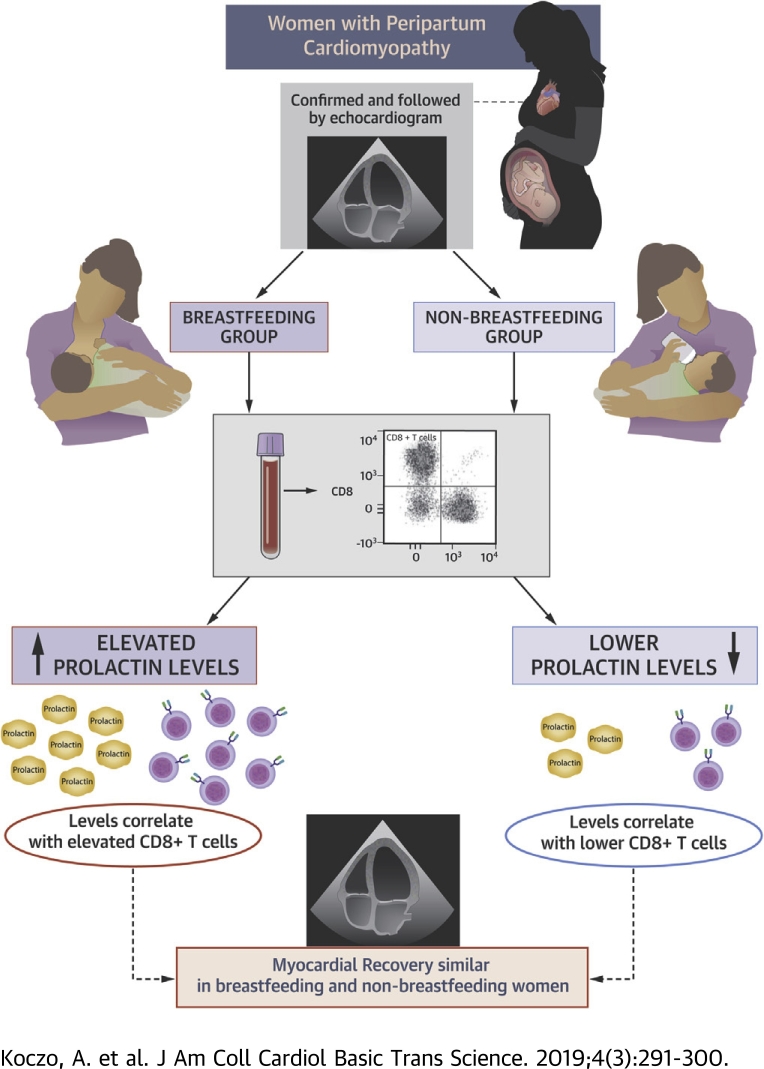
Visual Abstract
Key Words: breastfeeding, immune activation, peripartum cardiomyopathy
Abbreviations and Acronyms: BF, breastfeeding; LVEF, left ventricular ejection fraction; NBF, nonbreastfeeding; PPCM, peripartum cardiomyopathy
Highlights
-
•
The impact of breastfeeding on prolactin, cellular immune activation, and myocardial recovery was analyzed in 100 women with peripartum cardiomyopathy
-
•
Cardiac function was assessed by echocardiography at presentation and at serial intervals over the first year postpartum
-
•
The levels of circulating prolactin were assessed by ELISA, and cellular immunophenotyping by flow cytometry, and compared between breastfeeding and nonbreastfeeding women
-
•
Prolactin levels were higher in breastfeeding women and correlated with significant increases in CD8+ T cells
-
•
Despite significantly higher prolactin levels and increased CD8+ cells, myocardial recovery was similar in breastfeeding and nonbreastfeeding women
Summary
The etiology of peripartum cardiomyopathy remains unknown. One hypothesis is that an increase in the 16-kDa form of prolactin is pathogenic and suggests that breastfeeding may worsen peripartum cardiomyopathy by increasing prolactin, while bromocriptine, which blocks prolactin release, may be therapeutic. An autoimmune etiology has also been proposed. The authors investigated the impact of breastfeeding on cellular immunity and myocardial recovery for women with peripartum cardiomyopathy in the IPAC (Investigations in Pregnancy Associated Cardiomyopathy) study. Women who breastfed had elevated prolactin, and prolactin levels correlated with elevations in CD8+ T cells. However, despite elevated prolactin and cytotoxic T cell subsets, myocardial recovery was not impaired in breastfeeding women.
Peripartum cardiomyopathy (PPCM) is a rare complication of pregnancy that remains a major cause of maternal morbidity and mortality. PPCM is classically defined as a nonischemic cardiomyopathy presenting toward the end of pregnancy or in the months following delivery, without previously known structural heart disease (1). An examination of the Nationwide Inpatient Sample database analysis of PPCM in the United States found that the incidence ranges from approximately 1 in 1,000 to 1 in 4,000 live births (2). It is more prevalent in Africa and Asia, with an incidence of about 1 in 1,000 live births. There are also particular “hot spots” of PPCM, including Haiti, in which the incidence of PPCM may be closer to 1 in 300 live births (3).
Much research has been dedicated to understanding the pathophysiology of PPCM, but the etiology remains unknown. Several hypotheses have been proposed, from hemodynamic stress to viral myocarditis and underlying autoimmune processes 3, 4, 5. A theory of unbalanced oxidative stress and hormonal interaction leading to vasculopathy was proposed in a 2007 study, which postulated that the cathepsin-cleaved 16-kDa form of prolactin may be crucial to the development of the condition. It further showed that the inhibition of prolactin with bromocriptine, a dopamine D2 receptor agonist, inhibited the development of PPCM in a murine model (6). Recent studies have suggested that inhibition of prolactin with bromocriptine improves myocardial recovery 7, 8, 9. Given this postulate, a recent European study group recommendation advised against breastfeeding (BF) in women with PPCM, but these recommendations regarding BF in women with PPCM remain controversial (10).
In addition to its role in lactation, prolactin plays an important role in resetting maternal immunity in the peripartum and early postpartum periods. Despite the potential impact of prolactin on cellular immunity and the autoimmune hypothesis, there has been little investigation regarding the impact of BF on maternal cellular immunity in patients with PPCM. It also remains unclear as to whether prolactin-induced alterations of cellular immunity adversely affect recovery in these patients. We investigated the impact of BF and prolactin on cellular immunity and myocardial recovery in the prospective, multicenter IPAC (Investigations in Pregnancy Associated Cardiomyopathy) study.
Methods
Cohort
One hundred women with newly diagnosed PPCM were enrolled within the first 13 weeks postpartum at 30 centers (Supplemental Material) between December 2009 and September 2012. All women were at least 18 years of age, had no histories of cardiac disease, had estimated left ventricular ejection fractions (LVEF) of ≤45% at the time of enrollment, and had evaluations consistent with recent-onset nonischemic cardiomyopathy presenting in late pregnancy or early postpartum without evidence of pre-existing structural heart disease. Women with significant valvular disease, coronary disease (>50% stenosis of a major epicardial vessel or positive results on noninvasive study), evidence of ongoing bacterial septicemia (positive blood cultures), ongoing drug or alcohol abuse, history of chemotherapy or chest radiation within 5 years of enrollment, or histories of previous cardiomyopathy were excluded.
Protocol
The study protocol was approved by the Institutional Review Boards at all participating centers, and informed consent was obtained from all subjects. At the time of enrollment, demographic information including self-designated race, previous clinical evaluation, and current medical therapy were recorded. Women were followed until 1 year postpartum. All hospitalizations and major cardiac events including death, cardiac transplantation, and implantation of a left ventricular (LV) assist device were recorded.
LV function
All subjects underwent echocardiography to assess LVEF at entry and 6 and 12 months postpartum. Echocardiograms were reviewed in a core laboratory at the University of Pittsburgh for assessment of ventricular volumes and calculation of ejection fraction. LV volumes and LVEF were assessed using the biplane Simpson’s rule with manual tracing of digital images.
Flow cytometry
Patients with PPCM (n = 67) enrolled early (during the first 6 weeks postpartum) had immunophenotyping repeated at 2 and 6 months postpartum. The remaining 33 subjects, enrolled at 2 months postpartum, had immunophenotyping performed at 2 and 6 months postpartum. For the early time point, all 67 women had blood collected (postpartum 16.6 ± 10.6 days), while 73 women were sampled at 2 months (postpartum 62.1 ± 11.5 days) and 77 women at the 6-month time point (postpartum 179.2 ± 33.2 days).
Immunophenotyping of circulating cells was performed on whole blood collected and stabilized in Cyto-Chex BCT tubes, approximately 3 days prior to multicolor flow cytometry. Antibodies against CD3, CD4, CD8, CD16, and CD56 were used for determination of cellular subsets: overall T cells (CD3+), T helper cell subset (CD3+CD4+), cytotoxic T cells (CD3+CD8+), “double-negative” T cells (CD3+CD4−CD8−), classical monocytes (CD14+CD16−), nonclassical monocytes (CD14−CD16+), and natural killer cells (CD3−CD56+CD16+, CD3−CD56+CD16−). Cell “activation” status was assessed by expression of CD25, CD38, or human leukocyte antigen DR isotype. Antigen-specific and compensation antibodies used in flow cytometry were previously published (11). Flow cytometry data were acquired using a BD FACS ARIA 1 and analyzed using FACSDiva version 6.1.3 software (BD Biosciences, Ashland, Oregon). Data are presented as the percentage of all events within a particular immunophenotyping “gate.”
Biomarker assays
Serum was collected from 98 of 100 subjects at the time of entry, shipped overnight at room temperature to the core laboratory (University of Pittsburgh), and stored at −80°C until the time of analysis. Enzyme-linked immunosorbent assays for human prolactin were obtained from R&D Systems (Minneapolis, Minnesota), run with 50 μl of undiluted sample per well in duplicate, and read at 450 nm on a Packard Spectracount instrument (ALPCO, Salem, New Hampshire).
Statistical analysis
All analyses were done in SPSS version 24 (IBM, Armonk, New York). For analysis of clinical and demographic variables, Fisher exact tests were used to compare categorical variables by BF status, BF versus non-BF (NBF). The Mantel-Haenszel test for trend was used for comparison of New York Heart Association functional class by BF status. For continuous variables, we examined the distribution of data for normality using the Shapiro-Wilk test. Given the skewed distributions of several clinical variables (e.g., days postpartum, gravida, para), the nonparametric Mann-Whitney U test was used to compare groups for all continuous clinical variables. Given similar skewed distributions of cellular activation and biomarker data, the Mann-Whitney U test was also used for comparison of the percentage of circulating immune cells and prolactin levels in BF versus NBF subsets. To evaluate the role of prolactin in immune activation, a regression model with prolactin levels and percentage CD3+CD8+ at entry was used with percentage CD3+CD8+ as a continuous outcome variable and prolactin levels as the predictor to examine the relationship between these 2 variables. We examined this relationship first in the overall cohort and in the subset of women BF at entry. The impact of BF on myocardial recovery was examined by comparing LVEF at entry, 6 months, and 12 months and change in LVEF from entry to 6 and 12 months between the BF and NBF subsets. LVEF and the percentage of CD3+CD8+ cells were compared between BF and NBF women for the entire cohort and for the subset with complete data at all time points. The relationship of initial prolactin level and percentage CD3+CD8+ cell to subsequent myocardial recovery was examined by linear regression using both as predictors with 6- and 12-month LVEFs as the outcome variables.
Results
Comparison of BF and NBF cohorts
Of the overall IPAC cohort, 15 women were BF at time of entry, and the remaining 85 were not. There were no significant differences in age, race, body mass index, parity, or medical therapy on the basis of BF (Table 1).
Table 1.
Demographics and Clinical Phenotype of the Breastfeeding and Nonbreastfeeding Cohorts
| Breastfeeding at Entry (n = 15) | Nonbreastfeeding at Entry (n = 85) | p Value | |
|---|---|---|---|
| Age (yrs) | 32 ± 6 | 30 ± 6 | 0.23 |
| Race (black) | 27 | 31 | 0.76 |
| Days postpartum | 20 ± 16 | 33 ± 25 | 0.07 |
| Gravida | 3.1 ± 2.5 | 2.8 ± 1.8 | 0.89 |
| Para | 2.0 ± 1.4 | 2.2 ± 1.4 | 0.53 |
| NYHA functional class (I/II/III/IV) | 13/67/20/0 | 12/42/26/20 | 0.06 |
| LVEF (at entry) (%) | 0.39 ± 0.06 | 0.34 ± 0.10 | 0.06 |
| BP systolic (mm Hg) | 117 ± 12 | 111 ± 18 | 0.09 |
| BP diastolic (mm Hg) | 77 ± 12 | 69 ± 3 | 0.02 |
| HTN | 42 | 60 | 0.26 |
| BMI (kg/m2) | 27 ± 4 | 29 ± 8 | 0.30 |
| ACE inhibitor | 67 | 82 | 0.17 |
| Beta-blocker | 80 | 89 | 0.38 |
Values are mean ± SD or %, unless otherwise indicated.
ACE = angiotensin-converting enzyme; BMI = body mass index; BP = blood pressure; HTN = hypertension; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association.
Women who breastfed tended to present earlier postpartum (days postpartum: BF, 20 ± 16; NBF, 33 ± 25; p = 0.07) and also demonstrated a nonsignificant trend toward higher LVEF at entry (p = 0.06) as well as a lower New York Heart Association functional class (p = 0.06). Diastolic blood pressure (p = 0.02) was higher in BF women, but the difference in systolic blood pressure was not significant (p = 0.09). The percentage of women treated with beta-blockers (BF, 80%; NFB, 89%; p = 0.38) and angiotensin-converting enzyme inhibitors (BF, 67%; NFB, 82%; p = 0.17) was similar between groups.
Differences in BF and NBF circulating immune cells
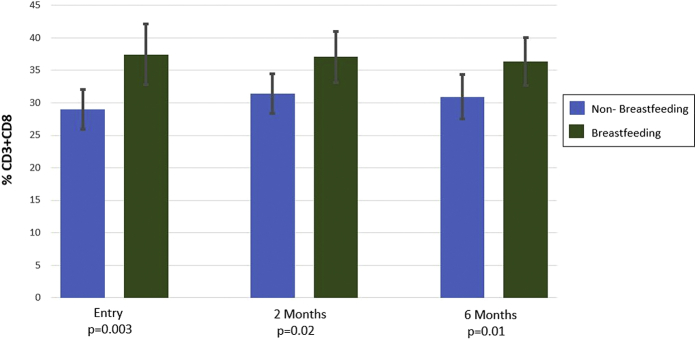
Comparison of cellular subsets revealed a significant increase in the percentage of CD3+CD8+ cells in BF women (Table 2). This was evident at entry (p = 0.003) and remained significant at 2 (p = 0.02) and 6 (p = 0.01) months postpartum (Figure 1). When evaluated only in women with complete cellular data at all 3 time points, the mean values of percentage CD3+CD8+ cells were similar and remained significantly higher in BF women (n = 41; BF vs. NBF percentage CD3+CD8+ cells at entry, 40.7 ± 7.3 vs. 28.8 ± 6.3 [p < 0.001]; at 2 months, 38.0 ± 8.6 vs. 30.1 ± 5.9 [p = 0.01]; and at 6 months, 36.8 ± 6.9 vs. 30.3 ± 6.6 [p = 0.02]). In comparison, percentage CD3+CD4+ T helper cells were not significantly different at entry (p = 0.08) but were lower in BF women at 2 (p = 0.02) and 6 (p = 0.03) months. When evaluated in women with complete data at the 3 time points (n = 39), the mean values of percentage CD3+CD4+ cells were significantly lower in BF women at all time points (BF vs. NBF percentage CD3+CD4+ cells at entry, 51.0 ± 7.7 vs. 56.8 ± 13.2 [p = 0.04]; at 2 months, 49.3 ± 10.7 vs. 57.5 ± 10.1 [p = 0.03]; at 6 months, 53.6 ± 8.8 vs. 61.0 ± 8.3 [p = 0.03]). The percentage of nonclassical monocytes (CD14+CD16+) was significantly lower (p = 0.005) and the percentage of classical monocytes (CD14+CD16−) higher (p = 0.005) in the BF cohort at entry. Similar trends remained at 2 months, which remained significant for classical monocytes (p = 0.04) but not nonclassical monocytes (p = 0.07). These differences were no longer significant at 6 months.
Table 2.
Flow Cytometry Analysis of Circulating Cells From Breastfeeding Women and Nonbreastfeeding Women
| Cell Subset | Entry |
2 Months |
6 Months |
||||||
|---|---|---|---|---|---|---|---|---|---|
| BF (n = 13) | NBF (n = 54) | p Value | BF (n = 12) | NBF (n = 61) | p Value | BF (n = 14) | NBF (n = 63) | p Value | |
| T cells | |||||||||
| CD3+ | 54.7 ± 14.1 | 49.8 ± 15.1 | 0.24 | 65.6 ± 7.1 | 56.3 ± 14.7 | 0.02 | 59.9 ± 13.1 | 54.8 ± 13.7 | 0.04 |
| CD3+CD4+ | 54.7 ± 9.0 | 59.1 ± 11.1 | 0.08 | 49.7 ± 9.1 | 56.6 ± 10.6 | 0.02 | 54.6 ± 8.8 | 59.8 ± 8.3 | 0.03 |
| CD3+CD4+HLA-DR+ | 2.8 ± 1.6 | 2.5 ± 1.9 | 0.33 | 3.0 ± 2.1 | 2.5 ± 1.3 | 0.77 | 2.4 ± 1.8 | 3.0 ± 3.2 | 0.63 |
| CD3+CD4+CD38+ | 47.6 ± 9.3 | 44.4 ± 12.4 | 0.52 | 46.3 ± 9.7 | 43.1 ± 13.2 | 0.60 | 51.2 ± 8.2 | 43.3 ± 14.0 | 0.03 |
| CD3+CD4+CD25+ | 3.1 ± 2.0 | 4.1 ± 3.0 | 0.38 | 4.3 ± 4.4 | 4.3 ± 4.2 | 0.89 | 5.9 ± 4.0 | 5.3 ± 6.1 | 0.14 |
| CD3+CD8+ | 37.4 ± 9.3 | 29.0 ± 6.1 | 0.003 | 37.0 ± 7.8 | 31.4 ± 6.1 | 0.02 | 36.4 ± 7.3 | 31.0 ± 7.0 | 0.01 |
| CD3+CD8+HLA-DR+ | 7.9 ± 5.9 | 6.2 ± 7.7 | 0.29 | 6.7 ± 6.3 | 4.7 ± 3.3 | 0.66 | 6.5 ± 9.1 | 5.0 ± 4.3 | 0.86 |
| CD3+CD8+CD38+ | 33.6 ± 13.9 | 31.1 ± 13.2 | 0.48 | 32.6 ± 17.0 | 28.3 ± 14.2 | 0.44 | 35.0 ± 15.1 | 28.4 ± 14.1 | 0.10 |
| CD3+CD8+CD25+ | 0.3 ± 0.4 | 0.3 ± 0.5 | 0.85 | 0.3 ± 0.3 | 0.4 ± 1.2 | 0.44 | 0.4 ± 0.3 | 0.5 ± 1.3 | 0.16 |
| CD3+CD4+/CD8+ | 1.6 ± 0.7 | 2.2 ± 0.7 | 0.01 | 1.4 ± 0.4 | 1.9 ± 0.6 | 0.01 | 1.6 ± 0.5 | 2.1 ± 0.7 | 0.01 |
| CD3+CD4−CD8− | 9.6 ± 16.0 | 7.4 ± 6.6 | 0.67 | 10.0 ± 6.7 | 9.1 ± 7.5 | 0.33 | 6.2 ± 3.5 | 7.6 ± 4.8 | 0.43 |
| CD3+CD4-8−HLA-DR+ | 5.7 ± 5.2 | 4.1 ± 5.4 | 0.26 | 4.1 ± 5.1 | 3.8 ± 2.8 | 0.54 | 4.2 ± 4.2 | 4.0 ± 3.6 | 0.98 |
| CD3+CD4-8−CD38+ | 28.4 ± 16.7 | 23.6 ± 11.2 | 0.41 | 23.2 ± 15.5 | 19.8 ± 10.2 | 0.70 | 24.2 ± 11.6 | 20.1 ± 11.5 | 0.15 |
| CD3+CD4-8−CD25+ | 0.6 ± 1.3 | 0.4 ± 0.5 | 0.82 | 0.4 ± 0.6 | 0.4 ± 0.8 | 0.98 | 1.0 ± 1.7 | 0.4 ± 0.5 | 0.26 |
| CD3+CD56+ | 4.2 ± 8.5 | 2.2 ± 2.5 | 0.69 | 3.6 ± 3.2 | 2.8 ± 2.6 | 0.36 | 1.9 ± 0.9 | 3.7 ± 4.7 | 0.38 |
| CD3+CD56+CD8+ | 1.6 ± 2.2 | 1.2 ± 1.8 | 0.41 | 2.3 ± 2.5 | 1.8 ± 1.7 | 0.64 | 1.3 ± 0.9 | 2.4 ± 3.1 | 0.80 |
| Monocytes | |||||||||
| CD14+ | 15.9 ± 6.4 | 15.1 ± 8.1 | 0.60 | 10.4 ± 3.4 | 13.2 ± 5.9 | 0.06 | 13.3 ± 2.3 | 14.5 ± 6.1 | 0.62 |
| CD14+CD16− | 91.4 ± 2.9 | 86.0 ± 7.8 | 0.005 | 89.4 ± 9.2 | 87.6 ± 5.2 | 0.04 | 88.3 ± 3.3 | 86.4 ± 8.4 | 0.97 |
| CD14+CD16-HLA-DR+ | 48.8 ± 9.5 | 45.6 ± 21.1 | 0.34 | 60.0 ± 16.6 | 52.7 ± 17.6 | 0.18 | 64.6 ± 18.1 | 60.7 ± 15.6 | 0.51 |
| CD14+CD16−CD38+ | 92.4 ± 11.3 | 91.8 ± 15.4 | 0.32 | 93.8 ± 6.0 | 91.7 ± 12.2 | 0.92 | 96.2 ± 5.1 | 95.1 ± 7.7 | 0.82 |
| CD14+CD16+ | 8.9 ± 3.1 | 13.7 ± 6.0 | 0.005 | 11.0 ± 9.3 | 12.5 ± 5.4 | 0.07 | 12.0 ± 3.6 | 14.0 ± 8.7 | 0.93 |
| CD14+CD16+HLA-DR+ | 65.0 ± 12.0 | 58.2 ± 20.7 | 0.34 | 74.4 ± 17.6 | 62.1 ± 19.4 | 0.03 | 71.3 ± 23.0 | 70.0 ± 17.1 | 0.55 |
| CD14+CD16+CD38+ | 79.5 ± 16.3 | 80.5 ± 17.0 | 0.57 | 78.3 ± 19.1 | 75.0 ± 18.1 | 0.42 | 79.1 ± 16.5 | 80.8 ± 14.5 | 0.82 |
| NK cells | |||||||||
| CD3−CD56+CD16+ | 8.2 ± 4.2 | 6.2 ± 3.9 | 0.11 | 8.5 ± 3.0 | 8.5 ± 4.9 | 0.80 | 8.8 ± 4.0 | 8.8 ± 4.8 | 0.94 |
| CD3−CD56+CD16+HLA-DR+ | 7.3 ± 5.3 | 7.8 ± 7.1 | 0.86 | 8.4 ± 4.9 | 6.1 ± 3.8 | 0.10 | 7.9 ± 4.9 | 8.3 ± 6.8 | 0.91 |
| CD3−CD56+CD16+CD38+ | 93.4 ± 5.6 | 93.5 ± 13.5 | 0.47 | 91.9 ± 3.9 | 91.5 ± 7.4 | 0.49 | 90.4 ± 7.3 | 89.2 ± 9.4 | 0.87 |
| CD3−CD56+CD16− | 2.6 ± 0.8 | 2.2 ± 1.5 | 0.08 | 3.1 ± 1.7 | 2.3 ± 1.3 | 0.11 | 2.0 ± 0.7 | 2.2 ± 1.1 | 0.34 |
Values are mean ± SD. Values with p < 0.05 are in bold.
BF = breastfeeding; NBF = nonbreastfeeding; NK = natural killer.
Figure 1.
Percentage CD3+CD8+ T Cells at Entry and 2 and 6 Months for the Breastfeeding and Nonbreastfeeding Cohorts
CD3+CD8+ cytotoxic T cells in breastfeeding women were significantly higher at entry (p = 0.003) and remained significant at 2 (p = 0.02) and 6 (p = 0.01) months postpartum.
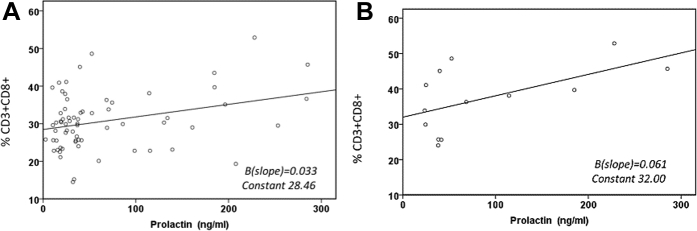
A prior analysis (11) comparing circulating immune cells from women with PPCM in the IPAC cohort with healthy postpartum women revealed a significant reduction in natural killer cells (CD3−CD56+CD16+) and an increase in CD3+CD4−CD8− double-negative T-cells in patients with PPCM. There was no observed difference in the percentage of either natural killer cells or double-negative T cells between the NBF and BF subsets in the present analysis. Prolactin levels at entry were significantly higher in BF women (NBF 50 ± 59 ng/ml vs. BF 82 ± 84 ng/ml; p = 0.02). Higher levels of prolactin at entry correlated with a greater percentage of CD3+CD8+ cells overall (n = 66; p = 0.01) (Figure 2A), and this remained significant when this analysis was limited to the smaller BF subset (n = 13; p = 0.04) (Figure 2B).
Figure 2.
Correlation of the Percentage CD3+CD8+ T Cells With Prolactin Levels in the Overall and Breastfeeding Peripartum Cardiomyopathy Cohorts
(A) In the overall peripartum cardiomyopathy cohort, higher serum levels of prolactin at entry were associated with a higher percentage of CD3+CD8+ cells (n = 98; p = 0.01). (B) In the smaller breastfeeding subset, higher serum prolactin levels remained significantly associated with a higher percentage of CD3+CD8+ cells (n = 13; p = 0.04).
Myocardial recovery: impact of BF, prolactin, and CD3+CD8+ cells
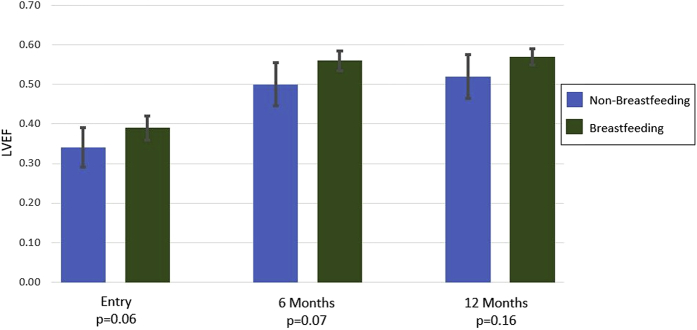
BF women had a trend toward higher LVEF at entry (BF 39 ± 6% vs. NBF 34 ± 10%; p = 0.06), with a similar difference at 6 months (BF 56 ± 5% vs. NBF 50 ± 11%; p = 0.07) and 12 months (BF 57 ± 4% vs. NBF 52 ± 11%; p = 0.16) postpartum (Figure 3). When evaluated only in women with complete LVEF data at all time points, the mean values of LVEF and p values were very similar to the analysis of the overall cohort (n = 71; BF vs. NBF at entry, 39 ± 6% vs. 34 ± 9% [p = 0.07]; 6 months, 56 ± 5% vs. 50 ± 12% [p = 0.07]; and 12 months, 57 ± 4% vs. 52 ± 12% [p = 0.19]). There were no differences noted by BF status in the mean change increase in LVEF from entry to 6 months (ΔLVEF: BF, 17 ± 9%; NBF, 16 ± 11%; p = 0.46) or in the mean change increase in LVEF from entry to 12 months postpartum (ΔLVEF: BF, 18 ± 8%; NBF, 17 ± 11%; p = 0.68). Analysis of linear regression models demonstrated that prolactin levels at entry did not predict subsequent LVEF at either 6 (p = 0.47) or 12 (p = 0.40) months. In a similar fashion, the percentage of CD3+CD8+ cells at entry also did not predict subsequent LVEF at 6 (p = 0.59) or 12 (p = 0.84) months.
Figure 3.
Left Ventricular Ejection Fraction at Entry and 6 and 12 Months for the Breastfeeding and Nonbreastfeeding Cohorts
Mean left ventricular ejection fraction (LVEF) at entry displayed a trend toward a higher mean LVEF in the breastfeeding subset (p = 0.06), which was significant at 6 months (p = 0.07) but not 12 months (p = 0.16) postpartum.
Discussion
This study revealed that in the women enrolled in the IPAC study who breastfed, there was a significantly higher percentage of circulating CD3+CD8+ cells at entry, and this higher percentage persisted through 6 months postpartum. Prolactin appears to be the driving force for this elevation, as there was also a linear relationship between percentage CD3+CD8+ cells and levels of prolactin for both the BF subset as well as the whole IPAC cohort. Despite the impact of prolactin and BF on maternal immunity, there was no clear evidence that BF (or prolactin) had any impact on subsequent LVEF at 6 or 12 months. Not surprisingly, the subset of women who breastfed had higher ejection fractions at entry and tended to be less ill than women who did not breastfeed. This difference persisted at 6 and 12 months. Overall in the IPAC study, there was no evidence that BF had any adverse impact on subsequent myocardial recovery.
The diagnosis of PPCM is made in late antepartum or early postpartum at a time when the adaptive down-regulation of maternal cellular immunity allowing fetal tolerance is ending and maternal cellular immunity is being restored. Prior studies have found differences in peripheral circulating subsets of T cell populations in women with PPCM compared with healthy postpartum women (11). One preliminary study found that patients with PPCM had marked reductions of CD4+CD25lo+ T regulatory cells during the third trimester compared with normal healthy pregnant patients, which persisted for more than 1 month postpartum (6). CD4+CD25+ cells, also known as professional suppressor T cells, are a subset of regulatory T cells that have been found to suppress T cell activation in an antigen-independent manner. CD4+CD25+ cells are thought to have profound suppressive effects on CD3+CD8+ cells. Therefore, persistent low levels of regulatory T cells may be one mechanism for the greater numbers of CD3+CD8+ cells among BF women, which was demonstrated in our study.
Prolactin receptors are expressed on a number of immune cells, including T and B lymphocytes and thymic epithelial cells. Interestingly, some studies suggest that prolactin up-regulates Th1-type cytokines, which play a role in stimulating CD3+CD8+ cells 12, 13, 14, as found in our study. One study on patients with systemic lupus erythematous revealed that prolactin receptors were expressed on both CD4+CD25+ regulatory and effector T cells (15). Another study showed that when adding prolactin to cocultures of regulatory and effector T cells, prolactin seemed to impair regulatory T cell suppression of effector T cells via increased production of Th1 cytokines (16). In patients with PPCM who have marked reduction in CD4+CD25+ T regulatory cells, prolactin may play a role in enhancing the Th1 cytokine response, which could result in up-regulation of CD3+CD8+ T cells, as seen in our study.
Although BF and prolactin appear to affect CD3+CD8+ levels, there was no evidence in our study that this had clinical impact in terms of LVEF at presentation or subsequent myocardial recovery. Our data do not support a significant role for cytotoxic T cells in the pathogenesis of PPCM or subsequent recovery. Recently, we reported that a decrease in natural killer cells was evident in patients with PPCM compared with healthy postpartum control subjects, but in contrast, circulating cytotoxic T cell and T helper cell levels were not significantly different between the 2 cohorts (11). The present study found higher circulating cytotoxic T cells in patients with PPCM who breastfed than those who did not. Overall, the analysis of circulating cellular subset data did not support the autoimmune hypothesis. Consistent with the data from circulating cells, examination of myocardial inflammation in a subset of 39 women from IPAC who underwent cardiac magnetic resonance imaging revealed little evidence of myocardial inflammation for the majority of women (17). Although the prevalence of myocarditis on endomyocardial biopsy studies in PPCM varies from 10% to 62%, the pathological evidence mirrors what is seen in other forms of nonischemic cardiomyopathies and is positive only in a minority of subjects 18, 19.
A pilot study of prolactin inhibition with bromocriptine in 20 South African women suggested that this strategy improved outcomes (20). A recent German study comparing high-dose versus low-dose bromocriptine in 63 women showed benefit compared with historical control subjects but did not show differences between the treatment groups and was limited by the absence of a control group not treated with bromocriptine (9). In addition, a German registry comprising 96 patients with PPCM showed that 67% (64 of 96) were treated with bromocriptine and revealed no difference in major adverse events, including advanced therapies, transplantation, or mortality among treatment subgroups (21). A randomized controlled trial enrolled 96 women in Burkina Faso in West Africa and revealed significant improvements in LVEF and end-diastolic LV diameter in the bromocriptine-treated group (8), though the control group had a lower recovery rate compared with European and U.S. cohorts. Despite limitations noted in each study, a recent publication from the Heart Failure Association of the European Society of Cardiology Study Group on PPCM discouraged BF and recommended the use of bromocriptine to block prolactin in patients with PPCM (10). Our study, in which women who breastfed had significantly higher LVEFs at 6 months, does not support this recommendation, nor does a retrospective Internet-based study in the United States that showed better outcomes among women with PPCM who breastfed (22). In addition, a recent single-center study showed that 27 of 63 patients with PPCM who breastfed had no significant difference in recovery status at 1 year compared with their NBF counterparts (23).
BF in postpartum women provides numerous maternal (24) and newborn benefits that may affect health far beyond the months spent actually BF, particularly in parts of Africa, Asia, and Haiti, where PPCM is endemic. In developing countries, where PPCM is more common than the United States, BF is of essential importance, not only as food and nutrition but also for neonatal immunity (25). Indeed, a recent report from the World Health Organization stressing the importance of BF for neonatal health in the developing world stated that BF has the potential to save 800,000 lives for children in developing countries every year. The negative health effects on infants of women with PPCM who are prohibited from BF remains high 26, 27, 28.
Study limitations
The 16-kDa prolactin fragment was not measured in the present analysis, and although we found no evidence that prolactin influenced myocardial recovery, we cannot address whether an increase in the 16-kDa fragment might be associated with poorer recovery. However, the present study uncovered no evidence that enhancing prolactin levels by continuing to breastfeed had any adverse impact on subsequent LVEF. Additionally, although CD4+CD25+ cells were measured, staining with antibodies for Foxp3, the most specific marker for regulatory T cells, was not done (29). Finally, the subset of women from IPAC who breastfed was small (15%), and in general they represent a healthier subset of IPAC with a trend toward a higher LVEF and lower New York Heart Association functional class. This healthier subset would be expected to do better than the more acutely ill subset that either could not, or chose not, to breastfeed. Comparisons of outcomes between these different subsets is limited; however, we can still confidently report that no hazard was evident in the BF group.
Conclusions
This study is the first to demonstrate the impact of BF on maternal cellular immunity in a cohort of women with PPCM and that this change in cellular immunity (increased cytotoxic T cells) was correlated with prolactin. To the extent that PPCM is an autoimmune form of myocarditis, one would expect this change in cellular immunity to affect outcomes. The fact that BF did not seem to affect outcomes argues against the inflammatory hypothesis. Indeed, the absence of any hazard for BF in IPAC argues against a significant role for prolactin as a mediator and bromocriptine as a therapy. For women presenting with PPCM who are well compensated, we find no evidence to support a recommendation against BF. Women with PPCM who are more gravely ill at the time of diagnosis may potentially benefit from prohibition of BF via bromocriptine therapy, but a recommendation regarding the use of bromocriptine in these patients with PPCM should be based on a rigorous large randomized controlled study comparing the use of bromocriptine versus placebo in patients with PPCM who are at higher risk for poor outcomes, all of whom should also be concomitantly treated with guideline-directed heart failure therapies.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: This study provides further evidence that BF does not adversely affect myocardial recovery for patients with PPCM. This result has implications on an international scale, particularly in resource-limited countries, where PPCM has been shown to be more prevalent. Bromocriptine has been promoted as a treatment for PPCM. However, a randomized placebo-controlled trial regarding the efficacy of bromocriptine for treatment of all, or a certain subset of, patients with PPCM must be completed before any recommendations regarding the use of bromocriptine for treatment of PPCM can be confidently supported.
TRANSLATIONAL OUTLOOK: In this study we explored the impact of BF has on cellular immunity, which is likely mediated by prolactin. Our study found that neither CD3+CD8+ cells nor prolactin has an impact on myocardial recovery in patients with PPCM. This argues against an autoimmune or inflammatory hypothesis for the etiology of PPCM. More research is needed to further explore alternative etiologies of this condition.
Contributor Information
Dennis M. McNamara, Email: mcnamaradm@upmc.edu.
IPAC Investigators:
Dennis M McNamara, James D. Fett, Jessica Pisarcik, Charles McTiernan, Karen Hanley-Yanez, John Gorcsan, III, Erik Schelbert, Rami Alharethi, Kismet Rasmusson, Kim Brunisholz, Amy Butler, Deborah Budge, A.G. Kfoury, Benjamin Horne, Joe Tuinei, Heather Brown, Julie Damp, Allen J. Naftilan, Jill Russell, Darla Freehardt, Eileen Hsich, Cynthia Oblak, Greg Ewald, Donna Whitehead, Jean Flanagan, Anne Platts, Uri Elkayam, Jorge Caro, Stephanie Mullin, Michael M. Givertz, M. Susan Anello, Navin Rajagopalan, David Booth, Tiffany Sandlin, Wendy Wijesiri, Leslie T. Cooper, Lori A. Blauwet, Joann Brunner, Mary Phelps, Ruth Kempf, Kalgi Modi, Tracy Norwood, Joan Briller, Decebal Sorin Griza, G. Michael Felker, Robb Kociol, Patricia Adams, Gretchen Wells, Vinay Thohan, Deborah Wesley-Farrington, Sandra Soots, Richard Sheppard, Caroline Michel, Nathalie Lapointe, Heather Nathaniel, Angela Kealey, Marc Semigran, Maureen Daher, John Boehmer, David Silber, Eric Popjes, Patricia Frey, Todd Nicklas, Jeffrey Alexis, Lori Caufield, John W. Thornton, III, Mindy Gentry, Vincent J.B. Robinson, Gyanendra K. Sharma, Joan Holloway, Maria Powell, David Markham, Mark Drazner, Lynn Fernandez, Mark Zucker, David A. Baran, Martin L. Gimovsky, Natalia Hochbaum, Bharati Patel, Laura Adams, Gautam Ramani, Stephen Gottlieb, Shawn Robinson, Stacy Fisher, Joanne Marshall, Jennifer Haythe, Donna Mancini, Rachel Bijou, Maryjane Farr, Marybeth Marks, Henry Arango, Biykem Bozkurt, Mariana Bolos, Paul Mather, Sharon Rubin, Raphael Bonita, Susan Eberwine, Hal Skopicki, Kathleen Stergiopoulos, Ellen McCathy-Santoro, Jennifer Intravaia, Elizabeth Maas, Jordan Safirstein, Audrey Kleet, Nancy Martinez, Christine Corpoin, Donna Hesari, Sandra Chaparro, Laura J. Hudson, Jalal K. Ghali, Zora Injic, and Ilan S. Wittstein
Appendix
References
- 1.Hilfiker-Kleiner D., Sliwa K. Pathophysiology and epidemiology of peripartum cardiomyopathy. Nat Rev Cardiol. 2014;11:364–370. doi: 10.1038/nrcardio.2014.37. [DOI] [PubMed] [Google Scholar]
- 2.Kolte D., Khera S., Aronow W.S. Temporal trends in incidence and outcomes of peripartum cardiomyopathy in the United States: a nationwide population-based study. J Am Heart Assoc. 2014;3:e001056. doi: 10.1161/JAHA.114.001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arany Z., Elkayam U. Peripartum cardiomyopathy. Circulation. 2016;133:1397–1409. doi: 10.1161/CIRCULATIONAHA.115.020491. [DOI] [PubMed] [Google Scholar]
- 4.McNamara D.M., Elkayam U., Alharethi R. Clinical outcomes for peripartum cardiomyopathy in North America: results of the IPAC Study (Investigations of Pregnancy-Associated Cardiomyopathy) J Am Coll Cardiol. 2015;25;66:905–914. doi: 10.1016/j.jacc.2015.06.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ansari A., Fett J.D., Carraway R.E. Autoimmune mechanisms as the basis for human peripartum cardiomyopathy. Clin Rev Allergy Immunol. 2002;23:301–324. doi: 10.1385/CRIAI:23:3:301. [DOI] [PubMed] [Google Scholar]
- 6.Hilfiker-Kleiner D., Kaminski K., Podewski E. A cathepsin cleaved 16kDa form of prolactin mediates postpartum cardiomyopathy. Cell. 2007;128:589–600. doi: 10.1016/j.cell.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 7.Haghikia A., Podewski E., Berliner D. Rationale and design of a randomized, controlled multicentre clinical trial to evaluate the effect of bromocriptine on left ventricular function in women with peripartum cardiomyopathy. Clin Res Cardiol. 2015;104:911–917. doi: 10.1007/s00392-015-0869-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yaméogo N., Kagambèga L., Seghda A. Bromocriptine in management of peripartum cardiomyopathy: a randomized study on 96 women in Burkina Faso. J Cardiol Clin Res. 2017;5:1098. [Google Scholar]
- 9.Hilfiker-Kleiner D., Haghikia A., Berliner D. Bromocriptine for the treatment of peripartum cardiomyopathy: a multicentre randomized study. Eur Heart J. 2017;38:2671–2679. doi: 10.1093/eurheartj/ehx355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sliwa K., Petrie M.C., Hilfiker-Kleiner D. Long-term prognosis, subsequent pregnancy, contraception and overall management of peripartum cardiomyopathy: practical guidance paper from the Heart Failure Association of the European Society of Cardiology Study Group on Peripartum Cardiomyopathy. Eur J Heart Fail. 2018;20:951–962. doi: 10.1002/ejhf.1178. [DOI] [PubMed] [Google Scholar]
- 11.McTiernan C., Morel P., Cooper L.T. Circulating T-cell subsets, monocytes, and natural killer cells in peripartum cardiomyopathy: results from the multicenter IPAC study. J Card Fail. 2018;24:33–42. doi: 10.1016/j.cardfail.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peeva E., Zouali M. Spotlight on the role of hormonal factors in the emergence of autoreactive B-lymphocytes. Immunol Lett. 2005;101:123–143. doi: 10.1016/j.imlet.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Lahat N., Miller A., Shtiller R., Touby E. Differential effects of prolactin upon activation and differentiation of human B lymphocytes. J Neuroimmunol. 1993;47:35–40. doi: 10.1016/0165-5728(93)90282-4. [DOI] [PubMed] [Google Scholar]
- 14.Orbach H., Shoenfeld Y. Hyperprolactinemia and autoimmune diseases. Autoimmun Rev. 2007;6:537–542. doi: 10.1016/j.autrev.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Legorreta-Haquet M.V., Chavez-Rueda K., Chavez-Sanchez L. Function of Treg cells decreased in patients with systemic lupus erythematosus due to the effect of prolactin. Medicine (Baltimore) 2016;95:e2384. doi: 10.1097/MD.0000000000002384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Legorreta-Haquet M.V., Chauvez-Rueda K., Montoya-Diaz E. Prolactin down-regulates CD4+CD25hiCD127low regulatory T cell function in humans. J Mol Endocrinol. 2012;48:77–85. doi: 10.1530/JME-11-0040. [DOI] [PubMed] [Google Scholar]
- 17.Schelbert E., Elkayam U., Cooper L.T. Myocardial damage detected by late gadolinium enhancement cardiac magnetic resonance is uncommon in peripartum cardiomyopathy. J Am Heart Assoc. 2017;6:e005472. doi: 10.1161/JAHA.117.005472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bultmann B.D., Klingel K., Nabauer M., Wallweiner W., Kandolf R. High prevalence of viral genomes and inflammation in peripartum cardiomyopathy. Am J Obstet Gynecol. 2005;193:263–265. doi: 10.1016/j.ajog.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 19.Hilfiker-Kleiner D., Haghikia A., Nonhaff J., Bauersachs J. Peripartum cardiomyopathy: current management and future perspectives. Eur Heart J. 2015;7;36:1090–1097. doi: 10.1093/eurheartj/ehv009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karen S., Blauwet L., Tibazarwa K. Evaluation of bromocriptine in the treatment of acute severe peripartum cardiomyopathy: a proof-of-concept pilot study. Circulation. 2010;121:1465–1473. doi: 10.1161/CIRCULATIONAHA.109.901496. [DOI] [PubMed] [Google Scholar]
- 21.Haghikia A., Podewski E., Libhaber E. Phenotyping and outcome on contemporary management in a German cohort of patients with peripartum cardiomyopathy. Basic Res Cardiol. 2013;108:366. doi: 10.1007/s00395-013-0366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Safirstein J.G., Ro A.S., Grandhi S. Predictors of left ventricular recovery in a cohort of peripartum cardiomyopathy patients recruited via the Internet. Int J Cardiol. 2012;154:27–31. doi: 10.1016/j.ijcard.2010.08.065. [DOI] [PubMed] [Google Scholar]
- 23.Kawamoto K., Langen E., Jackson E.A. Breastfeeding is not associated with worse outcomes for women with peripartum cardiomyopathy (abstr) Circ Cardiovasc Qual Outcomes. 2017;10:A237. [Google Scholar]
- 24.Perrine C., Nelson J., Corbelli J. Lactation and maternal cardio-metabolic health. Annu Rev Nutr. 2016;36:627–645. doi: 10.1146/annurev-nutr-071715-051213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Black R.E., Victora C.G., Walker S.P. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–451. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- 26.Binns C.W., Lee M.K. Exclusive breastfeeding for six months: the WHO six months recommendation in the Asia Pacific region. Asia Pac J Clin Nutr. 2014;23:344–350. doi: 10.6133/apjcn.2014.23.3.21. [DOI] [PubMed] [Google Scholar]
- 27.Victora C.G., Bahl R., Barros A.J. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387:475–490. doi: 10.1016/S0140-6736(15)01024-7. [DOI] [PubMed] [Google Scholar]
- 28.Fett J.D., Murphy J.G. Infant survival in Haiti after maternal death from peripartum cardiomyopathy. Int J Obstet Gynecol. 2006;94:135–136. doi: 10.1016/j.ijgo.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Rudensky A. Regulatory T cells and Foxp3. Immunol Rev. 2011;241:260–268. doi: 10.1111/j.1600-065X.2011.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.