Abstract
OBJECTIVE
Lifestyle intervention remains the cornerstone of management of type 2 diabetes mellitus (T2DM). However, adherence to physical activity (PA) recommendations and the impact of that adherence on cardiorespiratory fitness in this population have been poorly described. We sought to investigate adherence to PA recommendations and its association with cardiorespiratory fitness in a population of patients with T2DM.
RESEARCH DESIGN AND METHODS
A cross-sectional analysis of baseline data from a randomized clinical trial (NCT00424762) was performed. A total of 150 individuals with medically treated T2DM and atherosclerotic cardiovascular disease (ASCVD) or risk factors for ASCVD were recruited from outpatient clinics at a single academic medical center. All individuals underwent a graded maximal exercise treadmill test to exhaustion with breath-by-breath gas exchange analysis to determine VO2peak. PA was estimated using a structured 7-Day Physical Activity Recall interview.
RESULTS
Participants had a mean ± SD age of 54.9 ± 9.0 years; 41% were women, 40% were black, and 21% were Hispanic. The mean HbA1c was 7.7 ± 1.8% and the mean BMI, 34.5 ± 7.2 kg/m2. A total of 72% had hypertension, 73% had hyperlipidemia, and 35% had prevalent ASCVD. The mean ± SD reported daily PA was 34.3 ± 4 kcal/kg, only 7% above a sedentary state; 47% of the cohort failed to achieve the minimum recommended PA. Mean ± SD VO2peak was 27.4 ± 6.5 mL/kg fat-free mass/min (18.8 ± 5.0 mL/kg/min).
CONCLUSIONS
On average, patients with T2DM who have or are at risk for ASCVD report low levels of PA and have low measured cardiopulmonary fitness. This underscores the importance of continued efforts to close this therapeutic gap.
Introduction
Lifestyle interventions, including weight loss and physical activity (PA), are cornerstones in treating type 2 diabetes mellitus (T2DM) and preventing associated atherosclerotic cardiovascular disease (ASCVD)–related complications (1). Weight loss and PA have long been promoted as the first line of treatment to prevent many of the chronic complications associated with T2DM (2). Higher levels of PA and cardiorespiratory fitness are associated with a lower risk of mortality (3,4) and adverse cardiovascular events (5,6) among patients living with T2DM. Furthermore, improvements in PA level and cardiorespiratory fitness prevent weight regain (7), improve glycemic control (8,9), improve quality of life (10), and reduce the risk of heart failure (11). Many of these favorable effects occur regardless of changes in BMI (12,13).
Several organizations publish guidelines that set forth minimum recommended levels of PA required to favorably affect health (14–16). For example, the Physical Activity Guidelines for Americans recommend that all adults—including those with chronic conditions—do at least 150 cumulative minutes of moderate-intensity aerobic PA or at least 75 min of vigorous aerobic PA per week in order to achieve health benefits (16). Although these recommendations are well established, only 10–23% of Americans meet these recommendations at the population level (17,18). The pattern of adherence to guideline-recommended PA levels has been less well characterized among a diverse population of patients with T2DM who could gain substantial morbidity- and mortality-related benefits from increasing adherence to PA (19), especially those with or at high risk for ASCVD and across sex and race/ethnicity strata. Furthermore, the association of adherence to guideline-recommended PA levels with objective measures of cardiorespiratory fitness has not been well described in this patient population. Given these prescribed minimum PA recommendations and the evidence to support their application as the minimum standard of care in treating T2DM, the purpose of this study was to describe self-reported levels of PA and adherence to the PA guideline recommendations, in conjunction with direct measurement of fitness through the use of cardiopulmonary exercise testing, in a diverse (sex and race/ethnicity) group of medically treated ambulatory patients with T2DM who have or are at high risk for ASCVD.
Research Design and Methods
Study Design and Population
This is a cross-sectional analysis of the baseline data from a previous single-center randomized trial evaluating the cardiovascular effects of rosiglitazone (clinical trials reg. no. NCT00424762, ClinicalTrials.gov); the trial design and primary trial results have been previously reported (20,21). Patients were recruited from among those with data in an existing research database and those who attended outpatient cardiology and diabetes clinics at Parkland Hospital and Health System and the University of Texas Southwestern Medical Center, and by public advertisement. Participants were eligible if they had medically treated T2DM and either prevalent ASCVD (coronary artery disease [CAD], myocardial infarction, revascularization, cerebrovascular accident, transient ischemic attack, carotid artery disease, or peripheral arterial disease) or had at least one risk factor for ASCVD (smoking, hypertension [HTN], hypercholesterolemia, albuminuria, family history of premature CAD, or documented hs-CRP >3 mg/L). T2DM was defined as a prior clinical diagnosis of the disease and current use of antihyperglycemic therapy. Men and women aged >18 years were eligible. Targeted recruitment aimed to obtain an ethnically diverse cohort comprising about one-third each of non-Hispanic white, black, and Hispanic participants. Criteria for exclusion from the original study included treatment with a thiazolidinedione within the prior 6 months, documented intolerance of thiazolidinediones, history or evidence of heart failure, or serum liver transaminases greater than three times the upper limit of normal. The University of Texas Southwestern Medical Center Institutional Review Board approved the study protocol. All participants provided written informed consent before data collection.
Evaluations
All participants underwent physical examination, during which waist and hip circumferences, height (inches), and weight (kilograms) were measured. Body weight was measured to the nearest 0.1 kg on a calibrated scale. Height was obtained with a standard stadiometer and reported to the nearest 0.1 cm. Waist circumference at the midpoint between the iliac crest and the last lower ribs was measured with an anthropometric tape to the nearest 0.1 cm. Hip circumference at the widest part of the buttocks was measured with an anthropometric tape to the nearest 0.1 cm. Trained research staff estimated percentage body fat using skinfold thickness testing with calipers over four sites (the subscapular, biceps, triceps, and suprailiac areas), and duplicate measures were averaged (22). Participants completed the investigator-administered 7-Day Physical Activity Recall (PAR) interview (23). A graded cardiopulmonary exercise treadmill test to exhaustion allowed us to calculate VO2peak.
PAR Interview
The PAR interview is a validated method for estimating PA. A certified interviewer administers the survey, which requires recall of activity over the prior 7 days, consisting of 5 weekdays and 2 weekend days (23–25). For our purposes, each day was broken into morning, afternoon, and evening time brackets. Participants were asked to recall the amount of time spent sleeping and doing moderate, hard, and very hard activities. We classified moderate activities as those comparable to walking at a regular pace. Very hard–intensity activities were comparable to running. Hard activities included activities that were in-between moderate and very hard intensity. Light activities were assumed to make up the remainder of the time not accounted for and included activities such as activities of daily living, walking <5 min, standing, sitting, and so on. Only activities with a duration ≥5 min were recorded. All time was summed and recorded as total hours. Total weekly time in hours was converted to MET units (METs) using the following equation (23): Total weekly energy expenditure (kcal/kg) = Sleep (1.0 * METs) + Light activity (1.5 * METs) + Moderate activity (4.0 * METs) + Hard activity (6.0 * METs) + Very hard activity (10 * METs).
Cardiopulmonary Exercise Testing
The cardiopulmonary exercise test consisted of a graded treadmill test to exhaustion and integrated measurement of oxygen consumption, which was measured by using the MedGraphics Cardiopulmonary Exercise System (CPX/D; Medical Graphics Corp., St. Paul, MN). Ventilatory and gas exchange variables were measured with breath-by-breath monitoring through a mouthpiece module connected to the CPX system. The system was calibrated against known gas concentrations and volumes before each test. A 12-lead echocardiogram (Formula System; Biosound, Indianapolis, IN) was used for continuous cardiac monitoring. The initial treadmill speed was chosen on the basis of individual participant comfort during a warm-up before starting the test on the CPX system; beginning at 1.7 mph at 0% grade, the speed was gradually increased until the participant’s heart rate was at 60–70% of the age-predicted maximal rate or until the participant reported a rate of perceived exertion (RPE) of 11–13 based on the Borg scale. This speed was maintained for 4 min. After a 5- to 10-min rest, patients performed a maximal graded treadmill test using a modified Balke protocol at test speed, during which the grade increased 2% every 2 min.
Heart rate and RPE were recorded during the final 15 s of each 2-min stage. Blood pressure was measured manually and recorded during the 2nd minute of each stage. VO2 and respiratory exchange ratio over 30-s intervals were averaged and recorded. Peak heart rate and RPE were the maximal values achieved during the test. Peak values for gas exchange variables were calculated as the two highest consecutive 30-s values within the last 2 minutes. Given the high prevalence of obesity among patients in the cohort, we scaled peak oxygen consumption to fat-free mass (VO2peak-FFM), because this method accounts more precisely for the confounding effect of adiposity, which is not trivial in this population (26). Fat-free mass was calculated by using (100% − % body fat) × total body weight (kg). We report VO2peak instead of VO2max because confirming VO2max requires specific objective criteria that were not obtained in this study; these criteria include but are not limited to demonstrating leveling, that is, achieving a VO2 plateau with an increase in workload that is not associated with additional increase in VO2; respiratory exchange ratio >1.1; ventilatory equivalent for oxygen uptake (VE/VO2) >35; arterialized lactate >6 mmol/L; and respiratory rate >30 (26).
Statistical Methods
These analyses are restricted to baseline data collected at trial entry. Baseline characteristics are reported as the mean ± 1 SD, and categorical variables are presented as number (percentage). The mean levels of reported activity had a positive skew, so we report median values in addition to mean values. We used Spearman ρ correlations and multiple linear regression models to test for associations between VO2peak-FFM and METs. The linear regression models were built sequentially, first adjusting for age, sex, and race, and then adding BMI, then glycosylated hemoglobin (HbA1c), and finally diabetes duration. Recommended PA was compared among participants in each subgroup. We determined significance using the χ2 test, and we tested for the heterogeneity of association by determining the formal sex-by-race/ethnicity interaction; we performed similar interaction testing for VO2peak-FFM. Statistical significance was defined as a two-sided P value ≤0.05. All statistical testing was performed by using SAS version 9.2 (SAS Institute Inc., Cary, NC).
Results
Baseline characteristics of the 150 participants who underwent baseline testing are presented in Table 1. The mean ± SD age was 54.9 ± 9.0 years; 41% of the cohort were women, 40% were black, 33% were non-Hispanic white, and 21% were Hispanic. Study participants had a mean ± SD BMI of 34.5 ± 7.2 kg/m2; HTN (72%) and hyperlipidemia (73%) were prevalent. Over one-third of participants (35%) had a history of ASCVD. Less than half of participants (41%) were taking insulin, and 66% reported metformin use. Mean ± SD HbA1c was 7.7 ± 1.8%, and the median duration of T2DM was 7 years (interquartile range [IQR] 4, 12 years).
Table 1.
Baseline characteristics of participants
| Overall(n = 150) | Men(n = 88) | Women(n = 62) | Black(n = 60) | White(n = 49) | Hispanic(n = 32) | |
|---|---|---|---|---|---|---|
| Women, n (%) | 62 (41) | – | – | 29 (48) | 21 (43) | 10 (31) |
| Age (years), mean (SD) | 54.9 (9.0) | 55.0 (9.3) | 54.7 (8.7) | 54.7 (8.9) | 56.2 (8.4) | 53.7 (9.8) |
| Duration of T2DM (years), median (IQR) | 7 (4, 12) | 8 (4, 12) | 6 (3, 10) | 8 (5, 14) | 6 (3, 10) | 5.5 (4, 9) |
| Race/ethnicity, n (%) | ||||||
| Black | 60 (40) | 31 (35) | 29 (47) | – | – | – |
| White | 49 (33) | 28 (32) | 21 (34) | – | – | – |
| Hispanic | 32 (21) | 22 (25) | 10 (16) | – | – | – |
| Other | 9 (6) | 7 (8) | 2 (3) | – | – | – |
| Anthropometrics, mean (SD) | ||||||
| Weight (kg) | 99.5 (21.6) | 100.6 (21.8) | 97.9 (21.5) | 100.5 (21.6) | 103.8 (21.3) | 97.3 (20.6) |
| BMI (kg/m2) | 34.5 (7.2) | 32.9 (5.9) | 36.9 (8.2) | 35.2 (7.8) | 34.6 (6.3) | 35.2 (7.1) |
| Body fat (%) | 31.5 (7.5) | 28.4 (6.4) | 36.0 (6.5) | 31.7 (7.5) | 32.7 (7.1) | 30.7 (7.5) |
| Blood pressure (mmHg) | ||||||
| Systolic | 147.2 (23.3) | 148.1 (22.6) | 146 (24.4) | 153.1 (27.7) | 143.1 (18.7) | 145.1 (21.8) |
| Diastolic | 82.7 (12.7) | 84.8 (12) | 79.8 (13) | 85.7 (14.1) | 80.6 (11.7) | 81.2 (12.2) |
| Comorbidities, n (%) | ||||||
| Current smoking | 25 (17) | 16 (18) | 9 (15) | 12 (20) | 8 (16) | 4 (13) |
| HTN | 108 (72) | 64 (73) | 44 (71) | 47 (78) | 35 (71) | 22 (69) |
| Hyperlipidemia | 110 (73) | 65 (74) | 45 (73) | 44 (73) | 37 (76) | 21 (66) |
| Prior ASCVD | 52 (35) | 31 (35) | 21 (34) | 20 (33) | 16 (33) | 13 (41) |
| Medication use, n (%) | ||||||
| Aspirin | 64 (43) | 40 (45) | 24 (39) | 28 (47) | 22 (45) | 11 (34) |
| β-Blocker | 50 (33) | 29 (33) | 21 (34) | 22 (37) | 17 (35) | 10 (31) |
| ACE inhibitor or ARB | 90 (60) | 49 (56) | 41 (66) | 39 (65) | 30 (61) | 17 (53) |
| Statin | 83 (55) | 50 (57) | 33 (53) | 32 (53) | 28 (57) | 18 (56) |
| Insulin | 62 (41) | 39 (44) | 23 (37) | 31 (52) | 20 (41) | 9 (28) |
| Metformin | 99 (66) | 52 (59) | 47 (76) | 37 (62) | 34 (69) | 23 (72) |
| Sulfonylurea | 45 (30) | 27 (31) | 18 (29) | 17 (28) | 13 (27) | 12 (38) |
| Laboratory values, mean (SD) | ||||||
| HbA1c (%) | 7.7 (1.8) | 7.8 (1.9) | 7.5 (1.7) | 8 (1.8) | 7.5 (1.9) | 7.8 (1.8) |
| LDL cholesterol (mg/dL) | 105.4 (43.8) | 102.6 (42.9) | 109.4 (45) | 111.8 (42.7) | 99.1 (46.9) | 101.4 (37.4) |
| HDL (mg/dL) | 43.9 (9.7) | 41.5 (8.1) | 47.4 (10.6) | 45.4 (10.3) | 44.5 (10.1) | 42.2 (8.0) |
| TG (mg/dL) | 176.1 (122.5) | 190.8 (135.5) | 155.4 (98.6) | 138.2 (89.8) | 214.3 (145.8) | 193.7 (126.3) |
| Estimated GFR (mL/min)* | 90.3 (28.9) | 90.4 (25.6) | 90.1 (33.3) | 93.7 (34.2) | 81.5 (22.8) | 97.5 (27.4) |
ARB, angiotensin receptor inhibitor; GFR, glomerular filtration rate; TG, triglycerides.
*GFR was estimated through the use of the CKD-EPI equation.
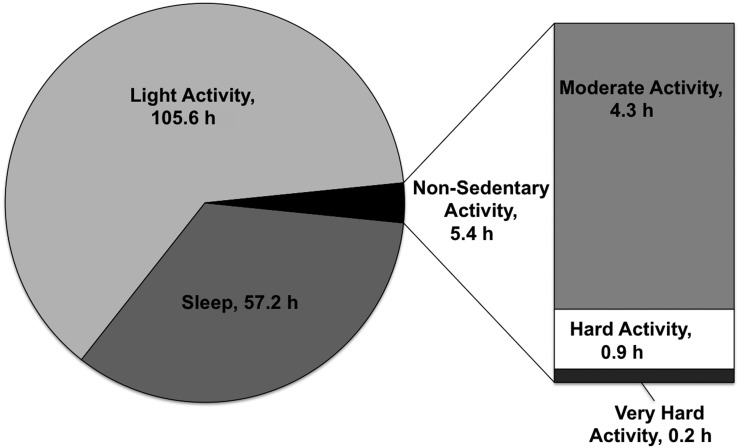
The PA patterns of participants in the sample are described in Supplementary Table 1. Overall, participants reported a median of 13 h (IQR 3.7, 30.3 h) of total moderate, hard, and very hard activities over the 7-day recall period. When we stratified activity by categories of exercise intensity, participants reported a median of 2.1 h (IQR 0.7, 5.2 h) of moderate-intensity exercise and almost no hard-intensity or very hard–intensity activity weekly. The majority of participants’ time was spent doing light-intensity activity (median 105.5 h [IQR 98.3, 114.8 h]) and sleeping (median 56.7 [IQR 48.8, 64 h]). Women spent less time than men doing moderate, hard, and very hard activities and more time doing light activities, although these differences were not statistically significant (P > 0.05). Among participants, 84% reported some moderate-intensity activity, whereas 21% reported some hard-intensity activity; only 8% reported some very hard–intensity activity. Only 68 of 150 participants (45%) reported weekly PA at or above the minimum recommended by the guidelines. On average, participants spent 97% of their time sleeping or doing light activity (Fig. 1).
Figure 1.
Breakdown of mean total time (hours) spent during each activity category over 1 week (total, 168 h/week). All hours are weekly totals.
The overall mean ± SD VO2peak-FFM was 27.4 ± 6.5 mL/kg FFM/min. Women had lower VO2peak-FFM than did men (overall, 24.7 ± 5.6 vs. 29.3 ± 6.4 mL/kg FFM/min, respectively; P < 0.001). When stratified by race and ethnicity, black participants had lower values than did whites and Hispanics (overall, 25.7 ± 6.0 vs. 27.6 ± 6.6 vs. 29.4 ± 6.2 mL/kg FFM/min, respectively; P = 0.0262). The breakdown by sex and race/ethnicity for activity achieved and CPX testing is presented in Table 2. Overall mean ± SD daily energy expenditure was 34.3 ± 4.1 kcal/kg. Men had an average mean daily energy expenditure of 35.2 ± 5.0 kcal/kg, whereas women expended 33.1 ± 2.0 kcal/kg/day (P = 0.12). We found no significant differences in mean daily energy expenditure by race/ethnicity.
Table 2.
Breakdown of activity achieved and aerobic measures by sex and race/ethnicity
| Peak VO2(mL/min) |
VO2peak(mL/kg/min) |
VO2peak-FFM(mL/kg FFM/min) |
Daily energy expenditure(kcal/kg) |
Total activity(METs∗h/week)§ |
|
|---|---|---|---|---|---|
| Overall | 1,836.0 (530.4) | 18.8 (5.0) | 27.4 (6.5) | 34.3 (4.1) | 25.0 (37.0) |
| Men | |||||
| Overall | 2,065.5 (518.4) | 20.9 (4.9) | 29.3 (6.4) | 35.2 (5.0) | 30.0 (43.4) |
| Black | 2,018.8 (447.3) | 20.1 (4.3) | 28.0 (5.8) | 35.7 (1.5) | 39.4 (57.2) |
| White | 2,128.4 (506.8) | 19.7 (4.6) | 28.6 (6.3) | 34.9 (3.5) | 23.9 (36.3) |
| Hispanic | 2,114.9 (678.1) | 22.7 (4.7) | 31.2 (6.7) | 34.9 (5.9) | 25.7 (31.1) |
| P value | 0.7560 | 0.0419 | 0.1932 | 0.847 | 0.4549 |
| Women | |||||
| Overall | 1,512.6 (349.7) | 17.6 (4.8) | 24.7 (5.6) | 33.1 (2.0) | 18.0 (24.2) |
| Black | 1,431.7 (295.8) | 14.8 (3.5) | 23.2 (5.0) | 32.8 (1.5) | 11.5 (10.7) |
| White | 1,607.9 (419.4) | 16.9 (4.0) | 26.3 (7.0) | 33.6 (2.9) | 31.1 (35.3) |
| Hispanic | 1,621.0 (264.0) | 16.1 (2.4) | 25.8 (3.0) | 32.8 (1.2) | 11.2 (14.8) |
| P value | 0.1820 | 0.1403 | 0.1652 | 0.523 | 0.1456 |
Data are mean (SD) unless otherwise indicated.
§Total hours of activity per week exclude sleep and light activity.
Results from the univariable and multivariable linear regression models are shown in Supplementary Table 2. In univariable analysis, MET estimations as determined by the PAR interview correlated poorly with measured VO2peak-FFM (R2 = 0.048; P = 0.004). Age (β = −0.22; P < 0.0001) and male sex (β = 3.60; P < 0.001) were the most significant univariable predictors of VO2peak-FFM in the fully adjusted multivariable analysis. Throughout iterative multivariable analyses, estimated METs remained a significant independent predictor (β = 0.03; P = 0.033) in the fully adjusted model. We found no significant sex-by-race/ethnicity interactions.
Table 3 lists the Spearman correlation coefficients for various activity levels between the PAR interview results and VO2peak-FFM. Moderate activities (P = 0.002), hard activities (P = 0.002), and very hard activities (P = 0.002) were the most significant predictors of measured VO2peak-FFM. The correlation between activity and VO2peak-FFM combining the hard and very hard categories was highly significant (P < 0.001); the Spearman ρ of 0.313 reflects a modest correlation.
Table 3.
Spearman correlations with VO2peak-FFM
| PAR activity level (METs∗h/week) | Spearman ρ | P value |
|---|---|---|
| Light | 0.097 | 0.241 |
| Light + moderate | 0.164 | 0.047 |
| Moderate | 0.250 | 0.002 |
| Hard | 0.259 | 0.002 |
| Very hard | 0.255 | 0.002 |
| Hard + very hard | 0.313 | <0.001 |
Conclusions
In this study of 150 patients with medically treated T2DM who have or are at risk for prevalent ASCVD, who are significantly diverse with regard to race/ethnicity and sex, and who were recruited at a single academic medical center, almost 50% of participants did not meet minimum PA recommendations despite lifestyle recommendations being the foundation for treating T2DM and preventing ASCVD progression. Additionally, on average, our population had alarmingly low cardiopulmonary fitness, a measure that has been independently predictive of premature mortality and other adverse clinical consequences (27,28). On average, participants spent 97% of their time sleeping or doing light activities such as sitting. Among participants, 79% reported no time spent doing hard activities; only 8% of participants reported any time spent doing very hard activities. Levels of cardiopulmonary fitness as measured by VO2peak and VO2peak-FFM revealed a mean ± SD of 27.4 ± 6.5 mL/kg FFM/min; the lowest levels were found in women (24.7 ± 5.6 mL/kg FFM/min) and black participants (25.7 ± 6.0 mL/kg FFM/min). As a standard for comparison, a VO2peak-FFM <19 mL/kg FFM/min has been recommended as a threshold measurement for considering cardiac transplant in patients with heart failure, especially women and obese individuals (29). These findings are meaningful and highlight the need for more effective strategies targeting lifestyle interventions for the vulnerable population of patients with T2DM who are at high risk for ASCVD.
Despite guideline recommendations from professional societies, almost half of our study population failed to meet even the minimum PA requirements. This observation is consistent with that published by Zhao et al. (30), who used the Behavioral Risk Factor Surveillance System to determine proportions of patients meeting PA recommendations among 99,172 older adults, 18.5% of whom had T2DM. They found that adults with T2DM were 31–34% less likely than those without T2DM to meet the minimum PA recommendations (P < 0.001), a trend that held true even among those individuals who reported no disabilities (30). Predictors of low PA levels included age >75 years, female sex, non-Hispanic black participants, obesity, history of CAD, and having a disability. That study was limited by the nature of self-reported data as the source for the Behavioral Risk Factor Surveillance System database; even with a self-report bias, however, Zhao et al. found that only 42% of included adults met the minimum 2008 Physical Activity Guidelines for Americans recommended by the U.S. Department of Health and Human Services (31), and as few as 25% met the somewhat more intensive minimum PA recommendations endorsed by the American Diabetes Association (32). Our high-risk study participants, who had prevalent ASCVD or risk factors for ASCVD, would probably benefit substantially from adhering PA recommendations given their higher risks for morbidity and mortality, yet almost half failed to meet even the minimum recommendation. More work is needed to understand strategies for overcoming barriers to achieving higher PA levels, and integrated strategies are needed within health care systems and within communities in order to encourage and reward healthy levels of PA.
Very low cardiorespiratory fitness, as seen in our population, is strongly associated with a high risk of premature death and other nonfatal cardiovascular adverse events (6,27,33). However, the utility of using cardiorespiratory fitness testing in the office setting is limited by the time and resources necessary to perform CPX testing, which has led to the widespread use of estimates of PA as a more useful measure (11,33,34). Although in our study levels of moderate, hard, and very hard PA had the strongest correlation with cardiorespiratory fitness, higher levels of total PA have been associated with a lower risk of adverse cardiovascular outcomes, irrespective of cardiorespiratory fitness measures, and have proven to be a more economical and meaningful population-level predictor of cardiometabolic risk (35). In a pooled analysis of 10 population-based cohorts of participants with diabetes, individuals with guideline-recommended levels of PA had an associated 38% lower risk of all-cause mortality and a 43% lower risk of cardiovascular mortality than did those who were inactive at baseline (36). Other cohort studies of individuals with diabetes have noted similar observations, which provide the basis for many guideline recommendations to support PA as an effective preventive intervention for patients with both T2DM and ASCVD.
Our study findings have important public health implications. Several exercise training and PA trials have demonstrated the beneficial effects of exercise and improved PA levels on cardiometabolic outcomes (11,37,38). Furthermore, recent studies have demonstrated that exercise training may be associated with improved glycemic control regardless of improvement in cardiorespiratory fitness (39). From a cardiovascular risk standpoint, higher levels of PA and cardiorespiratory fitness are more strongly associated with a lower risk of heart failure than atherosclerotic outcomes (33,35). Although intensive lifestyle interventions aimed at improving cardiorespiratory fitness and weight loss failed to improve atherosclerotic outcomes in the seminal Look AHEAD (Action for Health in Diabetes) trial, there was a trend toward reducing the risk of incident heart failure (40). Findings from the current study highlight the vast unmet need for promoting PA levels and exercise training interventions in patients with T2DM in order to improve their long-term cardiometabolic and cardiovascular outcomes. Adequately powered, long-term follow-up studies with intensive exercise training/PA interventions are needed to determine whether PA can improve the risk for heart failure in this patient population.
This study has noted limitations. First, its cross-sectional design does not allow us to determine causality. Second, the estimates of PA from the PAR interview are not as accurate as data measured directly, such as those from observed exercise training sessions or measured using accelerometers (24). Additionally, while the PAR interview has been validated in selected populations, it remains subject to recall bias. The strengths of this study include the diverse population of both men and women with T2DM who were recruited primarily from outpatient specialty clinics, where follow-up occurs at more regular intervals and where specialty care is expected to adhere most to disease-specific guideline recommendations and counseling. Additionally, we measured levels of fitness directly using CPX testing, the gold standard for determining aerobic capacity and fitness. Finally, the PAR interview was administered by an investigator trained in the survey methods and may have limited generalizability for administration by an untrained interviewer (23,24).
Conclusion
In this diverse cohort of outpatients with T2DM who have or are at high risk for ASCVD, fitness levels were strikingly low, with little volitional PA. In this population of patients who stand to gain the most from higher levels of PA, stronger efforts are needed to promote greater adherence to guidelines as the foundation of T2DM treatment.
Supplementary Material
Article Information
Funding. This work was supported by research grants from the Donald W. Reynolds Foundation and the U.S. Public Health Service General Clinical Research Center (grant no. M01-RR00633) at the National Institutes of Health/National Center Research Resources—Clinical Research. C.R.A. is supported in part by the National Heart, Lung, and Blood Institute (grant no. NIH-NHLBI 1 K23 HL092229-01-A1).
Duality of Interest. This work was supported by a research grant from GlaxoSmithKline. D.K.M. has received personal fees for trial leadership from GlaxoSmithKline, Janssen, Lexicon, AstraZeneca, Sanofi Aventis, Boehringer Ingelheim, Merck & Co., Pfizer, Novo Nordisk, Eisai Inc., Esperion, and Lilly USA and has received personal consultancy fees from AstraZeneca, Lilly USA, Boehringer Ingelheim, Merck & Co., Novo Nordisk, Metavant Sciences, Applied Therapeutics, and Sanofi Aventis. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. J.L.J. and D.K.M. wrote the manuscript, researched the data, and participated in the analyses. A.P. reviewed and edited the manuscript and contributed to the discussion. C.R.A. performed the analyses and edited the manuscript. J.M.M. and M.S. contributed to the discussion and edited the manuscript. J.D.B. and K.V.P. reviewed and edited the manuscript. J.L.J. and D.K.M. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Preliminary results were presented at the American Heart Association Quality of Care and Outcomes Research Scientific Sessions, Atlanta, GA, 9–11 May, 2012.
Footnotes
Clinical trial reg. no. NCT00424762, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc18-2634/-/DC1.
References
- 1.Lavie CJ, Johannsen N, Swift D, et al. Exercise is medicine - the importance of physical activity, exercise training, cardiorespiratory fitness and obesity in the prevention and treatment of type 2 diabetes. Eur Endocrinol 2014;10:18–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Diabetes Association 6. Obesity management for the treatment of type 2 diabetes:Standards of Medical Care in Diabetes—2016. Diabetes Care 2016;39(Suppl. 1):S47–S51 [DOI] [PubMed] [Google Scholar]
- 3.Sui X, LaMonte MJ, Laditka JN, et al. Cardiorespiratory fitness and adiposity as mortality predictors in older adults. JAMA 2007;298:2507–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei M, Kampert JB, Barlow CE, et al. Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese men. JAMA 1999;282:1547–1553 [DOI] [PubMed] [Google Scholar]
- 5.Sattelmair J, Pertman J, Ding EL, Kohl HW 3rd, Haskell W, Lee IM. Dose response between physical activity and risk of coronary heart disease: a meta-analysis. Circulation 2011;124:789–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah RV, Murthy VL, Colangelo LA, et al. Association of fitness in young adulthood with survival and cardiovascular risk: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. JAMA Intern Med 2016;176:87–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donnelly JE, Jacobsen DJ, Heelan KS, Seip R, Smith S. The effects of 18 months of intermittent vs. continuous exercise on aerobic capacity, body weight and composition, and metabolic fitness in previously sedentary, moderately obese females. Int J Obes Relat Metab Disord 2000;24:566–572 [DOI] [PubMed] [Google Scholar]
- 8.Ross R, Janssen I, Dawson J, et al. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obes Res 2004;12:789–798 [DOI] [PubMed] [Google Scholar]
- 9.Jarvie JL, Whooley MA, Regan MC, Sin NL, Cohen BE. Effect of physical activity level on biomarkers of inflammation and insulin resistance over 5 years in outpatients with coronary heart disease (from the Heart and Soul Study). Am J Cardiol 2014;114:1192–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ståhle A, Mattsson E, Rydén L, Unden A, Nordlander R. Improved physical fitness and quality of life following training of elderly patients after acute coronary events. A 1 year follow-up randomized controlled study. Eur Heart J 1999;20:1475–1484 [DOI] [PubMed] [Google Scholar]
- 11.Pandey A, Garg S, Khunger M, et al. Dose-response relationship between physical activity and risk of heart failure: a meta-analysis. Circulation 2015;132:1786–1794 [DOI] [PubMed] [Google Scholar]
- 12.Lee DC, Sui X, Artero EG, et al. Long-term effects of changes in cardiorespiratory fitness and body mass index on all-cause and cardiovascular disease mortality in men: the Aerobics Center Longitudinal Study. Circulation 2011;124:2483–2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pandey A, Cornwell WK III, Willis B, et al. Body mass index and cardiorespiratory fitness in mid-life and risk of heart failure hospitalization in older age: findings from the Cooper Center Longitudinal Study. JACC Heart Fail 2017;5:367–374 [DOI] [PubMed] [Google Scholar]
- 14.Haskell WL, Lee I-M, Pate RR, et al.; American College of Sports Medicine; American Heart Association . Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation 2007;116:1081–1093 [DOI] [PubMed] [Google Scholar]
- 15.Colberg SR, Sigal RJ, Fernhall B, et al.; American College of Sports Medicine; American Diabetes Association . Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care 2010;33:e147–e167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piercy KL, Troiano RP, Ballard RM, et al. The Physical Activity Guidelines for Americans. JAMA 2018;320:2020–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tucker JM, Welk GJ, Beyler NK. Physical activity in U.S.: adults compliance with the Physical Activity Guidelines for Americans. Am J Prev Med 2011;40:454–461 [DOI] [PubMed] [Google Scholar]
- 18.Blackwell DL, Clarke TC. State Variation in Meeting the 2008 Federal Guidelines for Both Aerobic and Muscle-Strengthening Activities Through Leisure-Time Physical Activity Among Adults Aged 18-64: United States, 2010-2015, Hyattsville, MD, National Center for Health Statistics, 2018 [PubMed] [Google Scholar]
- 19.Siegel KR, Bullard KM, Imperatore G, et al. Prevalence of major behavioral risk factors for type 2 diabetes. Diabetes Care 2018;41:1032–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGuire DK, See R, Abdullah SM, et al. The effect of rosiglitazone on integrated cardiovascular performance, cardiac structure, function and myocardial triglyceride: trial design and rationale. Diab Vasc Dis Res 2009;6:43–50 [DOI] [PubMed] [Google Scholar]
- 21.McGuire DK, Abdullah SM, See R, et al. Randomized comparison of the effects of rosiglitazone vs. placebo on peak integrated cardiovascular performance, cardiac structure, and function. Eur Heart J 2010;31:2262–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr 1974;32:77–97 [DOI] [PubMed] [Google Scholar]
- 23.Blair SN, Haskell WL, Ho P, et al. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol 1985;122:794–804 [DOI] [PubMed] [Google Scholar]
- 24.Richardson MT, Ainsworth BE, Jacobs DR Jr., Leon AS. Validation of the Stanford 7-day recall to assess habitual physical activity. Ann Epidemiol 2001;11:145–153 [DOI] [PubMed] [Google Scholar]
- 25.Washburn RA, Jacobsen DJ, Sonko BJ, Hill JO, Donnelly JE. The validity of the Stanford Seven-Day Physical Activity Recall in young adults. Med Sci Sports Exerc 2003;35:1374–1380 [DOI] [PubMed] [Google Scholar]
- 26.McGuire DK, Levine BD, Williamson JW, et al. A 30-year follow-up of the Dallas Bedrest and Training Study: I. Effect of age on the cardiovascular response to exercise. Circulation 2001;104:1350–1357 [PubMed] [Google Scholar]
- 27.Blair SN, Kohl HWI III, Paffenbarger RS Jr., Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 1989;262:2395–2401 [DOI] [PubMed] [Google Scholar]
- 28.Sobolski J, Kornitzer M, De Backer G, et al. Protection against ischemic heart disease in the Belgian Physical Fitness Study: physical fitness rather than physical activity? Am J Epidemiol 1987;125:601–610 [DOI] [PubMed] [Google Scholar]
- 29.Osman AF, Mehra MR, Lavie CJ, Nunez E, Milani RV. The incremental prognostic importance of body fat adjusted peak oxygen consumption in chronic heart failure. J Am Coll Cardiol 2000;36:2126–2131 [DOI] [PubMed] [Google Scholar]
- 30.Zhao G, Ford ES, Li C, Balluz LS. Physical activity in U.S. older adults with diabetes mellitus: prevalence and correlates of meeting physical activity recommendations. J Am Geriatr Soc 2011;59:132–137 [DOI] [PubMed] [Google Scholar]
- 31.U.S. Department of Health and Human Services 2008 Physical Activity Guidelines for Americans. Hyattsville, MD, U.S. Department of Health and Human Services, 2008 [Google Scholar]
- 32.American Diabetes Association 5. Lifestyle management: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019;42(Suppl. 1):S46–S60 [DOI] [PubMed] [Google Scholar]
- 33.Kulinski JP, Khera A, Ayers CR, et al. Association between cardiorespiratory fitness and accelerometer-derived physical activity and sedentary time in the general population. Mayo Clin Proc 2014;89:1063–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shuval K, Finley CE, Barlow CE, Gabriel KP, Leonard D, Kohl HW 3rd. Sedentary behavior, cardiorespiratory fitness, physical activity, and cardiometabolic risk in men: the Cooper Center Longitudinal Study. Mayo Clin Proc 2014;89:1052–1062 [DOI] [PubMed] [Google Scholar]
- 35.Sadarangani KP, Hamer M, Mindell JS, Coombs NA, Stamatakis E. Physical activity and risk of all-cause and cardiovascular disease mortality in diabetic adults from Great Britain: pooled analysis of 10 population-based cohorts. Diabetes Care 2014;37:1016–1023 [DOI] [PubMed] [Google Scholar]
- 36.Kodama S, Tanaka S, Heianza Y, et al. Association between physical activity and risk of all-cause mortality and cardiovascular disease in patients with diabetes: a meta-analysis. Diabetes Care 2013;36:471–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Church TS, Blair SN, Cocreham S, et al. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA 2010;304:2253–2262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Umpierre D, Ribeiro PA, Schaan BD, Ribeiro JP. Volume of supervised exercise training impacts glycaemic control in patients with type 2 diabetes: a systematic review with meta-regression analysis. Diabetologia 2013;56:242–251 [DOI] [PubMed] [Google Scholar]
- 39.Berry JD, Pandey A, Gao A, et al. Physical fitness and risk for heart failure and coronary artery disease. Circ Heart Fail 2013;6:627–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Look AHEAD Research Group; Wing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes [published correction appears in N Engl J Med 2014;370:1866]. N Engl J Med 2013;369:145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.