Abstract
Patterns of abdominal fat distribution (for example, a high vs. low visceral adipose tissue [VAT]/[VAT + subcutaneous adipose tissue (SAT)] ratio), independent of obesity, during adolescence carry a high risk for insulin resistance and type 2 diabetes. Longitudinal follow-up of a cohort of obese adolescents has recently revealed that a high ratio (high VAT/[VAT + SAT]) is a major determinant of fatty liver and metabolic impairment over time, with these effects being more pronounced in girls than in boys. To unravel the underlying metabolic alterations associated with the unfavorable VAT/(VAT + SAT) phenotype, we used the 2H2O labeling method to measure the turnover of adipose lipids and cells in the subcutaneous abdominal and gluteal/femoral adipose tissue (SAT) of weight-stable obese adolescent girls with a similar level of obesity but discordant VAT/(VAT + SAT) ratios. Girls with the unfavorable (high VAT/[VAT + SAT]) phenotype exhibited higher in vivo rates of triglyceride (TG) turnover (representing both lipolysis and synthesis at steady state), without significant differences in de novo lipogenesis in both abdominal and gluteal depots, compared with obese girls with the favorable phenotype. Moreover, mature adipocytes had higher turnover, with no difference in stromal vascular cell proliferation in both depots in the metabolically unfavorable phenotype. The higher TG turnover rates were significantly correlated with higher intrahepatic fat stores. These findings are contrary to the hypothesis that impaired capacity to deposit TGs or proliferation of new mature adipocytes are potential mechanisms for ectopic fat distribution in this setting. In summary, these results suggest that increased turnover of TGs (lipolysis) and of mature adipocytes in both abdominal and gluteal SAT may contribute to metabolic impairment and the development of fatty liver, even at this very early stage of disease.
Introduction
Adolescence is a critical period for the development of obesity (1). Significant increases in obesity among adolescent females aged 16–19 years from 2015 to 2016, compared with previous years, show that obesity is increasing in these subgroups (2). Even more concerning is the recent rising incidence in type 2 diabetes affecting particularly adolescent obese girls (3).
Patterns of abdominal fat distribution (for example, a high ratio of visceral fat depot to abdominal subcutaneous fat depot; high visceral adipose tissue [VAT]/[VAT + subcutaneous adipose tissue (SAT)]), more than overall obesity per se, during adolescence carries a high risk for insulin resistance (IR) and type 2 diabetes (4,5). Over the last decade, we formed a cohort (The Yale Study of Body Fat Patterning and Insulin Resistance in Obese Adolescents; NCT01966627) to assess the role of body fat distribution as a potential modifier of glucose and insulin metabolism. Using MRI imaging of abdominal fat distribution and intrahepatic fat accumulation coupled with deep metabolic phenotyping of glucose and lipid metabolism in obese adolescents recruited from this cohort, we described cross-sectionally a distinct “endophenotype” characterized by a thin superficial layer of abdominal SAT, increased VAT, fatty liver, and marked IR (6). More recently, the longitudinal follow-up of this cohort revealed that the high VAT/(VAT + SAT) ratio is a major determinant of fatty liver, with these associations being more pronounced in girls than in boys (7).
To unravel the cellular/molecular mechanisms associated with this phenotype and its relations to IR, we have previously combined metabolic/imaging studies with measurements of adipocyte cellularity and transcription of genes regulating lipogenesis/adipogenesis and inflammation in the subcutaneous abdominal biopsies from obese adolescents matched for overall obesity but with distinct abdominal fat distribution patterns (8,9). We found in obese adolescents an association between the abdominal subcutaneous depots and the transcription of key genes regulating lipogenesis/adipogenesis (8). Despite their novelty, these studies did not provide insights into the underlying metabolic lipid fluxes associated with the high VAT/(VAT + SAT) phenotype.
Impaired capacity of SAT to store and retain triglyceride (TG) has been postulated to explain ectopic fat distribution in liver, muscle, pancreas, and other organs and thereby contributes to metabolic impairment and fatty liver (10–16), but the dynamic metabolic basis of impaired SAT storage capacity could have more than one explanation. In particular, either a reduced capacity to synthesize and deposit TG (lower synthesis rates) or increased breakdown of stored TG (higher lipolysis rates) could contribute to higher exposure of nonadipose tissues to free fatty acids (FFAs). Similarly, either a reduced capacity to proliferate new mature adipocytes (lower cell proliferation rates) or increased turnover of mature adipocytes perhaps due to increased cell death could contribute to the lower capacity to expand mature adipocyte populations. Data for both dynamic explanations have been reported in adults (14–16), but this has not been studied in obese adolescents with discordant VAT/(VAT + SAT) ratios.
To fill this knowledge gap, we used the 2H2O labeling method to measure in vivo the dynamic fluxes of adipose TG, de novo lipogenesis (DNL), and adipocytes in obese adolescents (17). By measuring incorporation of 2H into TG-glycerol and TG-palmitate, followed by gas chromatography–mass spectrometry (GCMS) and application of mass isotopomer distribution analysis (MIDA), direct in vivo measures were made of adipose TG turnover and DNL flux in adipose tissue. Moreover, 2H incorporation into DNA provides a direct measure of mature adipocyte turnover and preadipocyte proliferation (17). Whereas this technique has been used in adults (15,18,19), to the best of our knowledge, there are no studies that have used this method to study in vivo lipid fluxes and adipocyte turnover during adolescence, a life cycle period with high biological plasticity during which changes in body fat accretion and distribution occur dramatically and at the same time (20–22).
Herein, we tested the following questions. 1) Is the high VAT/(VAT + SAT) ratio in obese adolescent girls linked to either significantly reduced synthesis or increased breakdown rates of TG in SAT, compared with obese adolescent girls with a similar level of obesity but discordant VAT/(VAT + SAT) ratios? 2) Is the high VAT/(VAT + SAT) ratio in obese adolescent girls linked to significantly reduced cell proliferation rates in SAT? 3) Do altered lipid fluxes in adipose tissue in obese adolescent girls correlate with intrahepatic fat accumulation? These metabolic questions were tested in paired biopsies of subcutaneous abdominal and gluteal depots in obese adolescents with a high VAT/(VAT + SAT) ratio compared with a BMI, age, sex, ethnicity, and pubertal stage–matched group with a low VAT/(VAT + SAT) ratio.
Research Design and Methods
Experimental Model and Subject Details
The Metabolic Markers and Predictors of Childhood Obesity is a long-term project aimed at examining early alterations in glucose metabolism and insulin sensitivity in relation to body fat patterning in obese adolescents (NCT01967849). As part of this study, all subjects undergo a detailed assessment of abdominal fat distribution by MRI and total body composition by DEXA. As previously described (6), we found that the metabolic profile worsens with the increasing VAT/(VAT + SAT) ratio. Based on the distribution of the VAT/(VAT + SAT) ratio obtained in our entire multiethnic cohort of 88 obese girls, we used the median value (0.0972) as a cutoff value to recruit and enroll subjects in the current biopsy study. We recruited 15 obese adolescents between the ages of 12 and 21 years, who agreed to have a paired subcutaneous periumbilical and gluteal adipose tissue biopsy on the same day of the study. Using the median of the VAT/(VAT + SAT) ratio in the obese girls, we divided the subjects into the following two groups: low (<0.0972) and high (>0.0972) VAT/(VAT + SAT) ratio (7). Their clinical characteristics are described in Table 1. None of the subjects were receiving treatment with any medications or had any known disease. The nature and potential risks of the study were explained to all subjects before obtaining their written informed consent. The study was approved by the ethics committees of the Yale University Hospital.
Table 1.
Clinical and metabolic characteristics of the obese adolescents undergoing fat biopsy
| Low VAT/(VAT + SAT) (n = 7) | High VAT/(VAT + SAT) (n = 8) | P value | |
|---|---|---|---|
| Age (years) | 17.1 (13–20) | 16.1 (12–20) | 0.43 |
| Race (Caucasian/African American/Hispanic), n | 3/1/3 | 2/0/6 | 0.34 |
| Tanner stage (III/IV/V), n | 1/2/4 | 1/3/4 | 0.94 |
| Anthropometrics | |||
| Weight (kg) | 96.4 (66.4–135.6) | 96.0 (59.4–120.8) | 0.97 |
| Height (m) | 1.623 (1.49–1.70) | 1.632 (1.57–1.73) | 0.79 |
| BMI (kg/m2) | 36.6 (25.9–48.0) | 36.0 (23.6–45.5) | 0.88 |
| % fat | 45.9 (39.7–50.2) | 46.6 (38.9–55.2) | 0.80 |
| Lean body mass (kg) | 51.6 (38.3–70.7) | 49.7 (36.2–59.0) | 0.74 |
| Systolic blood pressure (mmHg) | 114.4 (91–127) | 115.8 (107–121) | 0.80 |
| Diastolic blood pressure (mmHg) | 72.4 (67–79) | 66.0 (50–80) | 0.13 |
| Body fat distribution | |||
| Visceral (cm2) | 58.8 (34.2–81.2) | 105.2 (57.4–172.8) | 0.02 |
| Subcutaneous (cm2) | 627.3 (374.8–843.8) | 577.0 (306.6–940.6) | 0.63 |
| Superficial subcutaneous (cm2) | 201.5 (122.6–266.3) | 165.9 (73.0–279.3) | 0.29 |
| Deep subcutaneous (cm2) | 192.9 (106.5–295.2) | 181.0 (100.7–317.4) | 0.45 |
| VAT/(VAT + SAT) ratio | 0.086 (0.060–0.100) | 0.168 (0.107–0.230) | 0.001 |
| Waist-to-hip ratio (cm) | 0.89 (0.87–0.97) | 0.92 (0.82–1.0) | 0.38 |
| Body composition | |||
| % total body fat | 47.9 (43.4–51.3) | 47.2 (44.1–52.1) | 0.68 |
| Total body fat (kg) | 43.6 (30.8–52.4) | 45.1 (27.4–62.8) | 0.81 |
| Lean body mass (kg) | 44.4 (34.3–49.3) | 47.5 (32.5–56.9) | 0.48 |
| Android fat mass (kg) | 3.64 (2.45–5.11) | 3.84 (2.48–6.14) | 0.77 |
| Android fat %1 | 8.23 (6.77–9.80) | 8.48 (7.55–9.78) | 0.65 |
| Gynoid fat mass (kg) | 7.44 (5.78–9.15) | 7.29 (4.25–10.97) | 0.90 |
| Gynoid fat %1 | 17.1 (15.9–18.8) | 15.9 (14.1–17.5) | 0.06 |
| Android-gynoid ratio2 | 1.02 (0.92–1.11) | 1.04 (0.97–1.08) | 0.57 |
| Metabolic measurements | |||
| Fasting glucose (mg/dL) | 90.9 (85–96) | 94.9 (86–125) | 0.47 |
| 2-h glucose (mg/dL) | 113.9 (96–133) | 126.4 (87–203) | 0.39 |
| Fasting insulin (μU/mL) | 19.2 (10–32) | 44.4 (12–101) | 0.03 |
| 2-h insulin (μU/mL) | 83.7 (26–137) | 201.9 (50–559) | 0.03 |
| Whole-body insulin sensitivity index | 2.47 (1.55–3.41) | 1.40 (0.45–2.55) | 0.02 |
| Adiponectin (ng/mL) | 6.38 (4.84–8.55) | 5.52 (4.35–7.26) | 0.25 |
| Leptin (ng/mL) | 58.9 (37.5–85.9) | 77.2 (29.4–188.6) | 0.42 |
| Lipids | |||
| Total cholesterol (mg/dL) | 167.8 (141–209) | 146.6 (117–222) | 0.22 |
| HDL (mg/dL) | 43.2 (30–67) | 35.0 (29–47) | 0.21 |
| LDL (mg/dL) | 117.0 (69.4–144) | 81.2 (67–98) | 0.02 |
| TG (mg/dL) | 97.3 (50–193) | 121.4 (49–183) | 0.39 |
| FFA (μmol/L) | 476 (338–685) | 435 (229–568) | 0.56 |
| Liver | |||
| HFF (%) | 0.19 (0–0.8) | 7.4 (2.6–21.0) | 0.002 |
| Alanine aminotransferase (units/L) | 17.2 (7–56) | 77.3 (11–232) | 0.14 |
| AST (units/L) | 19.8 (6–38) | 55.0 (11–174) | 0.19 |
Demographic, anthropometric, and metabolic characteristics are shown for 15 obese adolescent females undergoing abdominal and gluteal fat biopsies. Data are shown as mean (range) unless otherwise indicated, and values in boldface type are P values <0.05 using unpaired Student t test, adjusted for age, race, and BMI.
1Of total fat mass.
2This ratio is determined by dividing the percentages of fat per depot.
Imaging Studies
Abdominal MRI and Total Body Composition (DEXA)
Multislice abdominal MRI studies were performed on a Siemens Sonata 1.5 T system (6). Total body composition was measured by DEXA with a Hologic scanner (Hologic, Boston, MA). Liver fat content (hepatic fat fraction [% HFF]) was measured by MRI using the proton density fat fraction method (23).
Metabolic Studies
Visit 1: Oral Glucose Tolerance Test
After an overnight fast, each subject visited the Yale Center for Clinical Investigation (YCCI) to undergo an oral glucose tolerance test (OGTT), as previously described (6). The composite whole-body insulin sensitivity index was calculated using the formula described by Matsuda and DeFronzo (24).
Visit 2: 2H2O Water Labeling Protocol (NCT02395003)
A week after the OGTT, the subject returned to the YCCI to start the 8-week 2H2O labeling protocol prior to the biopsies. For 2H2O labeling, subjects received a total of 140 mL 2H2O at YCCI, given as divided doses (70 mL of 70% 2H2O, given every 3–4 h), to achieve ∼1.0% enrichment in body water pool. The 2H2O was purchased from Isotec (Miamisburg, OH) and dispensed in sterile containers. Thereafter, the subjects drank 40 mL of 70% 2H2O three times a day for 5 days and then 40 mL two times a day for the remainder of the 8-week labeling period. This protocol leads to near-plateau body 2H2O enrichments (1.5–2.0%) within 5–7 days in most subjects and was well tolerated (15,17–19). Adverse effects were mild (transient lightheaded feeling at the beginning of the labeling period while the subjects were under supervision at YCCI Hospital Research Unit). Subjects then received the 2H2O as individual aliquots (40 mL of 70% 2H2O) in plastic vials, which were stored in the refrigerator. Compliance with outpatient 2H2O intake was checked through weekly visits for urine collection by counting the returned vials. Urine samples were collected weekly for all subjects and frozen in closed containers. 2H2O enrichments in body water were measured from urine by GCMS.
Visit 3: Biopsy of Abdominal and Gluteal Subcutaneous Fat
The subject returned to the YCCI in the afternoon at 1:00 p.m., after having eaten a standard mixed meal (lunch) consisting of turkey sandwich, sliced apple, and milk (500 kcal), and both biopsies were performed by D.N. or A.V.-M., under sterile conditions, after having applied the EMLA cream 26 cm from the umbilicus and after administration of only 0.25 lidocaine for local anesthesia, as described by McLaughlin et al. (25). Thereafter, a 2-cm scalpel incision was made, from which 2 g of SAT was removed. The gluteal fat biopsy was taken from the superficial SAT on the outer upper quadrant of the gluteus. Fat biopsy specimens were taken to measure fat cell size and number, gene expression, and adipocyte and TG turnover (as described below).
In Vivo Lipid Dynamics Measured by 2H2O
The University of California at Berkeley (UC Berkeley) laboratory assesses TG synthesis based on the incorporation of 2H into the glycerol moiety of TGs, followed by GCMS and application of MIDA (17). The triose precursors of α-glycerol phosphate in TG-synthesizing cells incorporate deuterium from heavy water prior to esterification to fatty acids. Newly synthesized TG thereby becomes labeled in the glycerol moiety (17). This labeling approach also allows us to measure DNL from 2H2O using MIDA. In addition, DNA synthesis and thus adipocyte replacement (proliferation and turnover) rates can be measured in vivo because ribose precursors used in nucleoside synthesis incorporate deuterium from 2H2O. After isolating DNA from isolated adipocytes and stromal vascular fraction (SVF), we analyzed the deoxyribose moiety of adenosine for deuterium enrichment. Deuterium enrichment of DNA represents the production of a new cell, i.e., the product of a cell that had undergone S phase during 2H2O exposure (17).
In Vitro Proliferation and Differentiation
Cells were isolated by collagenase digestion, and an aliquot of SVF (60%) was reconstituted in DMEM/F12 (1:1) medium containing 10% FBS and cultured for 16 h. The plastic-adherent cells from the plated SVF were detached using 0.05% trypsin with EDTA (Invitrogen) and centrifuged, and the pellet was snap frozen in liquid nitrogen and shipped, together with the isolated adipocytes, to the UC Berkeley laboratory for measurements (see above). After collagenase digestion, the adipocytes (the floating cell fraction) were immunodepleted by incubation with a cocktail of biotinylated antibodies against markers of hematopoietic cells, CD45 (1:400; Biolegend), mesenchymal stem cells, CD34 (1:200; eBioscience), and CD31 (1:5; eBioscience) (15,17).
Cell Size and Number Measurements
From each biopsy, the abdominal and gluteal depot, two 20–30 mg samples were used immediately for adipose cell size distribution analysis by osmium fixation (Multisizer 3; Beckman Coulter, Miami, FL). We performed a curve-fitting analysis technique as previously described (8). In addition to determining the “peak diameter” of the large adipose cells as described, the “% of adipose cells above” (% large cells) and “% below” (% small cells) the nadir were calculated. A secondary end point, the number of subcutaneous adipose cells, was estimated by the following formula: cell number volume of subcutaneous abdominal adipose tissue/weighted volume per cell. The volume of abdominal SAT was obtained from MRI scans, and the average volume per cell was calculated as the weighted volume based on the relative number of cells per volume bin in the cell-volume histogram generated by the Multisizer software. We used the following formula: average volume per cell = Σ4/3π(di/2)3pi (the sum of the volumes corresponding to each bin times the relative frequency [p] of that bin [i] in which di is defined as diameter) (26). The number of large cells, or the abdominal cell count, was then calculated by applying the percentage of large cells to the total number of cells. As the MRI of the gluteal region was not assessed because of budgetary constraints, the numbers of large and small adipocytes from the gluteal depot were determined by dividing the gynoid fat mass (determined by DEXA) by the gluteal adipocyte size, as described by Tchoukalova et al. (27).
Lipid Dynamics in SAT Measured With 2H2O
TGs were isolated from adipocytes by use of the Folch technique (17). Fractional replacement of TG (representing synthesis and turnover at metabolic steady state) and DNL were calculated based on deuterium (2H) incorporation into TG-glycerol and TG-palmitate, respectively (17). Quantification of newly synthesized TG-glycerol by using the 2H2O labeling technique, representing net newly synthesized TG retained in adipose tissue over the course of deuterium exposure, has been documented in humans before (17,28). The subjects were weight stable over the 8-week labeling period, indicating that replacement of adipose, or, in other words, TG by synthesis, is balanced by turnover through lipolysis. The adipocyte and preadipocyte proliferation or half-lives were measured via 2H incorporation into the deoxyribose moiety of extracted mature adipocyte and preadipocyte DNA (Qiagen). To adjust for minor differences in labeling times between subjects, the measures were expressed as synthesis rate per day (k) (k = −ln [1 − f]/labeling time). Isotope enrichments were measured by GCMS analysis (models 5971 and 5973; Hewlett-Packard).
Analytical Methods
Plasma glucose levels were measured using the YSI 2700 STAT Analyzer (Yellow Springs Instruments), and lipid levels were measured using an autoanalyzer (model 747-200; Roche-Hitachi). Plasma insulin, adiponectin, and leptin levels were measured using radioimmunoassay (Linco, St. Charles, MO).
Statistics
Before the analysis, the data were tested for normality, and nonnormally distributed variables were log transformed. Weighted means for adipocytes were calculated for adipose cell size of the abdominal and gluteal fat. To test for association between VAT/(VAT + SAT) ratio and variables obtained from the abdominal and gluteal biopsy (e.g., glycerol turnover, DNL, etc.), given the presence of repeated measures, a general linear mixed model (PROC MIXED) was used. A linear regression was used to test for associations between VAT/(VAT + SAT) and adipose tissue lipolysis and lipogenesis independently in each depot (abdominal and gluteal). Weighted ranks of peak adipocyte size were calculated (PROC RANKS) and plotted to glycerol turnover, DNL, HFF, and total, subcutaneous, and visceral fat mass. Data are expressed as means and SD.
Results
Anthropometric, Clinical, and Metabolic Characteristics
As shown in Table 1, sex, age, Tanner stage, and ethnicity were similar in the two groups. Despite a similar degree of overall adiposity, total fat mass, and BMI, the volume of VAT and intrahepatic fat content (% HFF) were significantly increased in the high VAT/(VAT + SAT) group (P < 0.002), whereas the percent of gynoid fat tended to be lower in the high VAT/(VAT + SAT) group (P < 0.06). Due to the small sample size, the volume of the abdominal superficial SAT was not significantly different between the groups. Fasting and 2-h insulin levels (P < 0.03) were significantly higher and whole-body insulin sensitivity (P < 0.02) was lower in the high VAT/(VAT + SAT) group, indicating greater IR than the group with the low VAT/(VAT + SAT).
Differential Effects of High Versus Low VAT/(VAT + SAT) on Adipocyte Cell Size and Number
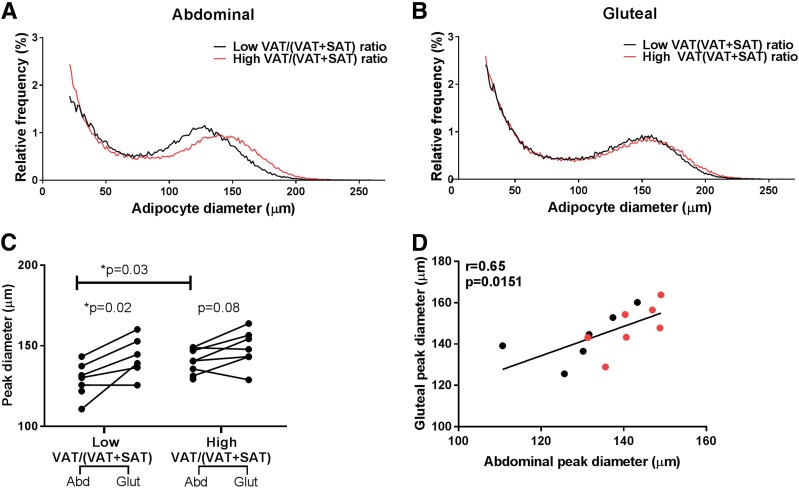
To determine the impact of altered abdominal fat distribution (i.e., high vs. low VAT/[VAT + SAT]) on the morphology of subcutaneous abdominal and gluteal adipocytes, immediately after the biopsy, we used osmium fixation of the cells, examined cell size with the Beckman Coulter Multisizer 3, and performed curve fitting analysis, as previously described (8). As illustrated in Fig. 1A, the abdominal adipocyte cell size curve was distinctly different in the high and low VAT/(VAT + SAT) groups, with a shift to the right in subjects with a high VAT/(VAT + SAT). Indeed, abdominal cell size was significantly greater in the high versus low VAT/(VAT + SAT) (P = 0.03) (Fig. 1C). Conversely, the distribution and shape of the gluteal adipocyte cell size curves were virtually identical in both the low and high VAT/(VAT + SAT) groups (Fig. 1B). Furthermore, paired comparison of each depot cell size parameter (Fig. 1C) shows that the gluteal adipocytes have a larger cell peak than the abdominal adipocytes (weighted mean gluteal cell peak in the low VAT/[VAT + SAT] 143.14 μm vs. abdominal low 128.87 μm, P = 0.02; weighted mean gluteal cell peak high VAT/(VAT + SAT) 148.23 μm vs. abdominal high 140.20 μm, P = 0.08). The cell sizes of abdominal and gluteal SAT were strongly correlated (r = 0.65, P = 0.015) (Fig. 1D). The total number of cells and of small and large cells in the gluteal SAT was higher than in the abdominal SAT depot (Supplementary Fig. 1). The abdominal and gluteal cell numbers were not affected significantly by the abdominal fat distribution, owing to the rather small sample of the groups. However, in the group with the high VAT/(VAT + SAT), the number of large cells tended to be lower in both the abdominal and gluteal depots (Supplementary Fig. 1C). A significant positive correlation was found between the number of large and small cells, particularly in the abdominal SAT depot (r = 0.77, P = 0.0009) (Supplementary Fig. 1D).
Figure 1.
Differences in adipose cell size parameters comparing girls with a low vs. a high VAT/(VAT + SAT) ratio. A: Multisizer abdominal adipose cell profiles using the mean parameters from the curve-fitting formula for subjects with low VAT/(VAT + SAT) ratio (n = 7, black) and subjects with a high VAT/(VAT + SAT) ratio (n = 8, red). B: Multisizer gluteal adipose cell profiles using the mean parameters from the curve-fitting formula for subjects with low VAT/(VAT + SAT) ratio (n = 6, black) and subjects with a high VAT/(VAT + SAT) ratio (n = 7, red). C: Peak diameter for subjects with low VAT/(VAT + SAT) ratio (n = 7) and subjects with a high VAT/(VAT + SAT) ratio (n = 8) for abdominal and gluteal depots. D: Correlation between abdominal and gluteal peak diameter in seven girls with low VAT/(VAT + SAT) ratio (black) and eight girls with high VAT/(VAT + SAT) ratio (red). *Student t test between two groups was significant at the <0.05 level. Abd, abdominal; Glut, gluteal.
Of particular note, the number of gluteal cells and the cell size are significantly greater than the abdominal SAT depot. To some extent, if the gluteal adipocytes can be compared with those from the thigh, our data are consistent with those from Tchoukalova et al. (27), showing that women have a large number of thigh (femoral) adipocytes. However, the size and cell number do not seem to be affected by the abdominal fat distribution. Further, these results raise the possibility that unlike the abdominal adipocytes, the size and number of gluteal adipocytes may not be limited in their capacity to expand.
Increased In Vivo Rates of TG Turnover (Higher Lipolysis and Synthesis Rates) Together With No Differences in DNL Rates in Both Abdominal and Gluteal SAT Depots in Obese Adolescents Girls With a High VAT/(VAT + SAT)
The 2H2O body water enrichment was stable over the course of the 8-week labeling protocol in each group, reaching an average enrichment of 1.57% in the high group and 1.60% in the low group (P = 0.93) (Supplementary Fig. 2). During the 8-week labeling period, subjects in both the high (baseline weight 95.4 ± 20.1 kg; after labeling 96.0 ± 20.1 kg) and low (baseline weight 97.1 ± 20.8 kg; after labeling 96.4 ± 20.6 kg) VAT/(VAT + SAT) groups maintained stable body weight.
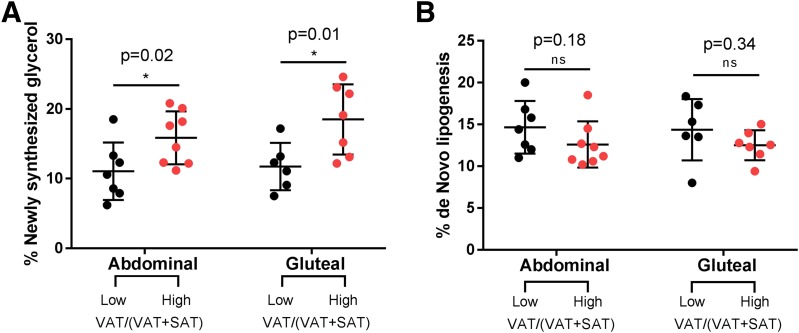
Deuterium incorporation into newly synthesized TG-glycerol was significantly different between the low and high VAT/(VAT + SAT) groups in both depots, with the high VAT/(VAT + SAT) group having higher fractional and absolute turnover rates (higher lipolysis and synthesis rates) in both the abdominal and gluteal depots (Fig. 2A). The absolute rate of TG synthesis being higher, not lower, in the high VAT/(VAT + SAT) group is consistent with the model that higher turnover (that is, reduced capacity of SAT to retain stored TG, not reduced capacity to deposit TG) is related to metabolic impairment (e.g., ectopic fat deposition). The fractional DNL, which represents new fat synthesis in the adipose organ rather than capacity to store fat from diet or liver, was not significantly different by body fat distribution in both the abdominal and gluteal depots (Fig. 2B).
Figure 2.
Differences in lipolysis and DNL comparing girls with a low vs. a high VAT/(VAT + SAT) ratio. A: Percentage of newly synthesized glycerol for subjects with low VAT/(VAT + SAT) ratio and subjects with a high VAT/(VAT + SAT) ratio in abdominal and gluteal depots. B: Percentage of new palmitate in TG for subjects with low VAT/(VAT + SAT) ratio and subjects with a high VAT/(VAT + SAT) ratio in abdominal and gluteal depots. *Student t test between two groups was significant at the <0.05 level.
Higher Turnover of Mature Adipocytes in the Abdominal but Not Gluteal SAT Depot in the High VAT/(VAT + SAT) Group
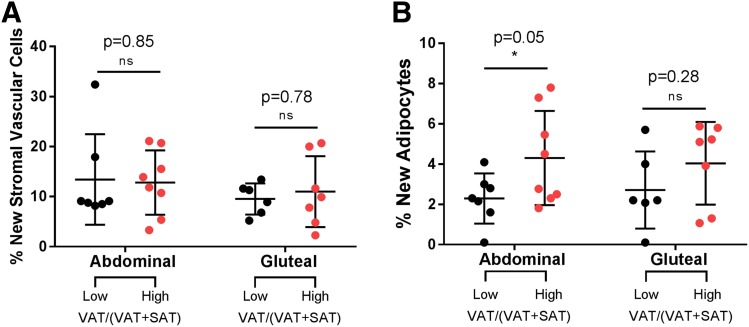
To assess whether adipocyte turnover is different between the two groups, in the two depots, we analyzed in vivo cell kinetics by measuring the incorporation of deuterium into DNA of stromal vascular cells and mature adipocytes. There were no differences in newly divided stromal vascular cells between the low and high VAT/(VAT + SAT) groups, in abdominal or gluteal depot (Fig. 3A). In contrast, the high VAT/(VAT + SAT) group shows a significantly higher turnover of mature adipocytes in the abdominal depot (Fig. 3B).
Figure 3.
Cell turnover in abdominal and gluteal depots for girls with a low VAT/(VAT + SAT) and a high VAT/(VAT + SAT) ratio. A: Percentage of new stromal vascular cells for subjects with low VAT/(VAT + SAT) ratio and subjects with a high VAT/(VAT + SAT) ratio in abdominal and gluteal depots. B: Percentage of new adipocytes for subjects with low VAT/(VAT + SAT) ratio and subjects with a high VAT/(VAT + SAT) ratio in abdominal and gluteal depots. *Student t test between two groups was significant at the <0.05 level.
Increased Turnover of TG (Lipolysis and Synthesis) in Both Abdominal and Gluteal SAT Depots Is Related to Fatty Liver in Obese Girls With the High VAT/(VAT + SAT)
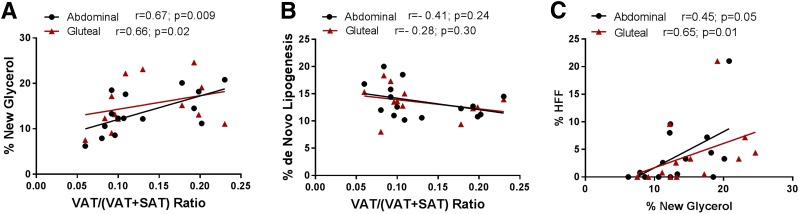
In the current study, the turnover rate (lipolysis and synthesis) of TG positively and strongly correlated with the VAT/(VAT + SAT) in both depots (Fig. 4A). Conversely, the contribution from DNL was inversely related to the VAT/(VAT + SAT) ratio in both depots; however, the correlation failed to achieve significance (Fig. 4B). These marked alterations of fluxes in lipids are seen in both subcutaneous depots in subjects with high VAT/(VAT + SAT), suggesting that inefficient storage of fat is not limited only to the abdominal subcutaneous white depot but extends also to the gluteal subcutaneous depot.
Figure 4.
Correlations between both percentage of new glycerol and DNL and VAT/(VAT + SAT) ratio and between HFF and lipolysis. A: Positive correlation of percentage of new glycerol with VAT/(VAT + SAT) ratio. B: Negative correlation of percentage of new palmitate in TG with VAT/(VAT + SAT) ratio. C: Positive correlation between log HFF and lipolysis (% new glycerol) in the abdominal (black dots) and gluteal (red triangles) depots in obese girls. n = 6 for gluteal with low VAT/(VAT + SAT); n = 8 for abdominal with high VAT/(VAT + SAT); n = 7 for gluteal with high VAT/(VAT + SAT).
To determine whether the increased turnover rates of TG in both the abdominal and gluteal SAT could affect accumulation of fat in the liver, we compared these metrics using Pearson correlation analysis, adjusting for BMI. Significant positive relationships were found between fractional turnover of TG (lipolysis and synthesis rates) and fatty liver (% HFF) in both the abdominal (r = 0.45, P = 0.05) and gluteal depots (r = 0.65, P = 0.01) (Fig. 4C). The association between intrahepatic fat content and fractional turnover of TG in the subcutaneous abdominal and gluteal depots was independent of VAT (P = 0.08 and P = 0.04, respectively).
Discussion
By using the method of metabolic labeling with deuterium into DNA and lipid moieties (17), we quantified for the first time in vivo TG synthetic fluxes and adipocyte turnover in paired biopsies of subcutaneous abdominal and gluteal depots in obese adolescent girls in relation to VAT/(VAT + SAT) ratio. We report several novel findings. 1) In obese adolescent girls with the unfavorable abdominal fat distribution phenotype (high VAT/[VAT + SAT] ratio), in vivo rates of adipose TG turnover (reflecting lipolysis) are higher in both abdominal and gluteal SAT depots than in obese adolescents with a low VAT/(VAT + SAT) ratio (matched for BMI, % total fat mass, and pubertal stage) (Fig. 2A). 2) These alterations of in vivo lipid fluxes, in both abdominal and gluteal depots, are linked to ectopic accumulation of fat in the liver, independent of the degree of abdominal visceral fat (Fig. 4C). 3) Alterations in abdominal body fat distribution did not significantly affect the dynamics in stromal vascular cell proliferation in either depot, but the group with high VAT/(VAT + SAT) ratio manifested a significantly higher adipocyte turnover rate in the abdominal depot (Fig. 3B).
In Vivo Dynamic Flux of TG in Abdominal and Gluteal SAT of Obese Girls
The most significant observation of the current study is the difference in the TG turnover rates between the two groups of similarly obese girls but with distinct abdominal fat distribution. This finding is, in some ways, contrary to the usual formulation of the “limited adipose expandability” hypothesis of ectopic fat deposition (15). The higher new TG-glycerol replacement rate reflects higher turnover of adipose TG, which at steady state, as evidenced by weight stability in these girls, reflects both higher lipolysis rates (shorter half-life of adipose TG stores) and higher synthesis rates. The absolute rate of TG synthesis was higher in the high VAT/(VAT + SAT) group, suggesting that the impaired capacity to retain TG in subcutaneous fat tissue plays a role in ectopic fat deposition, and metabolic abnormality is due to high lipolysis. One might call this model “reduced retention of TG.” However, fractional DNL, which represents additional TG burden on adipose tissue from endogenous synthesis of fatty acids, was not significantly different by body fat distribution in either the abdominal or gluteal depots (Fig. 2B). It is important to recognize a kinetic principle here: at steady-state, turnover rate constant (k) is a function of breakdown rate constant (half-life), not synthesis rate. A higher synthesis rate with no change in half-life will result in a larger pool size with no change in breakdown rate constant (half-life). So a higher turnover rate constant for TG-glycerol strongly implies IR of adipocytes to antilipolytic actions.
Measurements of altered TG synthesis in abdominal SAT by using the in vivo 2H2O water technique have been described by Allister et al. (19) and Tuvdendorj et al. (18) in obese adult males and, similarly, by White et al. (15) in obese adult women with the metabolic syndrome. In the current study, we provide clear evidence that whole-body fat distribution differences are associated with different TG dynamics in SAT of obese adolescent girls. We document here the impact of alteration in body fat distribution on lipolysis in obese girls during adolescence, a critical developmental period when accretion of adipose tissue is at its peak. Furthermore, we found that these altered dynamics in lipid and cell turnover are not merely restricted to the abdominal SAT but are also present in the gluteo/femoral depot in subjects with a central or unfavorable pattern of abdominal fat distribution phenotype. Before puberty, visceral (omental and mesenteric) fat comprises only ∼5% of total body fat, whereas the subcutaneous fat is the major fat compartment, forming more than 90% of total body fat (20–22). At puberty, adipose tissue growth is not only accelerated, but for the first time, there is a major change in body fat distribution greatly affecting the gluteo/femoral depot, particularly in girls. Indeed, striking sex differences arise at puberty when the typical android and gynoid fat distributions appear for the first time (29–32). This redistribution of fat is regulated by sex steroids (32), sex, and ethicity background (33). To reduce potential confounders between sexes on the dynamics of lipid turnover in the adipose tissue, we chose to study girls only, since the body fat distribution differences are more pronounced in girls than what is seen in boys at puberty.
The increased rates of TG lipolysis and synthesis found in both the abdominal and gluteal SAT depots of the obese girls with the high VAT/(VAT +SAT) are in contrast to the reduced rates in leg palmitate release despite the increase in leg fat mass reported in obese women with upper body obesity (34). These differences may be attributed to age differences, different methodology to measure lipid fluxes, differences between integrated around-the-clock fluxes over 8 weeks and fasting measurements, and assessment of body fat distribution (MRI vs. simple waist-to-hip ratio measurements). Our findings in the obese girls with different abdominal fat distribution are in contrast with findings from two more recent studies in adults (18,19) with different degrees of insulin sensitivity, in whom they found a reduced TG synthesis in the abdominal SAT of the adult obese subjects with IR (18,19), but are consistent with the results of White et al. (15) in obese adults. Again we interpret these differences as due to differences in age and perhaps the stage of pubertal-induced IR. In vitro studies (35) aimed at determining whether the effect of insulin to suppress lipolysis is impaired in adipocytes from white upper body obese women showed that both abdominal and gluteal fat cells from the upper body obese versus lower body obese group were less sensitive to the antilipolytic effects of insulin, which would support our findings here of in vivo exaggerated lipolysis in the obese girls with the high VAT/(VAT + SAT) ratio.
We found an increased rate of mature adipocyte turnover in the abdominal SAT of obese girls with the high VAT/(VAT + SAT) ratio. These results are consistent with our findings for triglyceride turnover, showing impaired tissue retention capacity for stored TG (increased lipolysis), not any impairment of TG synthesis. We were unable to detect any significant relationships between body fat distribution and preadipocyte stromal vascular cell proliferation in any of the two depots. Studies performed in obese adult females (15) reported a higher proliferation rate in stromal vascular cells in the subcutaneous gluteo/femoral depot compared with the subcutaneous abdominal one, suggesting that the subcutaneous gluteo/femoral depot may have a higher adipogenic capacity. Although we report overall rates in preadipocyte proliferation similar to those reported by White et al. (15), we did not find a depot difference in preadipocyte turnover, which may be due to the age difference and pubertal stage of development. It should be noted that although the two groups tended to have a significant difference in the percent gynoid fat (see Table 1), the clear “pear shape” was not seen. This may be due to the fact that these girls were in midpuberty, and this phenotype may fully develop later in life.
An important point that should be considered is that our obese girls were in puberty, a physiological state known to decrease insulin sensitivity. Recent comparative studies strongly showed that compared with equally obese adults, obese adolescent youths are remarkably more insulin resistant (36). Likely, the development of obesity during the critical period of puberty, compared with that of adulthood, may have long-lasting effects on white adipose tissue function and distribution that may contribute to the exacerbated metabolic dysfunctions associated with adolescent obesity. It is conceivable that due to the accelerated accretion of the adipose tissue and body fat distribution occurring during puberty, particularly in girls, the additive effect of obesity leads to increases in adipocyte cell number and size, which become hypertrophic and increasingly more insulin resistant as manifested by an augmented lipolytic rate shown in the current study. In contrast, the obesity that develops during adulthood is mainly the result of an augmented adipocyte cell number (hyperplasia) compared with that seen during youth-onset obesity (37). Thus, dysregulation of lipolysis may lead to the excessive FFA flux to the liver, muscle, and pancreas, thereby contributing to ectopic fat accumulation and the metabolic disturbances. Studies by Kim et al. (38) showed that obese youth with normal glucose tolerance have 32–45% higher fasting glycerol rate of appearance compared with what is reported in obese adults. Future studies should evaluate lipid metabolism between phenotypically matched adults and youth along the spectrum of glucose tolerance.
Limitations and Strengths
Our study limitations are due to the cross-sectional nature of the study, the small sample size, lack of more detailed assessment of whole-body insulin sensitivity, and that we have not been able to assess kinetics of lipid and adipocyte turnover in the visceral depot. We also did not include lean adolescent girls because of the high burden of these studies in healthy nonobese girls. In addition, this labeling approach cannot differentiate between newly synthesized fatty acids that originate from the liver and are transported to adipose tissue. Hepatic DNL contribution is, however, a much smaller number then adipose DNL (17), generating 1–2 g of new palmitate per day in liver compared with ∼10 g new palmitate per day in adipose tissue. Moreover, the inference of increased FFA flux to nonadipose tissue based on increased adipose TG lipolysis or turnover assumes that some of the lipolytic-derived FFAs escape the adipocyte and are exposed to tissues such as liver for uptake. The strengths of this study are due to the use of the labeled water to assess in vivo TG and adipocyte kinetics over several weeks in two metabolically divergent subcutaneous depots in obese girls with different patterns of body fat distribution. The detailed and robust measure of assessment of body composition, body fat distribution, and liver fat by MRI imaging is also notable.
In summary, the current findings provide direct, novel, in vivo evidence of altered lipid and cell dynamics in SAT depots of obese adolescent girls that is consistent with and potentially explanatory of the metabolic alterations associated with variations in body fat distribution. We find that higher turnover rates of TG and cells, not lower rates of turnover, are associated with the metabolically adverse fat distribution phenotype in weight-stable obese adolescent girls. SAT (abdominal and gluteal) of girls with high VAT/(VAT + SAT) ratio showed higher lipolytic and synthetic fluxes of stored TG and higher turnover of mature adipocytes. These findings suggest that increased turnover of TGs and mature adipocytes in SAT rather than reduced capacity to deposit TG or proliferate new adipocytes may contribute to the development of fatty liver and related metabolic impairment, even at this very early stage of disease.
Supplementary Material
Article Information
Acknowledgments. The authors thank all of the volunteers and research nurses at the Yale Hospital Research Unit for their skillful help in the study. D.N. is deceased.
Funding. This study was supported by the Robert E. Leet and Clara Guthrie Patterson Trust (Mentored Research Award to J.N.), the American Diabetes Association (DK-49230 and DK-085638 to G.I.S. and Distinguished Clinical Scientist Award to S.C.), the National Institutes of Health (NIH), the National Institute of Child Health and Human Development (R01-HD-28016, R01-HD-40787, and K24-HD-1464 to S.C.), the National Center for Research Resources (a component of the NIH) (Clinical and Translational Science Award UL1-RR-0249139), Bioimage Suite (R01-EB006494), and the Diabetes Research Center (P30-DK-045735).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.N. recruited all subjects, planned weekly visits, processed tissue and cells for 2H2O studies, performed the size measurements, and wrote the manuscript. M.F. and M.H. performed all measures related to the 2H2O studies. M.M. recruited the subjects and performed all OGTTs. N.S and B.G performed the statistical analyses. D.N. and A.V.-M. performed all subcutaneous biopsies. B.P. performed the MRI analysis. G.I.S. provided laboratory space and scientific knowledge with the design of the study, setup of the used methods, and interpretation of the results. S.C. initiated the concept of the study and wrote the manuscript. All authors contributed to the interpretation of the data. S.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db18-1162/-/DC1.
References
- 1.Dietz WH. Critical periods in childhood for the development of obesity. Am J Clin Nutr 1994;59:955–959 [DOI] [PubMed] [Google Scholar]
- 2.Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of obesity and severe obesity in US children, 1999-2016 [published correction appears in Pediatrics 2018;142]. Pediatrics 2018;141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayer-Davis EJ, Dabelea D, Lawrence JM. Incidence trends of type 1 and type 2 diabetes among youths, 2002-2012. N Engl J Med 2017;377:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss R. Fat distribution and storage: how much, where, and how? Eur J Endocrinol 2007;157(Suppl. 1):S39–S45 [DOI] [PubMed] [Google Scholar]
- 5.Weiss R, Dufour S, Taksali SE, et al. Prediabetes in obese youth: a syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet 2003;362:951–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taksali SE, Caprio S, Dziura J, et al. High visceral and low abdominal subcutaneous fat stores in the obese adolescent: a determinant of an adverse metabolic phenotype. Diabetes 2008;57:367–371 [DOI] [PubMed] [Google Scholar]
- 7.Umano GR, Shabanova V, Pierpont P, et al. A low visceral fat proportion, independent of total body fat mass, protects obese adolescent girls against fatty liver and glucose dysregulation: a longitudinal study. Int J Obes (Lond) 2019;43:673–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kursawe R, Eszlinger M, Narayan D, et al. Cellularity and adipogenic profile of the abdominal subcutaneous adipose tissue from obese adolescents: association with insulin resistance and hepatic steatosis. Diabetes 2010;59:2288–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kursawe R, Caprio S, Giannini C, et al. Decreased transcription of ChREBP-α/β isoforms in abdominal subcutaneous adipose tissue of obese adolescents with prediabetes or early type 2 diabetes: associations with insulin resistance and hyperglycemia. Diabetes 2013;62:837–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danforth E., Jr Failure of adipocyte differentiation causes type II diabetes mellitus? Nat Genet 2000;26:13. [DOI] [PubMed] [Google Scholar]
- 11.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest 2000;106:171–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ravussin E, Smith SR. Increased fat intake, impaired fat oxidation, and failure of fat cell proliferation result in ectopic fat storage, insulin resistance, and type 2 diabetes mellitus. Ann N Y Acad Sci 2002;967:363–378 [DOI] [PubMed] [Google Scholar]
- 13.Sethi JK, Vidal-Puig AJ. Wnt signalling at the crossroads of nutritional regulation. Biochem J 2008;416:e11–e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White U, Ravussin E. Dynamics of adipose tissue turnover in human metabolic health and disease. Diabetologia 2019;62:17–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White UA, Fitch MD, Beyl RA, Hellerstein MK, Ravussin E. Differences in in vivo cellular kinetics in abdominal and femoral subcutaneous adipose tissue in women. Diabetes 2016;65:1642–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White UA, Fitch MD, Beyl RA, Hellerstein MK, Ravussin E. Association of in vivo adipose tissue cellular kinetics with markers of metabolic health in humans. J Clin Endocrinol Metab 2017;102:2171–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strawford A, Antelo F, Christiansen M, Hellerstein MK. Adipose tissue triglyceride turnover, de novo lipogenesis, and cell proliferation in humans measured with 2H2O. Am J Physiol Endocrinol Metab 2004;286:E577–E588 [DOI] [PubMed] [Google Scholar]
- 18.Tuvdendorj D, Chandalia M, Batbayar T, et al. Altered subcutaneous abdominal adipose tissue lipid synthesis in obese, insulin-resistant humans. Am J Physiol Endocrinol Metab 2013;305:E999–E1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allister CA, Liu LF, Lamendola CA, et al. In vivo 2H2O administration reveals impaired triglyceride storage in adipose tissue of insulin-resistant humans. J Lipid Res 2015;56:435–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brambilla P, Manzoni P, Sironi S, et al. Peripheral and abdominal adiposity in childhood obesity. Int J Obes Relat Metab Disord 1994;18:795–800 [PubMed] [Google Scholar]
- 21.Huang TT, Johnson MS, Figueroa-Colon R, Dwyer JH, Goran MI. Growth of visceral fat, subcutaneous abdominal fat, and total body fat in children. Obes Res 2001;9:283–289 [DOI] [PubMed] [Google Scholar]
- 22.Siervogel RM, Roche AF, Himes JH, Chumlea WC, McCammon R. Subcutaneous fat distribution in males and females from 1 to 39 years of age. Am J Clin Nutr 1982;36:162–171 [DOI] [PubMed] [Google Scholar]
- 23.Tang A, Tan J, Sun M, et al. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology 2013;267:422–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 25.McLaughlin T, Craig C, Liu LF, et al. Adipose cell size and regional fat deposition as predictors of metabolic response to overfeeding in insulin-resistant and insulin-sensitive humans. Diabetes 2016;65:1245–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jo J, Gavrilova O, Pack S, et al. Hypertrophy and/or hyperplasia: dynamics of adipose tissue growth. PLOS Comput Biol 2009;5:e1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tchoukalova YD, Koutsari C, Karpyak MV, Votruba SB, Wendland E, Jensen MD. Subcutaneous adipocyte size and body fat distribution. Am J Clin Nutr 2008;87:56–63 [DOI] [PubMed] [Google Scholar]
- 28.Turner SM, Murphy EJ, Neese RA, et al. Measurement of TG synthesis and turnover in vivo by 2H2O incorporation into the glycerol moiety and application of MIDA. Am J Physiol Endocrinol Metab 2003;285:E790–E803 [DOI] [PubMed] [Google Scholar]
- 29.Wells JC. Sexual dimorphism of body composition. Best Pract Res Clin Endocrinol Metab 2007;21:415–430 [DOI] [PubMed] [Google Scholar]
- 30.Hattori K, Tahara Y, Moji K, Aoyagi K, Furusawa T. Chart analysis of body composition change among pre- and postadolescent Japanese subjects assessed by underwater weighing method. Int J Obes Relat Metab Disord 2004;28:520–524 [DOI] [PubMed] [Google Scholar]
- 31.Maynard LM, Wisemandle W, Roche AF, Chumlea WC, Guo SS, Siervogel RM. Childhood body composition in relation to body mass index. Pediatrics 2001;107:344–350 [DOI] [PubMed] [Google Scholar]
- 32.Roemmich JN, Rogol AD. Hormonal changes during puberty and their relationship to fat distribution. Am J Hum Biol 1999;11:209–224 [DOI] [PubMed] [Google Scholar]
- 33.Karastergiou K, Smith SR, Greenberg AS, Fried SK. Sex differences in human adipose tissues - the biology of pear shape. Biol Sex Differ 2012;3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin ML, Jensen MD. Effects of body fat distribution on regional lipolysis in obesity. J Clin Invest 1991;88:609–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dowling HJ, Fried SK, Pi-Sunyer FX. Insulin resistance in adipocytes of obese women: effects of body fat distribution and race. Metabolism 1995;44:987–995 [DOI] [PubMed] [Google Scholar]
- 36.RISE Consortium Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: I. Observations using the hyperglycemic clamp. Diabetes Care 2018;41:1696–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holtrup B, Church CD, Berry R, et al. Puberty is an important developmental period for the establishment of adipose tissue mass and metabolic homeostasis. Adipocyte 2017;6:224–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim JY, Nasr A, Tfayli H, Bacha F, Michaliszyn SF, Arslanian S. Increased lipolysis, diminished adipose tissue insulin sensitivity, and impaired β-cell function relative to adipose tissue insulin sensitivity in obese youth with impaired glucose tolerance. Diabetes 2017;66:3085–3090 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.