Abstract
OBJECTIVE
This study evaluated whether regression from impaired glucose regulation (IGR) to normal glucose regulation (NGR) after 1 year of a lifestyle intervention reduces diabetes risk in American Indians and Alaska Natives (AI/ANs). In addition, we sought to identify predictors for regression to NGR and understand possible mechanisms for the association between NGR and future diabetes risk.
RESEARCH DESIGN AND METHODS
Data from participants enrolled from 2006 to 2009 in the Special Diabetes Program for Indians Diabetes Prevention Program with IGR at baseline and an oral glucose tolerance test at year 1 were analyzed (N = 1,443). Cox regression models were used to estimate the subsequent diabetes risk (year 1 to year 3) by year 1 glucose status. Mediation analysis was used to estimate the proportions of the association between year 1 glycemic status and diabetes risk explained by specific factors.
RESULTS
Those who reverted to NGR at year 1 (38%) had lower diabetes risk than those with sustained IGR (adjusted hazard ratio 0.28, 95% CI 0.12–0.67). The lower risk associated with regression to NGR was explained by both baseline risk factors and differences in weight loss. Metformin use, weight loss, and an increase in exercise were modifiable risk factors associated with higher odds of regression to NGR.
CONCLUSIONS
Patients with prediabetes who reverted to NGR had a reduced risk of developing type 2 diabetes over the next 2 years. Both baseline and modifiable risk factors explained the risk reduction associated with NGR.
Introduction
Type 2 diabetes is a costly public health burden associated with many devastating comorbidities and is the seventh leading cause of death in the U.S. (1). Prediabetes, a precursor to type 2 diabetes, has been estimated to affect 84 million adults in the U.S. (1,2). Like diabetes, prediabetes is also associated with many costly comorbidities (3–10) and mortality (11,12). Diabetes and prediabetes both disproportionately affect disadvantaged populations (13). In particular, American Indians and Alaska Natives (AI/ANs) have the highest age-adjusted diabetes prevalence (15.1% in 2015) among all racial and ethnic groups in the U.S. (1,13). To reduce the daunting diabetes disparities borne by AI/ANs, effective intervention strategies are urgently needed to prevent diabetes in this special population.
Numerous studies, including the U.S. Diabetes Prevention Program (DPP), reported that preventing or delaying the development of diabetes through lifestyle changes or medication interventions could be efficacious (14–16) and cost-effective (2). Further, among those receiving a lifestyle intervention, regression to normal glucose regulation (NGR) was associated with a substantially reduced risk of developing type 2 diabetes (17–19). For example, the Diabetes Prevention Program Outcomes Study (DPPOS) found those who regressed to NGR any time during the DPP had a 56% lower risk of developing type 2 diabetes during the first 6 years of follow-up (20). Another study of Asian Indian men with prediabetes found a risk reduction of 75% during 1.5 years of follow-up among those who reverted to NGR at 6 months after initiation of a motivational text messages lifestyle intervention to prevent diabetes (18). More recently, the U.K.-based ADDITION (Anglo-Danish-Dutch Study of Intensive Treatment in People with Screen Detected Diabetes in Primary Care)-Prediabetes Cohort Study of a rudimentary lifestyle education program found that regression to NGR at year 1 reduced the risk of developing type 2 diabetes by 80% over the next 4 years (19). In all of these studies, weight loss was associated with regression to NGR (18,19,21). However, these previous studies did not investigate what proportion of the reduced risk associated with regression to NGR was explained by participants’ baseline diabetes risk and what proportion was explained by the effects of weight loss or other lifestyle changes induced by participating in the intervention.
The Special Diabetes Program for Indians Diabetes Prevention (SDPI-DP) Program (22) translated the DPP lifestyle intervention into AI/AN communities to prevent or delay the development of diabetes. From the long-term outcomes of the SDPI-DP participants during a follow-up period up to 10 years, we found moderate weight loss achieved through an intensive lifestyle intervention could substantially decrease the risk of type 2 diabetes among AI/ANs (23). Using the first 3 years of data from the SDPI-DP, the current study evaluated whether regression to NGR in this high-risk population also translated into a reduced risk of developing type 2 diabetes and estimated the proportions of the diabetes risk reduction associated with regression to NGR explained by baseline diabetes risk factors and the proportions explained by lifestyle changes. In addition, this study investigated which variables predicted regression to NGR 1 year after initiation of the intervention.
Research Design and Methods
Study Population
Data from the SDPI-DP Program were used. The details of this project are described elsewhere (22). Briefly, 36 AI/AN local health care programs were funded to implement the DPP lifestyle intervention in their communities. Programs were required to implement the 16-session Lifestyle Balance Curriculum, the primary goal of which was to achieve and maintain a 7% weight loss through healthy food choices and increased physical activity. The SDPI-DP program comprehensively evaluated the translation of this proven intervention into diverse settings across AI/AN communities.
Between January 2006 and July 2009, 3,310 participants enrolled in SDPI-DP. Eligibility criteria for the program included being AI/AN, at least 18 years of age with no previous diagnosis of diabetes, and having a previous diagnosis of prediabetes or impaired fasting glucose (IFG, fasting blood glucose [FBG] 100–125 mg/dL) and/or impaired glucose tolerance (IGT, 2-h glucose 140–199 mg/dL) based on a 75-g oral glucose tolerance test (OGTT) at baseline. Exclusion criteria included being pregnant, having end-stage renal disease, and any condition that would impede successful participation based on provider judgment. The current study included only participants with baseline isolated IFG or IGT, or both, who did not convert to diabetes by year 1 and had an OGTT at year 1. A total of 1,443 SDPI-DP participants remained in the final analytical sample (Supplementary Fig. 1). The SDPI-DP program was approved by the University of Colorado Denver and the Indian Health Service Institutional Review Boards. All participants were consented and provided Health Insurance Portability and Accountability Act authorization.
Data Collection
Participants’ demographic, clinical, and behavioral characteristics were collected at baseline and reassessed annually throughout the project. In addition, FBG levels were obtained at semiannual visits. Baseline demographic characteristics included age and sex. Clinical measures were BMI (kg/m2), weight (pounds), waist circumference (inches), systolic (mmHg) and diastolic blood pressure (mmHg), LDL cholesterol (LDL-C, mg/dL), HDL cholesterol (HDL-C, mg/dL), triglycerides (TGs, mg/dL), FBG and 2-h glucose (mg/dL), self-reported previous or current diagnosis of hypertension, self-administered comorbidity questionnaire (24), and family history of diabetes. Lipids and glucose were measured at local and regional laboratories using standard assays. A self-administered questionnaire determined smoking status, frequency of healthy and unhealthy food consumption (25), and the Rapid Assessment of Physical Activity (RAPA) (26). Medication data, including metformin use, were collected at baseline and annually. Grantee staff listed all current medications, which were confirmed by pharmacy records and patient history/medication bottles when possible.
The primary outcome of this study was incident diabetes between year 1 and year 3, diagnosed via annual OGTT or semiannual FBG, based on American Diabetes Association criteria of FBG ≥126 mg/dL and/or 2-h glucose ≥200 mg/dL, with confirmation by a second test or provider judgment. The major exposure variable of the present analysis was each participant’s glycemic status at year 1. OGTT results at year 1 were used to classify participants into four categories: NGR (FBG <100 mg/dL and 2-h glucose <140 mg/dL), isolated IFG (iIFG; FBG 100–125 mg/dL and 2-h glucose <140 mg/dL), isolated IGT (iIGT; FBG <100 mg/dL and 2-h glucose 140–199 mg/dL), or both IFG and IGT (IFG/IGT, FBG 100–125 mg/dL and 2-h glucose 140–199 mg/dL). All continuous variables were rescaled by dividing the values by the variable’s SD; therefore, the interpretation for continuous variables is per SD. Comorbidities were zero inflated and right skewed; therefore, they were categorized as none, one or two, and three or more comorbidities. TGs were right skewed and log-transformed for analysis. Smoking was defined as current smoker or not.
To evaluate whether changes in clinical or behavioral characteristics from baseline to year 1 were different among glycemic status groups and whether they were associated with outcomes, change variables were constructed by subtracting each participant’s baseline value from his or her year 1 value. Similarly, percentage differences were calculated by dividing the change variables by baseline values and then multiplying by 100. A negative change value indicates improvement for most measurements; the exceptions are HDL-C, healthy diet score, and RAPA, for which a positive change indicates improvement.
Statistical Analysis
Participant baseline characteristics and changes in clinical and behavioral characteristics among year 1 glycemic status groups were compared using χ2 tests for categorical data and ANOVA for numerical variables with a Bonferroni correction for multiple pairwise comparisons. To determine the association of year 1 glycemic status (iIFG, iIGT, or IFG/IGT vs. NGR) with the risk of developing type 2 diabetes, Cox proportional hazards analysis was used. Proportionality was confirmed using time-by-variable interaction terms. The proportions of the association between diabetes risk and year 1 glycemic status explained by specific variables were estimated using an SAS macro that calculates the percentage explained by each variable (% mediate) (27,28). Multiple logistic regression was used to examine variables associated with regression to NGR at year 1. All analyses were conducted using SAS 9.3 software (SAS Institute, Cary, NC).
Variables considered in the initial model for both Cox proportional hazards analysis and logistic regression were age, sex, baseline weight, percentage weight loss from baseline to year 1, baseline systolic blood pressure, baseline HDL-C, change in HDL-C, baseline TGs, change in TGs, baseline FBG and 2-h glucose, history of hypertension, comorbidities, family history of diabetes, baseline smoking status, baseline healthy diet score, change in healthy diet score, baseline unhealthy diet score, change in unhealthy diet score, baseline RAPA, and change in RAPA. In addition, baseline metformin use was included in the logistic regression analysis. For the Cox proportional hazards analysis, metformin was modeled as a time-varying covariate from year 1 to year 3. Because medication data were not collected at midyear visits, a participant who was on a medication at the preceding annual visit was considered to be on that medication at the subsequent midyear assessment. Manual backward elimination was used for model selection; variables with a P value <0.20 were kept in the final model.
Results
As reported in Table 1, 38% (n = 551) of the participants regressed to NGR at year 1. Of those who continued having impaired glucose regulation (IGR), 601 (42%) were iIFG, 76 (5%) were iIGT, and 215 (15%) were IFG/IGT. Those who regressed to NGR at year 1 were similar in age to the iIGT group but significantly younger than the iIFG and IFG/IGT groups. Although baseline weight of the NGR group did not differ significantly from any of the other three groups, the iIGT group had the lowest mean weight, which was significantly lower than that of the iIFG group. There was an overall significant difference among the four groups for waist circumference and systolic blood pressure, but none of the pairwise comparisons between groups met a Bonferroni-corrected level of significance. TG levels were significantly lower in the NGR group than in the other three groups. The iIFG group had the highest proportion of men and hypertension diagnosis. The iIGT group had higher levels of comorbidities.
Table 1.
Baseline demographic, clinical, and behavioral characteristics by year 1 glucose status
| Characteristic | NGR(n = 551 [38%]) | iIFG(n = 601 [42%]) | iIGT(n = 76 [5%]) | IFG/IGT(n = 215 [15%]) | P value |
|---|---|---|---|---|---|
| Demographic | |||||
| Age (years), mean (SD) | 46.0 (12.5)b,d | 49.7 (11.6)a | 47.4 (13.3) | 49.5 (12.7)a | <0.0001 |
| Males, n (%) | 134 (24.3) | 183 (30.4) | 9 (11.8) | 33 (15.3) | <0.0001 |
| Clinical, mean (SD) | |||||
| BMI (kg/m2) | 35.1 (7.4) | 36.0 (7.7) | 35.2 (6.0) | 36.6 (7.5) | 0.0633 |
| Weight (pounds) | 213 (53) | 221 (55)c | 204 (42)b | 216 (51) | 0.0113 |
| Waist circumference (inches) | 43 (6) | 44 (6) | 43 (5) | 44 (6) | 0.0221 |
| Blood pressure | |||||
| Systolic (mmHg) | 125 (14) | 128 (16) | 129 (16) | 128 (15) | 0.0480 |
| Diastolic (mmHg) | 79 (9) | 79 (10) | 78 (12) | 79 (10) | 0.9897 |
| LDL-C (mg/dL) | 111 (33) | 111 (31) | 113 (30) | 111 (30) | 0.9695 |
| HDL-C (mg/dL) | 46 (12) | 44 (12) | 46 (11) | 45 (11) | 0.1752 |
| Log-transformed TG (mg/dL) | 4.9 (0.5)b,c,d | 5.0 (0.5)a | 5.1 (0.6)a | 5.0 (0.5)a | <0.0001 |
| Geometric mean TG (mg/dL) | 134.3 | 148.4 | 164.0 | 148.1 | |
| FBG (mg/dL) | 104 (7)b,c,d | 109 (8)a,c | 99 (9)a,b,d | 108 (8)a,c | <0.0001 |
| 2-h glucose (mg/dL) | 118 (32)c,d | 118 (33)c,d | 152 (30)a,b | 144 (30)a,b | <0.0001 |
| Baseline glucose status, n (%) | <0.0001 | ||||
| iIFG | 392 (72) | 447 (76) | 21 (28) | 87 (41) | |
| iIGT | 77 (14) | 25 (4) | 34 (45) | 18 (8) | |
| IFG/IGT | 78 (14) | 118 (20) | 20 (27) | 107 (50) | |
| Diagnosis of hypertension, n (%) | 160 (29) | 253 (42) | 25 (33) | 83 (39) | <0.0001 |
| Comorbidities, n (%) | 0.0001 | ||||
| 0 | 203 (38) | 155 (27) | 16 (22) | 56 (27) | |
| 1–2 | 151 (28) | 185 (32) | 19 (26) | 73 (35) | |
| >2 | 175 (33) | 242 (42) | 37 (51) | 77 (37) | |
| Family history of diabetes, n (%) | 433 (79) | 477 (80) | 66 (87) | 175 (81) | 0.4169 |
| Metformin use, n (%) | 21 (4) | 12 (2) | 0 (0) | 10 (5) | 0.0517 |
| Behavioral | |||||
| Current smoker, n (%) | 117 (23) | 137 (24) | 13 (19) | 43 (21) | 0.6729 |
| Completed all 16 DPP classes, n (%) | 480 (87.1) | 514 (85.5) | 68 (89.5) | 183 (85.1) | 0.6789 |
| Healthy diet score, mean (SD) | 3.41 (0.81) | 3.42 (0.80) | 3.39 (0.83) | 3.36 (0.83) | 0.7813 |
| Unhealthy diet score, mean (SD) | 2.86 (0.74) | 2.83 (0.71) | 2.85 (0.75) | 2.84 (0.71) | 0.9702 |
| Physical activity (RAPA), mean (SD) | 3.77 (1.09) | 3.82 (1.03) | 3.68 (1.09) | 3.68 (1.15) | 0.3517 |
aSignificantly different from the mean of NGR at P ≤ 0.008 (based on Bonferroni correction for multiple comparisons).
bSignificantly different from the mean of iIFG at P ≤ 0.008 (based on Bonferroni correction for multiple comparisons).
cSignificantly different from the mean of iIGT at P ≤ 0.008 (based on Bonferroni correction for multiple comparisons).
dSignificantly different from the mean of IFG/IGT at P ≤ 0.008 (based on Bonferroni correction for multiple comparisons).
In terms of glucose patterns (Table 1), those who regressed to NGR at year 1 had an elevated mean FBG (104 ± 7 mg/dL) but a normal mean 2-h glucose (118 ± 32 mg/dL) at baseline, and 72% of this group were classified as iIFG at baseline. Compared with the NGR group, the iIFG group had a significantly higher mean baseline FBG (109 ± 8 mg/dL), with a higher proportion classified as iIFG at baseline (76%), but their mean 2-h glucose was similar to the NGR group (118 ± 33 mg/dL). The iIGT group had a significantly lower mean FBG (99 ± 9 mg/dL) than the other three groups but the highest 2-h glucose level (152 ± 30 mg/dL) and greater than 70% of the iIGT group (at year 1) were iIGT or IFG/IGT at baseline. The IFG/IGT group showed a similar pattern as the iIGT group, but their baseline FBG level (108 ± 8 mg/dL) was significantly higher.
Diabetes incidence and changes in clinical and behavioral characteristics by year 1 glucose status are summarized in Table 2. The crude diabetes incidence rate from year 1 to year 3 was the lowest among those who regressed to NGR (1.3%), followed by the iIFG group (5.3%) and iIGT group (6.6%), with the highest rate in the IFG/IGT group (13.0%). The NGR group achieved the greatest improvements in weight, waist circumference, glucose, and RAPA. Conversely, the IFG/IGT group had the least improvements in those characteristics and experienced increases in their mean TG level and both glucose measurements. Compared with those who regressed to NGR, the iIFG group showed significantly less improvement in weight loss, waist circumference, and glucose measurements. The iIGT group only differed significantly in less improvement in glucose measurements. Mean 2-h glucose measurement increased in the iIGT group.
Table 2.
Changes in clinical and behavioral characteristics by year 1 glucose status
| Characteristic | NGR (n = 551) | iIFG (n = 601) | iIGT (n = 76) | IFG/IGT (n = 215) | P value |
|---|---|---|---|---|---|
| Diabetes incidence year 1 to year 3, n (%) | 7 (1.3) | 32 (5.3) | 5 (6.6) | 28 (13.0) | <0.0001 |
| Clinical changes (year 1–baseline) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Weight (pounds) | −9.5 (14.8)b,d | −5.4 (13.4)a,d | −5.7 (10.9) | −1.4 (11.9)a,b | <0.0001 |
| Change in weight (%) | −4.3 (6.1)b,d | −2.3 (5.8)a,d | −2.8 (5.2)d | −0.7 (4.9)a,b,c | <0.0001 |
| Waist circumference (inches) | −1.8 (3.2)b,d | −1.1 (3.2)a | −1.0 (3.0) | −0.4 (2.8)a | <0.0001 |
| Change in waist circumference (%) | −4.0 (7.4)b,d | −2.2 (7.2)a | −2.1 (7.0) | −0.9 (6.4)a | <0.0001 |
| Blood pressure | |||||
| Systolic (mmHg) | −2.6 (14.5) | −1.4 (16.5) | −1.8 (15.4) | 0.7 (17.9) | 0.0891 |
| Diastolic (mmHg) | −1.5 (10.8) | −1.6 (10.9) | −0.9 (11.5) | −0.2 (10.0) | 0.3824 |
| LDL-C (mg/dL) | −3.6 (23.7) | −2.1 (23.9) | −5.3 (27.0) | −4.1 (24.0) | 0.5200 |
| HDL-C (mg/dL) | 1.9 (9.3) | 0.9 (7.4) | 2.2 (11.2) | 0.3 (7.6) | 0.0579 |
| Log-transformed TG (mg/dL) | −0.06 (0.39)d | −0.06 (0.35)d | −0.11 (0.35) | 0.02 (0.35)a,b | 0.0130 |
| FBG (mg/dL) | −11.4 (9.0)b,c,d | −1.0 (8.3)a,c,d | −5.7 (11.6)a,b,d | 1.4 (10.1)a,b,c | <0.0001 |
| Change in FBG (%) | −10.6 (8.0)b,c,d | −0.6 (7.6)a,c,d | −5.0 (11.6)a,b,d | 1.8 (9.7)a,b,c | <0.0001 |
| 2-h glucose (mg/dL) | −20.5 (32.0)b,c,d | −12.9 (32.3)a,c,d | 11.7 (36.4)a,b | 16.8 (30.1)a,b | <0.0001 |
| Change in 2-h glucose (%) | −12.5 (28.1)b,c,d | −5.7 (28.3)a,c,d | 12.3 (29.2)a,b | 16.4 (28.0)a,b | <0.0001 |
| Behavioral changes (year 1–baseline) | |||||
| Healthy diet score | 0.19 (0.71) | 0.24 (0.68) | 0.20 (0.70) | 0.27 (0.76) | 0.5838 |
| Unhealthy diet score | −0.35 (0.65) | −0.33 (0.67) | −0.34 (0.55) | −0.32 (0.58) | 0.9289 |
| Physical activity (RAPA) | 0.40 (1.23)d | 0.24 (1.15) | 0.39 (1.11) | 0.11 (1.39)a | 0.0335 |
aSignificantly different from the mean of NGR at P ≤ 0.008 (based on Bonferroni correction for multiple comparisons).
bSignificantly different from the mean of iIFG at P ≤ 0.008 (based on Bonferroni correction for multiple comparisons).
cSignificantly different from the mean of iIGT at P ≤ 0.008 (based on Bonferroni correction for multiple comparisons).
dSignificantly different from the mean of IFG/IGT at P ≤ 0.008 (based on Bonferroni correction for multiple comparisons).
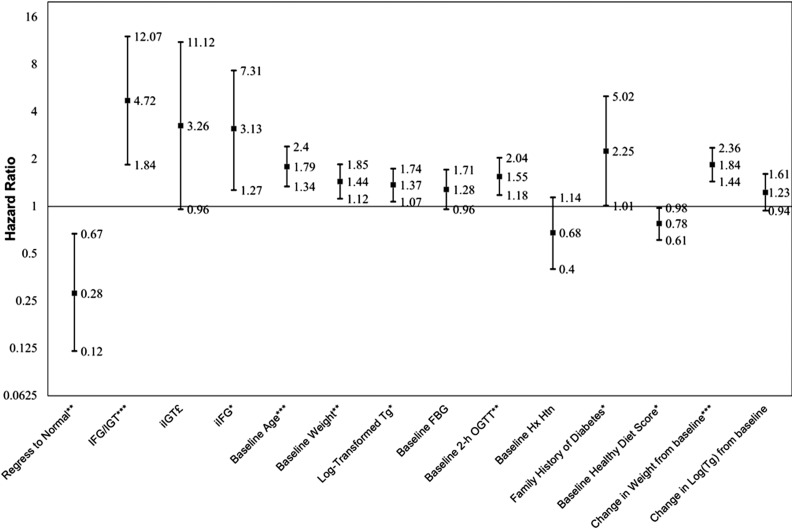
Figure 1 and Supplementary Table 1 report adjusted hazard ratios (HRs) generated by the Cox proportional hazards analysis, modeling the risk of glucose status at year 1 for developing diabetes during the subsequent 2 years while controlling potential confounders. When binary glucose status at year 1 (NGR group vs. all other participants with IGR) was included in the Cox model, regression to NGR was associated with 72% risk reduction for diabetes (adjusted HR 0.28, 95% CI 0.12–0.67). Alternatively, compared with the NGR group at year 1, IFG/IGT carried the highest risk of developing diabetes, with an adjusted HR of 4.72 (95% CI 1.84–12.07), followed by the iIGT group (HR 3.26, 95% CI 0.96–11.12; P = 0.059) and the iIFG group (HR 3.13, 95% CI 1.27–7.31). Per-decade increase in age was associated with an increased risk of diabetes (HR 1.59, 95% CI 1.26–2.01). Baseline weight and percentage weight gained at year 1, baseline TGs, and family history of diabetes were associated with an increased risk of developing diabetes. Furthermore, baseline 2-h glucose level was significantly associated with future diabetes risk independent of glycemic status at year 1 (HR 1.55 per SD, 95% CI 1.18–2.04). Those who started the intervention with a higher healthy diet score had a lower risk of diabetes (HR 0.78 per SD, 95% CI 0.61–0.98).
Figure 1.
HRs of glucose status at year 1 for diabetes incidence from year 1 to year 3. Hx Htn, history of hypertension. *P < 0.05, **P < 0.01, ***P < 0.001, £P = 0.059.
The proportions of the association between year 1 glycemic status and future diabetes risk explained by various covariates in the final model revealed different patterns for participants with iIFG, iIGT, and IFG/IGT compared with those with NGR at year 1 (Supplementary Table 2). Baseline 2-h glucose level explained 27.1% of the diabetes risk increase associated with iIGT and 16.2% of the risk increase associated with IFG/IGT but did not explain risk increase related to iIFG. Meanwhile, baseline FBG and age explained a significant proportion of increased risk of diabetes associated with iIFG and IFG/IGT (11.7% and 8.8% for FBG and 11.4% and 7.1% for age) but did not explain a significant proportion of the association between iIGT and future diabetes risk. Percentage weight loss at year 1 explained a similar proportion of diabetes risk increase associated with iIFG (20.6%) and IFG/IGT (20.1%) but only 11.7% of the iIGT-group risk increase. Further, baseline TGs explained a significant proportion of the diabetes risk increase associated with both iIGT and IFG/IGT (10.6% for iIGT and 6.9% for IFG/IGT). Family history of diabetes explained a significant proportion of increased diabetes risk associated with the iIGT group only (8.0%). A healthy diet score at baseline did not explain the association of diabetes risk with any of the glycemic groups.
Table 3 presents variables associated with regression to NGR at year 1. The only lifestyle modification factor significantly associated with regression to NGR was percentage weight loss from baseline to year 1, with each percentage less weight lost related to a 10% lower odds of regression to NGR. Metformin use at baseline was associated with greater odds of regressing to NGR, with an odds ratio (OR) of 2.54 (95% CI 1.16–5.57). An increase in RAPA was marginally associated with greater odds of regression to NGR (OR 1.19 per SD, P = 0.06). Other characteristics significantly associated with reduced odds of regression to NGR were older age, greater baseline weight, higher baseline TGs, and higher baseline FBG and 2-h glucose.
Table 3.
Factors predicting regression to NGR at year 1 (logistic regression)
| Variable | OR (95% CI) | P value |
|---|---|---|
| Baseline age (per 12.57 years) | 0.71 (0.61–0.83) | <0.0001 |
| Baseline weight (per 52.57 pounds) | 0.84 (0.73–0.975) | 0.0215 |
| Baseline log-transformed TG (per 0.49 mg/dL) | 0.80 (0.70–0.91) | 0.0011 |
| Baseline FBG (per 9.56 mg/dL) | 0.41 (0.34–0.49) | <0.0001 |
| Baseline 2-h glucose (per 34.71 mg/dL) | 0.64 (0.55–0.75) | <0.0001 |
| Baseline diagnosis of hypertension | 0.74 (0.55–1.003) | 0.0521 |
| Family history of diabetes | 0.79 (0.56–1.10) | 0.1662 |
| Baseline metformin use | 2.54 (1.16–5.57) | 0.0196 |
| Baseline physical activity (RAPA) (per 1.09) | 1.14 (0.96–1.37) | 0.1442 |
| Change in weight (per 5.97%) | 0.54 (0.46–0.63) | <0.0001 |
| Change in physical activity (RAPA) (per 1.22) | 1.19 (0.99–1.42) | 0.0635 |
C statistic = 0.769. ORs for continuous variables are per SD.
Conclusions
This translational study of diabetes prevention in an AI/AN population with prediabetes found that 38% of those with IGR at baseline reverted to NGR. This translates into a 72% reduction in the risk of developing type 2 diabetes over 2 years of follow-up compared with those who continued to have IGR at year 1, after adjusting for known diabetes risk factors. Similar observations have been reported by the DPP (17), a study of Asian Indian men (18), and the ADDITION-Prediabetes Cohort study (19). Moreover, this is the first study to find that lifestyle intervention participants whose glucose measurements remained in the prediabetes range had a different diabetes risk based on their glucose status at year 1. Compared with those who regressed to NGR, iIFG had the lowest risk, iIGT had a slightly higher risk, and IFG/IGT had the highest risk (adjusted HRs 3.13, 3.26, and 4.72, respectively). These risks are similar to the adjusted risks reported in the prediabetes population of a previous observational study of American Indians, the Strong Heart Study (29): adjusted HRs were 2.38, 3.47, and 4.06 for iIFG, iIGT and IFG/IGT, respectively, compared with NGR over an 8-year follow-up. This indicates the importance of obtaining NGR.
A large proportion of the risk reduction associated with regression to NGR was explained by lower baseline diabetes risk among participants who regressed. Specifically, compared with those who regressed to NGR, baseline FBG explained ∼10% of the excess risk associated with year 1 iIFG and IFG/IGT groups, and baseline age explained another 11% and 7% of the increased risk associated with iIFG and IFG/IGT, respectively. Neither baseline FBG nor age explained a significant amount of the association between iIGT and future diabetes risk. Conversely, 27% of the risk escalation associated with iIGT was explained by higher baseline 2-h glucose in that group, and 16% of the increased risk of the IFG/IGT group was explained by baseline 2-h glucose. However, little of the risk difference between the iIFG and NGR groups was explained by baseline 2-h glucose.
Baseline TG level explained a significant proportion of future diabetes risk for the iIGT and IFG/IGT groups but not the iIFG group. IGT has been reported to be more common in patients with nonalcoholic fatty liver disease (NAFLD) (30,31). Moreover, Borel et al. (32) found NAFLD was associated with iIGT but not iIFG. NAFLD is associated with increased TG production (33). Thus, the importance of TGs in the association between iIGT and future diabetes risk might imply NAFLD plays a role in this association. Although the current study did not measure insulin sensitivity/secretion or NAFLD, further investigation on the association of NAFLD with different types of prediabetes is warranted.
Family history of diabetes has been reported to be associated with diminished β-cell function, which is more strongly associated with IGT (2,34). In addition, genetic studies have identified several loci associated with abnormalities of β-cell function and a few with abnormalities in insulin activity (35,36), supporting the inheritance of susceptibility for iIGT. In this study, family history explained a significant proportion of future diabetes risk escalation in the iIGT group but not in the other two groups. This suggests a significant amount of the diabetes risk in those with iIGT might be explained by genetics.
In addition to baseline risk factors, a significant proportion of the risk reduction associated with regression to NGR was explained by percentage weight loss. Compared with those in the NGR group, ∼20% of the increased risk in the iIFG and IFG/IGT groups and 11% of the risk escalation in the iIGT group were explained by lack of weight loss. When weight loss was included in the regression model, the other intervention components (changes in physical activity and diet) were not significant predictors of future diabetes risk. This suggests that lifestyle intervention might only explain part of the risk decrease associated with regression to NGR observed in lifestyle intervention programs, with percentage weight loss being the dominant component explaining the additional risk reduction beyond the lower baseline diabetes risk in the NGR group. Therefore, establishing a healthy lifestyle early in life to prevent elevation of modifiable baseline risk factors might be an important strategy to stem the worldwide diabetes epidemic in the long term. Meanwhile, for those already at increased risk of diabetes, promoting weight loss is one of the key components that can facilitate regression to NGR and reduce risk of future diabetes.
The modifiable risk factors associated with an increased odds of regression to NGR were metformin use at baseline, change in weight, and change in exercise. Consistent with our findings, the DPP and the ADDITION-Prediabetes Cohort study both reported weight loss to be the dominant modifiable factor associated with regression to NGR (19,21). The DPP study found a marginal association (P = 0.06) of metformin use with a modest increased odds of reverting to NGR; however, the metformin group versus the control group had a significantly greater odds of regression to iIGT from IFG/IGT (21). Metformin suppresses the production of hepatic glucose and leads to a reduction in IFG (19,37), which may explain metformin’s stronger association with regression from IFG/IGT to iIGT in the DPP. Our sample had a large percentage of participants with iIFG (as opposed to IFG/IGT in the DPP), which may explain the strength and significance of the effects of metformin found in this study. Previous studies did not find exercise changes were associated with regression to NGR (18,19,21); however, the DPP regression models included intensive lifestyle intervention as a covariate, for which change in exercise is a component (19,21). Everyone received the intensive lifestyle intervention in the SDPI-DP; improvement in exercise was marginally associated with an adjusted increased likelihood (OR 1.15) of regression to NGR, suggesting exercise may play an important role in obtaining and possibly maintaining NGR.
Metabolic syndrome has been reported to predict incident diabetes (38,39). This study is the first to evaluate the association between components of the metabolic syndrome and regression to NGR. All metabolic syndrome components—higher baseline IFG and IGT levels, weight, history of hypertension, and high TG levels—were associated with a reduced adjusted odds of regression to NGR. A post hoc analysis found waist circumference had a similar relationship with regression to NGR as baseline weight and weight loss. DPP reported similar results for baseline FBG and 2-h glucose, but baseline weight was not associated with regression to normal. This lack of association could be due to controlling for insulin sensitivity in their model (21).
This study has several limitations. First, an OGTT was conducted every year, whereas FBG was assessed every 6 months. Because participants with iIGT were more likely to be diagnosed with diabetes based on 2-h glucose, the incident diabetes rate might have been underestimated in that group compared with those with iIFG. Second, diet and exercise were self-reported and thus subject to measurement error. This may partially explain the lack of association between changes in those behaviors and future diabetes risk in multivariate Cox regression models. Third, although SDPI-DP programs made every effort to assure that medication reporting was as accurate as possible, patient compliance was unknown. Fourth, the large number of dropouts between baseline and year 1 may have introduced unknown bias. The conclusions based on the small and potentially highly selected group of participants who completed year 1 assessments (Supplementary Table 1) might not be generalizable to all AI/ANs with prediabetes. Last, this study was conducted in a diverse population of AI/ANs only, which may not be generalizable to other populations.
In conclusion, this study supports previous findings that patients with prediabetes who revert to NGR have a reduced risk of developing type 2 diabetes in the subsequent 2 years; also, weight loss is an important determinant for both regression to NGR and decreased future diabetes risk. Participants who continued to have prediabetes after 1 year of lifestyle intervention had different levels of elevated diabetes risk based on their OGTT status, with those in the IFG/IGT group having the highest risk. Our findings support the heterogeneity of the disease process and the potential of using OGTT in a lifestyle intervention program to evaluate a participant’s glycemic status that might help tailor the intervention to different subtypes of prediabetes (6). Furthermore, as a significant addition to the regression to NGR literature, we found a large proportion of the reduced diabetes risk associated with regression to NGR was explained by various baseline diabetes risk factors, whereas only 12–20% of the risk reduction was explained by additional weight loss in that group. Thus, from a prevention perspective, establishing a healthy lifestyle early in life might be more critical than to intervene after detecting elevated risk factors. Finally, we identified metformin use, weight loss, and increase in exercise as modifiable risk factors associated with a higher odds of regression to NGR. Thus, adding personalized modifications such as a greater percent weight loss (40) or a combination of lifestyle and medication intervention strategies (20,40) might be needed for those who lack response to a standard lifestyle intervention.
Supplementary Material
Article Information
Acknowledgments. The authors are grateful to the Indian Health Service and to the tribal and urban Indian health programs and participants involved in the SDPI-DP Program. The authors also thank Drs. Richard F. Hamman, Department of Epidemiology, Colorado School of Public Health, LEAD Center, University of Colorado Anschutz Medical Campus, Aurora, Colorado, and James O. Hill, Department of Pediatrics & Medicine, Center for Human Nutrition, University of Colorado Anschutz Medical Campus, Aurora, Colorado, for their valuable scientific suggestions and comments.
Funding. Funding for the SDPI-DP Program was provided by the Indian Health Service (HHSI242200400049C, S.M.M.). Manuscript preparation was supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases (1P30-DK-092923, S.M.M., and R21-DK-108187, L.J.). Grant programs participating in the SDPI-DP Program: Cherokee Nation; Cheyenne River Sioux Tribe; Chickasaw Nation; Coeur d’Alene Tribe; Colorado River Indian Tribes; Colville Confederated Tribes; Fond du Lac Reservation; Gila River Health Care; Ho-Chunk Nation; Indian Health Board of Minneapolis; Kansas Consortium Diabetes Prevention Program with Haskell Health Center, Kickapoo Tribe, and Prairie Band of Potawatomi Nation; Kenaitze Indian Tribe IRA; Lawton Service Unit/7 Tribes Consortium; Menominee Indian Tribe of Wisconsin; Middle Rio Grande Pueblo Coalition with Pueblo of San Felipe, Pueblo of Santa Ana, and Pueblo of Cochiti; Mississippi Band of Choctaw Indians; Norton Sound Health Corporation; Pine Ridge IHS Service Unit; Quinault Indian Nation; Rapid City IHS Diabetes Program; Red Lake Comprehensive Health Services; Rocky Boy Health Board; SDPI/DPP Chehalis Tribes with Shoalwater Bay Tribe, Skokomish Indian Tribe, and Squaxin Island Tribe; Seneca Nation of Indians; Sonoma County Indian Health Project; South East Alaska Regional Health Consortium; Southcentral Foundation; Southern Oregon Tribal Diabetes Consortium with Cow Creek Band of Umpqua Tribe of Indians, Klamath Tribes, and Coquille Indian Tribe; Trenton Indian Service Area in consortium with Sac & Fox Tribe in Iowa; Tuba City Regional Health Care Corporation; United American Indian Involvement, Inc.; United Indian Health Services, Inc. in consortium with K’ima:w Medical Center (Hoopa Valley Tribe); Urban Native Diabetes Prevention Consortium with Indian Health Center of Santa Clara Valley, Native American Rehabilitation Association of the Northwest, Inc., and Hunter Health Clinic; Warm Springs Health & Wellness Center; Winnebago Tribe of Nebraska; Zuni Pueblo.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. K.A.P. researched the data, performed data analysis, and wrote, reviewed, and edited the manuscript. A.J. researched the data, contributed to the discussion, and reviewed and edited the manuscript. J.B. participated in the design of the SDPI-DP project, contributed to the discussion, and reviewed and edited the manuscript. A.B. contributed to the discussion and reviewed and edited the manuscript. S.M.M. conceptualized and designed the SDPI-DP project, contributed to the discussion, and reviewed and edited the manuscript. L.J. designed the study, researched the data, contributed to the discussion, and reviewed and edited the manuscript. L.J. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract and poster forms at the 79th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 7–11 June 2019.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc18-1964/-/DC1.
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Indian Health Service.
References
- 1.Blum R. Physicians’ assessment of deficiencies and desire for training in adolescent care. J Med Educ 1987;62:401–407 [DOI] [PubMed] [Google Scholar]
- 2.Khan T, Tsipas S, Wozniak G. Medical care expenditures for individuals with prediabetes: the potential cost savings in reducing the risk of developing diabetes. Popul Health Manag 2017;20:389–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diabetes Prevention Program Research Group The prevalence of retinopathy in impaired glucose tolerance and recent-onset diabetes in the Diabetes Prevention Program. Diabet Med 2007;24:137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A; KORA Study Group . Prevalence of polyneuropathy in pre-diabetes and diabetes is associated with abdominal obesity and macroangiopathy: the MONICA/KORA Augsburg Surveys S2 and S3. Diabetes Care 2008;31:464–469 [DOI] [PubMed] [Google Scholar]
- 5.Tapp RJ, Shaw JE, Zimmet PZ, et al. Albuminuria is evident in the early stages of diabetes onset: results from the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Am J Kidney Dis 2004;44:792–798 [PubMed] [Google Scholar]
- 6.Perreault L, Færch K. Approaching pre-diabetes. J Diabetes Complications 2014;28:226–233 [DOI] [PubMed] [Google Scholar]
- 7.Ford ES, Zhao G, Li C. Pre-diabetes and the risk for cardiovascular disease: a systematic review of the evidence. J Am Coll Cardiol 2010;55:1310–1317 [DOI] [PubMed] [Google Scholar]
- 8.Qiao Q, Pyörälä K, Pyörälä M, et al. Two-hour glucose is a better risk predictor for incident coronary heart disease and cardiovascular mortality than fasting glucose. Eur Heart J 2002;23:1267–1275 [DOI] [PubMed] [Google Scholar]
- 9.Goldberg RB, Temprosa M, Haffner S, et al.; Diabetes Prevention Program Research Group . Effect of progression from impaired glucose tolerance to diabetes on cardiovascular risk factors and its amelioration by lifestyle and metformin intervention: the Diabetes Prevention Program randomized trial by the Diabetes Prevention Program Research Group. Diabetes Care 2009;32:726–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orchard TJ, Temprosa M, Barrett-Connor E, et al.; Diabetes Prevention Program Outcomes Study Research Group . Long-term effects of the Diabetes Prevention Program interventions on cardiovascular risk factors: a report from the DPP Outcomes Study. Diabet Med 2013;30:46–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saydah SH, Loria CM, Eberhardt MS, Brancati FL. Subclinical states of glucose intolerance and risk of death in the U.S. Diabetes Care 2001;24:447–453 [DOI] [PubMed] [Google Scholar]
- 12.Kim NH, Kwon TY, Yu S, et al. Increased vascular disease mortality risk in prediabetic Korean adults is mainly attributable to ischemic stroke. Stroke 2017;48:840–845 [DOI] [PubMed] [Google Scholar]
- 13.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA 2015;314:1021–1029 [DOI] [PubMed] [Google Scholar]
- 14.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537–544 [DOI] [PubMed] [Google Scholar]
- 15.Tuomilehto J, Lindström J, Eriksson JG, et al.; Finnish Diabetes Prevention Study Group . Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 16.Knowler WC, Barrett-Connor E, Fowler SE, et al.; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perreault L, Temprosa M, Mather KJ, et al.; Diabetes Prevention Program Research Group . Regression from prediabetes to normal glucose regulation is associated with reduction in cardiovascular risk: results from the Diabetes Prevention Program Outcomes Study. Diabetes Care 2014;37:2622–2631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nanditha A, Ram J, Snehalatha C, et al. Early improvement predicts reduced risk of incident diabetes and improved cardiovascular risk in prediabetic Asian Indian men participating in a 2-year lifestyle intervention program. Diabetes Care 2014;37:3009–3015 [DOI] [PubMed] [Google Scholar]
- 19.Bodicoat DH, Khunti K, Srinivasan BT, et al. Incident Type 2 diabetes and the effect of early regression to normoglycaemia in a population with impaired glucose regulation. Diabet Med 2017;34:396–404 [DOI] [PubMed] [Google Scholar]
- 20.Perreault L, Pan Q, Mather KJ, Watson KE, Hamman RF, Kahn SE; Diabetes Prevention Program Research Group . Effect of regression from prediabetes to normal glucose regulation on long-term reduction in diabetes risk: results from the Diabetes Prevention Program Outcomes Study. Lancet 2012;379:2243–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perreault L, Kahn SE, Christophi CA, Knowler WC, Hamman RF; Diabetes Prevention Program Research Group . Regression from pre-diabetes to normal glucose regulation in the Diabetes Prevention Program. Diabetes Care 2009;32:1583–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang L, Manson SM, Beals J, et al.; Special Diabetes Program for Indians Diabetes Prevention Demonstration Project . Translating the Diabetes Prevention Program into American Indian and Alaska Native communities: results from the Special Diabetes Program for Indians Diabetes Prevention demonstration project. Diabetes Care 2013;36:2027–2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang L, Johnson A, Pratte K, Beals J, Bullock A, Manson SM; Special Diabetes Program for Indians Diabetes Prevention Program . Long-term outcomes of lifestyle intervention to prevent diabetes in American Indian and Alaska Native communities: the Special Diabetes Program for Indians Diabetes Prevention Program. Diabetes Care 2018;41:1462–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The self-administered comorbidity questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum 2003;49:156–163 [DOI] [PubMed] [Google Scholar]
- 25.Teufel-Shone NI, Jiang L, Beals J, et al. Demographic characteristics and food choices of participants in the Special Diabetes Program for American Indians Diabetes Prevention Demonstration Project. Ethn Health 2015;20:327–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Topolski TD, LoGerfo J, Patrick DL, Williams B, Walwick J, Patrick MB. The Rapid Assessment of Physical Activity (RAPA) among older adults (Abstract). Prev Chronic Dis 2006;3:A118. [PMC free article] [PubMed] [Google Scholar]
- 27.Spiegelman D. %mediate. SAS code to run mediate macro (SAS version 9 or above). Available from http://www.hsph.harvard.edu/donna-spiegelman/software/mediate/. Accessed 1 May 2017
- 28.Lin DY, Fleming TR, De Gruttola V. Estimating the proportion of treatment effect explained by a surrogate marker. Stat Med 1997;16:1515–1527 [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Shara NM, Calhoun D, Umans JG, Lee ET, Howard BV. Incidence rates and predictors of diabetes in those with prediabetes: the Strong Heart Study. Diabetes Metab Res Rev 2010;26:378–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hatziagelaki E, Karageorgopoulos DE, Chounta A, Tsiavou A, Falagas ME, Dimitriadis G. Predictors of impaired glucose regulation in patients with non-alcoholic fatty liver disease. Exp Diabetes Res 2012;2012:351974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kantartzis K, Machann J, Schick F, Fritsche A, Häring HU, Stefan N. The impact of liver fat vs visceral fat in determining categories of prediabetes. Diabetologia 2010;53:882–889 [DOI] [PubMed] [Google Scholar]
- 32.Borel AL, Nazare JA, Smith J, et al. Visceral, subcutaneous abdominal adiposity and liver fat content distribution in normal glucose tolerance, impaired fasting glucose and/or impaired glucose tolerance. Int J Obes 2015;39:495–501 [DOI] [PubMed] [Google Scholar]
- 33.Ahmed M. Non-alcoholic fatty liver disease in 2015. World J Hepatol 2015;7:1450–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cnop M, Vidal J, Hull RL, et al. Progressive loss of β-cell function leads to worsening glucose tolerance in first-degree relatives of subjects with type 2 diabetes. Diabetes Care 2007;30:677–682 [DOI] [PubMed] [Google Scholar]
- 35.Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet 2014;383:1068–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCarthy MI. Genomics, type 2 diabetes, and obesity. N Engl J Med 2010;363:2339–2350 [DOI] [PubMed] [Google Scholar]
- 37.Lachin JM, Christophi CA, Edelstein SL, et al.; DDK Research Group . Factors associated with diabetes onset during metformin versus placebo therapy in the Diabetes Prevention Program. Diabetes 2007;56:1153–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lorenzo C, Okoloise M, Williams K, Stern MP, Haffner SM; San Antonio Heart Study . The metabolic syndrome as predictor of type 2 diabetes: the San Antonio Heart Study. Diabetes Care 2003;26:3153–3159 [DOI] [PubMed] [Google Scholar]
- 39.Wilson PW, D’Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 2005;112:3066–3072 [DOI] [PubMed] [Google Scholar]
- 40.Maruthur NM, Ma Y, Delahanty LM, et al.; Diabetes Prevention Program Research Group . Early response to preventive strategies in the Diabetes Prevention Program. J Gen Intern Med 2013;28:1629–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.