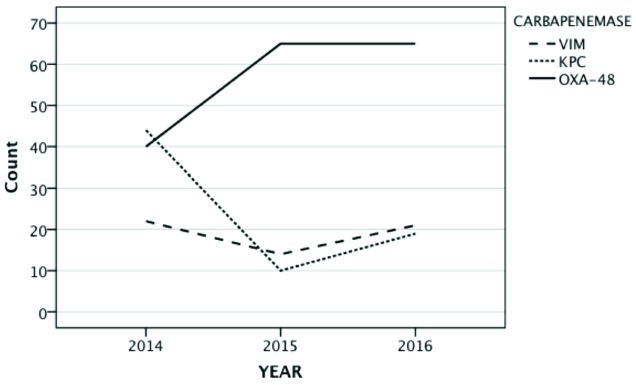
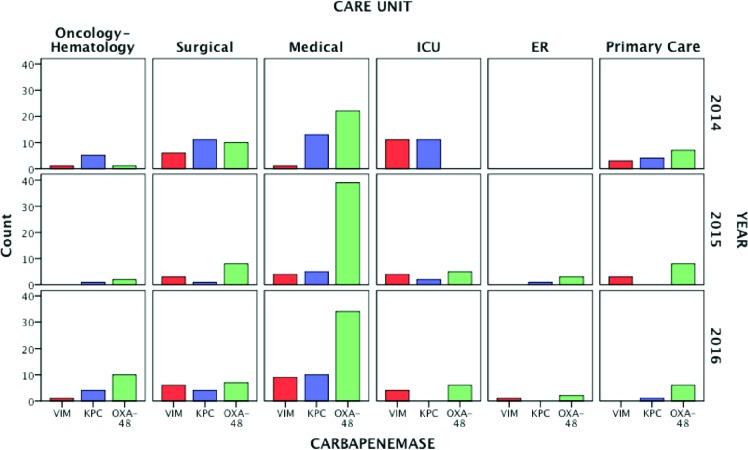
Regarding the types of carbapenemases detected (figure 2), 170 (56.7%) of them were blaOXA-48, 73 (24.3%) blaKPC and 57 (19%) blaVIM. By care units, most of isolates came from medicals wards, followed by surgical wards and the ICU. OXA-48 predominated in medical and surgical areas, and in patients whose samples came from primary care. On the other hand, KPC and VIM genotypes predominated in the ICU during the first year of the study. Isolations in surgical wards ranged from 27% in 2014 to 11% in 2016. In the ICU has decreased (21 isolations in 2014, 11 in 2015 and 9 in 2016). The complete distribution of carbapenemases isolated regarding the care unit, the year and the type of enzyme are depicted in figure 3.
Figure 2.
Frequency of carbapenemase isolates from 2014 to 2016
Figure 3.
Yearly distribution of carbapenemase isolates by care units
ICU: Intensive Care Unit; ER: Emergency Room.
In the univariate analysis, in 2014 we observed a significant association between the medical wards and the ICU with a higher prevalence of OXA-48 (OR 4.15; P<0.001) and VIM (OR 7.40; P<0.001). All the univariate analysis results are summarized in table 1. When we analysed the genotype in 2014 KPC was predominant and in 2016 OXA48 predominated (figure 2).
Table 1.
Risk of carbapenemase-producing Enterobacteriaceae isolation considering care unit and type of carbapenemase in the three years of the study
| 2014 | 2015 | 2016 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KPC | VIM | OXA-48 | KPC | VIM | OXA-48 | KPC | VIM | OXA-48 | ||||||||||
| OR (95% CI) | P-VALUE | OR (95% CI) | P-VALUE | OR (95% CI) | P-VALUE | OR (95% CI) | P-VALUE | OR (95% CI) | P-VALUE | OR (95% CI) | P-VALUE | OR (95% CI) | P-VALUE | OR (95% CI) | P-VALUE | OR (95% CI) | P-VALUE | |
| Oncology-Hematology | 3’846 (0’711-20’816) | 0’096 | 0’658 (0’075-5’784) | 0’704 | 0’256 (0’030-2’212) | 0’185 | 4’222 (0’347-51’330) | 0’222 | - | 0’443 | 0’742 (0’064-8’578) | 0’810 | 1’794 (0’503-6’400) | 0’363 | 0’246 (0’031-1’990) | 0’158 | 1’296 (0’409-4’113) | 0’659 |
| Surgical | 0’958 (0’394-2’330) | 0’925 | 1’219 (0’419-3’545) | 0’716 | 0’961 (0’389-2’372) | 0’931 | 0’677 (0’078-5’881) | 0’722 | 1’970 (0’460-8’435) | 0’354 | 0’714 (0’194-2’632) | 0’612 | 1’477 (0‘423-5’161) | 0’539 | 2’618 (0’838-8’184) | 0’090 | 0’368 (0’127-1’066) | 0’059 |
| Medical | 0’664 (0’291-1’515) | 0’329 | 0’068 (0’009-0’531) | 0’001 | 4’156 (1’779-9’705) | 0’001 | 0’814 (0’218-3’039) | 0’759 | 0’273 (0’078-0’951) | 0’033 | 2’600 (0’989-6’838) | 0’058 | 1’085 (0’401-2’938) | 0’872 | 0’665 (0’253-1’746) | 0’406 | 1’253 (0’568-2’764) | 0’577 |
| ICU | 1’545 (0.602-3.970) | 0.364 | 7.400 (2.551-21.469) | <0.001 | 0.524 (0.427-0.642) | <0.001 | 1.917 (0.351-10.471) | 0.446 | 3.829 (0.947-15.474) | 0.070 | 0.254 (0.069-0.932) | 0.030 | - | 0.116 | 3.020 (0.767-11.881) | 0.101 | 0.931 (0.246-3.526) | 0.916 |
| ER | - | - | - | - | - | - | 2.778 (0.261-29.609) | 0.379 | 0.833 (0.757-0.917) | 0.373 | 1.131 (0.112-11.435) | 0.917 | - | 0.406 | 2.025 (0.175-23.463) | 0.565 | 1.258 (0.110-14.342) | 0.853 |
| Primary care | 0’589 (0’169-2’050) | 0’401 | 0’708 (0’145-3’468) | 0’669 | 2’121 (0’658-6’836) | 0’201 | - | 0’229 | 2’610 (0’585-11’644) | 0’196 | 0’860 (0’203-3’636) | 0’837 | 0’889 (0’098-8’079) | 0’917 | - | 0’204 | 3’305 (0’372-29’379) | 0’258 |
ICU: Intensive Care Unit; ER: Emergency Room.
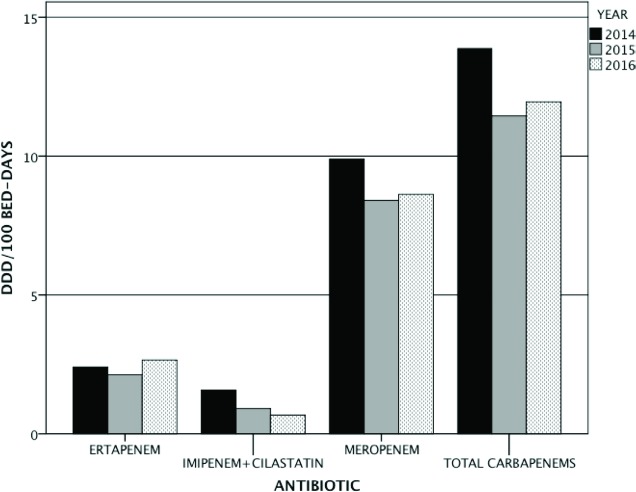
When we analysed the clinical significance of microbiological results after assessing our patients, 60% of isolates represented infection and 40% behaved as colonizers. One third of hospitalized patients with CPE isolation died within 30 days, regardless of whether they were colonized or infected. The global use of carbapenems, measured as DDD per 100 beddays, decreased slightly from 2014 to 2016, especially at the expense of meropenem and imipenem (figure 4). Only those units with more fragile patients such as internal medicine, oncology and hematology have maintained a similar level of carbapenem usage during the study period.
Figure 4.
Global DDD per 100 bed-days in our Hospital from 2014 to 2016
DISCUSSION
In the present study we analyse the epidemiological, clinical and outcome features of patients with colonisation and infection due to CPE. We observed that the overall prevalence of CPE increased during the study period, and this phenomenom was primarily associated with OXA-48. Our results showed that up to nine different species of Enterobacteriaceae showed enzyme production, mainly K. pneumoniae OXA-48. The most frequent types of clinical samples were urine, followed by exudates and respiratory samples. The majority of patients with CPE isolated from urinary samples usually correspond to patients with transient or permanent urological devices (catheter, pigtail, double J) and previous antimicrobial therapy, both factors constitute the perfect environment for a MDR selection, since the protection given by the urological devices counteracts the natural clearance mechanisms. At present time, UTIs due to CPE constitute a growing nosocomial infection [13-15].
The most predominant Enterobacteriaceae during the whole study period were Klebsiella spp (76% of the episodes), in particular K. pneumoniae. These data coincided with most of the studies, where it is considered to be the main reservoir of the carbapenemase genes, particularly OXA-48 [16, 17]. Other minority species were also detected in a slightly high percentage, including rare Enterobacteriaceae such as C. koseri. These results may reflect the efficient penetration of carbapenemase among the Enterobacteriaceae as it has occurred previously with the isolating producers of extended-spectrum β-lactamase (ESBL), in particular CTX-M. Distribution of these species was homogeneous throughout the study period, and the isolated CPE species diversity increased slightly from 2014 to 2016.
Most of the isolates came from medical wards, usually the length of stay, the number of medical procedures and the antimicrobial prescription were more frequent. This more complex profile of patients, along with a higher prevalence of comorbidities between them, has all been related to the increase of CPE isolates [5]. From the microbiological point of view, the increasing prevalence of OXA-48 both in the medical wards and in the hospital in general, could be due to the efficient dissemination of the blaOXA-48 gene between the enteric flora of hospitalized patients that facilitate a high colonization rate. This fact could lead to a greater dispersion of this enzyme that produces a complex epidemiological situation with a high number of colonized individuals [18, 19]. The blaOXA-48 gene is housed in a conjugated plasmid whose gene encodes a protein that inhibits bacterial conjugation and efficient propagation of the blaOXA-48 gene, although clonal dissemination also contributes to the success of OXA-48 [20]. We observed a constant maintenance of the number of isolates coming from primary care practices, this situation has the risk of dissemination of OXA-48 into the community. Several studies show the emergence of CPE as a cause of community-onset infections. This fact should be a matter of great concern, taking into account the latest trends detected and the increase in carbapenemase reservoirs worldwide [5, 6]. These changes in the epidemiology patterns suggest that the incidence of both nosocomial and community-acquired infections will increase in the next future, mainly due to the OXA-48 CPE [5, 6]. This situation might also be related to delayed clearance of CPE carrier status after hospital discharge. The intestinal microbiota can be a reservoir of CPE genes in the community since intestinal colonization can last for months [21, 22] allowing the transmission to close contacts and, therefore, increasing the prevalence of community-acquired CPE infections [23, 24].
On the other hand, in our serie a non-negligible number of CPE isolates were collected in patients admitted to the surgical areas, especially during 2014, without any enzymatic class predominating. In the two following years, due to a greater awareness in the contact measures and optimization in the antimicrobial treatment, KPC and VIM isolates number was reduced, keeping the OXA-48 level almost constant. Something similar happened in ICU, where the depletion in the isolation of VIM and KPC CPE was more evident, due to implementation of control measures such as selective bowel decontamination (SBD) and intensified by “Zero measures” [25, 26].
In a multicenter study performed by Oteo et al. [27] on Spanish ICU patients with rectal CPE colonization a low prevalence was also found. Nevertheless, it is important to know the data of the institution where you work since there is significant variability between the different Spanish hospital’s ICUs [27]. This low proportion could be in fact explained because a rectal CPE-carrier status analysis is carried out on every patient admitted to an ICU. This measure, which is not usually taken in the rest of the care units, allows the CPEcolonized patients to be subjected to special control protocols. In the case of our ICU, the CPE isolations decreased from 2014 to 2016.The above mentioned protocols, which include the SBD, were implemented in all ICU (neuro-polytrauma, medical-surgical and cardiovascular) from 2011 to 2015 and the “Zero measures” implemented from 2013 to 2015 (one intensive care specialist responsible of prescriptions, one nurse responsible of infection control measures, checklist of risk factors at admission, 4% chlorhexidine for hygiene in patients colonized or infected with MDR pathogens), were important to control the CPE dissemination in the ICU.
Carbapenem use is another important key point in order to understand the distribution trends and the predominance shift from one type of carbapenemase to another over time. Analysing the DDD per 100 bed-days employed on each of the main carbapenem-prescribing care units in our institution, we observed a slight decrease in the usage of this group of antibiotics during 2015 (figure 4). Nevertheless, since some of those care units, such as the medical wards, Oncology and Hematology, take care of the most fragile patients, it is sometimes difficult to restrict carbapenem prescription. Moreover, specific protocols or clinical guides usually exist in those care units promoting the empiric use of carbapenems in cases such as the febrile neutropenia. In that respect, many clinical guides have been recently modified regarding the empirical prescription of carbapenems in febrile neutropenia in an effort to prevent the selection of resistant strains. For example, the European Conference on infections in Leukemia (ECIL) guides now consider the carbapenems as an option only when the patient is in shock, maintaining piperacillin-tazobactam, cefepime or even ceftazidime as first options with an A-I level of evidence [28, 29]. The consultant experts on infectious diseases or the stewardship teams could advise the specialists from these care units whenever early de-escalation in stable patients is possible.
An explanation for the epidemiological change from KPC to OXA-48 in our hospital could be related to an OXA-48 CPEs disseminated throughout our country [18]. Therefore, our hospital would be representing just a minor part in what is in fact a nation-wide phenomenon. The epidemiological situation of the CPEs in Spain in 2014, was considered as an interregional dissemination where most CPE isolated were OXA48, which produced a rapid evolution. However, a significant number of KPC CPEs were isolated in 2014 in our institution. Our hypothesis to explain the high frequency of KPC isolations during that year is that probably it was an outbreak of the same clone of KPC K. pneumoniae. Prevalence of KPC genotype has been low in the majority of Spanish hospitals from the first isolations of KPC enzyme in 2010 [1, 30]. Nevertheless several outbreaks of K. pneumoniae KPC-2 and KPC-3 have been characterized in high-risk clones such as ST11, ST101 and ST512 [31, 32] and even colistin-resistant KPC-producing K. pneumoniae [8, 33].
VIM CPE isolations remained practically constant during the period studied, and it also was the genotype less frequently isolated. These data did not coincide with what happened at the same time in Spain, because OXA-48 and VIM were responsible for the major part of the isolates in the country. Currently, the strains of K. pneumoniae OXA-48 are responsible for the most CPE isolations and infections in Spain [18], while a great proportion of OXA-48 are colonizers.
Finally, 40% on CPE isolates were colonizations. Colonization precedes infection in most cases; an intestinal CPE-carrier status may be the source of subsequent clinical infection in approximately 9% of cases [34-38]. In addition, these patients can serve as a repository for the dissemination of CPE in health centers. The outbreaks of CPE are leading to an endemic situation where clinical infections are just the tip of the iceberg but most affected patients are asymptomatic intestinal carriers [7, 24, 39]. At present time, healthy carriers are usually considered as the most important reservoirs of CPE [40]. Also, being CPE colonized did not reduce the impact on the prognosis of these patients, since a third of these died in the following 30 days to the isolation, despite colonizer or pathogen.
In conclusion, we have observed an epidemiological change in the genotypes of our isolates that that could condition a change in the therapeutic protocols. The persistence of OXA48-producing Enterobacteriaceae in the medical wards is a serious problem, this evidence must make a position expressing the need for definitive and coordination action with strong control measures to contain this spread in this nosocomial environment.
FUNDING
None to declare
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Curiao T, Morosini MI, Ruiz-Garbajosa P, Robustillo A, Baquero F, Coque TM, et al. . Emergence of bla KPC-3-Tn4401a associated with a pKPN3/4-like plasmid within ST384 and ST388 Klebsiella pneu-moniae clones in Spain. J Antimicrob Chemother. 2010;65(8):1608-14. doi: 10.1093/jac/dkq174 [DOI] [PubMed] [Google Scholar]
- 2.Ruiz-Garbajosa P, Curiao T, Tato M, Gijon D, Pintado V, Valverde A, et al. . Multiclonal dispersal of KPC genes following the emer-gence of non-ST258 KPC-producing Klebsiella pneumoniae clones in Madrid, Spain. J Antimicrob Chemother. 2013;68(11):2487-92. doi: 10.1093/jac/dkt237 [DOI] [PubMed] [Google Scholar]
- 3.Branas P, Gil M, Villa J, Orellana MA, Chaves F. Molecular epidemi-ology of carbapenemase-producing Enterobacteriaceae infection/ colonisation in a hospital in Madrid. Enferm Infecc Microbiol Clin. 2018;36(2):100-3. doi: 10.1016/j.ijantimicag.2015.02.019 [DOI] [PubMed] [Google Scholar]
- 4.Oteo J, Domingo-Garcia D, Fernandez-Romero S, Saez D, Guiu A, Cuevas O, et al. . Abdominal abscess due to NDM-1-producing Kleb-siella pneumoniae in Spain. J Med Microbiol. 2012;61(Pt 6):864-7. doi: 10.1099/jmm.0.043190-0 [DOI] [PubMed] [Google Scholar]
- 5.Palacios-Baena ZR, Oteo J, Conejo C, Larrosa MN, Bou G, Fer-nandez-Martinez M, et al. . Comprehensive clinical and epidemi-ological assessment of colonisation and infection due to carbap-enemase-producing Enterobacteriaceae in Spain. J Infect. 2016; 72(2):152-60. doi: 10.1016/j.jinf.2015.10.008 [DOI] [PubMed] [Google Scholar]
- 6.Oteo J, Calbo E, Rodriguez-Bano J, Oliver A, Hornero A, Ruiz-Gar-bajosa P, et al. . [The threat of the carbapenemase-producing enterobacteriaceae in Spain: positioning report of the SEIMC study groups, GEIH and GEMARA]. Enferm Infecc Microbiol Clin. 2014;32(10):666-70. doi: 10.1016/j.eimc.2014.02.011 [DOI] [PubMed] [Google Scholar]
- 7.Calfee D, Jenkins SG. Use of active surveillance cultures to detect asymptomatic colonization with carbapenem-resistant Klebsiella pneumoniae in intensive care unit patients. Infect Control Hosp Epidemiol. 2008;29(10):966-8. doi: 10.1086/590661 [DOI] [PubMed] [Google Scholar]
- 8.Cano A, Gutierrez-Gutierrez B, Machuca I, Gracia-Ahufinger I, Perez-Nadales E, Causse M, et al. . Risks of Infection and Mortality Among Patients Colonized With Klebsiella pneumoniae Carbapen-emase-Producing K. pneumoniae: Validation of Scores and Pro-posal for Management. Clin Infect Dis. 2018;66(8):1204-10. doi: 10.1093/cid/cix991 [DOI] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute Performance Standards for Antimicrobial Susceptibility Testing. Twenty-second Informational Supplement M100-S22. 2012.
- 10.Tato M, Coque TM, Ruiz-Garbajosa P, Pintado V, Cobo J, Sader HS, et al. . Complex clonal and plasmid epidemiology in the first outbreak of Enterobacteriaceae infection involving VIM-1 met-allo-beta-lactamase in Spain: toward endemicity ? Clin Infect Dis. 2007;45(9):1171-8. doi: 10.1086/522288 [DOI] [PubMed] [Google Scholar]
- 11.Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakr-ishnan R, et al. . Emergence of a new antibiotic resistance mecha-nism in India, Pakistan, and the UK: a molecular, biological, and ep-idemiological study. Lancet Infect Dis. 2010; 10(9):597-602. PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunter PR, Gaston MA. Numerical index of the discriminatory abili-ty of typing systems: an application of Simpson’s index of diversity. J Clin Microbiol. 1988; 26(11):2465-6. PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez-Gonzalez L, Candel FJ, Vinuela-Prieto JM, Gonzalez-Del Cas-tillo J, Garcia AB, Pena I, et al. . Useful independent factors for distin-guish infection and colonization in patients with urinary carbapen-emase-producing Enterobacteriaceae isolation. Rev Esp Quimioter. 2017;30(6):450-7. PMid: [PubMed] [Google Scholar]
- 14.Shilo S, Assous MV, Lachish T, Kopuit P, Bdolah-Abram T, Yinnon AM, et al. . Risk factors for bacteriuria with carbapenem-resistant Kleb-siella pneumoniae and its impact on mortality: a case-control study. Infection. 2013;41(2):503-9. doi: 10.1007/s15010-012-0380-0 [DOI] [PubMed] [Google Scholar]
- 15.Zilberberg MD, Shorr AF. Secular trends in gram-negative resist-ance among urinary tract infection hospitalizations in the United States, 2000-2009. Infect Control Hosp Epidemiol. 2013;34(9):940-6. doi: 10.1086/671740 [DOI] [PubMed] [Google Scholar]
- 16.Oteo J, Saez D, Bautista V, Fernandez-Romero S, Hernandez-Mo-lina JM, Perez-Vazquez M, et al. . Carbapenemase-producing en-terobacteriaceae in Spain in 2012. Antimicrob Agents Chemother. 2013;57(12):6344-7. doi: 10.1128/AAC.01513-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coelho A, Piedra-Carrasco N, Bartolome R, Quintero-Zarate JN, Lar-rosa N, Cornejo-Sanchez T, et al. . Role of IncHI2 plasmids harbour-ing blaVIM-1, blaCTX-M-9, aac(6’)-Ib and qnrA genes in the spread of multiresistant Enterobacter cloacae and Klebsiella pneumoni-ae strains in different units at Hospital Vall d’Hebron, Barcelona, Spain. Int J Antimicrob Agents. 2012;39(6):514-7. doi: 10.1016/j.ijantimicag.2012.01.006 [DOI] [PubMed] [Google Scholar]
- 18.Oteo J, Hernandez JM, Espasa M, Fleites A, Saez D, Bautista V, et al. . Emergence of OXA-48-producing Klebsiella pneumoniae and the novel carbapenemases OXA-244 and OXA-245 in Spain. J Antimi-crob Chemother. 2013; 68(2):317-21. doi: 10.1093/jac/dks383 [DOI] [PubMed] [Google Scholar]
- 19.Glasner C, Albiger B, Buist G, Tambic Andrasevic A, Canton R, Car-meli Y, et al. . Carbapenemase-producing Enterobacteriaceae in Eu-rope: a survey among national experts from 39 countries, February 2013. Euro Surveill. 2013;18(28). PMid: [DOI] [PubMed] [Google Scholar]
- 20.Carrer A, Poirel L, Yilmaz M, Akan OA, Feriha C, Cuzon G, et al. . Spread of OXA-48-encoding plasmid in Turkey and beyond. An-timicrob Agents Chemother. 2010; 54(3):1369-73. doi: 10.1128/AAC.01312-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tumbarello M, Trecarichi EM, De Rosa FG, Giannella M, Giacobbe DR, Bassetti M, et al. . Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother. 2015; 70(7):2133-43. doi: 10.1093/jac/dkv086 [DOI] [PubMed] [Google Scholar]
- 22.Lopez-Cerero L, Almirante B. Epidemiology of infections caused by carbapenemase-producing Enterobacteriaceae: reservoirs and transmission mechanisms. Enferm Infecc Microbiol Clin. 2014;32 Suppl 4:10-6. doi: 10.1016/S0213-005X(14)70169-7 [DOI] [PubMed] [Google Scholar]
- 23.Pano-Pardo JR, Lopez Quintana B, Lazaro Perona F, Ruiz Carrascoso G, Romero-Gomez MP, Loeches Yague B, et al. . Community-Onset Bloodstream and Other Infections, Caused by Carbapenemase-Pro-ducing Enterobacteriaceae: Epidemiological, Microbiological, and Clinical Features. Open Forum Infect Dis. 2016; 3(3):ofw136. doi: 10.1093/ofid/ofw136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazaro-Perona F, Ramos JC, Sotillo A, Mingorance J, Garcia-Rodriguez J, Ruiz-Carrascoso G, et al. . Intestinal persistence of a plasmid harboring the OXA-48 carbapenemase gene after hospital discharge. J Hosp Infect. 2018. doi: 10.1016/j.jhin.2018.07.004 [DOI] [PubMed] [Google Scholar]
- 25.Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the im-pact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol. 2008. December;29(12):1099-106. doi: 10.1086/592412 [DOI] [PubMed] [Google Scholar]
- 26.Akova M, Daikos GL, Tzouvelekis L, Carmeli Y. Interventional strat-egies and current clinical experience with carbapenemase-produc-ing Gram-negative bacteria. Clin Microbiol Infect. 2012;18(5):439-48. PMid: [DOI] [PubMed] [Google Scholar]
- 27.Oteo J, Alcaraz R, Bou G, Conejo C, Diaz-Lamas AM, Fernan-dez-Martinez M, et al. . Rates of faecal colonization by carbapen-emase-producing Enterobacteriaceae among patients admitted to ICUs in Spain. J Antimicrob Chemother. 2015; 70(10):2916-8. doi: 10.1093/jac/dkv187 [DOI] [PubMed] [Google Scholar]
- 28.Averbuch D, Cordonnier C, Livermore DM, Mikulska M, Orasch C, Viscoli C, et al. . Targeted therapy against multi-resistant bacteria in leukemic and hematopoietic stem cell transplant recipients: guidelines of the 4th European Conference on Infections in Leu-kemia (ECIL-4, 2011). Haematologica. 2013;98(12):1836-47. PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bassetti M, Righi E. Multidrug-resistant bacteria: what is the threat ? Hematology Am Soc Hematol Educ Program. 2013;2013:428-32. PMid: [DOI] [PubMed] [Google Scholar]
- 30.Robustillo Rodela A, Diaz-Agero Perez C, Sanchez Sagrado T, Ruiz-Garbajosa P, Pita Lopez MJ, Monge V. Emergence and out-break of carbapenemase-producing KPC-3 Klebsiella pneumoniae in Spain, September 2009 to February 2010: control measures. Euro Surveill. 2012;17(7). PMid: [PubMed] [Google Scholar]
- 31.Lopez-Cerero L, Egea P, Gracia-Ahufinger I, Gonzalez-Padilla M, Rodriguez-Lopez F, Rodriguez-Bano J, et al. . Characterisation of the first ongoing outbreak due to KPC-3-producing Klebsiella pneumo-niae (ST512) in Spain. Int J Antimicrob Agents. 2014;44(6):538-40. doi: 10.1016/j.ijantimicag.2014.08.006 [DOI] [PubMed] [Google Scholar]
- 32.Oteo J, Perez-Vazquez M, Bautista V, Ortega A, Zamarron P, Saez D, et al. . The spread of KPC-producing Enterobacteriaceae in Spain: WGS analysis of the emerging high-risk clones of Klebsiella pneu-moniae ST11/KPC-2, ST101/KPC-2 and ST512/KPC-3. J Antimicrob Chemother. 2016;71(12):3392-9. doi: 10.1093/jac/dkw321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Machuca I, Gutierrez-Gutierrez B, Perez Cortes S, Gracia-Ahufinger I, Serrano J, Madrigal MD, et al. . Oral decontamination with amino-glycosides is associated with lower risk of mortality and infections in high-risk patients colonized with colistin-resistant, KPC-pro-ducing Klebsiella pneumoniae. J Antimicrob Chemother. 2016; 71(11):3242-9. doi: 10.1093/jac/dkw272 [DOI] [PubMed] [Google Scholar]
- 34.Schechner V, Kotlovsky T, Kazma M, Mishali H, Schwartz D, Nav-on-Venezia S, et al. . Asymptomatic rectal carriage of blaKPC pro-ducing carbapenem-resistant Enterobacteriaceae: who is prone to become clinically infected ? Clin Microbiol Infect. 2013;19(5):451-6. doi: 10.1111/j.1469-0691.2012.03888.x [DOI] [PubMed] [Google Scholar]
- 35.Borer A, Saidel-Odes L, Eskira S, Nativ R, Riesenberg K, Livshiz-Riven I, et al. . Risk factors for developing clinical infection with carbap-enem-resistant Klebsiella pneumoniae in hospital patients initially only colonized with carbapenem-resistant K pneumoniae. Am J In-fect Control. 2012;40(5):421-5. doi: 10.1016/j.ajic.2011.05.022 [DOI] [PubMed] [Google Scholar]
- 36.Wiener-Well Y, Rudensky B, Yinnon AM, Kopuit P, Schlesinger Y, Broide E, et al. . Carriage rate of carbapenem-resistant Klebsiella pneumoniae in hospitalised patients during a national outbreak. J Hosp Infect. 2010;74(4):344-9. doi: 10.1016/j.jhin.2009.07.022 [DOI] [PubMed] [Google Scholar]
- 37.Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, et al. . Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis. 2013; 13(9):785-96. doi: 10.1016/S1473-3099(13)70190-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pano Pardo JR, Serrano Villar S, Ramos Ramos JC, Pintado V. In-fections caused by carbapenemase-producing Enterobacteriaceae: risk factors, clinical features and prognosis. Enferm Infecc Microbiol Clin. 2014;32 Suppl 4:41-8. doi: 10.1016/S0213-005X(14)70173-9 [DOI] [PubMed] [Google Scholar]
- 39.Feldman N, Adler A, Molshatzki N, Navon-Venezia S, Khabra E, Co-hen D, et al. . Gastrointestinal colonization by KPC-producing Kleb-siella pneumoniae following hospital discharge: duration of car-riage and risk factors for persistent carriage. Clin Microbiol Infect. 2013;19(4):E190-6. doi: 10.1111/1469-0691.12099 [DOI] [PubMed] [Google Scholar]
- 40.Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. Carbapenemases in Klebsiella pneumoniae and other Entero-bacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev. 2012t;25(4):682-707. doi: 10.1128/CMR.05035-11 [DOI] [PMC free article] [PubMed] [Google Scholar]