Abstract
Systemic hyperuricemia (HyUA) in obesity/type 2 diabetes facilitated by elevated activity of xanthine oxidoreductase (XOR), which is the sole source of uric acid (UA) in mammals, has been proposed to contribute to the pathogenesis of insulin resistance/dyslipidemia in obesity. Here, the effects of hepatocyte-specific ablation of Xdh, the gene encoding XOR (HXO), and whole-body pharmacologic inhibition of XOR (febuxostat) on obesity-induced insulin resistance/dyslipidemia were assessed. Deletion of hepatocyte Xdh substantially lowered liver and plasma UA concentration. When exposed to an obesogenic diet, HXO and control floxed (FLX) mice became equally obese, but systemic HyUA was absent in HXO mice. Despite this, obese HXO mice became as insulin resistant and dyslipidemic as obese FLX mice. Similarly, febuxostat dramatically lowered plasma and tissue UA and XOR activity in obese wild-type mice without altering obesity-associated insulin resistance/dyslipidemia. These data demonstrate that hepatocyte XOR activity is a critical determinant of systemic UA homeostasis, that deletion of hepatocyte Xdh is sufficient to prevent systemic HyUA of obesity, and that neither prevention nor correction of HyUA improves insulin resistance/dyslipidemia in obesity. Thus, systemic HyUA, although clearly a biomarker of the metabolic abnormalities of obesity, does not appear to be causative.
Introduction
The long-described phenomenon of hyperuricemia (HyUA) in obesity/type 2 diabetes (T2D) (1–3) has led to speculation that uric acid (UA) plays a mechanistic role in the pathogenesis of the metabolic abnormalities associated with these diseases, specifically insulin resistance and dyslipidemia. UA is derived from the catabolism of purines, and xanthine oxidoreductase (XOR), which is the sole source of UA in mammals, catalyzes the terminal oxidation steps in this biochemical pathway by converting xanthine to UA. Thus, it has been proposed that the inhibition of XOR may correct HyUA in obesity and, in so doing, improve attendant metabolic abnormalities.
Few studies have reported on the metabolic consequences of XOR inhibition or deletion. Pharmacological interventions in rodents that reduce XOR activity (4–9) have produced inconsistent or inconclusive outcomes, based on some combination of the lack of a primary focus on metabolic outcomes (5,9), imprecise or absence of insulin sensitivity assessments (5,6,8,9), data interpretation issues related to body weight (6–8), and the use of a variety of models, including nonobese models (5). Use of genetic mouse models has been confounded by the premature death (before 30 days of age) of global XOR knockouts (10,11). Although heterozygote XOR knockouts develop glucose intolerance, they are more obese then than wild types (WT), and mothers exhibit lactation complications (12). In an effort to overcome these limitations, we have generated a hepatocyte-specific knockout of the XOR gene (Xdh) since the liver represents an abundant site of XOR activity (13) and is a crucial locus of metabolic regulation. To complement this model, we have chronically inhibited systemic XOR activity using febuxostat, a highly specific XOR inhibitor, allowing us to overcome the issues encountered in the genetic models of diminished whole-body XOR activity. Together, these models have allowed us to assess the impact of impaired liver XOR activity on UA homeostasis and the metabolic dysfunction associated with diet-induced obesity in mice.
Research Design and Methods
Animal Care and Maintenance
All mice were housed in the University of Pittsburgh facility under specific pathogen-free conditions with ad libitum access to water and food. C57BL/6J WT mice were obtained from The Jackson Laboratory. All experiments used only male mice and were conducted in compliance with National Institutes of Health guidelines, and all procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Generation of Hepatocyte-Specific Xdh Null Mice
Xdh floxed (FLX) mice were generated by Taconic. Balb/C blastocysts were injected with C57BL/6NTac embryonic stem cells containing a vector targeting the Xdh locus (Fig. 1B) and transferred to pseudopregnant females. First-generation chimeric offspring were bred with C57BL/6 Flp deleter mice to remove the Neo selection gene and generate C57BL/6Ntac mice containing the Xdh FLX allele. For all experiments, mice homozygous for the FLX Xdh allele (Xdhfloxed/floxed) were bred with Xdhfloxed/floxed mice heterozygous for Alb-1cre (B6.FVB(129)-Tg (alb1-cre)1Dlr/J; The Jackson Laboratory) to generate Xdhfloxed/floxedAlb-1Cre/Wt (hepatocyte-specific Xdh knockout [HXO]) and Xdhfloxed/floxedAlb-1Wt/Wt (littermate FLX controls). Genotyping was confirmed with PCR. For Xdh FLX, primers flanking the loxP sites in intron 1 (5′-GTATGGTCTGTAGTATGTCCACTGC-3′ and 5′-CCTTTCAAGACACGCATTCC-3′) and intron 2 (5′-TTGGGTGATCCTAGGCTCC-3′ and 5′-CTTCTTCTGGTCTCTCTGGACC-3′) were used. For Alb-1-cre, primers flanking the Cre transgene (5′-CCAGGCTAAGTGCCTTCTCTACA-3′ and 5′-AATGCTTCTGTCCGTTTGCCGGT-3′) were used.
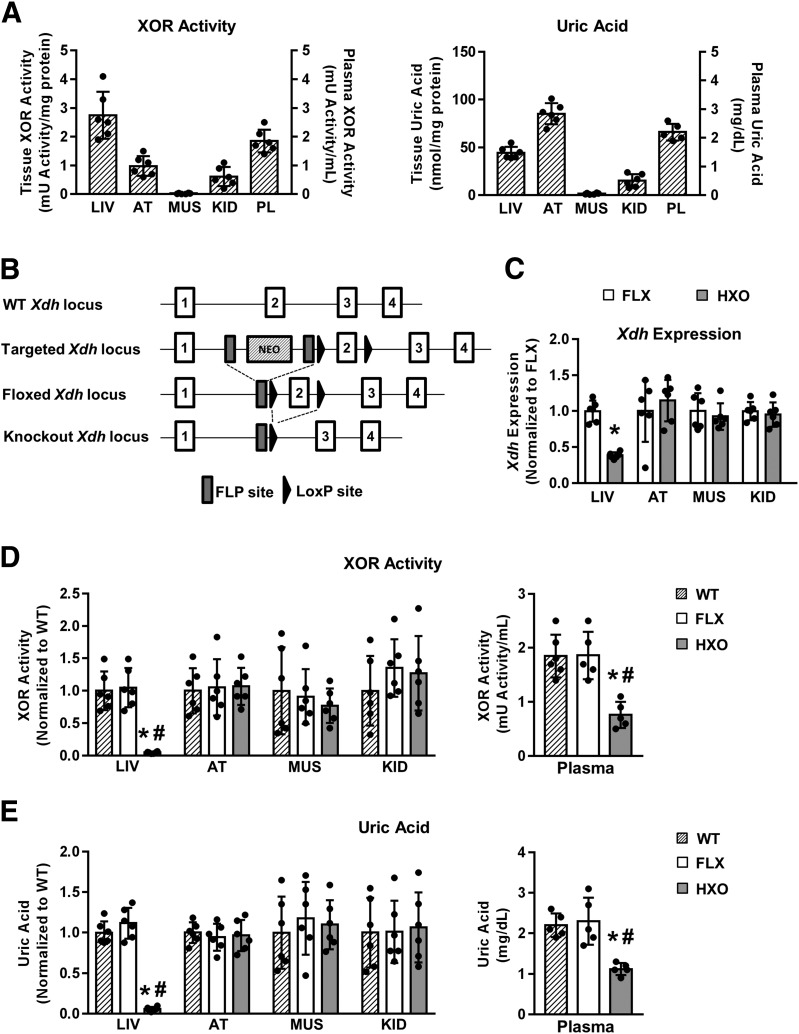
Figure 1.
Deletion of hepatocyte Xdh reduces liver and plasma XOR and UA. A: Absolute XOR activity (left panel) and UA concentration (right panel) in WT mice (n = 5–6). Left y-axis indicates liver (LIV), adipose tissue (AT), muscle (MUS), and kidney (KID) values; right y-axis indicates plasma (PL) values. B: Generation of HXO mouse. C: Relative tissue Xdh expression in FLX and HXO mice (n = 6). D: Relative tissue XOR activity (left panel) and absolute plasma XOR activity (right panel) in WT, FLX, and HXO mice (n = 5–6). E: Relative tissue UA concentration (left panel) and absolute plasma UA concentration (right panel) in WT, FLX, and HXO mice (n = 5–6). #P < 0.05 compared with WT; *P < 0.05 compared with FLX.
Diet-Induced Obesity Studies
For HXO obesity studies, mice were fed either a 41% fat diet (96001; Teklad) or a low-fat control diet (110340; Teklad) for 26 weeks. For febuxostat studies, obesity was induced in WT mice through high-fat (60% kcal from fat, D12492; Research Diets) feeding for 13 weeks. Mice were then continued on the same diet and treated with febuxostat (Axon Medchem) (50 mg/L in drinking water; ∼5–6 mg/kg per day) or vehicle (standard drinking water) for seven additional weeks (weeks 14–20). The 41% fat diet was selected for the study of progressive obesity to allow for the discrimination of subtle metabolic differences between the mouse models, whereas the 60% fat diet was selected for the reversal study where a robust starting metabolic phenotype was the prime consideration in the experimental design.
Biochemical Analysis
Quantitative RT-PCR was performed as described previously (14) using Xdh-specific primers spanning two exons (5′-CCGCCTTCAGAACAAGATCG-3′ and 5′-CCTTCCACAGTTGTCACAGC-3′). XOR activity (1 unit activity = 1 μmol UA/min) was assessed by electrochemical detection (ESA Coul-Array System) of UA generation using reverse-phase high-performance liquid chromatography (see Supplementary Data for detailed methodology). Xanthine, hypoxanthine, and inosine were measured by gas chromatography–mass spectrometry, and adenosine was measured by liquid chromatography–tandem mass spectrometry (15). Glutathione (GSH)/glutathione disulfide (GSSG) ratios were determined using the GSSG/GSH Quantification Kit (Dojindo). NADPH oxidase activity was assessed by O2•− production in the presence of NADPH (16). Total (free + esterified) 8-iso-PGF2α and PGF2α quantitation and subsequent calculations were performed as we described previously (17). Plasma free fatty acids (FFA) were quantified using a colorimetric kit (Wako Diagnostics). Liver and plasma triglycerides were assessed as previously described (18). Plasma insulin was determined using a chemiluminescence ELISA (ALPCO).
Metabolic Analysis
For glucose tolerance tests (GTTs), 5 h–fasted mice were injected i.p. with either 1.0 g/kg glucose (early GTT) or 0.75 g/kg glucose (late GTT). Blood glucose was assessed every 15 min for 2 h postinjection. Lean and fat mass were analyzed using nuclear magnetic resonance (EchoMRI). VO2, heat, and activity were assessed with a Comprehensive Lab Animal Monitoring System (CLAMS; Columbus Instruments). Euglycemic clamps were performed as previously described (19), with minor modifications. Mice received jugular vein catheters 1 week prior to study. Basal glucose turnover was measured after a 6-h morning fast using a [3-3H]glucose infusion (0.05 μCi/min; 120 min). Next, a primed/continuous infusion of insulin (11 mU ⋅ kg−1 ⋅ min−1; 4.5 mU ⋅ kg−1 ⋅ min−1) and [3-3H]glucose (0.1 μCi/min) and variable infusion of 20% dextrose was given to evaluate insulin sensitivity and rates of glucose turnover. Glucose turnover was measured over the last 40 min of the 120-min clamp.
Statistical Analysis
Data are expressed as mean ± SD. Statistical significance was determined by Student t test or one-way ANOVA, with the Tukey multiple comparisons post hoc test used where appropriate. P < 0.05 was considered significant. Statistical analysis was performed using GraphPad Prism (GraphPad) and SPSS (IBM).
Results
Hepatocyte-Specific Xdh Ablation Decreases Liver and Plasma XOR and UA
Baseline XOR activity and UA concentration were assessed in liver, adipose tissue, muscle, kidney, and plasma of 6- to 8-week-old WT mice. The liver demonstrated the highest XOR activity and relatively high UA concentration when compared with the other tissues examined (Fig. 1A). Hepatocyte Xdh ablation (HXO) (Fig. 1B) resulted in a significant reduction (∼60%) in hepatic Xdh expression compared with unrecombined floxed FLX littermate controls, whereas Xdh expression in other tissues was unaltered (Fig. 1C). This reduction in Xdh expression in HXO mice led to a >95% diminution of XOR activity in the liver with a corresponding ∼60% reduction in plasma XOR activity compared with WT and FLX littermate controls (Fig. 1D). Importantly, similar effects were observed with UA whereby the liver (∼95%) and plasma (∼50%) demonstrated significant reductions (Fig. 1E). XOR activity and UA concentration were not altered in adipose tissue, skeletal muscle, or kidney in HXO mice. Together, these data identify hepatocyte XOR expression as necessary for UA accumulation in the liver and demonstrate that hepatocyte XOR contributes substantially to blood XOR activity and UA homeostasis.
Deletion of Hepatocyte Xdh Prevents HyUA, Alters Hepatic Purine Metabolism, and Has No Effect on Oxidative Stress in Obesity
Obesity is associated with substantial increases in blood UA and XOR activity (1,2). To address the effects of hepatocyte deletion of Xdh on this phenotype, HXO and FLX mice were exposed to an obesogenic diet for 26 weeks. Tissue and plasma XOR activity and UA concentration, liver purine metabolites, and liver oxidative stress status were then assessed. As expected, obesity resulted in significant increases in plasma XOR activity and UA concentration in FLX mice, with variable effects in other tissues examined (Fig. 2A and B). Notably, the systemic effects of obesity on XOR and UA were absent in HXO mice. Indeed, plasma XOR activity in obese HXO was below that of lean WT mice, whereas obese HXO plasma UA concentration was similar to the concentration observed in lean WT (Fig. 2A and B). Overall, this equated to 7.5-fold and 2.5-fold reductions in plasma XOR activity and UA concentration, respectively, in obese HXO compared with obese FLX (Fig. 2A and B).
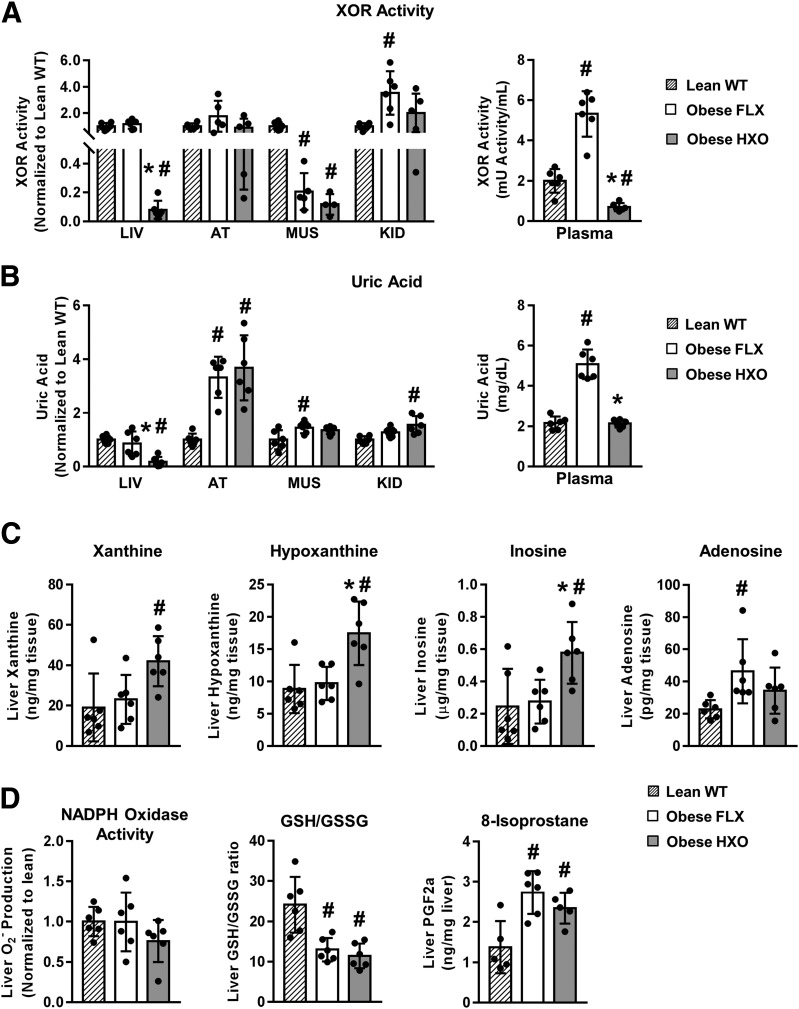
Figure 2.
Deletion of hepatocyte Xdh prevents HyUA, alters hepatic purine metabolism, and has no effect on oxidative stress in obesity. Obesity was induced in HXO and FLX control mice by high-fat (41% kcal from fat) feeding for 26 weeks. Lean, age-matched WT mice were included as controls. A: Relative tissue XOR activity (left panel) and absolute plasma XOR activity (right panel) (n = 4–6). B: Relative tissue UA concentration (left panel) and absolute plasma UA concentration (right panel) (n = 4–6). C: Purine catabolites in liver (n = 6). D: Markers of oxidative stress in liver. AT, adipose tissue; KID, kidney; LIV, liver; MUS, muscle; PGF2a, prostaglandin F2-α. #P < 0.05 compared with WT lean; *P < 0.05 compared with obese FLX.
Since deletion of hepatocyte XOR introduces a biochemical block to the purine catabolic pathway, and XOR activity can have pro-oxidant consequences, we also assessed the metabolites of the purine pathway and indices of oxidant stress in the liver. Obesity per se had no effect on liver xanthine, hypoxanthine, or inosine, because their levels were similar in lean WT and obese FLX (Fig. 2C). However, adenosine was increased in obese FLX compared with lean WT (Fig. 2C). Livers of obese HXO mice demonstrated elevated xanthine, hypoxanthine, and inosine but no alteration in adenosine compared with obese FLX (Fig. 2C). The effects of hepatocyte Xdh deletion on indices of liver oxidant load in obesity were assessed next. In short, although obesity per se had a varying impact on three oxidative stress readouts (NAPDH oxidase activity, the GSH/GSSG ratio, and total 8-isoprostanes), the effects were similar in FLX and HXO mice (Fig. 2D). Collectively, these findings demonstrate that in obesity, deletion of hepatocyte Xdh prevents elevation in plasma XOR activity and UA concentration and leads to buildup of purine metabolites in the liver but does not impact hepatic oxidative stress.
Obese HXO Mice Are Not Protected Against Metabolic Dysfunction
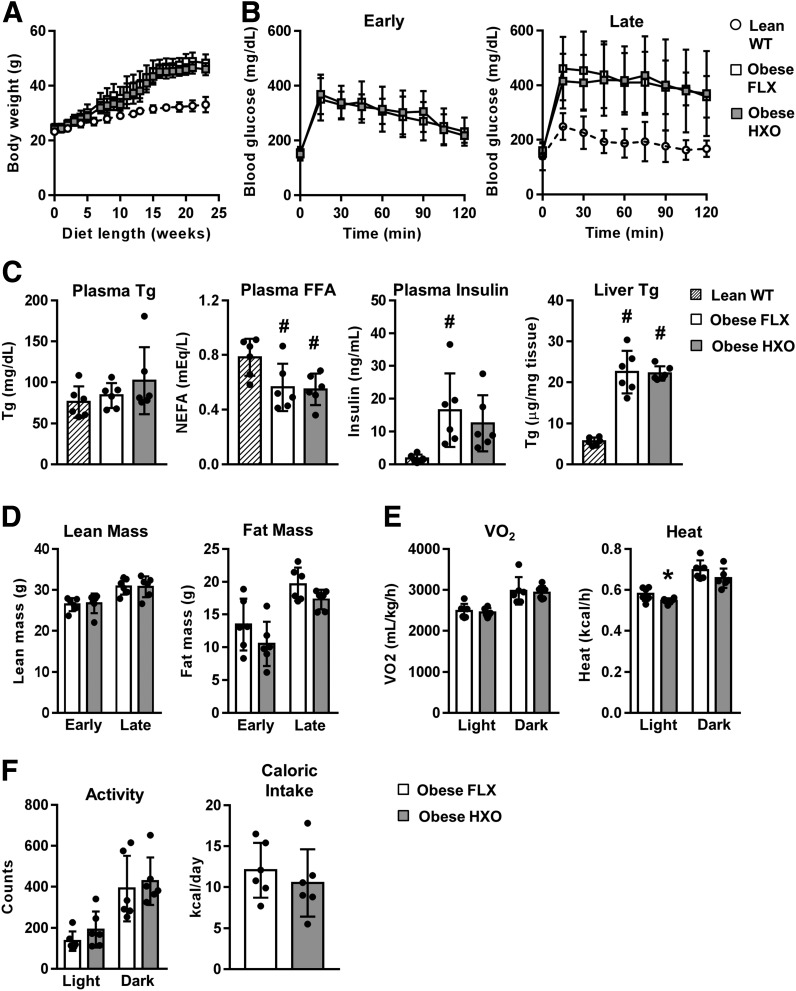
Additional obesity studies were performed in HXO and FLX mice to test whether depleted UA through loss of hepatocyte Xdh expression protects against obesity-associated metabolic dysfunction. During the course of the study, HXO mice showed no deviation from FLX controls in body weight gain (Fig. 3A) or glucose tolerance (Fig. 3B). Blood lipids, insulin, and liver triglycerides were also similar (Fig. 3C), as were lean and fat mass (Fig. 3D). Although metabolic cage assessment revealed a modest decrease in heat production during the light cycle, no differences in oxygen consumption, activity, or caloric intake were observed (Fig. 3E and F). These findings demonstrate that deletion of hepatocyte Xdh has no effect on metabolic readouts that are altered in obesity.
Figure 3.
Obese HXO mice are not protected against metabolic dysfunction. Obesity was induced in HXO and FLX control mice through high-fat (41% kcal from fat) feeding for 26 weeks (n = 6 for all groups). Where indicated, age-matched lean WT mice were included as controls (n = 6 for all groups). A: Body weight progression. B: GTT during early (8–12 weeks of diet) and late (18–21 weeks of diet) stages of obesity. C: Plasma triglyceride (Tg), FFA, and insulin, and liver Tg at euthanasia. D: Lean and fat mass at early and late stages of obesity. E: VO2 and heat production at late stages of obesity. F: Activity and caloric intake at late stages of obesity. NEFA, nonesterified fatty acid. #P < 0.05 compared with WT lean; *P < 0.05 compared with obese FLX.
Pharmacologic Inhibition of XOR Activity Reverses HyUA in Obesity but Does Not Impact Insulin Sensitivity or Lipid Homeostasis
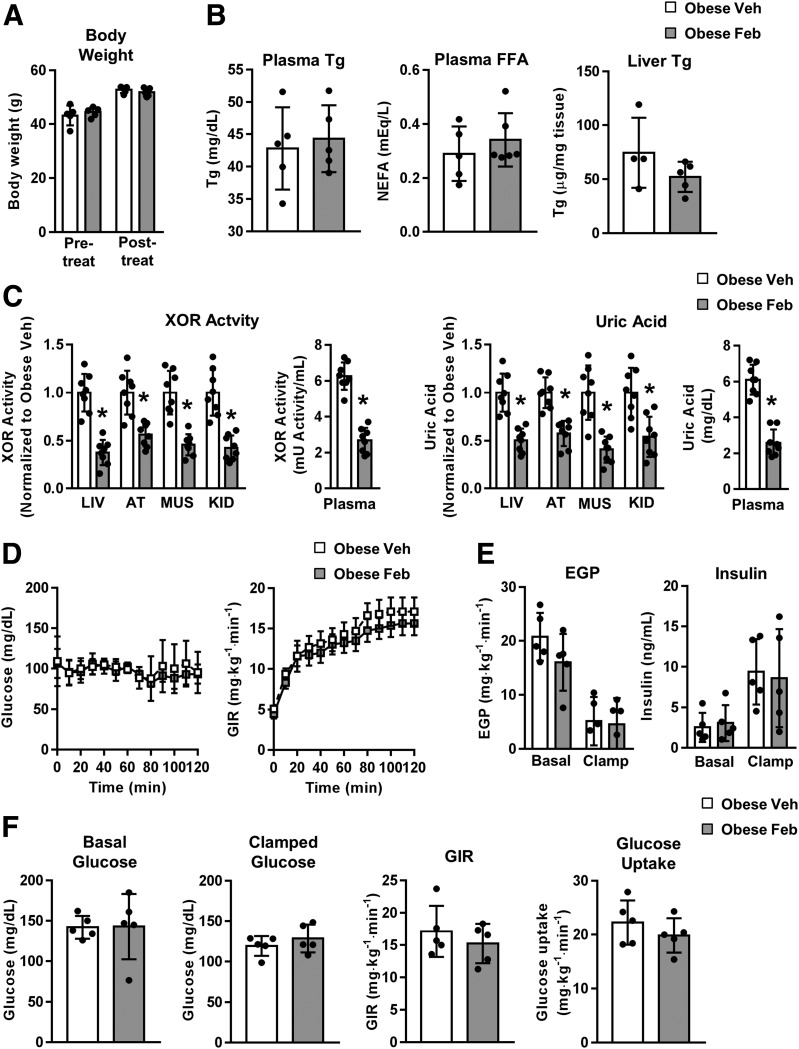
As XOR is also produced by tissues other than liver, we sought to test whether pharmacologic inhibition of XOR improves the metabolic state in the context of obesity. Once obesity was established in WT mice by high-fat feeding, mice were continued on the diet and treated with either the XOR inhibitor febuxostat or vehicle for 7 weeks. Febuxostat had no impact on body weight (Fig. 4A), blood lipids (Fig. 4B), or liver triglycerides (Fig. 4B). As expected, febuxostat reduced XOR activity and UA concentration in all tissues analyzed (Fig. 4C). Importantly, blood XOR activity and UA concentration were elevated in obesity, and febuxostat treatment reduced both to levels comparable to those observed in lean WT mice (compare Fig. 4C with Fig. 2A and B). However, insulin sensitivity as assessed by euglycemic clamp was not affected (Fig. 4D–F). These findings are consistent with results from the HXO mouse and further demonstrate that inhibition of XOR activity depletes UA but is insufficient to improve metabolic dysfunction associated with obesity.
Figure 4.
Pharmacologic inhibition of XOR activity reverses HyUA in obesity but does not impact insulin sensitivity or lipid homeostasis. Obesity was induced in WT mice through high-fat (60% kcal from fat) feeding for 13 weeks. Mice were then continued on the same diet and treated with febuxostat (50 mg/L in drinking water) or vehicle (standard drinking water) for seven additional weeks (weeks 14–20). A: Body weight pre- and post-treatment (n = 5). B: Plasma triglyceride (Tg) and FFA, and liver Tg at euthanasia (n = 4–5). C: Relative tissue and absolute plasma XOR activity (left panels); relative tissue and absolute plasma UA concentration (right panels) (n = 8). D–F: Data from euglycemic clamp studies (n = 5 all groups). D: Blood glucose and glucose infusion rate (GIR) time course. E: Endogenous glucose production (EGP) and plasma insulin. F: Basal and clamped glucose, GIR, and glucose uptake. AT, adipose tissue; Feb, febuxostat; KID, kidney; LIV, liver; MUS, muscle; NEFA, nonesterified fatty acid; Veh, vehicle. *P < 0.05 compared with obese vehicle.
Discussion
The current study was undertaken to determine the effects of preventing or decreasing HyUA on the metabolic abnormalities of obesity, a question of substantial clinical relevance that to our knowledge has not been sufficiently addressed. To accomplish our goal, a combined genetic (hepatocyte-specific ablation) and pharmacologic approach (febuxostat) was used in mice. A number of novel observations were made, leading to the central conclusion that plasma HyUA, although being positively correlated with obesity and the allied metabolic dysregulation, does not appear to be causative. Thus, the data demonstrate that hepatocyte Xdh expression is a critical determinant of systemic XOR and UA homeostasis and that deletion of hepatocyte Xdh is sufficient to prevent the systemic HyUA of obesity, but that neither prevention nor correction of HyUA improves insulin resistance/dyslipidemia in obesity.
Although liver XOR has been implicated in the determination of systemic XOR activity and UA homeostasis, it has not been possible to address this hypothesis to date because of the lack of appropriate models. Our findings that there are substantial reductions in plasma XOR activity and UA concentration in both lean and obese HXO mice demonstrate categorically an important role for hepatocyte Xdh expression in the regulation of not only systemic XOR activity but also systemic UA homeostasis. One particularly noteworthy aspect of these conclusions is that other tissues with relatively robust levels of XOR activity, specifically adipose tissue and kidneys, are unable to fully compensate for the loss of hepatocyte-derived XOR in respect of systemic XOR and UA homeostasis. Indeed, in the case of UA, adipose tissue has a substantially higher concentration compared with liver in lean mice, which becomes even more pronounced in the context of obesity. That these increases do not alter systemic UA raises questions of potential differences in the cell biology of liver and adipocyte UA and XOR homeostasis.
Data from our study are the first to categorically demonstrate that prevention or correction of HyUA in the context of diet-induced obesity, where body weight is well controlled, does not improve insulin sensitivity and indices of dyslipidemia. Many studies in humans performed over decades have identified the association of HyUA with BMI, waist circumference, hyperlipidemia, insulin resistance, the metabolic syndrome, and the development of T2D, but causative evidence has been lacking (20,21). Meta-analysis of multiple clinical studies shows a 17% risk increase in developing T2D with each 1 mg/dL increase in UA; however, each 1 mg/dL elevation also associates with a 1 kg/m2 increase in BMI (21), suggesting that obesity may be the variable explaining this increased risk. Interestingly, Mendelian randomization studies that focused on genetic variants associated with UA, thereby removing confounding factors such as obesity, showed that elevated UA could not independently predict T2D development (22,23). Although a number of studies in rodents have reported metabolic variables after genetic or pharmacological interventions to reduce XOR activity (4–12), the data are inconsistent or inconclusive, based on a combination of factors. Genetic models of Xdh deletion suffer from issues of premature death (homozygous null), obesity (heterozygous null), and lactation impairments influencing pup nutrition (heterozygous null), making them unsuitable for precisely addressing the causal relationship between UA and metabolic dysregulation in obesity. Of the pharmacologic intervention studies, some were not focused on metabolic outcomes (5,9), the measures of insulin sensitivity were rudimentary (fasting insulin and HOMA of insulin resistance) or absent (5,6,8,9), the important variable of body weight was not well controlled (6–8), or the model was unsuitable (5). Specifically, Xu et al. (8) showed that allopurinol treatment reduced hepatic triglyceride levels in high-fat diet–fed mice, but this study did not provide any measure of insulin sensitivity. Also, body weights, which are an independent determinant of the degree of steatosis and insulin sensitivity, were not reported. Sánchez-Lozada et al. (6) and Nakagawa et al. (7), using high-fructose diet rat models, evaluated the metabolic effects of inhibition of XOR by allopurinol and febuxostat, respectively. Both studies report that inhibiting XOR reduced circulating triglycerides and fasting insulin, but Nakagawa et al. (7) found no differences in glucose tolerance. Importantly, these two studies reported lower weights in rats receiving the XOR inhibitor, adding a confounding variable to the interpretation of the data. A study from Nakatsu et al. (5) focused on mouse models of NASH, but does contain a single HOMA-IR that supports our findings that XOR inhibition has no effect on insulin sensitivity. They also report that liver triglycerides are decreased after XOR inhibition, which contrasts with our findings. Also, in a relevant model (rats fed a diet high in fat and fructose) where body weight was well controlled, El-Bassossy and Shaltout (9) showed that allopurinol had no effect on blood insulin or glucose levels. However, Baldwin et al. (4) reported improved insulin sensitivity (as assessed by insulin tolerance test) in allopurinol-treated pound mice, a genetic model of obesity. In short, studies using febuxostat (or allopurinol) have yielded inconsistent results and/or results that are open to more than one interpretation based on confounding factors. Furthermore, dynamic measurement of insulin sensitivity (insulin tolerance test or GTT) was used in only two studies (4,7). Our study is the first to use a precisely targeted genetic intervention (the HXO mouse) to lower plasma UA in obesity to the concentration found in lean mice and the gold standard technique for the measurement of insulin sensitivity (euglycemic clamps in the febuxostat studies) to assess the role of HyUA in insulin resistance and dyslipidemia.
In summary, we have developed the first hepatocyte-specific deletion of Xdh and demonstrate that hepatocyte Xdh expression is an important determinant of liver and systemic UA homeostasis, as well as plasma XOR activity. Additionally, we show through both genetic and pharmacologic inhibition that although hepatocyte XOR activity is required for HyUA allied to obesity, reduction of hepatic and plasma UA alone is insufficient to improve insulin sensitivity or dyslipidemia. These findings confirm HyUA as a biomarker of obesity but indicate that plasma UA does not directly influence metabolic homeostasis.
Supplementary Material
Article Information
Funding. This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK102839 and T32 DK007052 to R.M.O. and R01 DK114012 to M.J.J.), the National Institute on Aging (P01 AG043376-02S1 to E.E.K.), the National Institute of General Medical Sciences (P20 GM109098 to E.E.K.), the National Institute on Alcohol Abuse and Alcoholism (R37 AA010422 to G.E.H.), the National Eye Institute (R01 EY026030 to J.D.), and the National Heart, Blood, and Lung Institute (R01HL079207 and P01HL103455-01 to P.J.P.). D.B.H. was supported by T32 DK007052.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. D.B.H. and E.E.K. contributed to the study concept and design; the acquisition, analysis, and interpretation of data; statistical analysis; and the writing of the manuscript. W.K.M., I.J.S., N.D., S.E.L., J.T.E., J.D., Y.W., B.R.H., P.J.P., E.C.-P., G.E.H., and T.J.V.E. contributed to the acquisition, analysis, and interpretation of data. M.S.-R. contributed to the study concept and design and interpretation of data. M.J.J. contributed to the study concept and design; the acquisition, analysis, and interpretation of data; and statistical analysis. R.M.O. contributed to the study concept and design, the analysis and interpretation of data, and the writing of the manuscript. R.M.O. and E.E.K. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in oral form at the Society for Redox Biology and Medicine's 25th Annual Conference, Chicago, IL, 14–17 November 2018.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db18-1198/-/DC1.
References
- 1.Battelli MG, Bortolotti M, Polito L, Bolognesi A. The role of xanthine oxidoreductase and uric acid in metabolic syndrome. Biochim Biophys Acta Mol Basis Dis 2018;1864:2557–2565 [DOI] [PubMed] [Google Scholar]
- 2.Soltani Z, Rasheed K, Kapusta DR, Reisin E. Potential role of uric acid in metabolic syndrome, hypertension, kidney injury, and cardiovascular diseases: is it time for reappraisal? Curr Hypertens Rep 2013;15:175–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson RJ, Nakagawa T, Sanchez-Lozada LG, et al. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes 2013;62:3307–3315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldwin W, McRae S, Marek G, et al. Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome. Diabetes 2011;60:1258–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakatsu Y, Seno Y, Kushiyama A, et al. The xanthine oxidase inhibitor febuxostat suppresses development of nonalcoholic steatohepatitis in a rodent model. Am J Physiol Gastrointest Liver Physiol 2015;309:G42–G51 [DOI] [PubMed] [Google Scholar]
- 6.Sánchez-Lozada LG, Tapia E, Bautista-García P, et al. Effects of febuxostat on metabolic and renal alterations in rats with fructose-induced metabolic syndrome. Am J Physiol Renal Physiol 2008;294:F710–F718 [DOI] [PubMed] [Google Scholar]
- 7.Nakagawa T, Hu H, Zharikov S, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol 2006;290:F625–F631 [DOI] [PubMed] [Google Scholar]
- 8.Xu C, Wan X, Xu L, et al. Xanthine oxidase in non-alcoholic fatty liver disease and hyperuricemia: one stone hits two birds. J Hepatol 2015;62:1412–1419 [DOI] [PubMed] [Google Scholar]
- 9.El-Bassossy HM, Shaltout HA. Allopurinol alleviates hypertension and proteinuria in high fructose, high salt and high fat induced model of metabolic syndrome. Transl Res 2015;165:621–630 [DOI] [PubMed] [Google Scholar]
- 10.Ohtsubo T, Matsumura K, Sakagami K, et al. Xanthine oxidoreductase depletion induces renal interstitial fibrosis through aberrant lipid and purine accumulation in renal tubules. Hypertension 2009;54:868–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung KJ, Tzameli I, Pissios P, et al. Xanthine oxidoreductase is a regulator of adipogenesis and PPARgamma activity. Cell Metab 2007;5:115–128 [DOI] [PubMed] [Google Scholar]
- 12.Murakami N, Ohtsubo T, Kansui Y, et al. Mice heterozygous for the xanthine oxidoreductase gene facilitate lipid accumulation in adipocytes. Arterioscler Thromb Vasc Biol 2014;34:44–51 [DOI] [PubMed] [Google Scholar]
- 13.Parks DA, Granger DN. Xanthine oxidase: biochemistry, distribution and physiology. Acta Physiol Scand Suppl 1986;548:87–99 [PubMed] [Google Scholar]
- 14.Mantell BS, Stefanovic-Racic M, Yang X, Dedousis N, Sipula IJ, O’Doherty RM. Mice lacking NKT cells but with a complete complement of CD8+ T-cells are not protected against the metabolic abnormalities of diet-induced obesity. PLoS One 2011;6:e19831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chao JR, Knight K, Engel AL, et al. Human retinal pigment epithelial cells prefer proline as a nutrient and transport metabolic intermediates to the retinal side. J Biol Chem 2017;292:12895–12905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson AR, Yousefzadeh MJ, Rozgaja TA, et al. Spontaneous DNA damage to the nuclear genome promotes senescence, redox imbalance and aging. Redox Biol 2018;17:259–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van’t Erve TJ, Lih FB, Jelsema C, et al. Reinterpreting the best biomarker of oxidative stress: the 8-iso-prostaglandin F2α/prostaglandin F2α ratio shows complex origins of lipid peroxidation biomarkers in animal models. Free Radic Biol Med 2016;95:65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stefanovic-Racic M, Perdomo G, Mantell BS, Sipula IJ, Brown NF, O’Doherty RM. A moderate increase in carnitine palmitoyltransferase 1a activity is sufficient to substantially reduce hepatic triglyceride levels. Am J Physiol Endocrinol Metab 2008;294:E969–E977 [DOI] [PubMed] [Google Scholar]
- 19.Jurczak MJ, Lee AH, Jornayvaz FR, et al. Dissociation of inositol-requiring enzyme (IRE1α)-mediated c-Jun N-terminal kinase activation from hepatic insulin resistance in conditional X-box-binding protein-1 (XBP1) knock-out mice. J Biol Chem 2012;287:2558–2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonçalves JP, Oliveira A, Severo M, Santos AC, Lopes C. Cross-sectional and longitudinal associations between serum uric acid and metabolic syndrome. Endocrine 2012;41:450–457 [DOI] [PubMed] [Google Scholar]
- 21.Kodama S, Saito K, Yachi Y, et al. Association between serum uric acid and development of type 2 diabetes. Diabetes Care 2009;32:1737–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sluijs I, Holmes MV, van der Schouw YT, et al.; InterAct Consortium . A Mendelian randomization study of circulating uric acid and type 2 diabetes. Diabetes 2015;64:3028–3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfister R, Barnes D, Luben R, et al. No evidence for a causal link between uric acid and type 2 diabetes: a Mendelian randomisation approach. Diabetologia 2011;54:2561–2569 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.