Abstract
OBJECTIVE
Sleep disturbances and circadian misalignment (social jet lag, late chronotype, or shift work) have been associated with worse glycemic control in type 2 diabetes (T2D). Whether these findings apply to adults with prediabetes is yet unexplored. We hypothesized that self-reported short sleep, poor sleep quality, and/or circadian misalignment are associated with higher glycemia, BMI, and blood pressure (BP) in adults with prediabetes or recently diagnosed, untreated T2D.
RESEARCH DESIGN AND METHODS
Our cohort included 962 overweight/obese adults ages 20–65 years with prediabetes or recently diagnosed, untreated T2D who completed a 2-h oral glucose tolerance test and validated sleep questionnaires. Independent associations of sleep and circadian variables with glycemia, BMI, and BP were evaluated with regression models.
RESULTS
The multiethnic cohort was 55% men, with mean ± SD age 52.2 ± 9.5 years and BMI 34.7 ± 5.5 kg/m2. Mean sleep duration was 6.6 ± 1.3 h. Poor sleep quality was reported by 54% and high risk for obstructive sleep apnea by 64%. HbA1c was significantly higher in those reporting <5 or >8 h sleep per night. Sleep duration >8 h was also associated with higher fasting glucose and <6 h with higher BMI. Shift work was also associated with higher BMI. Social jet lag and delayed chronotype were associated with higher BP.
CONCLUSIONS
In our cohort, self-reported short and long sleep were both associated with adverse measures of glycemia, and short sleep and shift work were associated with higher BMI. Further research using objective measures of sleep is needed to better delineate the relationship between sleep and glycemia in adults with prediabetes or T2D.
Introduction
The obesity epidemic has led to an increase in type 2 diabetes (T2D) (1). There are ∼30 million individuals with T2D and nearly 90 million with prediabetes in the U.S. (2). In parallel, there has been an increase in the prevalence of sleep disturbances (3). Chronic partial sleep loss due to bedtime restriction is increasingly prevalent in our modern society. Moreover, 24-h access to light allows people to engage in behaviors that are inappropriately timed relative to the endogenous circadian rhythm, leading to circadian misalignment. Nowadays, nearly 20% of working adults are shift workers, an extreme form of circadian misalignment (4).
Over the last decade, there has been mounting evidence from a large number of prospective epidemiologic studies that short and long sleep duration, poor sleep quality, obstructive sleep apnea (OSA), and circadian misalignment are associated with T2D (5,6). Whether sleep or circadian disturbances impact glycemic control in individuals with prediabetes and T2D, independently of BMI and other confounders, has been examined in fewer studies (7,8). This question is important, since these lifestyle factors may compromise the efficacy of treatment and accelerate the progression of the disease and the development of complications. Two cross-sectional studies from Thailand have reported an association of circadian misalignment with higher glucose concentrations and BMI in patients with prediabetes (7,8). To date, however, these associations have not been explored in individuals from other ethnic/racial backgrounds. To that end, we aimed to quantify the associations between self-reported sleep duration, sleep quality, and circadian misalignment (e.g., social jet lag, late chronotype, or shift work) and dysglycemia, higher BMI, and increased blood pressure (BP) in a cohort of adults with prediabetes or recently diagnosed, untreated T2D.
Research Design and Methods
Participants
This is a cross-sectional analysis of data obtained during the screening phase of the Restoring Insulin Secretion (RISE) Consortium randomized controlled trials. Between 2013 and 2017 we screened 1,355 overweight/obese men and women ages 20–65 years for RISE. Participants were recruited from the active patient populations and communities at four RISE adult centers: 1) University of Chicago and the Jesse Brown Veterans Affairs (VA) Medical Center, 2) Indiana University, 3) University of Washington and the VA Puget Sound Health Care System, and 4) University of Southern California. Recruitment techniques included referral from colleagues and screening from the investigators’ clinics, internal and external advertising, social and public media, and mailings to individuals identified via electronic medical records. Prescreening of electronic and clinical medical records was used, when possible, to identify patients at risk based on BMI and HbA1c. Additional details on participant recruitment and eligibility criteria have previously been described (9), and detailed information is available on the RISE website (https://rise.bsc.gwu.edu/web/rise/collaborators). The study was approved by the institutional review boards of all participating centers, and all participants provided written informed consent prior to initiation of any study-related activities.
Data Collection
Individuals who met preliminary inclusion/exclusion criteria underwent a 75-g oral glucose tolerance test (OGTT) and HbA1c measurement (n = 1,069). Fasting blood samples for HbA1c and plasma glucose were obtained; blood was also collected at 2 h of the OGTT for measurement of plasma glucose. For the current analyses, we included participants who met the American Diabetes Association definition of prediabetes (fasting plasma glucose 100–125 mg/dL, 2-h plasma glucose 140–199 mg/dL, or HbA1c 5.7–6.4%) as well as recently diagnosed (<1 year), untreated participants with existing T2D (fasting plasma glucose ≥126 mg/dL, 2-h plasma glucose ≥200 mg/dL, or HbA1c ≥6.5%) (10).
Sex and race/ethnicity were self-reported. Anthropometric measurements were performed with participants wearing light clothing without shoes. Height was measured in a fully vertical position with heels together using a calibrated stadiometer. Weight was measured using a calibrated electronic scale, zeroed before each measurement. Height and weight measurements were performed twice, with the average value reported.
BP was measured with a calibrated automated BP machine with appropriately sized arm cuffs; readings were obtained with the participant in a seated position with feet touching the floor or otherwise supported after at least 5 min of rest in a quiet room, with outer clothing removed and sleeves rolled to the shoulder. The cuff was placed at heart level, and two measurements were taken 5 min apart. Only the second measurement was used as the reported value. At screening, blood samples for HbA1c, fasting plasma glucose, and OGTT 2-h plasma glucose were collected. All blood samples were immediately placed on ice, separated by centrifugation, and frozen at −80°C prior to shipment to the central biochemistry laboratory at the University of Washington (Northwest Lipid Metabolism and Diabetes Research Laboratories, University of Washington, Seattle, WA). Plasma glucose concentrations were measured by the glucose hexokinase method using Roche reagent on a Roche c501 autoanalyzer. The method interassay coefficients of variation on quality control samples with low, medium, and high glucose were 2.0%, 1.7%, and 1.3%, respectively. HbA1c was measured by ion-exchange high-performance chromatography on a Tosoh G8 analyzer (Tosoh Bioscience, South San Francisco, CA). The interassay coefficients of variation on low- and high-quality control samples were 1.9% and 1.0%, respectively.
Sleep and Circadian Assessments
To assess sleep quality independent of sleep duration, we used a modified Pittsburgh Sleep Quality Index (PSQI) questionnaire to determine self-reported usual bedtime, wake time, and sleep duration on workdays and days off work during the prior month (11,12). Our modified PSQI starts by asking whether the responder is employed or not employed. Those who are employed are asked for how many days per week and whether their schedule involves a form of shift work. Sleep duration was derived from the following question: “During the past month, how many hours of actual sleep did you get at night? (This may be different than the number of hours you spent in bed)”; when appropriate, this question was asked separately for workdays and days off work, and average self-reported sleep duration was calculated as the weighted average of reported sleep duration on workdays and days off work. Self-reported sleep duration was analyzed categorically (<5 h, 5 to <6 h, 6 to <7 h, 7 to <8 h, and ≥8 h). A score of ≥5 on the PSQI (minimum score of 0 and maximum score of 21) indicated poor sleep quality (13). Sleep debt was calculated as the difference between preferred hours of sleep per night and the weighted average of sleep duration. Chronotype quantifies individual preference for bedtime. The metric of chronotype or midsleep time on free days with adjustment for sleep debt (MSFsc) was derived from midsleep time on days off work (or weekend nights in unemployed individuals) with further adjustment for the sleep debt taking into account the sleep duration average of days off work or weekends and weekdays as follows: MSFsc = midsleep time on days off work or weekend nights – 0.5 * [SDF − (no. of days off work * SDW + no. of days off work * SDF)/7], where SDF is the calculated sleep duration on days off work or weekend nights and SDW is the calculated sleep duration on workdays or weekday nights (14). In individuals who were unemployed, we used 2 days for weekends. In individuals who reported to be working, we specifically asked for the number of days each individual was off of work per week. Therefore, the weighing of “days off work” in the calculation of chronotype was individualized. For multivariate analyses, chronotype was transformed into values relative to midnight (−12 to 12) with 0 representing midnight. Social jet lag, the misalignment of biological and social time, was calculated based on the absolute difference between midsleep time on workdays and days off work (15). Absolute social jet lag was analyzed as a categorical variable (<1 h, 1–2 h, >2 h). The Berlin Questionnaire was used to classify the risk of OSA into high or low risk (16). The Epworth Sleepiness Scale (ESS) (scores 0–24) was used to assess subjective daytime sleepiness. Scores of >10 are considered indicative of daytime sleepiness (17). Shift work was assessed based on three questions: starting work before 6:00 a.m., working overnight shifts, and working rotating night and day shifts. Shift work was analyzed as a dichotomous variable (yes/no).
Statistical Analysis
Data were stored and managed centrally, and analyses were performed according to a prespecified analytic plan. All analyses were cross-sectional. Outcomes of interest were glycemic variables (HbA1c and fasting and OGTT 2-h plasma glucose), BMI, and BP. Continuous variables were expressed as mean ± SD for normally distributed data; categorical variables were summarized as percentages. Unadjusted group comparisons were performed using ANOVA for normally distributed continuous variables. Pearson χ2 test was used to compare categorical variables. Multiple linear regression models were used to explore the independent association of glycemic variables and BP with sleep variables (sleep duration, sleep quality, sleepiness, and OSA risk) and circadian measures (chronotype, social jet lag, and shift work) after adjustment for age, sex, race/ethnicity, and BMI, as well as the association of BMI with sleep and circadian measures after adjustment for age, sex, and race/ethnicity. All statistical calculations were performed without correction for multiple testing. Analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Results
Of the 1,355 adults who participated in screening OGTTs for RISE, 1,069 had measurements of complete fasting and 2-h OGTT plasma glucose concentrations as well as HbA1c values. Validated sleep questionnaire data were available in 1,042 participants. After exclusion of those with normal glucose and HbA1c values, there were 962 participants with complete OGTT and sleep data (704 with prediabetes and 258 with recently diagnosed, untreated T2D). Table 1 summarizes baseline characteristics of the participants. The cohort had nearly equal numbers of men and women, from diverse racial/ethnic backgrounds. Shift work was reported by 24.2% of the cohort. Table 2 summarizes sleep questionnaire measures. There were no significant differences in self-reported sleep or circadian measures between participants with prediabetes and T2D. The mean ± SD habitual sleep duration of the entire cohort was 6.6 ± 1.3 h with a sleep debt of 1.5 ± 1.3 h. Average sleep duration of ≤6 h per night was reported by 32% of the participants. Close to one-third (34.0%) of the cohort reported excessive daytime sleepiness based on an ESS score of >10. Poor sleep quality (54.1%) and high risk for OSA (64.9%) were highly prevalent. Bedtimes and wake times occurred later on days off work (or on weekends in the unemployed), leading to an absolute social jet lag of 1.2 ± 1.5 h. Nearly half of the participants (48.5%) had absolute social jet lag of 1–2 h, while 32.2% had <1 h and 19.3% had >2 h. Chronotype varied substantially, with 13.3% having a chronotype before 2:00 a.m. and 22.9% having a chronotype after 4:00 a.m. The remaining 63.8% had chronotypes between 2:00 and 4:00 a.m.
Table 1.
Descriptive characteristics of participants at RISE screening
| All (N = 962) | Prediabetes (N = 704) | Diabetes (N = 258) | P† | |
|---|---|---|---|---|
| Demographic | ||||
| Age, years | 52.2 ± 9.5 | 51.8 ± 9.5 | 53.2 ± 9.3 | 0.046 |
| Age category (years) | 0.574 | |||
| 20–39 | 111 (11.5) | 83 (11.8) | 28 (10.9) | |
| 40–49 | 224 (23.3) | 169 (24.0) | 55 (21.3) | |
| 50–66 | 627 (65.2) | 452 (64.2) | 175 (67.8) | |
| Sex | 0.231 | |||
| Men | 525 (54.6) | 376 (53.4) | 149 (57.8) | |
| Women | 437 (45.4) | 328 (46.6) | 109 (42.2) | |
| Race/ethnicity | 0.611 | |||
| White | 400 (41.6) | 288 (40.9) | 112 (43.4) | |
| Black | 348 (36.2) | 262 (37.2) | 86 (33.3) | |
| Hispanic | 155 (16.1) | 109 (15.5) | 46 (17.8) | |
| Asian | 32 (3.3) | 23 (3.3) | 9 (3.5) | |
| American Indian | 27 (2.8) | 22 (3.1) | 5 (1.9) | |
| Employment | ||||
| Employed | 579 (71.3) | 431 (70.3) | 148 (74.4) | 0.271 |
| Start work before 6:00 a.m. | 105 (13.7) | 80 (13.7) | 25 (13.7) | 0.977 |
| Overnight shifts | 82 (10.7) | 59 (10.2) | 23 (12.5) | 0.374 |
| Rotating night and day shifts | 81 (10.6) | 53 (9.1) | 28 (15.4) | 0.016 |
| Shift worker* | 188 (24.2) | 136 (23.1) | 52 (28.0) | 0.173 |
| Screening anthropometrics | ||||
| BMI (kg/m2) | 34.7 ± 5.5 | 34.5 ± 5.6 | 35.3 ± 5.3 | 0.053 |
| Weight (kg) | 100.8 ± 20.1 | 99.9 ± 20.0 | 103.3 ± 20.2 | 0.017 |
| Systolic BP (mmHg) | 127.9 ± 14.3 | 127.4 ± 14.2 | 129.3 ± 14.3 | 0.062 |
| Diastolic BP (mmHg) | 77.2 ± 9.8 | 76.9 ± 10.0 | 77.9 ± 9.1 | 0.151 |
| Screening glucose measurements | ||||
| HbA1c (%) | 5.8 ± 0.4 | 5.7 ± 0.3 | 6.1 ± 0.5 | <0.001 |
| Fasting plasma glucose (mg/dL) | 111.5 ± 13.1 | 106.6 ± 8.4 | 124.8 ± 14.4 | <0.001 |
| 2-h plasma glucose (mg/dL) | 160.5 ± 48.4 | 139.8 ± 30.6 | 217.6 ± 42.4 | <0.001 |
Data are n (%) or mean ± SD.
†P value comparing prediabetes with diabetes.
*The percentage of shift workers is not the sum of all three categories of shift work, as some participants had multiple types of shift work (i.e., starting work before 6:00 a.m., working overnight shifts, and working rotating night and day shifts).
Table 2.
Self-reported sleep and circadian measures
| All | Prediabetes | Diabetes | P† | |
|---|---|---|---|---|
| Sleep measures | ||||
| Habitual sleep duration (h) | 6.6 ± 1.3 | 6.6 ± 1.3 | 6.7 ± 1.4 | 0.673 |
| Sleep duration ≤6 h | 303 (32.1) | 221 (31.9) | 82 (32.5) | 0.850 |
| Sleep efficiency, % | 85.8 ± 14.2 | 85.6 ± 14.2 | 86.4 ± 14.3 | 0.485 |
| PSQI global score | 6.7 ± 3.9 | 6.8 ± 3.9 | 6.4 ± 3.9 | 0.140 |
| Poor sleep quality | 511 (54.1) | 386 (55.8) | 125 (49.6) | 0.092 |
| High apnea risk | 618 (64.6) | 447 (63.9) | 171 (66.3) | 0.504 |
| Sleep debt (h) | 1.5 ± 1.3 | 1.5 ± 1.3 | 1.4 ± 1.3 | 0.304 |
| Daytime sleepiness | 327 (34.0) | 246 (34.9) | 81 (31.4) | 0.303 |
| ESS | 8.1 ± 4.9 | 8.3 ± 5.0 | 7.6 ± 4.7 | 0.045 |
| Circadian measures | ||||
| Bedtime workdays (h:min) | 22:31 ± 2:01 | 22:31 ± 01:59 | 22:31 ± 02:06 | 0.998 |
| Bedtime days off work (h:min) | 23:15 ± 1:29 | 23:16 ± 01:30 | 23:15 ± 01:24 | 0.868 |
| Wake time workdays (h:min) | 06:31 ± 2:15 | 06:30 ± 02:07 | 06:36 ± 02:38 | 0.604 |
| Wake time days off work (h:min) | 07:43 ± 1:42 | 07:43 ± 01:44 | 07:43 ± 01:38 | 0.988 |
| Chronotype (h:min)* | 03:31 ± 02:33 | 03:35 ± 02:43 | 03:20 ± 01:57 | 0.280 |
| Social jet lag (h) | 1.2 ± 1.5 | 1.2 ± 1.5 | 1.3 ± 1.7 | 0.406 |
| Shift work | 188 (24.2) | 136 (23.1) | 52 (28.0) | 0.173 |
Data are n (%) or mean ± SD.
†P value comparing prediabetes with diabetes.
*The MSFsc was derived from midsleep time on days off work (or weekend nights in unemployed individuals) with further adjustment for the sleep debt taking into account the sleep duration average of days off work or weekends and weekdays as follows: MSFsc = midsleep time on days off work or weekend nights – 0.5 * [SDF − (no. of days off work * SDW + no. of days off work * SDF)/7], where SDF is the calculated sleep duration on days off work or weekend nights and SDW is the calculated sleep duration on workdays or weekday nights.
Association Between Sleep Measures and Outcomes
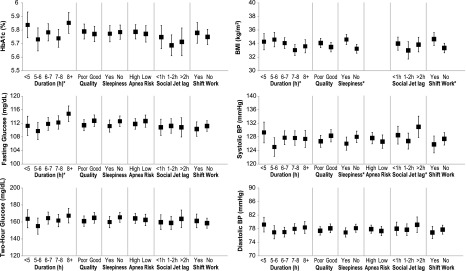
Associations of measures of glycemic control, BMI, and BP with sleep and circadian measures are shown in Fig. 1. After adjustment for age, sex, race/ethnicity, and BMI, there was a U-shaped relationship between categories of sleep duration and HbA1c, with those reporting <5 h (mean 5.84% [95% CI 5.74, 5.93]) and >8 h (mean 5.85% [95% CI 5.78, 5.93]) of sleep having significantly higher HbA1c values compared with those with 7–8 h of sleep (mean 5.74% [95% CI 5.67, 5.80]). Fasting glucose was directly associated with sleep duration as a continuous variable. For each hour of additional sleep, the adjusted fasting glucose was 0.79 mg/dL higher (95% CI 0.15, 1.42; P = 0.015). We did not detect significant associations between self-reported sleep duration and OGTT 2-h plasma glucose levels. Further, our measures of sleep quality, daytime sleepiness, and OSA risk were not significantly associated with HbA1c, fasting glucose, or 2-h glucose.
Figure 1.
Association between self-reported sleep measures and outcomes. Adjusted means from multiple linear regression models. Data are adjusted means and 95% CIs. “Quality” is sleep quality, “sleepiness” is daytime sleepiness, and “duration” is sleep duration. Models adjusted for age, sex, and race/ethnicity. HbA1c, fasting glucose, 2-h glucose, and BP models are also adjusted for BMI. The association between apnea risk and BMI was not quantified because we used the Berlin Questionnaire to asses for the risk of sleep apnea. In this questionnaire, a BMI >30 kg/m2 is one of the three categories to assign high risk of apnea. *ANOVA P < 0.05.
Consistent with existing epidemiologic evidence, BMI was inversely associated with sleep duration after adjustment for age, sex, and race/ethnicity (P = 0.028). For each hour of additional sleep, the adjusted BMI was 0.3 kg/m2 lower (95% CI −0.56, −0.03). Poorer sleep quality, measured by the global PSQI score, and excessive daytime sleepiness, as assessed by the ESS, were also significantly associated with higher BMI (P = 0.048 and P = 0.0024, respectively). For each 1-point increase on the ESS score, the adjusted BMI increased by 0.1 kg/m2 (95% CI 0.04, 0.18), and similarly, for each 1-point increase on the global PSQI score, the adjusted BMI increased by 0.09 kg/m2 (95% CI 0.001, 0.18). Compared with participants who reported good sleep quality, those who reported poor sleep quality had a trend toward a higher BMI (0.6 kg/m2 [95% CI −0.09, 1.28]; P = 0.09).
Sleep duration, sleep quality, and high risk of OSA were not associated with BP. Daytime sleepiness, however, was independently associated with systolic BP (P = 0.037). Compared with participants without daytime sleepiness, those who reported sleepiness had 2.0 mmHg lower systolic BP (95% CI −3.867, −0.128).
Association Between Circadian Measures and Outcomes
Social jet lag and shift work were not associated with HbA1c, fasting plasma glucose, or OGTT 2-h plasma glucose after adjustment for age, sex, race/ethnicity, BMI, and chronotype.
Chronotype and social jet lag were not associated with BMI. In contrast, shift work was associated with a higher BMI. In a fully adjusted model, shift work was associated with 1.32 kg/m2 higher BMI (95% CI 0.42, 2.23; P = 0.0043).
Both later chronotype and social jet lag were independently associated with higher BP. Chronotype was significantly associated with both systolic (P = 0.0004) and diastolic BP (P = 0.0120). For every hour of later chronotype, systolic BP was 1.28 mmHg higher (95% CI 0.58, 1.98) and diastolic BP was 0.66 mmHg higher (95% CI 0.15, 1.17). Compared with a social jet lag of <2 h, social jet lag of >2 h was associated with a significantly higher systolic BP (adjusted mean 127.4 mmHg [95% CI 125.3, 129.6] and 131.0 mmHg [95% CI 127.9, 134.2], respectively, P = 0.014). Shift work was not associated with BP.
In a sensitivity analysis that excluded shift workers, chronotype and social jet lag were not associated with measures of glycemia and BMI (data not shown).
Conclusions
In the present cross-sectional analysis of a large, ethnically diverse cohort of overweight/obese adults with prediabetes or recently diagnosed, untreated T2D, we demonstrated that both short and long self-reported sleep durations were associated with higher measures of glycemia after we controlled for BMI and other demographic characteristics. Short sleep duration and shift work were also associated with higher BMI. Chronotype and social jet lag, on the other hand, were not associated with measures of glycemia or BMI but instead were independently associated with BP.
Multiple laboratory-based studies involving experimental sleep manipulations, including sleep restriction and sleep fragmentation, have been performed to evaluate the role of sleep in the control of energy balance and glucose metabolism. These studies have revealed that short-term sleep restriction can decrease leptin levels, increase ghrelin levels, and increase endocannabinoid levels, leading to increases in hunger, appetite, and hedonic food intake and, simultaneously, leading to a decrease in glucose tolerance (18). Although the pathophysiologic and causal links between sleep disturbances and glucose dysregulation are not fully understood, multiple mechanistic pathways are likely to be involved. Sleep restriction can increase sympathetic nervous system activity, leading to a decrease in insulin sensitivity (19−21). Activation of the hypothalamic-pituitary-adrenal axis with elevation of cortisol can also decrease insulin sensitivity (5,18). However, it is important to note that the studies of sleep manipulation have been short-term in nature and performed primarily in healthy young individuals. It is less clear whether sleep manipulation impacts glycemic measures in patients with prediabetes or T2D.
At a population level, several cross-sectional studies from various geographic regions have reported an association between self-reported short sleep duration and impaired fasting glucose (22−24). Other studies have found associations between self-reported short sleep duration and prevalent prediabetes or progression to T2D (25−29). Although most epidemiologic and laboratory-based studies have focused on the association between short sleep duration and dysglycemia or obesity, a few prospective studies have suggested that self-reported sleep duration has a U-shaped association, with increased risk of developing T2D with both short (<5–6 h per night) and long (>8–9 h per night) sleep (30,31). The mechanisms by which long sleep duration leads to increased risk of obesity and T2D are not fully understood. Long sleep may reflect a more sedentary lifestyle and, similar to short sleepers, long sleepers engage in more snacking (32). Although both long and short sleep have been associated with worse glycemic control in patients with established T2D (33), there is a paucity of data on the impact of sleep duration or quality on glycemic control in prediabetes. Moreover, most studies have focused on either fasting glucose or HbA1c, without performing an OGTT.
Several studies have experimentally induced extreme circadian misalignment to better elucidate its role in glucose metabolism dysregulation. These studies demonstrated that circadian misalignment mimicking shift work led to reduced glucose tolerance in healthy humans (34−36). In these studies, both fasting and postprandial glucose concentrations increased. Moreover, circadian misalignment led to a change in appetite-regulating hormones, a decrease in energy expenditure, and an increase in BP (34,36). However, it is important to point out that these studies used short-term extreme circadian misalignment. A few population-based studies have reported an association between milder forms of circadian misalignment, such as later chronotype or social jet lag, and prediabetes and T2D (37,38), but the effect of circadian misalignment on actual measures of glycemia in people with prediabetes has not been well studied. Only one study explored the association between chronotype and HbA1c in prediabetes. This study was performed in a clinical cohort of participants with prediabetes in Thailand and found a small effect of chronotype on HbA1c (7). For each 2 h of later chronotype, HbA1c increased by only 0.04% (0.4 mmol/mol). There was not a significant relationship between social jet lag and HbA1c. This may have been in part due to a very narrow range of social jet lag in their patient population (7). Similarly, we did not find an association between later chronotype or social jet lag and measures of glycemia. However, we did find an association between these variables and higher BP. Although in our cohort shift work was not associated with measures of glycemia, it was associated with higher BMI, as has been shown by others. Shift work has been associated with increased risk of obesity (39), particularly abdominal obesity (40), and the risk of developing T2D (6).
Our study has several strengths. First and foremost, we studied a large number of adults with prediabetes/recently diagnosed, untreated T2D. By virtue of our identification of participants in a study screening program, none of the participants had been previously treated with any confounding glucose-lowering medications or medications known to affect glucose metabolism. The cohort was ethnically diverse, and both sexes were well represented, thereby increasing the generalizability of our findings. Moreover, the proportion of participants who reported shift work was similar to that in the U.S. workforce (4). Second, all participants underwent a 2-h OGTT as well as HbA1c measurements on the same day the sleep questionnaires were completed. Plasma glucose and HbA1c from all participating centers were measured by a centralized laboratory. Lastly, we used standardized sleep questionnaires across all centers. Notwithstanding the strengths, our study has several important limitations. Due to its cross-sectional design, the direction of causality cannot be ascertained. Another important weakness is lack of objective measures of sleep and circadian markers. We did not measure several important confounders such as total calorie consumption, macronutrient dietary composition, meal timing, and amount of physical activity and, as such, cannot control for these covariates in our analysis. Information about treatment of OSA was not collected during the screening phase of RISE, and thus we cannot account for any potential effect of apnea treatment on glycemic control. We also did not collect data on antihypertensives at the time of screening. Lastly, lack of a control group not at increased risk for diabetes is another limitation of our study.
In summary, sleep duration was independently associated with HbA1c in adults with prediabetes/recently diagnosed, untreated T2D. This relationship was most pronounced in those who reported <5 h or >8 h of sleep per night (U-shaped relationship). Both short sleep duration and shift work were also independently associated with higher BMI in this population. Later chronotype and social jet lag were associated with higher BP. Further research using objective measures of sleep and circadian markers is needed to better delineate the relationship between sleep disturbances, circadian misalignment, and cardiometabolic factors in prediabetes and T2D in order to determine whether intervention studies targeting these novel lifestyle factors to decrease the rate of conversion from prediabetes to diabetes are warranted.
Supplementary Material
Article Information
Acknowledgments. The RISE Consortium thanks the RISE Data and Safety Monitoring Board and Barbara Linder, the National Institute of Diabetes and Digestive and Kidney Diseases Program Official for RISE (Rockville, MD), for support and guidance. The Consortium also thanks the participants who, by volunteering, are furthering the ability to reduce the burden of diabetes.
Funding and Duality of Interest. RISE is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (U01-DK-094406, U01-DK-094430, U01-DK-094431, U01-DK-094438, U01-DK-094467, P30-DK-017047, P30-DK-020595, P30-DK-045735, P30-DK-097512, UL1-TR-000430, UL1-TR-001082, UL1-TR-001108, UL1-TR-001855, UL1-TR-001857, UL1-TR-001858, and UL1-TR-001863), the Department of Veterans Affairs, and Kaiser Permanente Southern California. In addition, the National Heart, Lung, and Blood Institute provided support for the RISE Sleep Ancillary study to B.M. (R01HL119161). Additional financial and material support was received from the American Diabetes Association, Allergan, Apollo Endosurgery, Abbott Laboratories, and Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. Members of the RISE Consortium recruited participants and collected study data. B.M. and E.V.C. proposed the analysis. A.H.T. and S.L.E. performed the analysis. B.M. interpreted data and wrote the first draft. K.A.T., K.M.U., K.J.N., T.S.H., S.S., E.B., S.M., D.A.E., and E.V.C. reviewed and edited the manuscript. The RISE Steering Committee reviewed and edited the manuscript and approved its submission. B.M. and A.H.T. are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 22–26 June 2018.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-0298/-/DC1.
This article is featured in a podcast available at http://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
A complete list of the RISE Consortium Investigators can be found in Supplementary Data.
Contributor Information
Collaborators: The RISE Consortium, David A. Ehrmann, Karla A. Temple, Abby Rue, Elena Barengolts, Babak Mokhlesi, Eve Van Cauter, Susan Sam, M. Annette Miller, Steven E. Kahn, Karen M. Atkinson, Jerry P. Palmer, Kristina M. Utzschneider, Tsige Gebremedhin, Abigail Kernan-Schloss, Alexandra Kozedub, Brenda K. Montgomery, Emily J. Morse, Kieren J. Mather, Tammy Garrett, Tamara S. Hannon, Amale Lteif, Aniket Patel, Robin Chisholm, Karen Moore, Vivian Pirics, Linda Pratt, Kristen J. Nadeau, Susan Gross, Philip S. Zeitler, Jayne Williams, Melanie Cree-Green, Yesenia Garcia Reyes, Krista Vissat, Silva A. Arslanian, Kathleen Brown, Nancy Guerra, Kristin Porter, Sonia Caprio, Mary Savoye, Bridget Pierpont, Thomas A. Buchanan, Anny H. Xiang, Enrique Trigo, Elizabeth Beale, Fadi N. Hendee, Namir Katkhouda, Krishan Nayak, Mayra Martinez, Cortney Montgomery, Xinhui Wang, Sharon L. Edelstein, John M. Lachin, Ashley Hogan Tjaden, Santica Marcovina, Jessica Harting, John Albers, Dave Hill, Peter J. Savage, and Ellen W. Leschek
References
- 1.Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet 2012;379:2279–2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017 [Internet], 2017. Available from https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed 1 February 2018
- 3.Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med 2015;3:310–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMenamin T. A time to work: recent trends in shift work and flexible schedules. Mon Labor Rev 2007;130:3–15 [Google Scholar]
- 5.Reutrakul S, Mokhlesi B. Obstructive sleep apnea and diabetes: a state of the art review. Chest 2017;152:1070–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anothaisintawee T, Reutrakul S, Van Cauter E, Thakkinstian A. Sleep disturbances compared to traditional risk factors for diabetes development: systematic review and meta-analysis. Sleep Med Rev 2016;30:11–24 [DOI] [PubMed] [Google Scholar]
- 7.Anothaisintawee T, Lertrattananon D, Thamakaison S, Knutson KL, Thakkinstian A, Reutrakul S. Later chronotype is associated with higher hemoglobin A1c in prediabetes patients. Chronobiol Int 2017;34:393–402 [DOI] [PubMed] [Google Scholar]
- 8.Anothaisintawee T, Lertrattananon D, Thamakaison S, Thakkinstian A, Reutrakul S. The relationship among morningness-eveningness, sleep duration, social jetlag, and body mass index in Asian patients with prediabetes. Front Endocrinol (Lausanne) 2018;9:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.RISE Consortium Restoring Insulin Secretion (RISE): design of studies of β-cell preservation in prediabetes and early type 2 diabetes across the life span. Diabetes Care 2014;37:780–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Diabetes Association Standards of medical care in diabetes—2014. Diabetes Care 2014;37(Suppl. 1):S14–S80 [DOI] [PubMed] [Google Scholar]
- 11.Knutson KL, Ryden AM, Mander BA, Van Cauter E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med 2006;166:1768–1774 [DOI] [PubMed] [Google Scholar]
- 12.Pilz LK, Keller LK, Lenssen D, Roenneberg T. Time to rethink sleep quality: PSQI scores reflect sleep quality on workdays. Sleep (Basel) 2018;41 [DOI] [PubMed] [Google Scholar]
- 13.Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213 [DOI] [PubMed] [Google Scholar]
- 14.Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms 2003;18:80–90 [DOI] [PubMed] [Google Scholar]
- 15.Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr Biol 2012;22:939–943 [DOI] [PubMed] [Google Scholar]
- 16.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med 1999;131:485–491 [DOI] [PubMed] [Google Scholar]
- 17.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14:540–545 [DOI] [PubMed] [Google Scholar]
- 18.Reutrakul S, Van Cauter E. Sleep influences on obesity, insulin resistance, and risk of type 2 diabetes. Metabolism 2018;84:56–66 [DOI] [PubMed] [Google Scholar]
- 19.Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes 2010;59:2126–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robertson MD, Russell-Jones D, Umpleby AM, Dijk DJ. Effects of three weeks of mild sleep restriction implemented in the home environment on multiple metabolic and endocrine markers in healthy young men. Metabolism 2013;62:204–211 [DOI] [PubMed] [Google Scholar]
- 21.Broussard JL, Ehrmann DA, Van Cauter E, Tasali E, Brady MJ. Impaired insulin signaling in human adipocytes after experimental sleep restriction: a randomized, crossover study. Ann Intern Med 2012;157:549–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rafalson L, Donahue RP, Stranges S, et al. Short sleep duration is associated with the development of impaired fasting glucose: the Western New York Health Study. Ann Epidemiol 2010;20:883–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engeda J, Mezuk B, Ratliff S, Ning Y. Association between duration and quality of sleep and the risk of pre-diabetes: evidence from NHANES. Diabet Med 2013;30:676–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim CR, Song YM, Shin JY, Gim W. Association between sleep duration and impaired fasting glucose in Korean adults: results from the Korean National Health and Nutrition Examination Survey 2011-2012. Korean J Fam Med 2016;37:51–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan M, Fu Z, Qin T, et al. Associations of sleep duration and prediabetes prevalence in a middle-aged and elderly Chinese population with regard to age and hypertension: the China Health and Retirement Longitudinal Study baseline survey. J Diabetes 2018;10:847–856 [DOI] [PubMed] [Google Scholar]
- 26.Kim CW, Chang Y, Sung E, Ryu S. Sleep duration and progression to diabetes in people with prediabetes defined by HbA1c concentration. Diabet Med 2017;34:1591–1598 [DOI] [PubMed] [Google Scholar]
- 27.Nakajima K, Suwa K, Toyama K. Age-dependent changes in the association between sleep duration and impaired glucose metabolism. World J Diabetes 2017;8:397–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nuyujukian DS, Beals J, Huang H, et al.; Special Diabetes Program for Indians Diabetes Prevention Demonstration Project . Sleep duration and diabetes risk in American Indian and Alaska native participants of a lifestyle intervention project. Sleep 2016;39:1919–1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kowall B, Lehnich AT, Strucksberg KH, et al. Associations among sleep disturbances, nocturnal sleep duration, daytime napping, and incident prediabetes and type 2 diabetes: the Heinz Nixdorf Recall Study. Sleep Med 2016;21:35–41 [DOI] [PubMed] [Google Scholar]
- 30.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 2010;33:414–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shan Z, Ma H, Xie M, et al. Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care 2015;38:529–537 [DOI] [PubMed] [Google Scholar]
- 32.Tan X, Chapman CD, Cedernaes J, Benedict C. Association between long sleep duration and increased risk of obesity and type 2 diabetes: a review of possible mechanisms. Sleep Med Rev 2018;40:127–134 [DOI] [PubMed] [Google Scholar]
- 33.Lee SWH, Ng KY, Chin WK. The impact of sleep amount and sleep quality on glycemic control in type 2 diabetes: a systematic review and meta-analysis. Sleep Med Rev 2017;31:91–101 [DOI] [PubMed] [Google Scholar]
- 34.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A 2009;106:4453–4458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leproult R, Holmbäck U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes 2014;63:1860–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McHill AW, Melanson EL, Higgins J, et al. Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proc Natl Acad Sci U S A 2014;111:17302–17307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koopman ADM, Rauh SP, van ’t Riet E, et al. The association between social jetlag, the metabolic syndrome, and type 2 diabetes mellitus in the general population: the New Hoorn Study. J Biol Rhythms 2017;32:359–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu JH, Yun CH, Ahn JH, et al. Evening chronotype is associated with metabolic disorders and body composition in middle-aged adults. J Clin Endocrinol Metab 2015;100:1494–1502 [DOI] [PubMed] [Google Scholar]
- 39.Liu Q, Shi J, Duan P, et al. Is shift work associated with a higher risk of overweight or obesity? A systematic review of observational studies with meta-analysis. Int J Epidemiol 2018;47:1956–1971 [DOI] [PubMed] [Google Scholar]
- 40.Sun M, Feng W, Wang F, et al. Meta-analysis on shift work and risks of specific obesity types. Obes Rev 2018;19:28–40 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.