ABSTRACT
Objectives
The aim of this study is to analyze the effect, safety and tolerability of a specific probiotic combination of Lactobacillus and Bifidobacterium strains (Pearls IC©) on antibiotic associated diarrhea due to amoxicillin-clavulanic acid treatment.
Patients and methods
Pilot, unicentric, randomized, double-blind, parallel group, placebo-controlled study (probiotic vs. placebo for 30 days). Target population: Adult patients, both sexes treated with amoxicillin-clavulanic acid (850mg / 125mg every 8h /orally) for 7 days who attended the Emergency Department (Dexeus Hospital, Barcelona) between January and April of 2018 with prior signed informed consent with a follow up at 30 days. Variables: The differences between day 0 and day 30 of the number of daily stools and duration of diarrhea were evaluated; Stool consistency according to Bristol Stool Form, Quality of intestinal life. Subjective evaluation and evaluation of adverse effects of the product through a specifically designed questionnaire.
Results
Thirty-six subjects were included (18 per group) 25 females and 11 males, average age of 38.5 years (range 19-65 years). Pearls IC© delayed between 4 and 5 days the appearance of the diarrheic episode vs. placebo (p <0.001). The results of the quality of life assessment showed an improvement at the end of the 30 days period but without difference vs placebo. The results of the subjective assessment were in favor of the probiotic with higher rate of like responses than placebo.
Conclusions
Pearls IC© demonstrated its beneficial effect on antibiotic associated diarrhea by delaying the onset of diarrhea and showed a tendency to decrease the number of daily stools vs. placebo.
Keywords: Lactobacillus, Bifidobacterium, antibiotic-associated diarrhea, amoxicillin-clavulanic acid
RESUMEN
Introducción
El objetivo es analizar el efecto y la seguridad de una combinación probiótica específica de lactobacilos y bifidobacterias (Pearls IC©) en la diarrea asociada a antibióticos debida al tratamiento con amoxicilina-ácido clavulánico.
Pacientes y métodos
Estudio piloto, unicéntrico, aleatorizado, doble ciego, paralelo, controlado con placebo. Población objetivo: pacientes adultos, ambos sexos tratados con amoxicilina-ácido clavulánico (850mg / 125mg cada 8h / oral) durante 7 días que asistieron a un Servicio de Urgencias entre enero y abril de 2018 con consentimiento informado previo firmado con un seguimiento a los 30 días. Variables: se evaluaron las diferencias entre el día 0 y el día 30 del número de deposiciones diarias y la duración de la diarrea; Consistencia de las heces según la forma de heces de Bristol, calidad de vida intestinal. Evaluación subjetiva y evaluación de los efectos adversos del producto a través de un cuestionario específicamente diseñado.
Resultados
Se incluyeron 36 sujetos (18 por grupo), 25 mujeres y 11 hombres, con una edad promedio de 38,5 años (rango 19-65 años). Pearls IC© retrasó entre 4 y 5 días la aparición del episodio diarreico versus placebo (p<0,001). Los resultados de la evaluación de la calidad de vida mostraron una mejoría al final del período de estudio, pero sin diferencias frente a placebo. Los resultados de la evaluación subjetiva fueron a favor del probiótico con una tasa más alta de respuestas similares que el placebo.
Conclusiones
Pearls IC© demostró su efecto beneficioso sobre la diarrea asociada a antibióticos al retrasar el inicio de la diarrea y mostró una tendencia a disminuir el número de deposiciones diarias versus placebo.
Palabras clave: Lactobacillus, Bifidobacterium, diarrea asociada a antibióticos, amoxicilina-ácido clavulánico
INTRODUCTION
Antibiotic-associated diarrhea (AAD) is defined as diarrhea that occurs in relation to the administration of antibiotics [1] and that is not explained by other causes, must be clinically significant and have more than 3 liquid stools per day [2]. Its frequency oscillates between 5% and 25% of patients and varies depending on the type of antibiotic administered, 5-10% with ampicillin, 15-20% with cefixime and 10-25% with amoxicillin-clavulanic acid [1].
The AAD is produced mainly by three mechanisms [3]: Selection or overgrowth of enteropathogenic bacteria, by the suppression of the endogenous intestinal flora that alters the metabolism of bile acids and of non-absorbed carbohydrates and due to the direct pharmacological effect of antibiotics.
Amoxicillin-clavulanic acid is one of the most widely used antibiotics and one of the most frequent causes of AAD and other gastrointestinal disorders [4, 5].
Probiotics help to maintain the balance of the intestinal microbiota and they have been used for many years for the treatment and prevention of AAD [6-11].
The objective of this pilot study is to analyze the effect and safety of a specific combination of probiotic strains, Pearls IC© on the prevention and evolution of diarrhea associated with amoxicillin-clavulanic acid.
PATIENTS AND METHODS
Pilot, randomized, double-blind, placebo-controlled study involving patients over 18 years of both sexes who had signed informed consent and who had been prescribed amoxicillin-clavulanic acid for 7 days at a dose of 850mg / 125mg every 8 hours. It was not allowed the inclusion in the study of patients with other gastrointestinal pathologies, with hypersensitivity to any of the study products, in treatment with other medications that could alter the results of the study, or if they were taking laxatives, suffering a process that would condition the occurrence of diarrhea or being pregnant.
One group was given Pearls IC© from the beginning of the antibiotic treatment until completing 30 days at a dose of 1 capsule per day and placebo the other one. Pearls IC© is a probiotic from Schwabe Farma Ibérica that contains a combination of six strains: Lactobacillus acidophilus NCFM, Lactobacillus rhamnosus Lr-32, Bifidobacterium breve M-16V, Bifidobacterum longum BB536, Bifidobacterium lactis BI-04 and Bifidobacterium bifidum Bb-02, with 109 colony forming units per capsule.
The study was carried out in the Emergency Department of the Hospital Universitari Dexeus-Quirónsalud Group of Barcelona and was previously approved by the Center’s Ethical Committee.
The number of daily stools and values of the stool scale Bristol Stool Form (BSS) were analyzed (from 1-7 according to the consistency) and recorded in the diary provided to the patient at visit 1. For the evaluation of diarrhea (presence or absence), the day of occurrence of the first diarrheal event and the number of days with diarrheal episodes, four criteria for assessment were used: score above 5 in the BSS after the 3rd day of antibiotic treatment, 3 or more stools from a previous value after the 3rd day of antibiotic treatment, presence of one or the other and the combination of one and the other.
Quality of life was assessed through the modified Quality of intestinal life GIQLI questionnaire in visit 1 and visit 30 (end of the study), the questions were grouped into five areas for evaluation separately: Symptoms, Emotional, Physical, Treatment and Social. The characteristics of the product were evaluated through a subjective questionnaire, completed by the patient at the final visit (T30). The safety of the product was evaluated through the monitoring of the adverse effects.
For the statistical analysis we used the software SPSS version 23 and the free software R version 3.3.2 (2016-10-31) “Sincere Pumpkin Patch” Copyright (C) 2016.
For the estimation of the sample size it was considered that the prevalence of the effect of the antibiotic in the stools was 75% and an expected reduction with the probiotic was 35%, so for a power of 80 % and a confidence of 95% a sample size of 40 patients was estimated.
RESULTS
Between January and April 2018, 40 patients were recruited who met the inclusion criteria, 4 of whom were lost to follow-up. Of the 36 remaining patients, 18 were assigned to the Pearls IC© group and 18 to the placebo one. There was a predominance of women over men homogeneously in both arms, in total 25 women (13 with probiotics and 12 with placebo) over 11 men (5 vs 6, respectively). The mean age and age range of the patients included in the group treated with Pearls IC© was 40.72 (19-65) and in the placebo group of 36.83 (19-62).
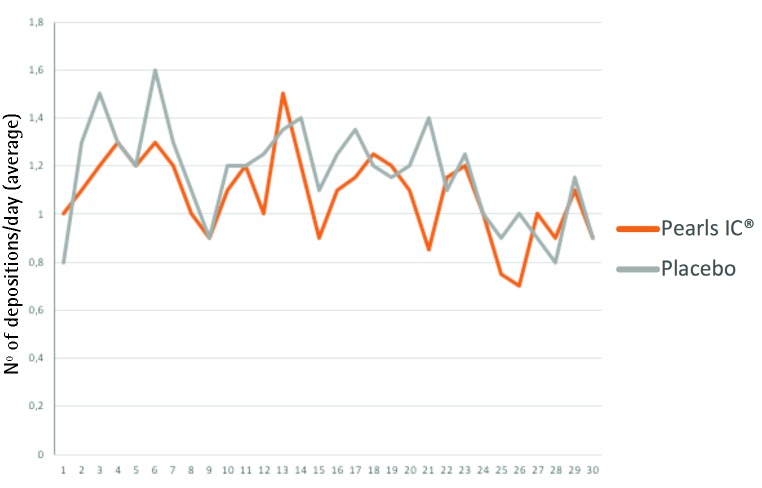
It was found that the probiotic was able to delay the occurrence of diarrhea in a statistically significant and consistent manner over three days using the four endpoints (table 1). Using the most demanding criterion (stool consistency criterion and the number of stools) Pearls IC© delayed the appearance of diarrhea in 5.39 days (p = 0.002). On the other hand, no statistically significant differences were found between the two treatment arms in terms of the percentage of diarrhea occurrence or in relation to the number of days with diarrhea, although there was a tendency towards a lower number of daily stools without statistical significance (figure 1).
Table 1.
Effect of Pearls IC© over diarrhea using the four endpoints: consistency, number of stools, consistency and/or number of stools.
| Diarrhea onset daya | Pearls IC© | Placebo | Difference | Mann-Whitney | P value |
| Consistency (1) | 7.55 | 4.44 | 3.10 | 78.5 | 0.010* |
| Depositions, n (2) | 9.17 | 3.00 | 6.17 | 27.4 | 0.008* |
| (1) or (2) | 8.25 | 4.44 | 3.81 | 87.5 | 0.006* |
| (1) and (2) | 9.83 | 4.44 | 5.39 | 93.5 | 0.002* |
| Diarrheab | Pearls IC© (n) | Placebo (n) | Odds ratio | IC 95% | |
| Consistency (1) | 61.1% (11) | 50% (9) | 1.57 | (0.42; 5.90) NS | |
| Depositions, n (2) | 33.3% (6) | 27.8% (5) | 1.3 | (0.31; 5.39) NS | |
| (1) or (2) | 66.7% (12) | 50.0% (9) | 2 | (0.52; 7.69) NS | |
| (1) and (2) | 27.8% (5) | 27.8% (5) | 1 | (0.23; 4.30) NS | |
| Nº of days with diarrheab | Pearls IC© | Placebo | Diferencia | Mann-Whitney | P value |
| Consistency (1) | 3.50 | 2.17 | 1.33 | 191.5 | 0.16 |
| Depositions, n (2) | 0.78 | 2.17 | -1.39 | 164.5 | 0.45 |
| (1) or (2) | 3.56 | 3.44 | 0.11 | 190.5 | 0.17 |
| (1) and (2) | 0.72 | 0.89 | -0.17 | 158.5 | 0.43 |
aThe probiotic delays the onset of diarrhea compared with placebo in more than 3 days, in a statistically significant way (*)
bNo statistically significant differences were found in the percentage of patients with diarrhea and the average number of daily stools between the two treatment groups.
Figure 1.
Average number of daily stools, where a tendency to a lower mean is observed in the arm treated with the probiotic.
In relation to the quality of life assessment, there was no differences between arms at the end of the study (improvements of 4.2 points for Pearls IC© and 5.03 for placebo, p= ns). The improvements were greater with the probiotic than in the placebo arm in the Symptoms area (0.05 vs 0.03) and in the Emotional one (0.13 vs 0.03), although it was not reached statistical significance.
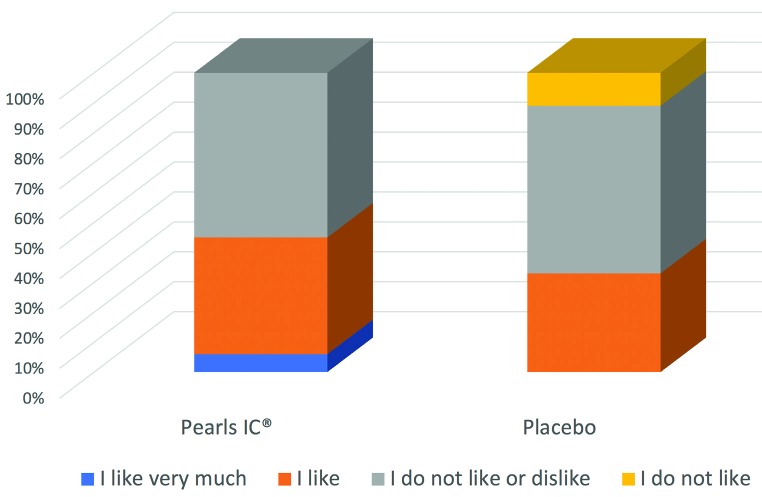
The subjective evaluations found that 45% of the probiotic evaluations were clearly positive compared to 33% in the case of the group treated with placebo, which included 11% with a very negative assessment (figure 2). No adverse effects were registered in either of the two treatment arms.
Figure 2.
Subjective ratings showed a higher percentage of patients with a positive rating than in the placebo arm.
DISCUSSION
Probiotics are live microorganisms that are able to reach the intestinal mucosa in sufficient quantity to confer benefits to the host, as they help maintain normal intestinal flora and reduce the colonization of pathogenic organisms by competitive inhibition of epithelial and mucosal adhesion [12]. It has been shown to secrete acetic and lactic acids that lower intraluminal pH and inhibit the growth of some pathogens, including enterohemorrhagic Escherichia coli [13-15]. Probiotics can also inhibit the growth of other pathogenic bacteria through the production of antimicrobial molecules, including shortchain fatty acids, bacteriocins and microcines [15], in addition to their immunomodulatory effect that can counteract the proinflammatory effect of pathogenic bacteria [16].
In view of the lack of clinical evidence demonstrating the beneficial effect of the administration of probiotics during antibiotic treatment, this study was carried out with a specific combination of 6 strains, Pearls IC©, to analyze its effect on the AAD. To this end, amoxicillin-clavulanic acid was selected because of its wide use and because diarrhea is one of its most frequent adverse effects, around 20% [4] and patients were included who were to receive this antibiotic for at least a week, without taking into account the site of the infection and was compared with masked placebo.
One of the limitations of the study, besides it was a pilot study, is the small sample size, only 18 patients in each treatment group, which has not allowed to show differences compared to the group treated with placebo in some of the variables studied.
Four definitions of diarrhea were included, in order to achieve a greater objectivity in the assessment, including the combination of the consistency of feces and the number of stools. This criterion showed a delay of more than 5 days in the beginning of diarrhea vs the placebo treatment arm showing statistical significance, although a reduction in the percentage of patients who developed diarrhea could not be demonstrated, at least during the period of the 30 days of the study. A tendency to a lower number of daily stools could be seen in the group treated with the probiotic compared to placebo, although without statistical significance, probably due to the small sample size.
Regarding the quality of life, there were no significant differences between the two groups, although there was a tendency towards greater improvement in the areas of Symptoms and Emotional in favor of the probiotic, as well as in the subjective assessment, which favored the treatment with Pearls IC©, which was better valued. Regarding safety, no adverse events were reported in any of the two arms, which confirms the safety of the product.
As conclusions, Pearls IC© significantly delayed the first diarrheic episode between 4 and 5 days compared to placebo and showed a tendency to decrease the number of daily stools compared with placebo, although not statistically significant, improved quality of life and specifically some areas and subjective assessment by patients was favorable compared to placebo, so that Pearls IC© seems to have a beneficial effect on the imbalance of intestinal flora induced by antibiotics, which should be demonstrated in studies with larger sample size.
ACKNOWLEDGEMENTS
The results of this study have been presented in poster format in the congress of the SEPYP (Probiotic and Prebiotic Spanish Society) that was held in Las Palmas, February 6-8, 2019.
FUNDING
This study was funded by Schwabe Farma Ibérica.
CONFLICTS OF INTEREST
Two authors are employees of Schwabe Farma Ibérica. No other conflicts of interest declared.
REFERENCES
- 1.Bartlett JG. Antibiotic-associated diarrhea. N Engl J Med. 2002; 346(5):334-339. PMid: [DOI] [PubMed] [Google Scholar]
- 2.Hogenauer C, Hammer HF, Krejs GJ, et al. Mechanisms and man-agement of antibiotic-associated diarrhea. Clin Infect Dis. 1998; 27(4):702-710. PMid: [DOI] [PubMed] [Google Scholar]
- 3.Varughese CA, Vakil NH, Phillips KM. Antibiotic-associated diar-rhea: a refresher on causes and possible prevention with probiot-ics--continuing education article. J Pharm Pract. 2013;26(5):476-82. PMid: [DOI] [PubMed] [Google Scholar]
- 4.Amoxicilina y Ácido Clavulánico: MedlinePlus medicinas [Internet]. [cited 2017 May 8]. Available from: https://medlineplus.gov/spanish/druginfo/meds/a685024-es.html
- 5.Rodríguez-Varón A, Muñoz OM, Pulido-Arenas J, Amado SB, To-bón-Trujillo M. Diarrea asociada a antibióticos: características clínicas y presencia de Clostridium difficile. Rev Gastroenterol Mex. 2017; 82(2):129-133. doi: 10.1016/j.rgmx.2016.10.003 [DOI] [PubMed] [Google Scholar]
- 6.Evans M, Salewski RP, Christman MC, Girard S-A, Tompkins TA. Effectiveness of Lactobacillus helveticus and Lactobacillus rhamnosus for the management of antibiotic-associated di-arrhoea in healthy adults: a randomised, double-blind, place-bo-controlled trial. Br J Nutr. 2016;116(1):94-103. doi: 10.1017/S0007114516001665. [DOI] [PubMed] [Google Scholar]
- 7.Kołodziej M, Szajewska H. Lactobacillus reuteri DSM 17938 in the prevention of antibiotic-associated diarrhoea in children: protocol of a randomised controlled trial. BMJ Open. 2017;7(1): e013928 DOI: 10.1136/bmjopen-2016-013928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engelbrektson A, Korzenik JR, Pittler A, Sanders ME, Klaenhammer TR, Leyer G, Kitts CL. Probiotics to minimize the disruption of fae-cal microbiota in healthy subjects undergoing antibiotic therapy. J Med Microbiol. 2009; 58: 663–670. DOI 10.1099/jmm.0.47615-0 [DOI] [PubMed] [Google Scholar]
- 9.Johnston BC, Ma SS, Goldenberg JZ, Thorlund K, Vandvik PO, Loeb M, Guyatt GH. Probiotics for the prevention of Clostridium difficile–Associated Diarrhea. Ann Intern Med. 2012;157(12):878-88. PMid: [DOI] [PubMed] [Google Scholar]
- 10.Pattani R, Palda VA, Hwang SW, Shah PS. Probiotics for the pre-vention of antibiotic-associated diarrhea and Clostridium difficile infection among hospitalized patients: systematic review and me-ta-analysis. Open Med. 2013;7(2):e56-67. eCollection 2013. [PMC free article] [PubMed] [Google Scholar]
- 11.McFarland LV, Ship N, Auclair J, Millette M. Primary prevention of Clostridium difficile infections with a specific probiotic combin-ing Lactobacillus acidophilus, L. casei, and L. rhamnosus strains: assessing the evidence. J Hosp Infect. 2018;99(4):443-452. PMid: . [DOI] [PubMed] [Google Scholar]
- 12.Sherman PM, Johnson-Henry KC, Yeung HP, et al. Probiotics re-duce enterohemorrhagic Escherichia coli O157: H7 and enter-opathogenic E.coli O127: H6-induced changes in polarized T84 epithelial cell monolayers by reducing bacterial adhesion and cy-toskeletal rearrangements. Infect Immun. 2005;73(8):5183-5188. PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogawa M, Shimizu K, Nomoto K, et al. Inhibition of in vitro growth of Shiga toxin-producing Escherichia coli O157: H7 by probiotic lactobacillus strains due to production of lactic acid. Int J Food Mi-crobiol. 2001;68(1-2):135-140. PMid: [DOI] [PubMed] [Google Scholar]
- 14.Alvarez-Olmos MI, Oberhelman RA. Probiotic agents and infectious diseases: a modern perspective on a traditional therapy. Clin Infect Dis. 2001;32(11):1567-1576. PMid: [DOI] [PubMed] [Google Scholar]
- 15.Doron S, Gorbach SL. Probiotics: their role in the treatment and prevention of disease. Expert Rev Anti Infect Ther. 2006; 4(2): 261-275. PMid: [DOI] [PubMed] [Google Scholar]
- 16.Morrow LE, Gogineni V, Malesker MA. Probiotics in the intensive care unit. Nutr Clin Pract. 2012;27(2):235-241. PMid: . [DOI] [PubMed] [Google Scholar]