Abstract
Fibroblast growth factor 1 (FGF1) has been shown to reverse hyperglycemia in diabetic rodent models through peripheral and central administration routes. Previous studies demonstrated that insulin is required for central and peripheral FGF1 metabolic improvements; however, it is unknown if FGF1 targets insulin secretion at the islet level. Here we show for the first time that FGF1 increases islet insulin secretion in diabetic mouse models. FGF1 was administered via a single intracerebroventricular or multiple subcutaneous injections to leptin receptor-deficient (db/db), diet-induced obese, and control mice; pancreatic islets were isolated 7 days later for analysis of insulin secretion. Central and peripheral FGF1 significantly lowered blood glucose in vivo and increased ex vivo islet insulin secretion from diabetic, but not control, mice. FGF1 injections to the cisterna magna mimicked intracerebroventricular outcomes, pointing to a novel therapeutic potential. Central effects of FGF1 appeared dependent on reductions in food intake, whereas peripheral FGF1 had acute actions on islet function prior to significant changes in food intake or blood glucose. Additionally, peripheral, but not central, FGF1 increased islet β-cell density, suggesting that peripheral FGF1 may induce long-term changes in islet structure and function that are not present with central treatment.
Introduction
Fibroblast growth factor 1 (FGF1) has been identified as a potential type 2 diabetes therapeutic (1–5). FGF1 is a 15-kDa mitogenic factor involved in several processes, including embryonic development, cell proliferation, and neurogenesis (6–8). In humans, low serum levels of FGF1 are associated with an increased risk of weight gain and obesity (9,10). In rodents, FGF1 knockout mice on a high-fat diet (HFD) exhibit more severe hyperglycemia and insulin resistance than their wild-type counterparts (1,11). Whereas the absence of FGF1 is associated with adverse metabolic outcomes, administration of FGF1 has been shown to reverse hyperglycemia in diabetic rodent models (2,4). When administered peripherally, FGF1 reduced blood glucose in diabetic rodents, without hypoglycemic events or weight gain (2). Unlike peripheral administration, which requires multiple injections to reverse diabetes, a single central injection of FGF1 restored blood glucose to normal levels for up to 18 weeks in the ob/ob leptin-deficient diabetic mouse model (4). Although the mechanisms for FGF1-mediated normoglycemia are unclear, central FGF1 is likely to target the hypothalamus, demonstrated by increased activation of third ventricle tanycytes and hypothalamic synaptophysin content upon intracerebroventricular administration (4). In contrast to central administration of FGF1, it is unknown whether peripheral administration of FGF1 affects the brain.
Although central and peripheral administration of FGF1 have various effects on metabolic systems, both routes lower blood glucose via an insulin-dependent mechanism. When administered peripherally to insulin-deficient streptozotocin-induced mice, FGF1 was unable to lower blood glucose; however, upon the addition of insulin, FGF1 function was fully restored (2). Similarly, central administration of FGF1 was unsuccessful at reducing hyperglycemia in mice treated with an insulin receptor antagonist (4). Although these findings demonstrate that insulin is required for FGF1-mediated metabolic outcomes, the effects of FGF1 on islet insulin secretion are unknown. To address this question, we administered FGF1 via a single intracerebroventricular or multiple subcutaneous injections to control, diet-induced obese (DIO), and db/db mice and then isolated pancreatic islets 7 days later to measure glucose-stimulated insulin secretion (GSIS). Here we demonstrate for the first time that central and peripheral administration of FGF1 increase islet insulin secretion in diabetic mouse models.
Research Design and Methods
Animals
All animal procedures were approved by the Oregon National Primate Research Center Institutional Animal Care and Use Committee. Mice were singly housed in ventilated cages at 22°C with free access to water and food (standard chow, 5001 [LabDiets, St. Louis, MO], or 60% HFD, D12492 [Research Diets, New Brunswick, NJ]), unless otherwise noted. Mice were maintained on a 12-h light/dark cycle. Male 12- to 18-week-old control (C57BL/6J), db/db [B6.BKS(D)-Leprdb/J], and 22-week-old DIO mice (C57BL/6J maintained on HFD for 16 weeks) were purchased from The Jackson Laboratory (Bar Harbor, ME).
Intracerebroventricular Cannulation Surgeries
Lateral-ventricle cannulations were performed under 3% isoflurane in oxygen delivered by nose cone. Using a stereotax (David Kopf Instruments, Tujunga, CA), mice were implanted with a cannula (PlasticsOne, Roanoke, VA) in the lateral ventricle: −0.7 mm posterior to bregma, −1.2 mm lateral to the midsagittal suture, and −2.0 mm below the skull surface. Mice were allowed to recover for at least 7 days prior to testing. For verification of cannula placement, angiotensin II (2.5 µg/mouse, 5-µL injection volume; Sigma-Aldrich) was administered via intracerebroventricular injection, and water intake was recorded over the course of an hour. Mice that drank <1 mL were excluded from the study.
Cisterna Magna Injection Surgeries
Surgeries were performed under isoflurane anesthesia as previously described (12). In brief, mice were placed in a stereotax with the head bent 120°. An incision was made at the nape of the neck to expose the sagittal suture of the cranium. A 27-gauge needle was bent 40° 2.5 mm from the tip and attached to a 5-µL microsyringe (Hamilton Company, Reno, NV) using PE20 plastic tubing. The needle was inserted into the cleft between the occiput and atlas vertebra. FGF1 (3 µg, mFGF1; Prospec, Ness Ziona, Israel) or vehicle (saline) was injected over 30 s in 4 µL.
Intracerebroventricular and Subcutaneous Injections
For intracerebroventricular injections, FGF1 (3 µg, dissolved in sterile saline) or vehicle (saline) was injected over 10 s in a final volume of 4 µL using an injector with a 1-mm projection. For subcutaneous injections every other day, FGF1 was administered at a dose of 0.5 mg/kg body weight. All injections were done in the morning.
Intraperitoneal Glucose Tolerance Tests
Intraperitoneal glucose tolerance tests (ipGTTs) were conducted at 10:30 a.m. in 4 h–fasted animals, 6 days after initial FGF1 or vehicle injections. Mice received an intraperitoneal injection of glucose (50% dextrose) at a dose of 2 mg/kg (DIO and db/db) or 1 mg/kg (control). Blood glucose was measured at baseline and 15, 30, 60, 120, and 180 min postglucose injection. For plasma insulin, blood was collected in EDTA-coated capillary tubes (Sarstedt, Nümbrecht, Germany), separated into plasma, and assayed using a Mouse Insulin ELISA kit (ALPCO, Salem, NH).
Measurements of Metabolic Parameters
Measurements were taken in the morning at the same time daily. Blood was collected from tail bleeds using a 5-µL capillary tube, placed into glucose/lactate system and hemolyzing solution (EKF Diagnostics, Cardiff, U.K.), and analyzed using a Biosen Glucose Analyzer (EKF Diagnostics) (13).
Metabolic Chambers
DIO mice were individually housed in a Comprehensive Laboratory Animal Monitoring System (CLAMS; Columbus Instruments, Columbus, OH) for 24 h to acclimate. After 24 h, mice received a single intracerebroventricular injection or subcutaneous injections every other day of FGF1 or vehicle and were monitored for 72 h. Food intake was measured hourly. Additionally, the CLAMS generated hourly data for VO2 (volume of oxygen consumed, mL/kg/h) and respiratory exchange ratio (respiratory exchange ratio). After 72 h, mice were transferred to standard housing. Seven days after initial injections, intracerebroventricular-injected animals were placed back in the CLAMS for 36 h.
Isolation of Mouse Pancreatic Islets
Mice were euthanized by cervical dislocation under isoflurane anesthesia. The bile duct was clamped off near the junction with the liver, and pancreata were inflated with collagenase P (0.5 mg/mL) (Sigma-Aldrich) via cannulation of the pancreatic duct. Pancreata were digested for 15 min at 37°C in a collagenase solution and separated using a histopaque gradient (Sigma-Aldrich). Islets from individual animals of the same treatment group were pooled together and cultured overnight in supplemented RPMI 1640 media (Sigma-Aldrich) at 37°C and 5% CO2.
GSIS Assay
Islets were transferred into prepared columns and placed in a perifusion system (PERI-4.2; Biorep Technologies, Miami Lakes, FL) maintained at 37°C. Islets were preincubated in Krebs-Ringer bicarbonate HEPES buffer (KRBH) containing 2.8 mmol/L glucose for 1 h at a flow rate of 100 µL/min. After preincubation, islets underwent four 15-min washes in 2.8 or 16.7 mmol/L glucose-supplemented KRBH for a total of 60 min, with collections every 3 min. Conditioned media collections were stored at 4°C and processed the same day. All perifusion GSIS assays were done in triplicate. Insulin was assayed using a Mouse Insulin ELISA kit and normalized to the number of islets.
c-Fos Immunohistochemistry
Mice received a single intracerebroventricular or subcutaneous injection of FGF1 or vehicle and 90 min later were anesthetized with ketamine/xylazine and perfused with 0.9% saline followed by 4% paraformaldehyde. Brains were removed, cryoprotected in 20% sucrose, frozen, and cut at 25 µm in a 1:6 series using a sliding microtome. Sections were stained for c-Fos using a polyclonal rabbit anti–c-Fos antibody (1:10,000, SC-52; Santa Cruz Biotechnology), amplified (PK-4000; Vectastain ABC HRP kit), and visualized with nickel-DAB (DAB Kit, SK-4100; Vector Laboratories) or anti–rabbit-Alexa Flour 488 (Thermo Fisher Scientific). Sections were mounted on slides and imaged with an Olympus brightfield slide scanner. c-Fos immunoreactivity–positive cells were counted by hand in multiple anatomical areas while viewed in ImageJ software. Monoclonal mouse anti–S-100B antibody (1:500, S2532; Sigma Aldrich) was used as a marker for α-tanycytes (14).
Pancreatic Tissue Immunohistochemistry
Pancreata were fixed in 10% zinc formalin overnight and then transferred to 70% ethanol before being paraffin embedded and cut into 5-µm-thick sections. Tissue (one slide per animal) was incubated overnight with guinea pig anti-insulin (1:50, ab7842; Abcam) and rabbit anti-glucagon (1:1,000, 20076; Immunostar) antibodies. Immunoreactivity was detected by incubation for 1 h with anti–guinea pig-Alexa Fluor 568 and anti–rabbit-Alexa Fluor 488, and tissue was counterstained with DAPI to label nuclei. Images of each slide were acquired at 20× with an Olympus VS1000 fluorescence slide scanner and analyzed using Visiopharm software. Regions of interest were drawn around the entire pancreas section (total area) as well as each individual islet (islet area), as defined by insulin and/or glucagon immunoreactivity. The α- and β-cells were counted by hand from 10 average-sized islets per pancreas using ImageJ.
Statistical Analyses
A two-way ANOVA followed by Sidak post hoc test with multiple comparisons was used to evaluate differences between treatment and time. An unpaired Student t test was used to evaluate differences between treatments. All SPSS were performed using Prism (version 7.0; GraphPad, La Jolla, CA) software.
Results
Metabolic Effects of Central and Peripheral FGF1
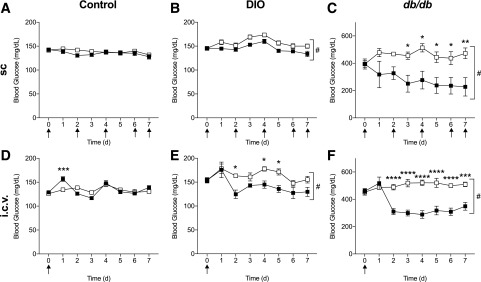
To assess the effects of FGF1 on pancreatic islet insulin secretion and metabolic parameters, we administered FGF1 or vehicle via a single intracerebroventricular or subcutaneous injections every other day to control, DIO, and db/db mice. Both central and peripheral administration of FGF1 significantly lowered blood glucose over 7 days in DIO and db/db mice but failed to lower blood glucose in control mice (Fig. 1). Central and peripheral treatment of FGF1 also induced a transient decrease in food intake (Supplementary Fig. 1), whereas body weights were only affected in centrally treated control and db/db mice (Supplementary Fig. 2). Furthermore, central administration of FGF1 to DIO mice housed in metabolic chambers caused a reduction in respiratory exchange ratio that was maintained for the duration of the 7-day study (Supplementary Fig. 3). The volume of oxygen consumed (VO2) appeared increased during the daytime after central administration of FGF1. Peripheral treatment of FGF1 had no significant effect on metabolic rate in DIO mice.
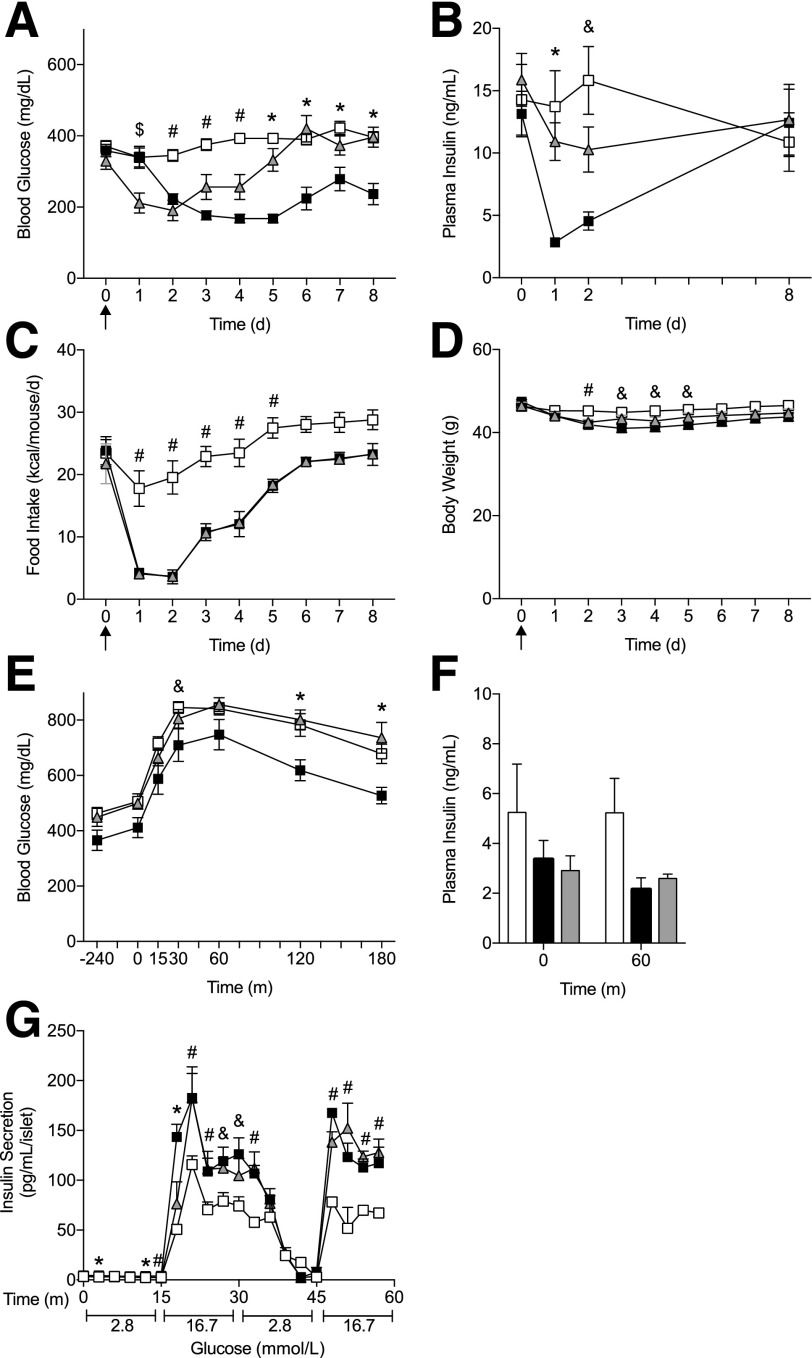
Figure 1.
Central and peripheral FGF1 reduced hyperglycemia in diabetic mouse models. Blood glucose levels after subcutaneous injections every other day of FGF1 (filled squares) or vehicle (open squares) in control (n = 6) (A), DIO (n = 6) (B), and db/db mice (n = 6) (C). Blood glucose levels after a single intracerebroventricular injection of FGF1 or vehicle in control (n = 5 for vehicle; n = 6 for FGF1) (D), DIO (n = 6) (E), and db/db mice (n = 8 for vehicle; n = 9 for FGF1) (F). Recombinant mouse FGF1 (0.5 mg/kg body weight for subcutaneous administration; 3 µg/mouse for intracerebroventricular administration) or vehicle (saline) was administered to ad libitum fed mice. Arrows represent injection time course. Data are expressed as mean ± SEM. P values were determined by two-way ANOVA. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; #P < 0.05 for overall treatment effect. d, days; i.c.v., intracerebroventricular; sc, subcutaneous.
After 6 days of exposure to FGF1 or vehicle, we performed an ipGTT in fasted mice (Supplementary Fig. 4). Four hours prior to the ipGTT, the peripheral treatment groups received injections of FGF1 or vehicle. Although glucose tolerance was improved in peripherally treated control mice and centrally treated db/db mice, when data were corrected for differences in basal glucose, these improvements were no longer significant (data not shown). Plasma insulin was unaffected by glucose stimulation in vivo.
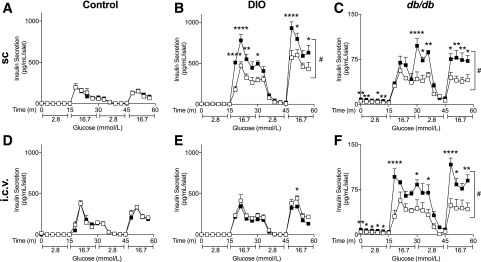
Chronic Central and Peripheral FGF1 Increase Insulin Secretion From Diabetic Mouse Islets
After 7 days of treatment, we isolated pancreatic islets for analysis of insulin secretion ex vivo (Fig. 2). Chronic peripheral FGF1 significantly increased insulin secretion from DIO and db/db islets but had no effect on insulin secretion from control mouse islets (Fig. 2A–C). Central administration of FGF1 also increased insulin secretion from isolated islets, but only in the db/db mouse model where we observed the largest reduction in blood glucose (Fig. 2D–F). It is important to note that for both central- and peripheral-treated db/db mice, ex vivo insulin secretion was significantly increased under basal and glucose-stimulated conditions. Moreover, fold change of insulin secretion between basal and glucose-stimulated conditions was not significant between FGF1- and vehicle-treated db/db islets (data not shown), suggesting that FGF1 may not improve islet sensitivity to glucose but rather overall islet function.
Figure 2.
Chronic central and peripheral FGF1 increased ex vivo insulin secretion from diabetic, but not control, mouse islets. GSIS from control (A), DIO (B), and db/db (C) mouse islets after 7 days of every other day subcutaneous FGF1 (filled squares) or vehicle (open squares) administration. GSIS from control (D), DIO (E), and db/db (F) mouse islets 7 days after a single intracerebroventricular injection of FGF1 or vehicle. Isolated islets were cultured overnight prior to GSIS assay using a perifusion system. Data are expressed as mean ± SEM. P values were determined by two-way ANOVA. *P < 0.05; **P < 0.01; ****P < 0.0001; #P < 0.05 for overall treatment effect. i.c.v., intracerebroventricular; m, minutes; sc, subcutaneous.
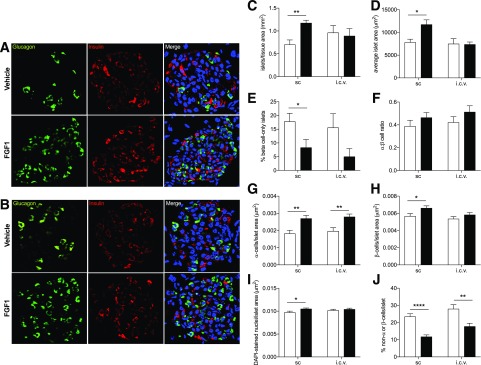
Chronic Peripheral, but Not Central, FGF1 Increases β-Cell Density in db/db Mice
Given that chronic central and peripheral FGF1 increased insulin secretion in db/db islets under basal glucose conditions, we next investigated whether chronic FGF1 treatment altered islet microanatomy. After 7 days of exposure to central or peripheral FGF1, pancreata from db/db mice were harvested and fixed for immunohistochemical analysis (Fig. 3). Central and peripheral FGF1 significantly increased α-cell density, whereas only peripheral FGF1 improved β-cell density in db/db mice (Fig. 3G and H). Peripheral FGF1 also increased overall cell density per islet, average islet area, and number of islets per tissue area, whereas central FGF1 had no effect on these parameters (Fig. 3C, D, and I).
Figure 3.
Chronic central and peripheral FGF1 improved islet microanatomy in db/db mice. Representative images of islet microanatomy after 7 days of FGF1 or vehicle treatment via subcutaneous injections every other day (A) or a single intracerebroventricular (i.c.v.) injection in db/db mice (B). C–J: Histological analysis of representative images from FGF1- (filled bars) and vehicle-treated (open bars) db/db mice. The following parameters were analyzed: islets/tissue area (mm2) (C), average islet area (μm2) (D), percent of β-cell–only islets (E), ratio of α- to β-cells (F), α-cells/islet area (μm2) (G), β-cells/islet area (μm2) (H), DAPI-stained nuclei/islet area (μm2) (I), and percent of non–α-cells or –β-cells/islet (J). Anti-insulin (red), antiglucagon (green), and DAPI (blue) were used for detection of β-cells, α-cells, and nuclei, respectively. Data are expressed as mean ± SEM. P values were determined by unpaired Student t test. *P < 0.05; **P < 0.01; ****P < 0.0001. i.c.v., intracerebroventricular; sc, subcutaneous.
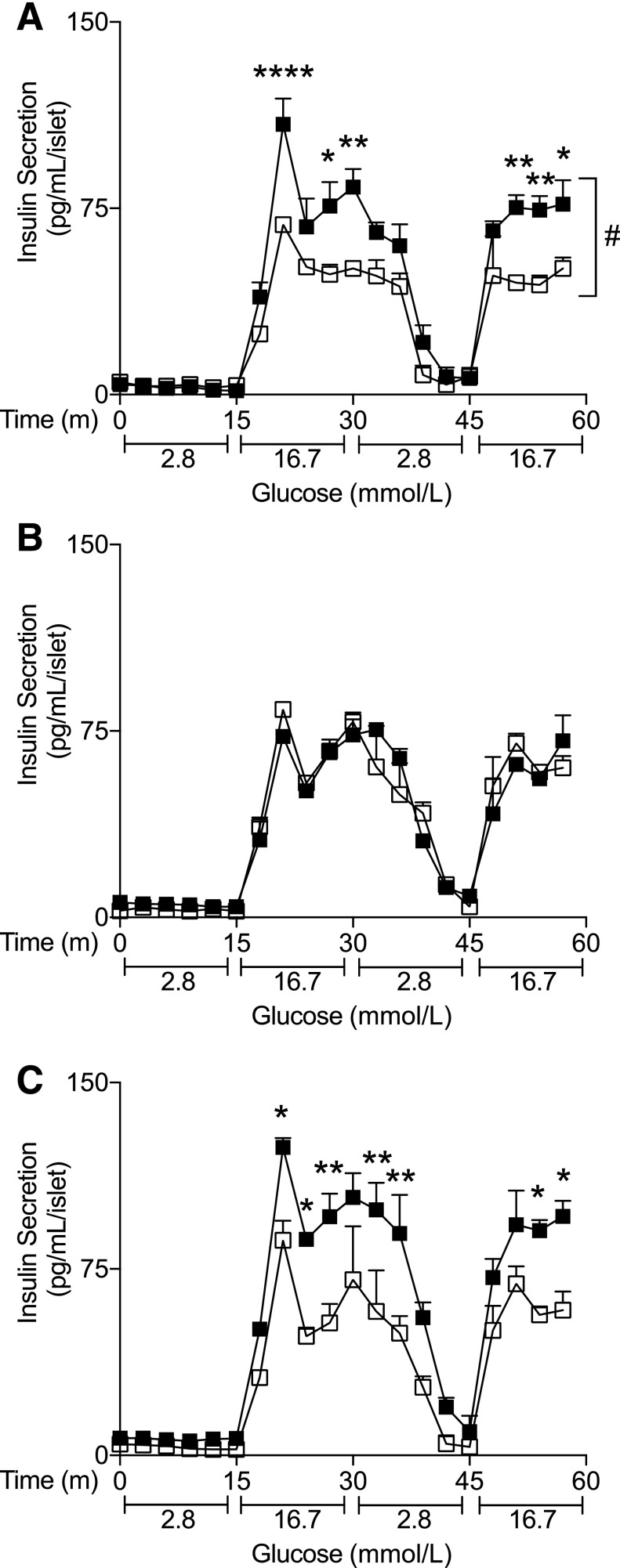
Acute Peripheral, but Not Central, FGF1 Increases Insulin Secretion in db/db Islets
Central and peripheral FGF1 increased islet insulin secretion under basal glucose conditions in db/db mice, but only peripheral FGF1 induced dramatic improvements in islet microanatomy. Given these findings, we hypothesized that peripheral FGF1 may directly target the pancreas, whereas central FGF1 effects on the pancreas may be secondary to sustained metabolic improvements. To examine the effects of central and peripheral FGF1 on islet insulin secretion independent of significant metabolic changes in vivo, we isolated islets 2 h after exposure to FGF1 or vehicle. After 2 h of peripheral FGF1 treatment, insulin secretion was significantly increased in db/db islets (Fig. 4A), independent of changes in blood glucose, food intake, and body weight (Supplementary Fig. 5). In contrast, acute central FGF1 had no effect on insulin secretion in db/db mice (Fig. 4B). Forty-eight hours of exposure to central FGF1 was required before we observed improvements in insulin secretion from isolated islets (Fig. 4C).
Figure 4.
Acute peripheral, but not central, FGF1 increased ex vivo insulin secretion from db/db mouse islets. A: GSIS from db/db islets isolated 2 h after subcutaneous administration of FGF1 (filled squares) or vehicle (open squares). GSIS from db/db islets isolated 2 h (B) or 48 h (C) after intracerebroventricular administration of FGF1 or vehicle. Isolated islets were cultured overnight prior to GSIS assay. n = 3 per group for in vivo treatment. Data are expressed as mean ± SEM. P values were determined by two-way ANOVA. *P < 0.05; **P < 0.01; ****P < 0.0001; #P < 0.05 for overall treatment effect. m, minutes.
To validate these findings in an additional model of obesity, we repeated this study in DIO mice. Similar to the db/db model, peripheral FGF1 increased insulin secretion from DIO islets after 2 h of treatment (Supplementary Fig. 6A). Surprisingly, acute central FGF1 also improved islet insulin secretion; however, these effects were lost after 48 h (Supplementary Fig. 6B and C), consistent with the long-term study demonstrating a lack of effect of central FGF1 on insulin secretion in DIO mice (Fig. 2E).
Improvements in Islet Insulin Secretion After Central FGF1 Are Dependent on Food Intake in db/db Mice
In db/db mice, peripheral FGF1 induced islet insulin secretion under acute conditions, independent of changes in blood glucose, food intake, and body weight (Fig. 4A and Supplementary Fig. 5); however, central FGF1 treatment failed to increase islet insulin secretion until after metabolic changes occurred. To examine if central FGF1-mediated improvements on the islet were independent of reductions in food intake, we pair-fed a cohort of vehicle-treated db/db mice to FGF1-treated mice for 8 days (Fig. 5). As expected, a single intracerebroventricular injection of FGF1 reduced blood glucose chronically, while reductions in food intake were transient (Fig. 5A and C). In the pair-fed cohort, blood glucose was initially reduced to levels that matched FGF1-treated mice, but this reduction was only temporary, consistent with previous findings (4). Additionally, we observed an improvement in glucose tolerance during an ipGTT after 6 days of exposure to FGF1 (Fig. 5E), but when corrected for basal glucose levels, the data were not significant (data not shown). Furthermore, plasma insulin during an ipGTT was unaffected by FGF1 and pair-feeding (Fig. 5F). Plasma insulin during the chronic study was significantly reduced after a central injection of FGF1 but returned back to baseline by the end of the study (Fig. 5B). After 8 days of exposure to central FGF1, we isolated islets for analysis of insulin secretion ex vivo. Surprisingly, islets from FGF1 and pair-fed cohorts had comparable levels of insulin secretion under glucose-stimulated conditions, demonstrating that central FGF1-mediated reductions in food intake are sufficient to drive improvements on the islet (Fig. 5G).
Figure 5.
Pair-feeding was sufficient to improve ex vivo insulin secretion from centrally treated db/db mice. A–D: db/db mice received a single intracerebroventricular injection of FGF1 (filled squares, n = 10) or vehicle (open squares, n = 10) and were fed ad libitum, and an additional cohort of vehicle-treated mice was pair-fed (gray triangles, n = 10) to FGF1-treated mice. Blood glucose (A), plasma insulin (B), food intake (C), and body weight (D) were measured for 8 days after treatment. Blood glucose levels (E) and plasma insulin (F) were measured during an ipGTT 6 days after a single intracerebroventricular injection of FGF1 or vehicle in separate cohorts of mice (n = 4). G: GSIS from FGF1- or vehicle-treated mouse islets 8 days after a single intracerebroventricular injection. Isolated islets were cultured overnight prior to GSIS assay using a perifusion system. Data are expressed as mean ± SEM. P values were determined by two-way ANOVA. A significance of P < 0.5 is represented by the following: * = FGF1 vs. vehicle (ad libitum) and vehicle (pair-fed); $ = vehicle (pair-fed) vs. FGF1 and vehicle (ad libitum); # = vehicle (ad libitum) vs. FGF1 and vehicle (pair-fed); and & = FGF1 vs. vehicle (ad libitum). d, days; m, minutes.
Central and Peripheral FGF1 Induce Hypothalamic c-Fos Activation
Our data have demonstrated that improvements in islet insulin secretion and microanatomy may be a necessary component for peripheral FGF1 action. In contrast, central FGF1-mediated effects on the islet are likely secondary to improvements in overall metabolism that could be driven by the central nervous system. To investigate this, we measured c-Fos activation in the hypothalamus from control, DIO, and db/db mice treated with central or peripheral FGF1. FGF1 induced robust activation of c-Fos across multiple hypothalamic areas in all groups (Supplementary Fig. 7), despite marked differences in FGF1’s ability to reduce blood glucose across these three groups. We only observed slight differences in c-Fos activation patterns between control, DIO and db/db mice. In particular, c-Fos activation was increased in the arcuate nucleus (ARH) of centrally treated control and DIO mice but not db/db mice (Supplementary Fig. 7E).
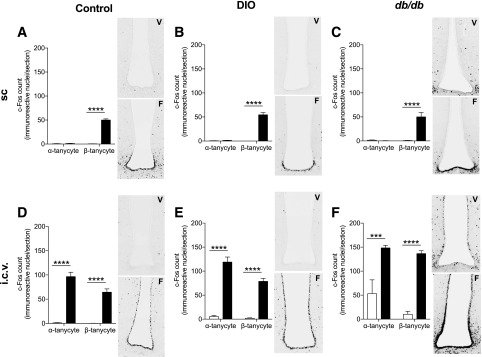
While FGF1 treatment induced robust c-Fos activation in several hypothalamic regions independent of mouse model, we were surprised to find a marked difference in tanycytic c-Fos activation between administration routes (Fig. 6). Central administration of FGF1 induced c-Fos expression in third ventricle α- and β-tanycytes, whereas peripheral administration of FGF1 only activated β-tanycytes in the third ventricle (Fig. 6 and Supplementary Fig. 8). This distinct tanycytic activation pattern is a novel finding and may contribute to the metabolic differences between centrally and peripherally administered FGF1.
Figure 6.
Central, but not peripheral, FGF1 induced c-Fos activation of third ventricle α-tanycytes. A–C: c-Fos activation of third ventricle tanycytes 90 min after subcutaneous administration of FGF1 (filled bars, F) or vehicle (open bars, V) in control (A), DIO (B), and db/db (C) mice. c-Fos activation of third ventricle tanycytes 90 min after intracerebroventricular injection of FGF1 or vehicle in control (D), DIO (E), and db/db (F) mice. n = 3 per group. Data are expressed as mean ± SEM. P values were determined by two-way ANOVA. ***P < 0.001; ****P < 0.0001. i.c.v., intracerebroventricular; sc, subcutaneous.
Cisterna Magna Administration of FGF1
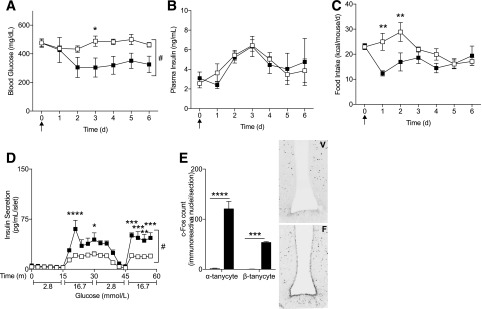
The ability of a single intracerebroventricular injection of FGF1 to restore blood glucose to normoglycemic levels in mice is clinically relevant (4), but intracerebroventricular drug administration in nonrodent models is highly variable. To examine if FGF1 yielded similar results via alternative intrathecal injection routes, we administered FGF1 into the cerebrospinal fluid through a single cisterna magna injection in db/db mice (15,16) (Fig. 7). FGF1 reduced blood glucose and improved ex vivo islet insulin secretion to levels comparable with our intracerebroventricular administration studies (Fig. 7A and D). Importantly, cisterna magna–administered FGF1 induced c-Fos activation of α- and β-tanycytes, demonstrating that injections into the cerebrospinal fluid via this route are sufficient for FGF1 delivery to the third ventricle of the hypothalamus (Fig. 7E).
Figure 7.
Cisterna magna administration of FGF1 improved metabolic outcomes in db/db mice. Daily blood glucose levels (A), plasma insulin (B), and food intake (C) after a single cisterna magna injection of FGF1 (filled squares, 3 µg, n = 5) or vehicle (open squares, n = 6) in db/db mice. D: GSIS 6 days after cisterna magna administration of FGF1 or vehicle. E: c-Fos activation of third ventricle tanycytes 90 min after cisterna magna administration of FGF1 (filled bars, F, n = 3) or vehicle (open bars, V, n = 3). Arrows represent injection time course. Data are expressed as mean ± SEM. P values were determined by two-way ANOVA. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; #P < 0.05 for overall treatment effect. d, days; m, minutes.
Discussion
Impaired islet insulin secretion is a major component to the progression of type 2 diabetes. In this study, we demonstrate that FGF1 is able to increase islet insulin secretion in diabetic mouse models. During nutrient excess, β-cells are required to secrete more insulin in order to maintain normoglycemic conditions. This heightened insulin demand induces β-cell insulin synthesis and secretion, induces β-cell proliferation, and increases overall β-cell mass (17). However, in states of prolonged overnutrition and hyperglycemia, β-cell dysfunction occurs. In humans and rodents, chronic high levels of glucose are toxic to the β-cell, resulting in apoptosis and impaired insulin secretion (18,19). Our findings demonstrate that FGF1 is able to partially ameliorate diabetes-induced β-cell dysfunction by improving basal insulin secretion at the islet level.
FGF1 is expressed in both α- and β-cells of embryonic and adult rodent pancreata (20). Unlike other FGFs, FGF1 ubiquitously binds to all FGF receptors (FGFRs) to initiate intracellular FGF signaling, which is critical for development and function of the pancreas (21,22). Specifically, FGFR1c has been identified as a key receptor for normal β-cell function. Pancreas-specific attenuation of FGFR1c in mice causes hyperglycemia after 12 weeks, with a marked reduction in total insulin production and β-cell number (22). In a zebrafish model of overnutrition, the absence of FGF1 failed to induce compensatory β-cell differentiation; however, when FGF1 was overexpressed in this model, β-cell differentiation was restored (23). Taken together with our finding that peripheral FGF1 increased β-cell density and islet mass, we postulate that peripheral FGF1-mediated metabolic outcomes may rely, in part, on FGF1’s ability to combat islet dysfunction in a diabetic model.
We have demonstrated that central and peripheral administration of FGF1 increased insulin secretion at the islet level in diabetic mice. Although both administration routes improved insulin secretion under basal and glucose-stimulated conditions ex vivo, further investigation revealed marked differences in islet response to central versus peripheral FGF1. When administered acutely, peripheral FGF1 significantly increased insulin secretion from isolated db/db islets within 2 h (Fig. 4A), whereas central FGF1 had no effect on islet insulin secretion after this short exposure in db/db mice (Fig. 4B). Importantly, improvements in islet insulin secretion from peripherally treated mice were independent of reductions in blood glucose, food intake, and body weight (Supplementary Fig. 5), demonstrating a potential direct effect of peripheral FGF1 on the islet. In contrast, central FGF1 administered to db/db mice only improved islet insulin secretion after reductions in blood glucose and food intake occurred (Fig. 4C), and upon further examination, central FGF1-mediated islet improvements were dependent on reductions in food intake (Fig. 5G).
In addition to variances in acute islet insulin secretion in response to central and peripheral FGF1, we also discovered marked differences in islet microanatomy. Immunohistochemical (IHC) analysis of pancreata after 7 days of treatment showed that peripherally treated db/db mice had dramatic improvements in islet microanatomy, including increased α- and β-cell density, increased islet area, and increased number of islets per tissue area (Fig. 3). In contrast, central FGF1 only increased α-cell density but not other cell types. We hypothesize that improvements at the islet level may be a necessary component for peripheral FGF1 action, whereas central FGF1-mediated islet response is likely an effect of chronic modifications in overall metabolism.
A limitation to our study was a lack of effect on circulating insulin levels, given that central and peripheral FGF1 increased insulin secretion in isolated islets. Whereas we observed an acute significant decrease in circulating insulin after a single intracerebroventricular injection of FGF1 in db/db mice (Fig. 5B), we failed to measure any significant changes in insulin during our chronic cisterna magna study (Fig. 7B), or after 6 days of exposure to FGF1 in the central and peripheral studies across all three models (Supplementary Fig. 4, baseline data). With that said, our central FGF1 data are consistent with previous findings that report a lack of effect of central FGF1 on circulating insulin levels after 7 days (24). Additionally, these findings support our data demonstrating that central FGF1-mediated effects on the islet are driven by reductions in food intake and are likely transient. For peripheral FGF1, we were surprised that circulating insulin remained unaffected given that FGF1 increased islet insulin secretion, β-cell density, and islet size. Furthermore, it was previously reported that peripheral administration of FGF1 significantly altered circulating insulin in ob/ob mice; however, this was observed after 4 weeks of treatment (2). Taken together, we postulate that peripheral FGF1 may alter circulating insulin in the db/db model with longer treatment time.
Another interesting finding is the differential response to acute central FGF1 between db/db and DIO mice. Whereas 48 h of exposure to central FGF1 was required before we observed improvements in db/db islet insulin secretion (Fig. 4C), these improvements were evident after only 2 h in DIO mice (Supplementary Fig. 6B). One possible explanation for this result is that acute central FGF1 increased c-Fos activation in the ARH of DIO, but not db/db, mice (Supplementary Fig. 7E). It was recently found that activation of glucose-sensing neurons within the ARH may have direct effects on insulin secretion from the pancreas (25). Additionally, it is well established that the absence of leptin signaling present in db/db mice, but to a lesser extent in DIO mice, results in dysregulation of neuronal populations within the ARH (26,27). It is possible that the acute FGF1 effects on DIO islets are due to transient activation of neuronal populations within the ARH that innervate islets, whereas in the db/db model, FGF1 may restore the function of dysregulated neuronal circuitry, which have sustained effects on metabolism.
Although we have demonstrated that peripheral FGF1 action likely involves alterations within pancreatic islets, we hypothesize that sustained central FGF1 action may rely on α-tanycytes to initiate blood glucose–lowering effects. Previous work by Scarlett et al. (4) demonstrated that intracerebroventricular administration of FGF1 induced c-Fos activation of α- and β-tanycytes of the third ventricle. In contrast, we found that peripheral FGF1 only activated β-tanycytes of the third ventricle (Fig. 6 and Supplementary Fig. 8). β-Tanycytes are located predominantly on the floor of the third ventricle, project into the median eminence and ARH, and come into contact with portal blood vessels within the median eminence (28). By contrast, α-tanycytes occupy the ventrolateral walls of the third ventricle, lack blood vessel contact, and have been identified as neural progenitors (28–30). We postulate that activation of α-tanycytes may be a necessary component of the sustained metabolic outcomes of central FGF1. Current hypotheses suggest that FGF1 restores hypothalamic circuits that are impaired in states of overnutrition by stimulating α-tanycyte signaling, which induces neurogenesis of critical glucose-sensing neurons (5).
In summary, we have demonstrated that FGF1 improves islet insulin secretion in diabetic mouse models via peripheral- and central-mediated mechanisms. These findings further support the therapeutic potential of peripheral FGF1 through elucidation of its mode of action on improving overall islet function.
Supplementary Material
Article Information
Acknowledgments. The authors thank their colleague, Dr. Charles Roberts (Oregon National Primate Research Center), for editorial assistance.
Funding. This work was supported in part by the National Institutes of Health Office of the Director (grant P51OD01192 for operation of the Oregon National Primate Research Center) and the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK079194 to P.K.).
Duality of Interest. P.K. receives research support from Novo Nordisk, Leidos Biomedical Inc., and Janssen Pharmaceuticals unrelated to the work presented in this article. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. K.G.T. conceived and designed the experiments, performed experiments, analyzed data, interpreted results of experiments, prepared figures, and drafted, edited, revised, and approved the final version of the manuscript. S.R.L. performed the c-Fos and metabolic cage and IHC experiments and analyzed c-Fos data. M.A.K. performed the c-Fos and IHC experiments and edited and revised the manuscript. C.T. interpreted results of experiments and edited and revised the manuscript. P.K. conceived and designed the experiments, analyzed data, interpreted results of experiments, and edited, revised, and approved the final version of the manuscript. P.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented as an oral abstract at the 100th Annual Meeting of the Endocrine Society, Chicago, IL, 17–20 March 2018.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db18-1175/-/DC1.
References
- 1.Jonker JW, Suh JM, Atkins AR, et al. . A PPARγ-FGF1 axis is required for adaptive adipose remodelling and metabolic homeostasis. Nature 2012;485:391–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suh JM, Jonker JW, Ahmadian M, et al. . Endocrinization of FGF1 produces a neomorphic and potent insulin sensitizer [published correction appears in Nature 2015;520:388]. Nature 2014;513:436–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu W, Struik D, Nies VJ, et al. . Effective treatment of steatosis and steatohepatitis by fibroblast growth factor 1 in mouse models of nonalcoholic fatty liver disease. Proc Natl Acad Sci U S A 2016;113:2288–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scarlett JM, Rojas JM, Matsen ME, et al. . Central injection of fibroblast growth factor 1 induces sustained remission of diabetic hyperglycemia in rodents. Nat Med 2016;22:800–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gasser E, Moutos CP, Downes M, Evans RM. FGF1 - a new weapon to control type 2 diabetes mellitus. Nat Rev Endocrinol 2017;13:599–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Itoh N, Nakayama Y, Konishi M. Roles of FGFs as paracrine or endocrine signals in liver development, health, and disease. Front Cell Dev Biol 2016;4:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Renaud F, El Yazidi I, Boilly-Marer Y, Courtois Y, Laurent M. Expression and regulation by serum of multiple FGF1 mRNA in normal transformed, and malignant human mammary epithelial cells. Biochem Biophys Res Commun 1996;219:679–685 [DOI] [PubMed] [Google Scholar]
- 8.Burgess WH, Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem 1989;58:575–606 [DOI] [PubMed] [Google Scholar]
- 9.Zhu J, Wang Y, Zhu K, et al. . Serum fibroblast growth factor 1 is associated with the decreased risk of obesity in human. Exp Clin Endocrinol Diabetes 2017;125:322–326 [DOI] [PubMed] [Google Scholar]
- 10.Saber GY, Kasabri V, Saleh MI, et al. . Increased irisin versus reduced fibroblast growth factor1 (FGF1) in relation to adiposity, atherogenicity and hematological indices in metabolic syndrome patients with and without prediabetes. Horm Mol Biol Clin Investig. 6 March 2019. [Epub ahead of print]. DOI: 10.1515/hmbci-2018-0063 [DOI] [PubMed] [Google Scholar]
- 11.Miller DL, Ortega S, Bashayan O, Basch R, Basilico C. Compensation by fibroblast growth factor 1 (FGF1) does not account for the mild phenotypic defects observed in FGF2 null mice. Mol Cell Biol 2000;20:2260–2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Imai H, Ito A, Saito N. Novel modified method for injection into the cerebrospinal fluid via the cerebellomedullary cistern in mice. Acta Neurobiol Exp (Warsz) 2013;73:304–311 [DOI] [PubMed] [Google Scholar]
- 13.Nowotny B, Nowotny PJ, Strassburger K, Roden M. Precision and accuracy of blood glucose measurements using three different instruments. Diabet Med 2012;29:260–265 [DOI] [PubMed] [Google Scholar]
- 14.Goodman T, Hajihosseini MK. Hypothalamic tanycytes-masters and servants of metabolic, neuroendocrine, and neurogenic functions. Front Neurosci 2015;9:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hinderer C, Bell P, Katz N, et al. . Evaluation of intrathecal routes of administration for adeno-associated viral vectors in large animals. Hum Gene Ther 2018;29:15–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Proescholdt MG, Hutto B, Brady LS, Herkenham M. Studies of cerebrospinal fluid flow and penetration into brain following lateral ventricle and cisterna magna injections of the tracer [14C]inulin in rat. Neuroscience 2000;95:577–592 [DOI] [PubMed] [Google Scholar]
- 17.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 2004;429:41–46 [DOI] [PubMed] [Google Scholar]
- 18.Finegood DT, McArthur MD, Kojwang D, et al. . Beta-cell mass dynamics in Zucker diabetic fatty rats. Rosiglitazone prevents the rise in net cell death. Diabetes 2001;50:1021–1029 [DOI] [PubMed] [Google Scholar]
- 19.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003;52:102–110 [DOI] [PubMed] [Google Scholar]
- 20.Theodoraki A, Hu Y, Poopalasundaram S, et al. . Distinct patterns of heparan sulphate in pancreatic islets suggest novel roles in paracrine islet regulation. Mol Cell Endocrinol 2015;399:296–310 [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem 2006;281:15694–15700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hart AW, Baeza N, Apelqvist A, Edlund H. Attenuation of FGF signalling in mouse beta-cells leads to diabetes. Nature 2000;408:864–868 [DOI] [PubMed] [Google Scholar]
- 23.Li M, Page-McCaw P, Chen W. FGF1 mediates overnutrition-induced compensatory β-cell differentiation. Diabetes 2016;65:96–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scarlett JM, Muta K, Brown JM, et al. . Peripheral mechanisms mediating the sustained antidiabetic action of FGF1 in the brain. Diabetes 2019;68:654–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosario W, Singh I, Wautlet A, et al. . The brain-to-pancreatic islet neuronal map reveals differential glucose regulation from distinct hypothalamic regions. Diabetes 2016;65:2711–2723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elias CF, Aschkenasi C, Lee C, et al. . Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron 1999;23:775–786 [DOI] [PubMed] [Google Scholar]
- 27.Coppari R, Ichinose M, Lee CE, et al. . The hypothalamic arcuate nucleus: a key site for mediating leptin’s effects on glucose homeostasis and locomotor activity. Cell Metab 2005;1:63–72 [DOI] [PubMed] [Google Scholar]
- 28.Rodríguez EM, Blázquez JL, Pastor FE, et al. . Hypothalamic tanycytes: a key component of brain-endocrine interaction. Int Rev Cytol 2005;247:89–164 [DOI] [PubMed] [Google Scholar]
- 29.De Juan Romero C, Borrell V. Coevolution of radial glial cells and the cerebral cortex. Glia 2015;63:1303–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu Y, Tamamaki N, Noda T, et al. . Neurogenesis in the ependymal layer of the adult rat 3rd ventricle. Exp Neurol 2005;192:251–264 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.