Abstract
OBJECTIVE
We sought to examine associations in older adults among diabetes, glycemic control, diabetes duration, and biomarkers of hyperglycemia with incident mild cognitive impairment (MCI) and incident dementia.
RESEARCH DESIGN AND METHODS
We conducted a prospective analysis of 5,099 participants from the Atherosclerosis Risk in Communities (ARIC) Study who attended the fifth (2011–2013) exam. Cognitive status was assessed during follow-up via telephone calls, death certificate codes, surveillance, and a follow-up examination (2016–2017). We defined incident cognitive impairment as incident MCI or incident dementia in persons dementia-free at the index examination; we also examined each outcome separately. Diabetes was defined using self-report, medications, or HbA1c ≥6.5%; poor glycemic control in persons with diabetes was defined as HbA1c ≥7%. We examined the following biomarkers of hyperglycemia: HbA1c, fructosamine, glycated albumin, and 1,5-anhydroglucitol.
RESULTS
Mean age at baseline was 76 years, 59% were female, and 21% were black. Diabetes (hazard ratio [HR] 1.14 [95% CI 1.00, 1.31]), poor glycemic control in persons with diabetes (HR 1.31 [95% CI 1.05, 1.63]), and longer diabetes duration (≥5 vs. <5 years; HR 1.59 [95% CI 1.23, 2.07]) were significantly associated with incident cognitive impairment. We found a J-shaped association between HbA1c and incident dementia. Glycated albumin and fructosamine were also associated with incident dementia, independently of HbA1c. HbA1c and fructosamine were also associated with incident MCI.
CONCLUSIONS
Diabetes status, poor glycemic control, and longer diabetes duration were associated with worse cognitive outcomes over a median follow-up of 5 years.
Introduction
The U.S. population is rapidly aging. In 2012, 14% of the U.S. population was 65 years and older, and this proportion is expected to climb to >20% by 2030, representing >70 million people (1). Among older adults, the prevalence of diabetes is 22%, and the prevalence of prediabetes is 24% (2). Studies have documented that persons with diabetes, particularly in midlife, have greater cognitive decline and risk of dementia than those without diabetes (3–6). Results from studies examining diabetes assessed in late life with subsequent risk of cognitive decline and dementia have been consistent, though more mixed (7–9), likely due to small sample sizes or shorter duration of follow-up. Additionally, data related to diabetes severity and documented duration in late life are limited, and few studies have examined whether diabetes is a risk factor for mild cognitive impairment (MCI) or progression to dementia in persons with MCI.
Measures of hyperglycemia have been of particular interest in relation to cognitive impairments. HbA1c is the standard measure used in clinical practice to monitor glycemic control. There is growing interest in fructosamine, glycated albumin (GA), and 1,5-anhydroglucitol (1,5-AG) as alternative or complementary biomarkers of hyperglycemia that may add additional prognostic information beyond HbA1c (10–13). These markers may provide additional insights into the association of hyperglycemia, such as the role of glucose excursions (14,15), with cognitive impairment.
In this study, we sought to examine associations among late-life diabetes, glycemic control as measured by HbA1c, diabetes duration, and biomarkers of hyperglycemia with incident MCI and incident dementia using data from the Atherosclerosis Risk in Communities (ARIC) Study.
Research Design and Methods
Study Population
ARIC is a community-based, prospective study of adults from four U.S. communities: Forsyth County, North Carolina; Jackson, Mississippi; suburbs of Minneapolis, Minnesota; and Washington County, Maryland. Participants were initially recruited in 1987–1989 (visit 1) and examined in 1990–1992 (visit 2), 1993–1995 (visit 3), 1996–1998 (visit 4), 2011–2013 (visit 5), and 2016–2017 (visit 6). Participants received annual follow-up telephone calls to assess changes in health status.
The baseline for the current study was visit 5, the first visit that included comprehensive cognitive measures. Of the 6,538 participants who attended visit 5, we excluded participants who were neither black nor white (n = 18) or were nonwhite from Minnesota or Maryland field centers (n = 24), were missing cognitive status (n = 14), had dementia (n = 342), were missing covariates of interest (n = 563), or had no follow-up time (n = 478), giving an analytic sample size of 5,099. For analyses of glycemic control, we additionally excluded persons missing HbA1c (n = 46).
Categorization of Diabetes, Glycemic Control, and Diabetes Duration
At visit 5, we defined diabetes if a participant self-reported a physician diagnosis, reported using glucose-lowering medication, or had an HbA1c of ≥6.5% (48 mmol/mol). Among persons with diabetes, we dichotomized HbA1c at 7% (53 mmol/mol), a treatment goal recommended by the American Diabetes Association (ADA) Standards of Medical Care for many adults (16), while individuals with complex health may have less stringent glycemic goals (such as 8–8.5% and 64–69 mmol/mol), 73% of ARIC participants with diabetes had an HbA1c <7% (53 mmol/mol). In secondary analyses, we examined associations across three levels of HbA1c: <7%, 7–7.9%, and ≥8% (<53, 53–63, and ≥64 mmol/mol), respectively.
We used information from visits 1–4 and annual follow-up calls following visit 4 to identify the date of first reported diabetes. We calculated diabetes duration as the difference between this date and the visit 5 date. Self-reported diabetes in ARIC has been shown to be reliable and highly specific (17). Participants who reported no diabetes at any visit or phone call prior to visit 5 but who met our definition of diabetes at visit 5 were categorized as having diabetes with 0 duration; participants with undiagnosed diabetes at visit 5 (i.e., no self-reported diagnosis or medication use at any visit with HbA1c ≥6.5% at visit 5) were excluded from this analysis. We dichotomized diabetes duration as <5 or ≥5 years.
Markers of Glycemia: HbA1c, Fructosamine, Glycated Albumin, and 1,5-AG
HbA1c was measured using a Tosoh G7 automated high-performance liquid chromatography analyzer (Tosoh Bioscience, South San Francisco, CA) standardized to the Diabetes Control and Complications Trial assay. Fructosamine was measured in serum on the Roche Modular P800 Chemistry Analyzer (Roche Diagnostics) using a colorimetric assay. GA was measured in serum using a complex method by Asahi Kasei Pharma adapted to the Roche Modular P800 Chemistry Analyzer (Roche Diagnostics). 1,5-AG was measured in serum using GlycoMark 1,5-AG reagent on a Roche Modular P800 Chemistry Analyzer (Roche Diagnostics). All assays were conducted in stored samples collected from ARIC participants at visit 5, which had been stored at −70°C in freezers at the University of Minnesota.
Categorization of Cognitive Status at Visit 5
ARIC participants’ cognitive function at visit 5 was categorized by committee review as part of the ARIC Neurocognitive Study (ARIC-NCS) (18), which added dementia surveillance, and cognitive status to visit 5 of the parent ARIC Study. Briefly, cognitive status was categorized as normal, MCI, or dementia using information from proxy interviews, change in cognitive scores on three cognitive tests administered at study visits since midlife, and visit 5 results from the Mini-Mental State Examination, the Clinical Dementia Rating (CDR) form, the Functional Activities Questionnaire (FAQ), and Z scores from a full battery of 10 neuropsychological tests. An algorithmic diagnosis was assigned for all possible combinations of diagnostic elements; an expert committee reviewed the algorithmic diagnosis and assigned final cognitive status as normal cognition, MCI, or dementia. Etiologic classification was added to the MCI and dementia cases, but etiology was not considered in the current study.
Categorization of Cognitive Status After Visit 5
Cognitive status after visit 5 was defined by expert committee review (19) using the same criteria as at visit 5, which has been previously described in detail (18). Briefly, dementia was categorized in a stepwise fashion based on five hierarchical, leveled dementia diagnosis variables (19) based on the availability of data: level 1 diagnosis was based on neuropsychological data collected from participants who attended visits 5 and/or 6; level 2a was based on the Telephone Interview for Cognitive Status and retrospective dementia surveillance from visit 5; levels 2b and 2c were based on the Six-Item Screener (SIS) or Ascertain Dementia 8-item (AD8) (20–22) informant questionnaire, both captured from the annual telephone calls; and level 3 was based on ARIC surveillance of hospitalizations and deaths. The sequential order from available data sources considered for dementia ascertainment assignment were 1) reviewer diagnosis at visit 5 or visit 6, 2) algorithmic diagnosis at visit 5 or visit 6, 3) AD8 result, 4) two SIS results, 5) one SIS result if the participant is lost to follow-up or deceased, 6) hospitalization discharge codes, and 7) death certificate codes.
Each of the leveled diagnosis variables had an associated date of diagnosis. If the participant was categorized as having dementia, the date corresponded to the earliest date that dementia was detected. If the participant was categorized as not having dementia, the corresponding date came from the visit 5 date, visit 6 date, AD8 date, or SIS date.
Participants who attended visit 6 were categorized as cognitively normal if their neuropsychological test scores in each of three domains (memory, language, and executive function) were higher than −1.5 SDs expected for their age, race, and education level, or they had little or no cognitive decline in the full ARIC cognitive battery (decline of <0.055 SDs per year [ARIC visit 6 Manual 17 (23)]). MCI was defined only in persons examined at study visits. Diagnosis required having at least one failed domain score (defined as a score <1.5 SDs below the participant’s expected score), a CDR sum of boxes between >0.5 and ≤3, an FAQ ≤5, and a decline on the full ARIC cognitive battery of >0.055 SDs per year. Dementia diagnoses in persons examined at visit 6 required cognitive decline >0.055 SDs/year, two or more failed cognitive domains and FAQ >5, or CDR sum of boxes >3.
Incident Cognitive Impairment
Incident cognitive impairment was defined to represent progression in cognitive status from visit 5 to visit 6 (median follow-up 5 years). The following two groups were categorized as having incident cognitive impairment: 1) persons categorized as cognitively normal at visit 5 and having MCI or dementia at visit 6 or dementia from surveillance for those who did not attend visit 6, and 2) persons categorized as having MCI at visit 5 and as having dementia at or before visit 6.
Covariates
We included the following confounders based on a priori knowledge: age; race and field-center (categorized into five groups as white adults from Minneapolis, Washington County, or Forsyth County or black adults from Forsyth or Jackson); sex; education (less than high school education; high school graduate, high school equivalency, or vocational school; or college or above); drinking status (current, former, or never); cigarette smoking (current, former, or never); hypertension (systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or blood pressure–lowering medication); apolipoprotein E genotype, number of e4 alleles (APOE4; zero or one or more alleles); history of stroke (yes/no); and history of coronary heart disease (CHD; yes/no). All variables were assessed at visit 5 except for education, which was determined at visit 1. For analyses of glycemic markers, we also examined associations additionally adjusted for HbA1c.
Statistical Analysis
We compared baseline characteristics by diabetes status using means (SD) and proportions. For analyzing incident events, we used Cox proportional hazards models to estimate hazard ratios (HRs) and 95% CIs. We verified the proportional hazards assumption using log-log plots. We present results unadjusted and fully adjusted using the covariates described above. We fit models for four separate cognitive outcomes: 1) incident dementia and 2) incident MCI, both among participants who were cognitively normal at baseline; 3) incident cognitive impairment among all baseline participants; and 4) incident dementia among participants with MCI at baseline.
To examine the association between each biomarker of hyperglycemia and incident dementia, we used restricted cubic splines (24) with knots at the 5th, 35th, 65th, and 95th percentiles; restricted cubic splines allow for potential nonlinear relationships between biomarker level and incident dementia. To allow for comparison across markers, we converted each participant’s biomarker values to Z scores by subtracting the mean and dividing by the SD.
In secondary analyses, we examined incident dementia in persons with diabetes by tertiles of age and diabetes duration in an effort to examine whether associations between diabetes duration with dementia differ by baseline age. Because of small sample sizes, this secondary model was adjusted only for age at study baseline, race-center, sex, and education level.
All analyses were done using Stata/SE 14.2 (StataCorp, College Station, TX). We report 95% CIs, and P values <0.05 were considered statistically significant.
Results
Study Characteristics
At study baseline (visit 5), the mean age of participants was 76 years, 59% were female, 21% were black, and 34% had diabetes (Table 1). Participants with diabetes were more likely to be black, to have lower levels of education, higher BMI, hypertension, a history of CHD, and history of stroke and were less likely to be current drinkers. Other characteristics were similar across the groups (Table 1).
Table 1.
Baseline (visit 5, 2011–2013) population characteristics by diabetes* status
| Total | No diabetes | Diabetes |
||
|---|---|---|---|---|
| HbA1c <7% | HbA1c ≥7% | |||
| N (%) | 5,099 | 3,318 (65.7) | 1,276 (25.3) | 459 (9.1) |
| Age, years, mean (SD) | 75.8 (5.0) | 75.8 (5.0) | 76.0 (5.1) | 75.1 (4.7) |
| Female | 59.2 | 60.0 | 58.3 | 55.3 |
| Black | 21.4 | 17.3 | 26.7 | 35.7 |
| Education | ||||
| Less than high school | 12.9 | 9.9 | 17.6 | 21.4 |
| High school | 42.5 | 41.9 | 44.0 | 42.5 |
| Higher than high school | 44.7 | 48.2 | 38.5 | 36.2 |
| Current smoking | 5.8 | 5.8 | 6.1 | 5.4 |
| Current drinking | 49.9 | 54.9 | 42.5 | 34.0 |
| BMI, kg/m2, mean (SD) | 28.8 (5.7) | 27.8 (5.2) | 30.2 (5.8) | 32.0 (6.2) |
| Hypertension | 74.0 | 68.1 | 84.0 | 88.3 |
| History of CHD | 13.7 | 11.6 | 17.8 | 17.7 |
| History of stroke | 3.2 | 2.4 | 4.0 | 7.0 |
| APOE e4 alleles | ||||
| 0 | 72.3 | 72.2 | 72.3 | 72.5 |
| 1 | 25.7 | 25.9 | 25.2 | 25.5 |
| 2 | 2.1 | 1.9 | 2.5 | 2.0 |
| Cognitive outcomes† after visit 5 | ||||
| Dementia | 11.1 | 10.0 | 12.5 | 15.5 |
| MCI | 16.8 | 16.2 | 16.6 | 23.2 |
| Cognitive impairment | 20.1 | 19.0 | 20.7 | 25.9 |
Data are percentages unless otherwise noted. The three-level categorization of diabetes and glycemic control excludes 46 persons missing HbA1c data.
*Diabetes was defined based on self-reported diagnosis, use of glucose-lowering medication, or HbA1c of ≥6.5% (48 mmol/mol). HbA1c of 7% is equivalent to 53 mmol/mol.
†Dementia was categorized using information from study visits, telephone calls with participants or their proxy, or surveillance of hospitalizations and deaths. MCI was defined only in persons examined at visit 6 based on neuropsychological testing. Cognitive impairment includes the following progression in cognitive status from visit 5 to 6: 1) cognitively normal at visit 5, MCI or dementia at visit 6, or dementia from surveillance, or 2) MCI at visit 5, dementia at visit 6, or dementia from surveillance.
Incident Dementia and MCI in Participants Cognitively Normal at Baseline
Median follow-up for incident events was ∼5 years (mean 4.8 years, SD 0.8). In participants who were cognitively normal at visit 5, there were 275 incident dementia and 455 incident MCI cases. Diabetes was not associated with incident dementia, and differences in incident dementia by glycemic control were nonsignificant (Table 2). In contrast, diabetes was significantly associated with incident MCI (HR 1.23 [95% CI 1.00, 1.51]) (Table 2). Persons with HbA1c <7% (53 mmol/mol) did not have significantly higher risk of developing MCI compared with persons without diabetes (HR 1.11 [95% CI 0.88, 1.40]). In contrast, those with HbA1c ≥7% (53 mmol/mol) compared with those with no diabetes had 1.73 times higher risk of MCI (95% CI 1.26, 2.38) (Table 2); additionally, comparing the glycemic control groups among persons with diabetes, those with HbA1c ≥7% (53 mmol/mol) had 1.56 times the risk of MCI (95% CI 1.10, 2.21) compared with persons with HbA1c <7% (53 mmol/mol). Longer duration of diabetes was associated with incident dementia (HR 1.91 [95% CI 1.09, 3.35]) (Table 2) and incident MCI (HR 1.58 [95% CI 1.04, 2.41]) (Table 2).
Table 2.
Adjusted HRs (95% CI) for cognitive outcomes by diabetes status, glycemic control, and diabetes duration among participants who were cognitively normal at visit 5
| Incident dementia |
Incident MCI |
|||
|---|---|---|---|---|
| n/N‡ (%) | Adjusted HR (95% CI) | n/N‡ (%) | Adjusted HR (95% CI) | |
| Diabetes | ||||
| No | 164/2,690 (6.1) | 1 (reference) | 302/1,938 (15.6) | 1 (reference) |
| Yes | 111/1,317 (8.4) | 1.08 (0.83, 1.39) | 153/882 (17.4) | 1.23 (1.00, 1.51)* |
| Diabetes and glycemic control | ||||
| No diabetes | 164/2,690 (6.1) | 1 (reference) | 302/1,938 (15.6) | 1 (reference) |
| Diabetes, HbA1c <7% | 77/968 (8.0) | 1.01 (0.76, 1.35) | 105/655 (16.0) | 1.11 (0.88, 1.40) |
| Diabetes, HbA1c ≥7% (compared with no diabetes) | 32/336 (9.5) | 1.16 (0.77, 1.74) | 48/218 (22.0) | 1.73 (1.26, 2.38)*** |
| Diabetes, HbA1c ≥7% (compared with HbA1c <7%) | 32/336 (9.5) | 1.15 (0.75, 1.77) | 48/218 (22.0) | 1.56 (1.10, 2.21)* |
| Diabetes duration† | ||||
| <5 years | 17/336 (5.1) | 1 (reference) | 30/226 (13.3) | 1 (reference) |
| ≥5 years | 89/921 (9.7) | 1.91 (1.09, 3.35)* | 118/617 (19.2) | 1.58 (1.04, 2.41)* |
Models are adjusted for age, race-center, sex, education level, cigarette smoking status (current/former/never), drinking status (current/former/never), APOE e4 (zero, one, or two alleles), hypertension (yes/no), history of stroke (yes/no), and history of CHD (yes/no). Diabetes was defined based on self-reported diagnosis, use of glucose-lowering medication, or HbA1c of ≥6.5% (48 mmol/mol). HbA1c of 7% is equivalent to 53 mmol/mol.
†Among persons with diabetes.
‡n/N: the number of incident cases (n) among the total number of participants in that group (N).
*P < 0.05; ***P < 0.001.
Incident Cognitive Impairment in All Study Participants and Incident Dementia in Persons With MCI
In analyses examining progression in cognitive status from visit 5 to visit 6, diabetes was significantly associated with incident cognitive impairment (HR 1.14 [95% CI 1.00, 1.31]) (Table 3). This was primarily driven by persons with diabetes and HbA1c ≥7% (HR 1.38 [95% CI 1.12, 1.69]); persons with diabetes and HbA1c <7% (53 mmol/mol) did not have significantly higher hazard of incident dementia compared with persons without diabetes (HR 1.05 [95% CI 0.91, 1.22]) (Table 3). Among persons with diabetes, those with diabetes duration of ≥5 years had 1.59 (95% CI 1.23, 2.07) times the hazard of cognitive impairment compared with persons of shorter diabetes duration (Table 3). Trends were similar for diabetes and glycemic control for incident dementia among persons with MCI at baseline (Table 3).
Table 3.
HRs (95% CI) for incident cognitive impairment and dementia by baseline cognitive status
| Incident cognitive impairment§ among participants dementia-free at baseline |
Incident dementia among participants with MCI at baseline |
|||
|---|---|---|---|---|
| n/N‡ (%) | Adjusted HR (95% CI) | n/N‡ (%) | Adjusted HR (95% CI) | |
| Diabetes | ||||
| No | 638/3,344 (19.1) | 1 (reference) | 169/639 (26.5) | 1 (reference) |
| Yes | 390/1,755 (22.2) | 1.14 (1.00, 1.31)* | 125/434 (28.8) | 1.15 (0.90, 1.47) |
| Diabetes and glycemic control | ||||
| No diabetes | 638/3,344 (19.1) | 1 (reference) | 169/639 (26.4) | 1 (reference) |
| Diabetes, HbA1c <7% | 264/1,276 (20.7) | 1.05 (0.91, 1.22) | 81/305 (26.6) | 1.02 (0.78, 1.35) |
| Diabetes, HbA1c ≥7% (compared with no diabetes) | 119/459 (25.9) | 1. 38 (1.12, 1.69)** | 39/122 (32.0) | 1.38 (0.96, 2.00) |
| Diabetes, HbA1c ≥7% (compared with HbA1c<7%) | 119/459 (25.9) | 1.31 (1.05, 1.63)* | 39/122 (32.0) | 1.35 (0.91, 2.00) |
| Diabetes duration† | ||||
| <5 years | 75/441 (17.0) | 1 (reference) | 27/103 (26.2) | 1 (reference) |
| ≥5 years | 302/1,234 (24.5) | 1.59 (1.23, 2.07)*** | 95/311 (30.6) | 1.40 (0.88, 2.20) |
Models are adjusted for age, race-center, sex, education level, cigarette smoking status (current/former/never), drinking status (current/former/never), APOE e4 (zero, one, or two alleles), hypertension (yes/no), history of stroke (yes/no), and history of CHD (yes/no). Diabetes was defined based on self-reported diagnosis, use of glucose-lowering medication, or HbA1c of ≥6.5% (48 mmol/mol). HbA1c of 7% is equivalent to 53 mmol/mol.
†Among persons with diabetes.
‡n/N: the number of incident cases (n) among the total number of participants in that group (N).
§Cognitive impairment includes incident MCI in persons cognitively normal at baseline and incident dementia in persons cognitively normal or MCI at baseline.
*P < 0.05; **P < 0.01; ***P < 0.001.
Glcyemic Markers and Incident Dementia
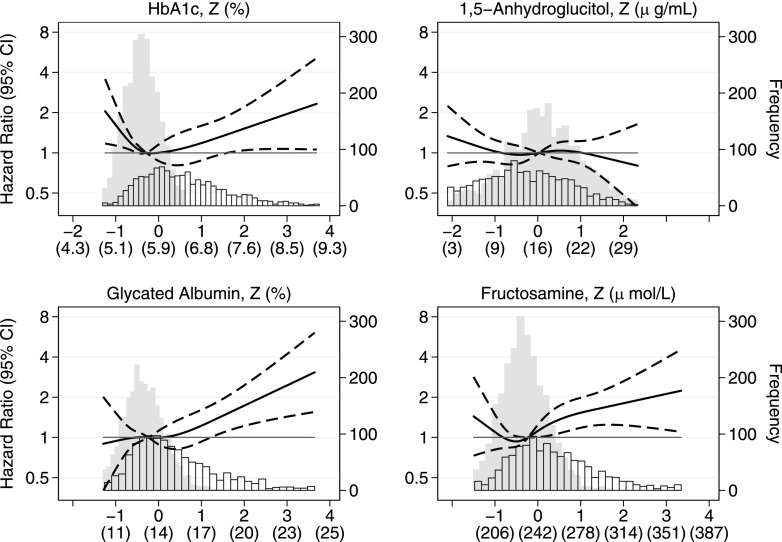
The continuous associations for each glycemic marker with incident dementia among participants dementia-free at baseline are shown in Fig. 1. For HbA1c, low (less than ∼5.8% and ∼40 mmol/mol) and high (greater than ∼7.5% and ∼58 mmol/mol) values were significantly associated with higher risk of incident dementia. Higher values of GA and fructosamine, values predominately in the diabetes range, were associated with higher risk of incident dementia (Fig. 1) and persisted after additional adjustment for HbA1c (Supplementary Fig. 1). We did not see an association with incident dementia for 1,5-AG. We found similar, though more modest, trends with incident MCI (Supplementary Fig. 2).
Figure 1.
Adjusted HRs (95% CI) for standardized (actual) HbA1c, 1,5-AG, GA, and fructosamine with incident dementia among persons dementia-free at baseline. Biomarker values are shown as Z scores (original units). HRs (solid line) and 95% CIs (dashed lines) are from Cox proportional hazard regression, adjusted for age, race, sex, education level, cigarette smoking status (current/former/never), drinking status (current/former/never), APOE e4 (zero, one, or two alleles), hypertension (yes/no), history of stroke (yes/no), and history of CHD (yes/no). Each biomarker was modeled using a restricted cubic spline, with knots at the 5th, 35th, 65th, and 95th percentiles, and centered at the median. Histograms of each biomarker are shown separately for persons without (solid bars) and with (outlined bars) diabetes.
Secondary Analyses
Our secondary analyses of incident dementia in dementia-free individuals with diabetes by age and diabetes duration are shown in Supplementary Table 1. Age appeared to be a stronger driver of associations rather than duration. After adjusting for demographic variables, persons who were older at baseline had a higher absolute risk of incident dementia, regardless of their diabetes duration (Supplementary Table 1). In the first tertile of baseline age (<72.8 years), persons in the highest tertile of diabetes duration had 2.49 times the risk of dementia compared with persons in the lowest tertile of duration (95% CI 1.23, 5.06). In persons in the middle tertile of age (72.9–77.9 years), those with the longest duration of diabetes had 1.47 times the risk of dementia compared with persons with shorter duration (95% CI 0.80, 2.72). There was no appreciable association with diabetes duration in the highest tertile of baseline age. Results from analyses examining three levels of HbA1c for glycemic control are shown in Supplementary Tables 2 and 3; trends across the three HbA1c groups were similar to when HbA1c was dichotomized.
Conclusions
In this study of older adults without dementia, we found that diabetes, poor glycemic control, and longer diabetes duration were associated with incident cognitive impairment; persons with well-controlled diabetes (HbA1c <7%, 53 mmol/mol) did not have significantly higher risk of cognitive impairment compared with persons without diabetes. We also found that GA and fructosamine were associated with incident cognitive impairment, even after adjustment for HbA1c. In persons who were cognitively normal at baseline, diabetes and glycemic control were strongly associated with incident MCI.
The overall association between diabetes and dementia has been well described (25,26); however, less is known about the association of diabetes in older adults on late-life incident MCI and progression to dementia from MCI. In our study, in which the median age was 76 years, we found relatively weak associations of diabetes and glycemic control with incident dementia in cognitively normal individuals over a median follow-up of 5 years. However, the strong associations with incident MCI and with progression in cognitive status imply that the effects of diabetes, glycemic control, and diabetes duration may have an impact on cognitive function along the continuum from normal cognition to MCI to dementia. These estimated overall associations between diabetes and incident dementia were similar to results reported in cognitively normal adults aged 65 and older with similar length of follow-up (27,28).
Studies have generally found stronger associations when vascular risk factors are ascertained in midlife compared with late life (7,9,29,30). In ARIC, diabetes measured in midlife (mean age 52 years) was associated with 1.77 times the risk of dementia (95% CI 1.53, 2.04) over 25 years compared with persons without diabetes (31). The ability in ARIC to examine both mid- and late-life exposures extends the literature by showing that late-life onset of diabetes, though associated with worse cognitive outcomes, is a weaker risk factor for dementia compared with midlife onset of diabetes. Our finding that longer diabetes duration was associated with higher risk of dementia only in the youngest tertile supports this hypothesis, though sample sizes were small, and survival bias may be contributing to these findings; more research in larger studies is needed to clarify these findings.
Studies examining diabetes in relation to MCI incidence and progression from MCI to dementia are more limited. One study examining MCI incidence in older adults (mean age 78 years) estimated an unadjusted risk of MCI over 4 years of 1.51 (95% CI 1.04, 2.20) in persons with diabetes compared with those without (32). In the Singapore Longitudinal Ageing Study (mean age 65 years), the estimated risk of MCI and progression to dementia in persons with diabetes were 2.84 (95% CI 1.92, 4.19) and 2.47 (95% CI 1.92, 4.19), respectively (33).
The association between HbA1c and cognitive impairment is complex. We have previously shown in ARIC that higher midlife HbA1c values in both persons with and without diabetes were associated with greater cognitive decline over 20 years (6). In the current study, higher HbA1c was associated with incident dementia at values in the diabetic range, though low levels (<5.8%, 40 mmol/mol) also appeared associated with higher risk of incident dementia. This contrasts with prior studies that found associations between HbA1c and brain atrophy in cognitively normal individuals (34) and with higher risk of MCI (35), though not all studies have found associations, particularly when HbA1c was assessed in combination with other markers of glycemia and insulin resistance (36,37). This may be in part related to J-shaped associations between HbA1c and death (and other outcomes) in adults with (38) and without (39) diabetes, which has been shown in a number of prior studies. Lastly, tight glycemic control increases the likelihood of hypoglycemic events. Prior work (40,41) has shown associations between severe hypoglycemia (identified through hospitalizations) and cognitive deficits, though the association is likely bidirectional. In our study, 73% of participants with diabetes had an HbA1c <7%, a treatment goal recommended by the ADA as a reasonable target for healthy adults. Although we observed similar trends in incident cognitive outcomes when we considered three categories of HbA1c, our sample sizes in these groups were small, and we were unable to identify mild cases of hypoglycemia. More research is needed to elucidate the association of HbA1c with cognitive function in older adults with complex health and to identify optimal targets that balance the risks and benefits of tight glycemic control with respect to cognition and other health outcomes.
In our study, fructosamine and GA, biomarkers that are positively correlated with HbA1c, were also strongly associated with incident dementia, even after additional adjustment for HbA1c. Fructosamine and GA can be used to better estimate short-term (2–3 weeks) glycemic control because the glycation of hemoglobin occurs more slowly than the glycation of albumin (13). Glycated albumin levels have also been shown to be higher in persons with diabetes and decreased insulin secretion (15); low insulin levels have been associated with incident dementia in a pathway suggested to be independent of diabetes and hyperinsulinemia (42,43). The additional prognostic value of these biomarkers for dementia, above and beyond HbA1c, suggests the complementary nature of these measures and the possible utility of measuring multiple glycemic biomarkers in a single blood sample to better classify hyperglycemia in older adults.
Our study has several strengths, including the large, well-characterized population-based cohort and the inclusion of both cognitively normal individuals and participants with MCI. Additionally, we were able to use a wealth of data to categorize diabetes, diabetes duration, and glycemic control, and cognitive status was adjudicated via expert committee review. There are a few notable limitations to our study. First, we lacked imaging and cerebrospinal fluid biomarkers to define dementia and MCI etiologic subtypes. Second, most dementia cases occurred in participants who did not attend visit 6; however, persons with cognitive impairments are more likely to drop out, so access to telephone interviews with participants and proxies provided opportunities to capture case subjects who would otherwise have been missed. Prior work in ARIC has shown this approach may not be prone to ascertainment or diagnostic bias (J.A. Deal, A. Alonso, K. Bandeen-Roche, P. Palta, K. Perryman, M.C.P., A.L.C. Schneider, A.R.S., L.M. Wruck, unpublished observations).
In conclusion, diabetes status, poor glycemic control, and longer diabetes duration remain associated with worse cognitive outcomes over a median follow-up of 5 years in persons evaluated at ages 66–90 years. In older adults with diabetes, maintaining glycemic control is an important avenue for mitigating cognitive impairments into older age.
Supplementary Material
Article Information
Acknowledgments. The authors thank the staff and participants of the ARIC Study for the important contributions.
Funding. The ARIC Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute (NHLBI) contracts (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, and HHSN268201700004I). Neurocognitive data are collected through National Institutes of Health (NIH) grants 2U01-HL-096812, 2U01-HL-096814, 2U01-HL-096899, 2U01-HL-096902, and 2U01-HL-096917 (from the NHLBI, National Institute of Neurological Disorders and Stroke, National Institute on Aging, and National Institute on Deafness and Other Communication Disorders) and with previous brain MRI examinations funded by NHLBI grant R01-HL-70825. Funding for laboratory testing and biospecimen collection at ARIC visit 6 was supported by NIH/National Institute of Diabetes and Digestive and Kidney Diseases grant R01-DK-089174. M.C.P. was supported by NIH/National Institute on Aging grant R01-AG-057869. Reagents for the 1,5-AG assays were donated by GlycoMark, Inc. Reagents for the GA (albumin) assays were donated by the Asahi Kasei Corporation.
Duality of Interest. B.G.W. has previously received reimbursements from Acadia Pharmaceuticals. D.S.K. serves on a Data Safety Monitoring Board for the Dominantly Inherited Alzheimer Network (DIAN) study; is an investigator in clinical trials sponsored by Biogen, Lilly Pharmaceuticals, and the University of Southern California; and receives research support from the NIH. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. A.M.R., A.R.S., and E.S. designed the study, researched the data, and drafted the manuscript. A.M.R., A.R.S., M.S.A., J.C., B.G.W., M.C.P., D.S.K., K.W., S.B., T.H.M., R.F.G., and E.S. provided interpretation of the data and meaningful contributions and revisions to the manuscript. A.M.R. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-0120/-/DC1.
References
- 1.Ortman JM, Velkoff VA, Hogan H. An Aging Nation: The Older Population in the United States. Washington, DC, U.S. Census Bureau, 2014 [Google Scholar]
- 2.Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in prevalence and control of diabetes in the United States, 1988-1994 and 1999-2010. Ann Intern Med 2014;160:517–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reijmer YD, van den Berg E, Ruis C, Kappelle LJ, Biessels GJ. Cognitive dysfunction in patients with type 2 diabetes. Diabetes Metab Res Rev 2010;26:507–519 [DOI] [PubMed] [Google Scholar]
- 4.Biessels GJ, van der Heide LP, Kamal A, Bleys RL, Gispen WH. Ageing and diabetes: implications for brain function. Eur J Pharmacol 2002;441:1–14 [DOI] [PubMed] [Google Scholar]
- 5.Kodl CT, Seaquist ER. Cognitive dysfunction and diabetes mellitus. Endocr Rev 2008;29:494–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rawlings AM, Sharrett AR, Schneider ALC, et al. Diabetes in midlife and cognitive change over 20 years: a cohort study. Ann Intern Med 2014;161:785–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 2005;64:277–282 [DOI] [PubMed] [Google Scholar]
- 8.Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J 2012;42:484–491 [DOI] [PubMed] [Google Scholar]
- 9.Xu W, Qiu C, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Mid- and late-life diabetes in relation to the risk of dementia: a population-based twin study. Diabetes 2009;58:71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armbruster DA. Fructosamine: structure, analysis, and clinical usefulness. Clin Chem 1987;33:2153–2163 [PubMed] [Google Scholar]
- 11.Yamanouchi T, Ogata N, Tagaya T, et al. Clinical usefulness of serum 1,5-anhydroglucitol in monitoring glycaemic control. Lancet 1996;347:1514–1518 [DOI] [PubMed] [Google Scholar]
- 12.Dungan KM. 1,5-Anhydroglucitol (GlycoMark) as a marker of short-term glycemic control and glycemic excursions. Expert Rev Mol Diagn 2008;8:9–19 [DOI] [PubMed] [Google Scholar]
- 13.Koga M. Glycated albumin: clinical usefulness. Clin Chim Acta 2014;433:96–104 [DOI] [PubMed] [Google Scholar]
- 14.Yoshiuchi K, Matsuhisa M, Katakami N, et al. Glycated albumin is a better indicator for glucose excursion than glycated hemoglobin in type 1 and type 2 diabetes. Endocr J 2008;55:503–507 [DOI] [PubMed] [Google Scholar]
- 15.Koga M, Murai J, Saito H, Kasayama S. Glycated albumin and glycated hemoglobin are influenced differently by endogenous insulin secretion in patients with type 2 diabetes. Diabetes Care 2010;33:270–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Diabetes Association Standards of Medical Care in Diabetes–2017. Diabetes Care 2017;40(Suppl. 1):S1–S13527979885 [Google Scholar]
- 17.Schneider ALC, Pankow JS, Heiss G, Selvin E. Validity and reliability of self-reported diabetes in the Atherosclerosis Risk in Communities Study. Am J Epidemiol 2012;176:738–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knopman DS, Gottesman RF, Sharrett AR, et al. Mild cognitive impairment and dementia prevalence: the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). Alzheimers Dement (Amst) 2016;2:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atherosclerosis Risk in Communities Study Exam 6 NCS Derived Variable Dictionary [Internet], 2018. Available from https://sites.cscc.unc.edu/aric/sites/default/files/public/manuals/ARIC%20V6NCSCOG61%20Derived%20Variable%20Dictionary_180724.pdf. Accessed 1 April 2019
- 20.Carpenter CR, DesPain B, Keeling TN, Shah M, Rothenberger M. The Six-Item Screener and AD8 for the detection of cognitive impairment in geriatric emergency department patients. Ann Emerg Med 2011;57:653–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology 2005;65:559–564 [DOI] [PubMed] [Google Scholar]
- 22.Galvin JE, Roe CM, Xiong C, Morris JC. Validity and reliability of the AD8 informant interview in dementia. Neurology 2006;67:1942–1948 [DOI] [PubMed] [Google Scholar]
- 23.Visit 6 Manual 17: ARIC Neurocognitive Exam [Internet]. Available from https://sites.cscc.unc.edu/aric/sites/default/files/public/manuals/ARIC%20MOP17%20170405.pdf. Accessed 1 April 2019
- 24.Harrell FE. Regression Modeling Strategies. New York, NY, Springer New York, 2001. (Springer Series in Statistics)
- 25.Cooper C, Sommerlad A, Lyketsos CG, Livingston G. Modifiable predictors of dementia in mild cognitive impairment: a systematic review and meta-analysis. Am J Psychiatry 2015;172:323–334 [DOI] [PubMed] [Google Scholar]
- 26.Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes—systematic overview of prospective observational studies. Diabetologia 2005;48:2460–2469 [DOI] [PubMed] [Google Scholar]
- 27.Peila R, Rodriguez BL, Launer LJ; Honolulu-Asia Aging Study . Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: the Honolulu-Asia Aging Study. Diabetes 2002;51:1256–1262 [DOI] [PubMed] [Google Scholar]
- 28.MacKnight C, Rockwood K, Awalt E, McDowell I. Diabetes mellitus and the risk of dementia, Alzheimer’s disease and vascular cognitive impairment in the Canadian Study of Health and Aging. Dement Geriatr Cogn Disord 2002;14:77–83 [DOI] [PubMed] [Google Scholar]
- 29.Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol 2005;62:1556–1560 [DOI] [PubMed] [Google Scholar]
- 30.Kivipelto M, Helkala EL, Laakso MP, et al. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ 2001;322:1447–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gottesman RF, Schneider AL, Zhou Y, et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA 2017;317:1443–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ganguli M, Fu B, Snitz BE, Hughes TF, Chang C-CH. Mild cognitive impairment: incidence and vascular risk factors in a population-based cohort. Neurology 2013;80:2112–2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ng TP, Feng L, Nyunt MSZ, et al. Metabolic syndrome and the risk of mild cognitive impairment and progression to dementia: follow-up of the Singapore longitudinal ageing study cohort. JAMA Neurol 2016;73:456–463 [DOI] [PubMed] [Google Scholar]
- 34.Enzinger C, Fazekas F, Matthews PM, et al. Risk factors for progression of brain atrophy in aging: six-year follow-up of normal subjects. Neurology 2005;64:1704–1711 [DOI] [PubMed] [Google Scholar]
- 35.Yaffe K, Blackwell T, Whitmer RA, Krueger K, Barrett Connor E. Glycosylated hemoglobin level and development of mild cognitive impairment or dementia in older women. J Nutr Health Aging 2006;10:293–295 [PubMed] [Google Scholar]
- 36.Byun MS, Kim HJ, Yi D, et al.; KBASE Research Group . Differential effects of blood insulin and HbA1c on cerebral amyloid burden and neurodegeneration in nondiabetic cognitively normal older adults. Neurobiol Aging 2017;59:15–21 [DOI] [PubMed] [Google Scholar]
- 37.Mukai N, Ohara T, Hata J, et al. Alternative measures of hyperglycemia and risk of Alzheimer’s disease in the community: the Hisayama Study. J Clin Endocrinol Metab 2017;102:3002–3010 [DOI] [PubMed] [Google Scholar]
- 38.Forbes A, Murrells T, Mulnier H, Sinclair AJ. Mean HbA1c, HbA1c variability, and mortality in people with diabetes aged 70 years and older: a retrospective cohort study. Lancet Diabetes Endocrinol 2018;6:476–486 [DOI] [PubMed] [Google Scholar]
- 39.Carson AP, Fox CS, McGuire DK, et al. Low hemoglobin A1c and risk of all-cause mortality among US adults without diabetes. Circ Cardiovasc Qual Outcomes 2010;3:661–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee AK, Rawlings AM, Lee CJ, et al. Severe hypoglycaemia, mild cognitive impairment, dementia and brain volumes in older adults with type 2 diabetes: the Atherosclerosis Risk in Communities (ARIC) cohort study. Diabetologia 2018;61:1956–1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yaffe K, Falvey CM, Hamilton N, et al.; Health ABC Study . Association between hypoglycemia and dementia in a biracial cohort of older adults with diabetes mellitus. JAMA Intern Med 2013;173:1300–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peila R, Rodriguez BL, White LR, Launer LJ. Fasting insulin and incident dementia in an elderly population of Japanese-American men. Neurology 2004;63:228–233 [DOI] [PubMed] [Google Scholar]
- 43.Mehlig K, Lapidus L, Thelle DS, et al. Low fasting serum insulin and dementia in nondiabetic women followed for 34 years. Neurology 2018;91:e427–e435 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.