Abstract
A three-arm, randomized, double-masked, placebo-controlled phase 2b trial performed by the Type 1 Diabetes TrialNet Study Group previously demonstrated that low-dose anti-thymocyte globulin (ATG) (2.5 mg/kg) preserved β-cell function and reduced HbA1c for 1 year in new-onset type 1 diabetes. Subjects (N = 89) were randomized to 1) ATG and pegylated granulocyte colony-stimulating factor (GCSF), 2) ATG alone, or 3) placebo. Herein, we report 2-year area under the curve (AUC) C-peptide and HbA1c, prespecified secondary end points, and potential immunologic correlates. The 2-year mean mixed-meal tolerance test–stimulated AUC C-peptide, analyzed by ANCOVA adjusting for baseline C-peptide, age, and sex (n = 82) with significance defined as one-sided P < 0.025, was significantly higher in subjects treated with ATG versus placebo (P = 0.00005) but not ATG/GCSF versus placebo (P = 0.032). HbA1c was significantly reduced at 2 years in subjects treated with ATG (P = 0.011) and ATG/GCSF (P = 0.022) versus placebo. Flow cytometry analyses demonstrated reduced circulating CD4:CD8 ratio, increased regulatory T-cell:conventional CD4 T-cell ratios, and increased PD-1+CD4+ T cells following low-dose ATG and ATG/GCSF. Low-dose ATG partially preserved β-cell function and reduced HbA1c 2 years after therapy in new-onset type 1 diabetes. Future studies should determine whether low-dose ATG might prevent or delay the onset of type 1 diabetes.
Introduction
Type 1 diabetes is characterized by autoimmune β-cell destruction and a lifelong dependence on exogenous insulin (1). As such, most efforts seeking to prevent or reverse the disease have used immunosuppressive or immunomodulatory drugs (2–8). Given the limited extended capacity of monotherapies to interdict the natural history of type 1 diabetes, we and others have long proposed combination therapy as a strategy toward this purpose (9–11).
To determine safe and effective therapeutic combinations, preclinical studies were performed using the NOD mouse model. The combination of low-dose murine anti-thymocyte globulin (ATG) plus granulocyte colony-stimulating factor (GCSF) demonstrated synergy and significant reversal of diabetes in NOD mice (12), with the premise of the ATG/GCSF synergy being that ATG depletes pathogenic T cells while GCSF promotes regulatory T cells (Tregs) (13,14). A subsequent pilot, randomized, placebo-controlled, single-masked clinical trial of low-dose ATG (2.5 mg/kg) and pegylated GCSF (6 mg subcutaneously every 2 weeks × six doses) in humans with established type 1 diabetes (duration 4–24 months) suggested that low-dose ATG/GCSF preserved C-peptide (15,16). Notably, higher doses of ATG (6.5 mg/kg) monotherapy failed to preserve C-peptide in new-onset type 1 diabetes (17,18). Flow cytometry of cells obtained from subjects who received low-dose ATG/GCSF or higher-dose ATG showed that although both approaches lowered the absolute numbers of Tregs, low-dose ATG/GCSF increased the proportion of Tregs to conventional CD4+ T cells (Tconvs) while higher-dose ATG decreased Tregs proportionally (16–18). As such, questions remained regarding the relative contributions of GCSF and low-dose ATG and the potential impact of combination treatment earlier in disease.
To explore the potential of low-dose ATG/GCSF and low-dose ATG monotherapy to preserve β-cell function in new-onset type 1 diabetes, the National Institutes of Health Type 1 Diabetes TrialNet Study Group (TrialNet) conducted a three-arm randomized, double-masked, placebo-controlled trial (low-dose ATG/GCSF, low-dose ATG, and placebo) in subjects with new-onset type 1 diabetes (duration <100 days). At the year 1 primary end point, we reported that low-dose ATG significantly preserved C-peptide area under the curve (AUC) following a mixed-meal tolerance test (MMTT) compared with the control group (P = 0.0003) (19). Notably, C-peptide AUC was not significantly preserved in patients treated with low-dose ATG/GCSF versus placebo (P = 0.031) (19). However, HbA1c was significantly reduced in both the low-dose ATG (P = 0.002) and the low-dose ATG/GCSF groups (P = 0.011) compared with placebo at 1 year (19). Herein, we report 2-year clinical end point data and mechanistic findings from flow cytometry studies performed on longitudinal samples obtained at baseline and during the 6 months following treatment.
Research Design and Methods
Study Design and Patients
As previously described, this study was registered as a clinical trial (19). All protocol and consent documents were approved by appropriate independent ethics committees or institutional review boards. All participants (or parents) provided written informed consent and, if <18 years of age, signed assent.
Screening and subsequent study visits took place at 14 TrialNet sites in the U.S. (Supplementary Data). Inclusion criteria were age 12–45 years, diagnosis with type 1 diabetes for <100 days confirmed by the presence of at least one type 1 diabetes–related autoantibody (microinsulin autoantibody, tested only if duration of insulin therapy was <7 days; GAD-65 autoantibody; islet cell antigen-512 autoantibody; zinc transporter 8 [ZnT8] autoantibody; or islet cell autoantibody [ICA]), and stimulated C-peptide ≥0.2 nmol/L during an MMTT conducted at least 21 days after diagnosis of type 1 diabetes and within 37 days of randomization. Subjects who screened positive for serum antibodies to hepatitis B surface antigen, hepatitis C, or HIV were excluded from participation. We screened 113 patients and enrolled 89 (from December 2014 to June 2016).
The first author proposed the trial, which was conducted under the auspices of TrialNet. Sanofi (Cambridge, MA) provided thymoglobulin (ATG) but was not involved with study management, data collection, data analysis, or manuscript preparation. Amgen (Thousand Oaks, CA) provided Neulasta (GCSF) and placebo for the study and similarly had no further investigational involvement. Roche Diabetes Care (Indianapolis, IN) provided glucose meters, test strips, and lancets for diabetes management.
Randomization and Masking
Subjects were randomly assigned in a 1:1:1 ratio stratified by participating site, with 29 randomized to receive experimental treatment with ATG/GCSF, 29 to receive ATG alone (and GCSF placebo), and 31 to receive both placebos. Randomization was conducted centrally by the TrialNet Coordinating Center. The study was double masked through the 2-year study visit. An independent data and safety monitoring board reviewed adverse events (AEs) and conducted study accrual and safety reviews every 6 months. An independent medical monitor (masked to treatment assignment) reviewed all accruing safety data.
Procedures
Dosing and premedication were performed as previously described (19). Briefly, ATG or placebo infusions were given, with ATG administered at a dose of 2.5 mg/kg as two intravenous infusions of 0.5 mg/kg and 2 mg/kg given on subsequent days (19). Premedication for ATG or placebo infusions included oral diphenhydramine and acetaminophen and intravenous methylprednisolone or placebo. Subjects who developed serum sickness were offered oral prednisone (19). GCSF or placebo was administered subcutaneously every 2 weeks for a total of six doses. All subjects received intensive diabetes management, coordinated by their primary endocrinologists, with the goal of achieving glycemic targets as recommended by the American Diabetes Association (20). Use of noninsulin pharmaceuticals affecting glycemic control was prohibited.
Laboratory Tests
Blood samples were analyzed centrally at TrialNet core laboratories (19). C-peptide was measured from frozen plasma by two-site immunoenzymometric assay (Tosoh Bioscience, South San Francisco, CA). HbA1c was measured using ion-exchange high-performance liquid chromatography (Variant II; Bio-Rad Laboratories, Hercules, CA). Reliability coefficients for each assay were >0.99 from split duplicate samples. Microinsulin, GAD-65, islet cell antigen-512, and ZnT8 autoantibodies were measured using radioimmunobinding assays, and ICAs were measured using indirect immunofluorescence. A routine chemistry panel was performed (Hitachi 917, reagents from Roche Diagnostics). HLA class II alleles were measured using PCR. CD4 and CD8 cell counts were measured in whole blood through FC 500 using four-color fluorescent monoclonal antibody reagents and software for automated analysis (Beckman Coulter, Indianapolis, IN).
Flow Cytometry
Samples from the first 6 months following randomization were available for mechanistic testing. In a blinded fashion, cryopreserved peripheral blood mononuclear cells (PBMCs) from the 0-, 2-, 12-, and 24-week study visits were assessed by flow cytometry. PBMCs were thawed and counted: 2 × 106 cells per stain were sequentially incubated with viability dye (LIVE/DEAD Fixable Blue Dead Cell Stain Kit; Thermo Fisher Scientific) and Human TruStain FcX (Fc Receptor Blocking Solution) (BioLegend) before staining with monoclonal antibodies for two panels (Supplementary Table 1). Poststaining, cells were fixed in 1% paraformaldehyde and stored at 4°C until sample acquisition on a BD LSRFortessa (BD Biosciences). Absolute T-cell counts were generated by multiplying lymphocyte complete blood count with flow cytometry–detected CD4 or CD8 T-cell percentages within the lymphocyte gate. CD4+ Tconv (CD127highFOXP3neg) and CD4+ Treg (CD127low FOXP3high) percentages within the parent total lymphocyte gate were similarly calculated. Longitudinal CD4:CD8 T-cell ratios, Treg:Tconv ratios, percent CD45RO+ memory Tregs, percent TIGIT+ of Tregs, and PD-1+ mean fluorescence intensity (MFI) on memory Tregs were also determined from samples obtained at the 0-, 2-, 12-, and 24-week study visits.
Quality control measures taken to achieve comparable data across multiple acquisition days were previously described (21). In brief, eight peak rainbow calibration beads (Spherotech, Lake Forest, IL) were used to adjust photomultiplying tube voltages, maintaining comparable MFIs between acquisitions. A technical control from one individual was run concomitantly on each acquisition day to monitor reproducibility over time. All longitudinal samples from a given subject (0, 2, 12, and 24 weeks) were thawed, stained, and assayed on the LSRFortessa on the same day. Criteria for analysis exclusion were <25 events for frequency and <50 events for MFI reporting. Data were analyzed using FlowJo version 9.9.6 software (Tree Star, Ashland, OR).
Statistical Methods
The statistical methods used for analysis of the primary and secondary end points were previously described (19). A prespecified secondary outcome of this trial was a comparison of the AUC-stimulated C-peptide response over the first 2 h of a 4-h MMTT conducted at the 2-year visit. End point analyses were based on the prespecified intention-to-treat cohort, defined as all subjects with measured 2-year C-peptide AUC regardless of treatment compliance. By August 2018, seven subjects had withdrawn from the study. Hence, 82 of the 89 randomized subjects completed their 2-year visit MMTT and were included in this outcome assessment. A multiple imputation method was used to evaluate the potential effect the additional five withdrawals between years 1 and 2 may have had on the comparison (22,23) (Supplementary Data). All figures of C-peptide means are predicted, and associated 95% CIs for each treatment group are model based using the means of the other covariates.
Flow markers were descriptively and graphically summarized at each time point by treatment arm. Actual values at each time point were summarized by treatment arm. Differences between treatment arms were evaluated using nonparametric tests (Wilcoxon rank sum test). Repeated-measures mixed-effects models were used to assess differences in these measures over time between the treatment arms, adjusting for age and mean AUC C-peptide at baseline. Given that these analyses were exploratory and hypothesis generating in nature, we did not correct for multiple comparisons. AEs were defined using Common Terminology Criteria for Adverse Events version 4.0 (24). The number of events and number of subjects experiencing any AE were tabulated. Analyses were conducted in TIBCO Spotfire S+ 8.2 Workbench or SAS 9.4.
Results
Patient Enrollment
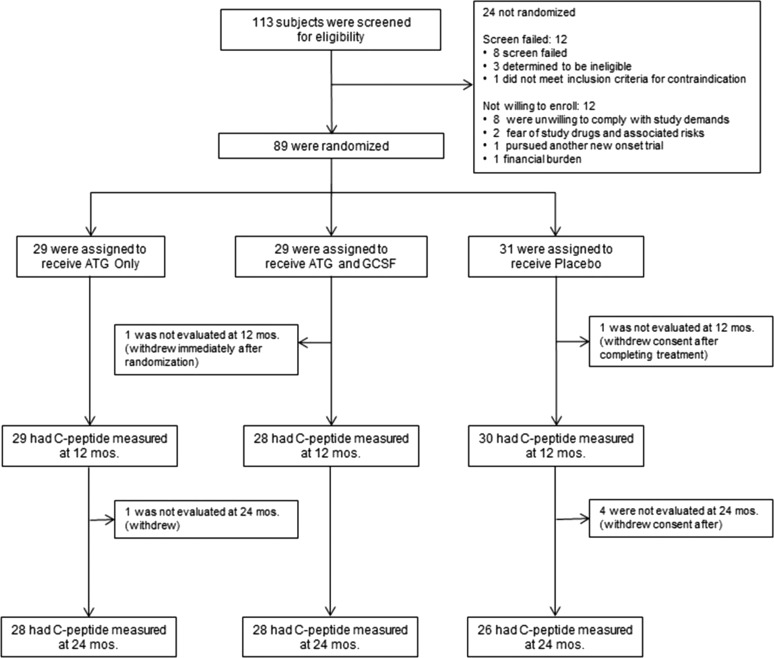
Of 113 patients screened for eligibility, 89 were randomized to one of three treatment arms: ATG/GCSF, ATG only, or placebo (Fig. 1). Clinical and demographic characteristics were similar among treatment groups (Table 1). Insulin pump and continuous glucose monitoring use did not differ among the three study groups. Compliance with the study protocol was high (19). One subject was randomized but withdrew consent before receiving any study drug. All remaining subjects received ATG or placebo infusions as specified in the protocol. Seven subjects failed to complete the 2-year visit. As prespecified, these were not included in the analyses.
Figure 1.
Consolidated Standards of Reporting Trials diagram. One hundred thirteen patients were screened for eligibility, of whom 89 were randomized and 82 completed the secondary outcome measure at 2 years. One randomized subject withdrew consent before receiving any study drug. All remaining subjects received ATG and placebo infusions as specified in the protocol. This included two subjects who received reduced doses per protocol specifications (one ATG and one placebo). mos., months.
Table 1.
Baseline patient characteristics
| Characteristic | ATG/GCSF (n = 29) | ATG only (n = 29) | Placebo (n = 31) |
|---|---|---|---|
| Age (years) | |||
| Mean ± SD | 17.2 ± 5.0 | 18.1 ± 6.9 | 16.9 ± 4.6 |
| Median | 16.4 | 15.5 | 15.0 |
| Range | 12.0–32.8 | 12.4–42.5 | 12.2–29.3 |
| Male sex | 16 (55.2) | 17 (58.6) | 17 (54.8) |
| Race | |||
| White | 28 (96.6) | 29 (100.0) | 29 (93.5) |
| Black | 1 (3.4) | 0 (0.0) | 2 (6.5) |
| Ethnicity | |||
| Not Hispanic and not Latino | 28 (96.6) | 27 (93.1) | 30 (96.8) |
| Autoantibody positive | |||
| GAD-65H | 23 (79.3) | 23 (79.3) | 23 (74.2) |
| IA-2H | 25 (86.2) | 23 (79.3) | 25 (80.6) |
| ICA* | 26 (92.9) | 25 (86.2) | 22 (71.0) |
| ZnT8 | 21 (72.4) | 21 (72.4) | 22 (71.0) |
| Number of autoantibodies positive* | |||
| 1 | 0 (0.0) | 3 (10.3) | 1 (3.2) |
| 2 | 3 (10.3) | 2 (6.9) | 3 (9.7) |
| 3 | 2 (6.9) | 1 (3.4) | 5 (16.1) |
| 4 | 11 (37.9) | 11 (37.9) | 13 (41.9) |
| 5 | 13 (44.8) | 12 (41.4) | 9 (29.0) |
| Days from diagnosis to randomization | |||
| Median | 83 | 81 | 84 |
| Range | 49–97 | 47–100 | 52–99 |
| Weight (kg) | |||
| Median | 62.3 | 66.4 | 62.0 |
| Range | 39.8–89.1 | 39.6–92.4 | 33.8–118 |
| BMI (kg/m2) | |||
| Median | 21.4 | 22.6 | 21.8 |
| Range | 16.6–27.7 | 15.2–32.8 | 14.3–34.3 |
| AUC for C-peptide (nmol/L) | |||
| Mean ± SD | 0.793 ± 0.321 | 0.878 ± 0.474 | 0.966 ± 0.503 |
| Median | 0.701 | 0.757 | 0.932 |
| Range | 0.338–1.78 | 0.211–2.15 | 0.144–2.08 |
| HbA1c | |||
| Median | |||
| % | 7.3 | 7.4 | 7.2 |
| mmol/mol | 56 | 57 | 55 |
| Range | |||
| % | 5.3–12.3 | 4.7–9.0 | 5.5–11.2 |
| mmol/mol | 34–111 | 28–75 | 35–99 |
| Total daily insulin dose at baseline* (units/kg) | |||
| Median | 0.339 | 0.315 | 0.306 |
| Range | 0–1.06 | 0–0.963 | 0–0.921 |
Data are n (%) unless otherwise indicated.
*Missing data: one patient was missing ICA status (had all four other autoantibodies positive), and three patients were missing baseline insulin dose.
Effectiveness
C-Peptide
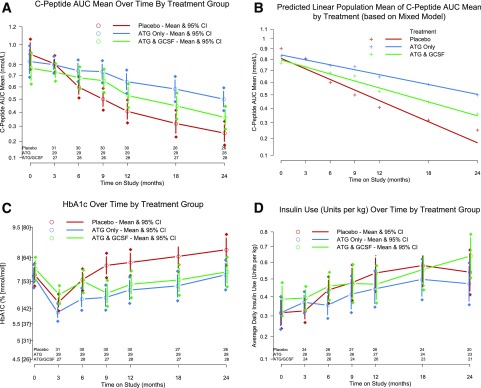
At 2 years, AUC C-peptide was higher in subjects treated with low-dose ATG versus placebo (P = 0.00005) (Fig. 2A). In those receiving ATG/GCSF, AUC C-peptide was not significantly different from placebo (P = 0.032, significance defined as P < 0.025 to adjust for multiple comparisons). At 2 years, AUC C-peptide geometric-like means (nmol/L) were as follows: ATG/GCSF 0.360 (95% CI 0.281, 0.445), ATG only 0.500 (95% CI 0.412, 0.594), and placebo 0.253 (95% CI 0.177, 0.334).
Figure 2.
Effects of low-dose ATG and low-dose ATG/GCSF on C-peptide (A), mixed-model predicted C-peptide (B), HbA1c (C), and insulin (D). C-peptide AUC mean over time by treatment group (A). Analysis at the 2-year end point: ATG alone vs. placebo P = 0.0005 and ATG/GCSF vs. placebo P = 0.032. Mixed-model predicted population mean of the C-peptide AUC mean by treatment over time (B). Two-year decline in mean C-peptide AUC mean: placebo −0.635 nmol/L, ATG −0.337 nmol/L, and ATG/GCSF −0.446 nmol/L. HbA1c over time by treatment group (C). Analysis at 2-year end point: ATG alone vs. placebo P = 0.011 and ATG/GCSF vs. placebo P = 0.022. Insulin use over time by treatment group (D). No significant differences at 2 years. A, C, and D show adjusted means and 95% CIs at each time point; B shows the mixed-model predicted population mean of the C-peptide AUC mean by treatment. Two-year decline was −0.635 nmol/L, −0.337 nmol/L, and −0.446 nmol/L in placebo-, ATG-, and ATG/GCSF-treated subjects, respectively.
A mixed model was used to fit all C-peptide measurements through 2 years (Fig. 2B). Consistent with the primary analysis by ANCOVA, the slope of C-peptide was statistically less steep in low-dose ATG than placebo. Over 2 years, the differences in rate of decline for the ATG/GCSF and low-dose ATG-only groups (relative to placebo) were 0.113 and 0.175 nmol/L, respectively. These estimates were adjusted for age and baseline C-peptide and change over time in the treatment groups (Supplementary Fig. 1).
HbA1c
Two years after therapy, HbA1c (adjusted for baseline HbA1c level, age, and sex using ANCOVA) was significantly lower in both ATG/GCSF (P = 0.022) and ATG only (P = 0.011) versus placebo (Fig. 2C).
Insulin Use
Reported insulin use (units/kg/day) adjusted for baseline insulin use, age, and sex by ANCOVA was not significantly different between either experimental treatment group and placebo at 2 years (Fig. 2D).
AEs
AEs observed in the first year following low-dose ATG and GCSF have been previously reported (19). Notably, between the 1- and 2-year end points, there were no increases in AEs in ATG- or ATG/GCSF-treated subjects (Supplementary Tables 2 and 3). There were nearly twice as many AEs reported in the placebo group than in either the ATG or the ATG/GCSF group between the 1- and 2-year end points. There were no cases of Epstein-Barr virus reactivation and no reported cases of severe hypoglycemia.
Mechanism
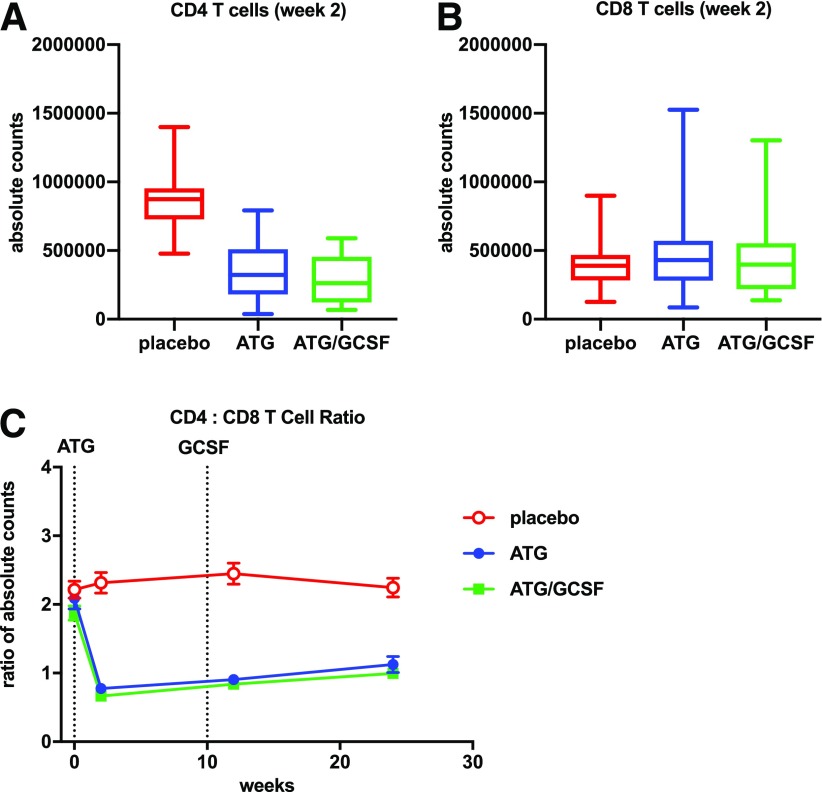
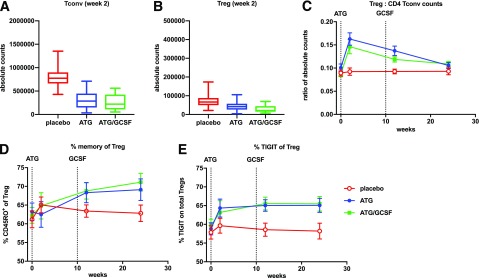
Flow cytometry performed on PBMCs demonstrated a reduction in the CD4:CD8 ratio (P < 0.001 for both ATG and ATG/GCSF vs. placebo) at all time points following therapy. There was selective depletion of CD4+ (P < 0.001 for both ATG and ATG/GCSF) and preservation of CD8+ T cells (P NS for both ATG and ATG/GCSF) versus placebo (Fig. 3). In the CD4+ T-cell compartment, absolute counts of Tconvs, defined as CD4+ non-Tregs, exhibited significant reduction following ATG and ATG/GCSF treatment. Absolute counts of Tregs were also reduced following ATG and ATG/GCSF treatment (Fig. 4A and B). However, an increase in the Treg:Tconv ratio was observed with ATG (vs. placebo: 2 weeks P < 0.001, 12 weeks P = 0.001, 24 weeks P = 0.05) and ATG/GCSF (vs. placebo: 2 weeks P = 0.003, 12 weeks P = 0.004, 24 weeks P = 0.018). At a population level, a linear mixed-effects model with repeated measures demonstrated significant changes in the Treg:Tconv ratio throughout the 6 months following ATG or ATG/GCSF (P = 0.006) (Fig. 4C). In addition, mixed-model analyses demonstrated that change in Treg:Tconv ratio at 12 weeks was significantly associated with 2-year C-peptide (ATG P = 0.042, ATG-GCSF P = 0.028).
Figure 3.
CD4:CD8 T-cell ratio declined with low-dose ATG and ATG/GCSF treatment. Absolute counts were generated at the 2-week time point by multiplying lymphocyte complete blood cell counts with flow cytometry–detected CD4+ (A) or CD8+ (B) T-cell percentages within the lymphocyte gate. Significant differences in CD4+ cells were identified between each of the treatment arms and placebo (P < 0.001 for both pairwise comparisons). No significant differences were observed for CD8+ cells. Longitudinal CD4:CD8 T-cell ratios (C) were determined using frequencies in the lymphocyte gate. Average measures with SDs by treatment arm are shown. Dotted lines in C denote initiation of ATG and last dose of GCSF. The CD4:CD8 T-cell ratios were significantly different at the postbaseline time points for those treated with ATG or ATG/GCSF vs. placebo (all P < 0.001).
Figure 4.
Low-dose ATG reduced CD4+ Tconv and increased Treg:Tconv ratios. A and B: Absolute counts of Tconv (CD127highFOXP3neg) and CD4+ Tregs (CD127low FOXP3high) for samples collected at the 2-week time point. C: Treg:Tconv ratios were calculated using values from A and B. D and E: Treg phenotyping is reported as % CD45RO+ memory cells and % TIGIT+ cells within the Treg gate. In C and E, average measures with SDs are shown; dotted lines denote initiation of ATG and last dose of GCSF. Treg:CD4 Tconv counts shown for placebo vs. ATG arm (2 weeks P < 0.001, 12 weeks P = 0.001, 24 weeks P = 0.05) and placebo vs. ATG/GCSF arm (2 weeks P = 0.003, 12 weeks P = 0.004, 24 weeks P = 0.018). Percent memory of Tregs was not significantly different between ATG and placebo but was significantly different between placebo and ATG/GCSF at 24 weeks (P = 0.04). Percent TIGIT+ Tregs shown for the ATG/GCSF arm vs. placebo (2 weeks P = 0.28, 12 weeks P = 0.009, 24 weeks P = 0.017) and the ATG arm vs. placebo (2 weeks P = 0.07, 12 weeks P = 0.017, 24 weeks P = 0.07).
Notable persistent effects of ATG and ATG/GCSF on Tregs included an increased percentage of Tregs expressing CD45RO+, indicating a memory Treg phenotype (P = 0.003) and increased percentage of TIGIT+ Tregs (Fig. 4D and E). The percent of Tregs expressing TIGIT reflected significant differences at the later time points for ATG/GCSF versus placebo (2 weeks P = 0.28, 12 weeks P = 0.009, 24 weeks P = 0.017) and ATG versus placebo (2 weeks P = 0.07, 12 weeks P = 0.017, 24 weeks P = 0.07). Using mixed-model analyses, fold-change in percent Tregs expressing TIGIT at 12 weeks was associated with 2-year C-peptide (ATG P < 0.001, ATG/GCSF P = 0.023). CD4+ Tconv memory subsets showed a transient increase with both treatment arms versus placebo (Supplementary Fig. 2).
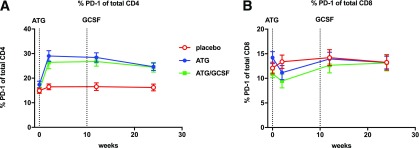
ATG with or without GCSF resulted in significant and persistent upregulation of PD-1 on total CD4+ (P = 0.0001) but not CD8+ (P = 0.12) T cells as determined by a linear mixed-effects model with repeated measures (Fig. 5). By comparison, PD-1 expression on CD4+ Tregs was transiently upregulated, with significance at only 2 weeks (Supplementary Fig. 2C). Increased PD-1 ligand expression on antigen-presenting cells was also transient yet differentially enhanced by the addition of GCSF to ATG versus ATG alone (Supplementary Fig. 3). Similarly, administration of ATG/GCSF, but not ATG alone, enhanced early and transient changes in innate cells (Supplementary Fig. 3). Neither treatment generated persistent changes in antigen-presenting cell populations that were observed for Tconv populations, supporting the T-cell–targeted nature of this therapy.
Figure 5.
Low-dose ATG enhances PD-1 expression on CD4+ but not CD8+ T cells. Percent of cells expressing PD-1 within the CD4+ (A) or CD8+ (B) T-cell gates are shown, with average measures at the longitudinal time points (0, 2, 12, and 24 weeks) by treatment arm with SDs. Dotted lines denote initiation of ATG and last dose of GCSF. Pairwise comparisons were performed at each time point for placebo vs. ATG (all time points P < 0.001) and vs. ATG/GCSF (all time points P < 0.01).
Discussion
Two years after a single course of low-dose ATG (2.5 mg/kg), subjects with new-onset type 1 diabetes (duration <100 days) in this clinical trial had a slower decline of β-cell function and reduction of HbA1c compared with subjects treated with placebo. These findings provide additional evidence that low-dose ATG meaningfully alters the natural course of type 1 diabetes. Given the early separation in C-peptide following treatment with low-dose ATG versus placebo, immunologic studies on longitudinal samples obtained in the 6 months following therapy were performed. Flow cytometry demonstrated a significant reduction of the CD4:CD8 ratio, and while absolute numbers of Tregs decreased, increases in Treg:Tconv ratios were observed. In contrast, flow cytometry data from a previous trial using higher-dose ATG (6.5 mg/kg) in new-onset type 1 diabetes demonstrated marked reductions in Treg:Tconv ratios (17,18). These observations support the hypothesis that low-dose ATG is immunologically distinct from higher-dose ATG.
Reduction of pathogenic lymphocytes with concurrent increases in Treg:Tconv ratios is a rational target in developing treatments for autoimmune diseases. Tregs from subjects treated with low-dose ATG with or without GCSF also displayed enhanced TIGIT, a Treg subset shown to suppress the T helper 1 and 17 cells found in patients with type 1 diabetes (25,26). Hence, our data support the hypothesis that low-dose ATG, in contrast to high-dose ATG, alters the lymphocyte composition to promote a potentially tolerogenic environment. This is consistent with increased Treg:Tconv ratios associated with beneficial clinical outcomes in at least two other recent-onset type 1 diabetes trials that used T-cell–targeting therapies (27,28). In fact, fold-change in 12-week posttreatment TIGIT expression and Treg:Tconv ratios showed significant relationships with 2-year C-peptide in subjects who received low-dose ATG or ATG/GCSF, supporting the use of Treg:Tconv ratio and TIGIT expression as immunologic biomarkers to predict long-term clinical/therapeutic response. While additional unmeasured factors may also be at play, these observations provide strong support for the hypothesis that increased Treg:Tconv ratios and reduction of pathogenic lymphocytes may play a role in the maintenance of β-cell function.
An unexpected but prominent mechanistic observation of this trial was the persistent increase of PD-1 on CD4+ Tconv following ATG and ATG/GCSF therapy. PD-1 expression on T cells can occur from recent activation or exposure to chronic stimulation that can induce anergy or exhaustion. PD-1 was transiently upregulated on Tregs, suggesting acute activation. By contrast, PD-1 was expressed and maintained on CD4+ Tconvs through 6 months, suggesting a phenotype of chronic stimulation that is likely maintained through contextual cues from both antigen and cytokines (29,30). PD-1 expression on CD8+ T cells was unaltered with treatment and time, highlighting the CD4+-specific skewing following low-dose ATG and ATG/GCSF treatment. Interestingly, increased PD-1 expression has also been observed following T-cell–targeted treatment (alefacept) of subjects with recent-onset type 1 diabetes (28). Nevertheless, mixed-model analyses did not reveal significant associations with PD-1 expression and metabolic outcomes. Whether PD-1+ T cells are functionally hyporesponsive or linked to therapeutic response remains to be determined.
`In addition to the key mechanistic observations noted above, there were no concerning safety signals observed in low-dose ATG- or ATG/GCSF-treated subjects during the second year of follow-up. In fact, between the 1- and 2-year end points, nearly twice as many AEs were reported in placebo-treated subjects than in either actively treated study group. While serum sickness was observed in the majority of subjects who received ATG, this was predictable and short-lived. Indeed, all subjects were treated successfully and without sequelae in this trial (19). Nevertheless, studies are being considered to determine whether additional reductions in the dose of ATG can preserve immunologic and clinical outcomes while further reducing the side effect profile. In addition, efforts are under way to develop humanized ATG products that might markedly reduce, if not eliminate, serum sickness and permit maintenance dosing (31).
In our previous pilot trial, low-dose ATG/GCSF treatment significantly preserved C-peptide AUC in subjects with established type 1 diabetes (duration >4 months to <2 years) (15). However, in this phase 2b study of patients with new-onset type 1 diabetes, we did not observe significant preservation of C-peptide AUC in the low-dose ATG/GCSF group compared with placebo. In contrast to our hypothesis, combining low-dose ATG and GCSF did not provide for synergistic benefit when compared with low-dose ATG monotherapy. At this time, our mechanistic studies do not explain why GCSF did not have synergistic effects on the clinical trial outcomes or why our findings differ from those generated through numerous NOD mouse studies. This further exposes our reliance on and the limitations of animal models in predicting responses in human disease. In addition, our observations highlight challenges associated with the design of combination therapies when mechanistic knowledge of the individual or combined therapeutics is incomplete.
In conclusion, low-dose ATG (2.5 mg/kg) provides for long-term preservation of β-cell function, reduction of HbA1c, and favorable changes in immune cell subsets compared with placebo, while the addition of GCSF may diminish the benefits provided by low-dose ATG. Additional studies are needed to elucidate the immunologic implications of low-dose ATG, and efforts to define responders and nonresponders using mechanistic data are under way. Ongoing follow-up of this cohort is warranted. Importantly, the side effect profile of low-dose ATG in this trial was predictable, manageable, and short-lived. Low-dose ATG should be considered as a potential means for preventing type 1 diabetes and preserving β-cell function in new-onset type 1 diabetes.
Supplementary Material
Article Information
Funding. The sponsor of the trial was the Type 1 Diabetes TrialNet Study Group. The Type 1 Diabetes TrialNet Study Group is a clinical trials network funded by the National Institutes of Health (NIH) through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development through the cooperative agreements U01-DK-061010, U01-DK-061034, U01-DK-061042, U01-DK-061058, U01-DK-085465, U01-DK-085461, UC4-DK-085466, U01-DK-085476, U01-DK-085499, U01-DK-085509, U01-DK-097835, U01-DK-103266, U01-DK-103282, U01-DK-106984, U01-DK-107014, and UC4-DK-106993; the National Center for Research Resources through Clinical Translational Science Awards UL1-TR-001085, UL1-TR-001427, UL1-TR-001863, UL1-TR-001082, UL1-TR-000114, UL1-TR-001857, UL1-TR-000445, UL1-TR-002529, UL1-TR-001872, UL1-TR-002243, and PO1-NIH-AI-42288; the JDRF; and the American Diabetes Association. The flow cytometry was supported by the Immune Tolerance Network and sponsored by the National Institute of Allergy and Infectious Diseases under award number UM1-AI-109565. Additional funding sources for this study were provided by The Leona M. and Harry B. Helmsley Charitable Trust.
Under the terms of the cooperative agreement funding mechanism used by the NIH, representatives from the sponsoring institutes of the NIH (National Institute of Diabetes and Digestive and Kidney Diseases) participated in the design and conduct of the study; interpretation of the data; preparation, review, approval of the manuscript; and the decision to submit the manuscript for publication. The sponsor did not have the right or ability to veto submission for publication.
Duality of Interest. Additional funding sources for this study were provided by Sanofi. Sanofi provided ATG but had no involvement with study management, data collection, data analysis, or manuscript preparation. Amgen provided Neulasta (GCSF) and placebo for the study and similarly had no further involvement. Roche Diabetes Care provided diabetes management supplies only for the study. As per the clinical trials agreement between Amgen and the NIH and separately between Sanofi and the University of Florida, Amgen and Sanofi were sent the draft manuscript before submission to Diabetes. Amgen and Sanofi did not comment or provide input on the manuscript. M.J.H. is a board member of SAB Biotherapeutics, which is developing a transchromosomic bovine–derived human ATG. M.A.A. holds patent US 8758761 B2, Combination Therapies for Treating Type 1 Diabetes, which includes the use of ATG in combination with GCSF for the treatment of type 1 diabetes. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. M.J.H., D.A.S., J.S.S., and C.J.G. conceived of the study, obtained funding, collected data, and wrote the manuscript. S.A.L. and J.L.B. collected data and wrote the manuscript. J.P.K. obtained funding, performed the data analyses, and wrote the manuscript. B.N.B., S.M.G., and M.V.W. performed the data analyses and wrote the manuscript. J.L.M., M.A.A., D.J.B., D.A.B., S.E.G., R.G., P.A.G., K.C.H., J.B.M., A.M., H.R., W.E.R., and D.M.W. collected data and edited the manuscript. M.J.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the Immunology of Diabetes Society Congress, London, U.K., 25–29 October 2018.
Footnotes
Clinical trial reg. no. NCT02215200, clinicaltrials.gov
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db19-0057/-/DC1.
A list of members of the Type 1 Diabetes TrialNet ATG-GCSF Study Group is included in the Supplementary Data.
Contributor Information
Collaborators: Type 1 Diabetes TrialNet ATG-GCSF Study Group, Carla J. Greenbaum, Mark A. Atkinson, David A. Baidal, Manuela Battaglia, Dorothy Becker, Penelope Bingley, Emanuele Bosi, Jane Buckner, Mark Clements, Peter G. Colman, Linda DiMeglio, Carmella Evans-Molina, Stephen E. Gitelman, Robin Goland, Peter Gottlieb, Kevan Herold, Mikael Knip, Jeffrey P. Krischer, Ake Lernmark, Wayne Moore, Antoinette Moran, Andrew Muir, Jerry Palmer, Mark Peakman, Louis Philipson, Philip Raskin, Maria Redondo, Henry Rodriguez, William Russell, Desmond A. Schatz, Jay M. Sosenko, Lisa Spain, John Wentworth, Diane Wherrett, Darrell M. Wilson, William Winter, Anette Ziegler, Mark Anderson, Peter Antinozzi, Richard Insel, Thomas Kay, Jennifer B. Marks, Alberto Pugliese, Bart Roep, Jay S. Skyler, Jorma Toppari, Carla J. Greenbaum, Jeffrey P. Krischer, Ellen Leschek, Lisa Spain, Katarzyna Bourcier, Richard Insel, John Ridge, Jay S. Skyler, Carla J. Greenbaum, Lisa Rafkin, Jay M. Sosenko, Jay S. Skyler, Irene Santiago, Jeffrey P. Krischer, Brian Bundy, Michael Abbondondolo, Timothy Adams, Ilma Asif, Jenna Bjellquist, Matthew Boonstra, Cristina Burroughs, Mario Cleves, David Cuthbertson, Meagan DeSalvatore, Christopher Eberhard, Steve Fiske, Julie Ford, Jennifer Garmeson, Susan Geyer, Brian Hays, Courtney Henderson, Martha Henry, Kathleen Heyman, Belinda Hsiao, Christina Karges, Beata-Gabriela Koziol, Lindsay Lane, Shu Liu, Jennifer Lloyd, Kristin Maddox, Jamie Malloy, Julie Martin, Cameron McNeill, Margaret Moore, Sarah Muller, Thuy Nguyen, Jodie Nunez, Ryan O’Donnell, Melissa Parker, M.J. Pereyra, Amy Roberts, Kelly Sadler, Christine Sullivan, Roy Tamura, Elon Walker-Veras, Megan V. Warnock, Keith Wood, Rebecca Wood, Ping Xu, Vanessa Yanek, Kenneth Young, Darlene Amado, Amanda Kinderman, Ashley Leinbach, Jessica Miller, Nichole Reed, Tina Stavros, Ellen Leschek, Lisa Spain, Emily Blumberg, Sean Aas, Gerald Beck, Rose Gubitosi-Klug, Lori Laffel, Robert Vigersky, Dennis Wallace, David Brillon, Robert Veatch, Brett Loechelt, Lindsey Baden, Peter Gottlieb, Michael Green, Ellen Leschek, Adriana Weinberg, Santica Marcovina, Jerry P. Palmer, Jay Tischfield, Adriana Weinberg, William Winter, Liping Yu, Annie Shultz, Emily Batts, Arielle Pagryzinski, Mary Ramey, Meghan Tobin, Kristin Fitzpatrick, Randy Guerra, Melita Romasco, Christopher Webb, Peter Gottlieb, Maya Barr, Mary Drye, Jordan Lykens, Aaron Michels, Allison Schauwecker, Andrea Steck, Paul Wadwa, Carla J. Greenbaum, Jane Buckner, Wei Hao, Sandra Lord, Marli McCulloch-Olson, Mary Ramey, Elaine Sachter, Jenna Snavely, Meghan Tobin, Corinna Tordillos, Dana VanBuecken, Robin Goland, Analia Alvarez, Magdalena Bogun, Rachelle Gandica, Natasha Leibel, Sarah Pollak, Barney Softness, Kristen Williams, Bryce Nelson, James Amrhein, Lisa Looper, Elaine Moreland, Andrew Smith, Beth Weir, Lori Wise, Linda DiMeglio, Carmella Evans-Molina, Manasa Mantravadi, Maureen Mullen, Vanessa Patrick, Maria Spall, Stephanie Woerner, Darrell M. Wilson, Nora Arrizon-Ruiz, Tandy Aye, Laura Bachrach, Karen Barahona, Trudy Esrey, Laura Nally, Stephen E. Gitelman, Mark Anderson, Glenna Auerback, Jeanne Buchanan, Christine T. Ferrara, Karen Ko, Srinath Sanda, Christine Torok, Rebecca Wesch, Michael J. Haller, Anastasia Albanese-O'Neill, Todd Brusko, Miriam Cintron, Jennifer Hosford, Laura M. Jacobsen, Henry Rohrs, Desmond A. Schatz, Janet Silverstein, Paula Towe, David A. Baidal, Carlos Blaschke, Della Matheson, Janine Sanchez, Natalia Sanders-Branca, Jay S. Skyler, Jay M. Sosenko, Antoinette Moran, Janice Leschyshyn, Jennifer McVean, Brandon Nathan, Brittney Nelson, Beth Pappenfus, Jessica Ruedy, Anne Street, Muna Sunni, Darcy Weingartner, Dorothy Becker, Kelli DeLallo, Ana Diaz, David Groscost, Mary Beth Klein, Ingrid Libman, Karen Riley, Henry Rodriguez, Sureka Bollepalli, Rachel Brownstein, Emily Eyth, Danielle Henson, Michele Laine, Dorothy Shulman, William Russell, Faith Brendle, Anne Brown, Brenna Dixon, Justin Gregory, Dan Moore, James Thomas, Kevan Herold, Laurie Feldman, and William Tamborlane
References
- 1.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet 2014;383:69–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silverstein J, Maclaren N, Riley W, Spillar R, Radjenovic D, Johnson S. Immunosuppression with azathioprine and prednisone in recent-onset insulin-dependent diabetes mellitus. N Engl J Med 1988;319:599–604 [DOI] [PubMed] [Google Scholar]
- 3.Herold KC, Gitelman SE, Masharani U, et al. . A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes 2005;54:1763–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ludvigsson J, Krisky D, Casas R, et al. . GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N Engl J Med 2012;366:433–442 [DOI] [PubMed] [Google Scholar]
- 5.Wherrett DK, Bundy B, Becker DJ, et al.; Type 1 Diabetes TrialNet GAD Study Group . Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. Lancet 2011;378:319–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moran A, Bundy B, Becker DJ, et al.; Type 1 Diabetes TrialNet Canakinumab Study Group; AIDA Study Group . Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicentre, randomised, double-blind, placebo-controlled trials. Lancet 2013;381:1905–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orban T, Bundy B, Becker DJ, et al.; Type 1 Diabetes TrialNet Abatacept Study Group . Costimulation modulation with abatacept in patients with recent-onset type 1 diabetes: follow-up 1 year after cessation of treatment. Diabetes Care 2014;37:1069–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pescovitz MD, Greenbaum CJ, Bundy B, et al.; Type 1 Diabetes TrialNet Anti-CD20 Study Group . B-lymphocyte depletion with rituximab and β-cell function: two-year results. Diabetes Care 2014;37:453–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schatz D, Gale EA, Atkinson MA. Why can’t we prevent type 1 diabetes? Maybe it’s time to try a different combination. Diabetes Care 2003;26:3326–3328 [DOI] [PubMed] [Google Scholar]
- 10.Haller MJ, Gottlieb PA, Schatz DA. Type 1 diabetes intervention trials 2007: where are we and where are we going? Curr Opin Endocrinol Diabetes Obes 2007;14:283–287 [DOI] [PubMed] [Google Scholar]
- 11.Ludvigsson J. Combination therapy for preservation of beta cell function in Type 1 diabetes: new attitudes and strategies are needed! Immunol Lett 2014;159:30–35 [DOI] [PubMed] [Google Scholar]
- 12.Parker MJ, Xue S, Alexander JJ, et al. . Immune depletion with cellular mobilization imparts immunoregulation and reverses autoimmune diabetes in nonobese diabetic mice. Diabetes 2009;58:2277–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohty M. Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia 2007;21:1387–1394 [DOI] [PubMed] [Google Scholar]
- 14.Zhao X-Y, Wang Y-T, Mo X-D, et al. . Higher frequency of regulatory T cells in granulocyte colony-stimulating factor (G-CSF)-primed bone marrow grafts compared with G-CSF-primed peripheral blood grafts. J Transl Med 2015;13:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haller MJ, Gitelman SE, Gottlieb PA, et al. . Anti-thymocyte globulin/G-CSF treatment preserves β cell function in patients with established type 1 diabetes. J Clin Invest 2015;125:448–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haller MJ, Gitelman SE, Gottlieb PA, et al. . Antithymocyte globulin plus G-CSF combination therapy leads to sustained immunomodulatory and metabolic effects in a subset of responders with established type 1 diabetes. Diabetes 2016;65:3765–3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gitelman SE, Gottlieb PA, Rigby MR, et al.; START Study Team . Antithymocyte globulin treatment for patients with recent-onset type 1 diabetes: 12-month results of a randomised, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol 2013;1:306–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gitelman SE, Gottlieb PA, Felner EI, et al.; ITN START Study Team . Antithymocyte globulin therapy for patients with recent-onset type 1 diabetes: 2 year results of a randomised trial. Diabetologia 2016;59:1153–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haller MJ, Schatz DA, Skyler JS, et al.; Type 1 Diabetes TrialNet ATG-GCSF Study Group . Low-dose Anti-Thymocyte Globulin (ATG) preserves β-cell function and improves HbA1c in new-onset type 1 diabetes. Diabetes Care 2018;41:1917–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Diabetes Association Erratum. Glycemic targets. Sec. 6. In standards of medical care in diabetes-2017. Diabetes Care 2017;40(Suppl. 1);S48-S56. Diabetes Care 2017;40:985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long SA, Thorpe J, Herold KC, et al. . Remodeling T cell compartments during anti-CD3 immunotherapy of type 1 diabetes. Cell Immunol 2017;319:3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shafer J. Analysis of Incomplete Multivariate Data. New York, Chapman & Hall/CRC, 1997 [Google Scholar]
- 23.Bundy BN, Krischer JP; Type 1 Diabetes TrialNet Study Group . A model-based approach to sample size estimation in recent onset type 1 diabetes. Diabetes Metab Res Rev 2016;32:827–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Cancer Institute. Common Terminology Criteria for Adverse Events v4.0. NCI N, DHHS. Bethesda, MD, National Institutes of Health, 2009 (NIH publ. no. 09–7473) [Google Scholar]
- 25.Joller N, Lozano E, Burkett PR, et al. . Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity 2014;40:569–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuhrman CA, Yeh WI, Seay HR, et al. . Divergent phenotypes of human regulatory T cells expressing the receptors TIGIT and CD226. J. Immunol 2015;195:145–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rigby MR, Harris KM, Pinckney A, et al. . Alefacept provides sustained clinical and immunological effects in new-onset type 1 diabetes patients. J Clin Invest 2015;125:3285–3296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daifotis AG, Koenig S, Chatenoud L, Herold KC. Anti-CD3 clinical trials in type 1 diabetes mellitus. Clin Immunol 2013;149:268–278 [DOI] [PubMed] [Google Scholar]
- 29.Crespo J, Sun H, Welling TH, Tian Z, Zou W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr Opin Immunol 2013;25:214–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bally AP, Austin JW, Boss JM. Genetic and epigenetic regulation of PD-1 expression. J Immunol 2016;196:2431–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuroiwa Y, Kasinathan P, Sathiyaseelan T, et al. . Antigen-specific human polyclonal antibodies from hyperimmunized cattle. Nat Biotechnol 2009;27:173–181 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.