Abstract
Energy homeostasis is coordinated by bidirectional communication pathways between the brain and peripheral organs, including adipose tissue, muscle, the pancreas, liver, and gut. Disruption of the integrated chemical, hormonal, and neuronal signals that constitute the gut–brain axis significantly contributes to disorders of metabolism and body weight. Initial studies of glucagon-like peptide-1 (GLP-1), a gut hormone released in response to the ingestion of nutrients, focused on its incretin actions to improve postprandial glucose homeostasis by enhancing meal-induced insulin secretion. However, GLP-1 is also a key player in the gut–brain regulatory axis with multiple effects on appetite and energy metabolism outside of its peripheral glucoregulatory actions. In this review, we explore the function of GLP-1 as a component of the gut–brain axis in the regulation of energy homeostasis, and consider the implications of this role for the development of therapeutic treatment options for obesity.
Keywords: appetite, energy homeostasis, glucagon-like peptide-1 (GLP-1), gut–brain axis
Introduction
Obesity, a complex multifactorial disease, has emerged as the leading global health care issue. The etiological complexity and its association with numerous comorbidities create unique management challenges when treating the patient with obesity. As a result, a clear knowledge of the neurohormonal regulation of appetite and metabolism is vital for an understanding of the mechanisms behind current and future medications for weight management.
Dysregulation of the homeostatic systems governing energy metabolism leads to unwanted weight gain and defense of a new, higher body weight in patients with overweight or obesity.1–3 Fundamentally, any weight-loss intervention must act either directly or indirectly to induce a state of negative energy balance.4 However, for this weight loss to then be sustained, successful treatment must interfere with the homeostatic metabolic, endocrine, and behavioral mechanisms intent on returning an individual with overweight or obesity to their former weight.5
Energy homeostasis is coordinated by the brain and involves the integration of chemical, hormonal, and neuronal signals from peripheral organs such as adipose tissue, pancreas, liver, and the gut, constituting the gut–brain axis.6,7 Peripheral signals, such as leptin and insulin, reflect the body's level of energy stores and various gut factors convey the types and availability of nutrients signifying the timing, frequency, caloric amount, and macronutrient content of ingested meals. Disruption of the signals constituting this axis is thought to be a common pathophysiological pathway that leads not only to unwanted weight gain and the development of obesity, but is now also recognized to be a key mediator in the development of metabolic disorders such as dyslipidemia, prediabetes, and type 2 diabetes.8
A key player in the gut–brain axis is glucagon-like peptide-1 (GLP-1), a hormone released in response to meal ingestion that was originally described for its incretin properties because of its capacity to enhance meal-related insulin secretion, suppress glucagon, and thus improve postprandial glucose homeostasis. However, outside of their glucoregulatory role, GLP-1 receptor agonists (GLP-1RAs) have also demonstrated effects on appetite and energy metabolism, likely by acting directly on specific areas of the brainstem and hypothalamus.7
In this review, we explore the role of GLP-1 as a component of the gut–brain axis involved in the regulation of energy homeostasis, and the implications of this role for the development of therapeutic treatment options for obesity.
The Gut–Brain Axis and GLP-1
The gut–brain axis is a bidirectional communication pathway linking the gut with the key centers of the peripheral nervous system (PNS) and central nervous system (CNS), contributing to regulation of energy homeostasis.9 More than a century ago, Pavlov reported that the sight, smell or taste of food without swallowing stimulates gastric secretions in dogs.10 Around the same time, Starling and Bayliss demonstrated that the intravenous infusion of scraped mucosa from the duodenum immediately stimulated pancreatic secretions.11 They postulated that this phenomenon was the result of factors present within the gut lining, referred to as secretin.11 It is now well recognized that the presence of nutrients detected in the mouth, stomach, and small intestine, often mediated through activation of G protein-coupled receptors (GPCRs),12 affects the secretion of numerous gut hormones. Among these known appetite-altering gut peptides, ghrelin is recognized as an orexigenic hormone due to its ability in increasing hunger and food intake, and peptides such as cholecystokinin, peptide YY, oxyntomodulin, and GLP-1 are recognized as satiety hormones, with anorexigenic (appetite suppressing) effects.13
GLP-1 is a 30 amino-acid peptide [GLP-1-(7-36)] derived from the preproglucagon (GCG) gene,14 which is expressed in the pancreatic α-cells and intestinal L-cells15 as well as in neurons located in the brainstem and the hypothalamus.16 Tissue-specific posttranslational processing of proglucagon peptide, as a result of differential activities of prohormone convertase 1/3 and 2 (PC1/3 and PC2),17 yields peptides specific to the pancreas, intestine, and brain.14,15 Glucagon is the main proglucagon-derived peptide in the pancreatic α-cell given much higher expression of PC2 compared with PC1/3 in this tissue, whereas PCI 1/3 in the intestinal L-cells and neurons of the nucleus of the solitary tract (NTS) of the medulla generate GLP-1.14
The majority of circulatory GLP-1 is secreted dose-dependently in response to nutrient ingestion by neuroendocrine L-cells located throughout the gastrointestinal (GI) tract (predominantly in the distal ileum and colon).14 Observation of a colocalization of PC1/3 and GCG expression in the caudal region of the NTS within the brain has also indicated that that GLP-1 is produced in this region.18,19 The stimuli responsible for the synthesis and secretion of this brain-derived GLP-1 remain largely unknown.
GLP-1 activity is mediated by the GLP-1R, a member of the B1 family of seven-transmembrane GPCRs.20 GLP-1Rs are expressed in pancreatic β-cells as well as various other tissues, including neurons in specific central brain regions, the peripheral afferent parasympathetic nervous system, atrial cardiac myocytes, pulmonary endothelial cells, kidney, and the GI tract,14 reflecting the potential for pleiotropic physiological effects of GLP-1 beyond its insulin secretory effect.
The assumption that physiologic actions of endogenous GLP-1 are entirely mediated by binding to organ-specific GLP-1Rs has been challenged. A significant amount of GLP-1 secreted from the gut is rapidly degraded by the enzyme dipeptidyl peptidase-4 (DPP-4) to an inactive component, GLP-1-(9-36), before reaching the portal vein.21 Thus, given its extremely short plasma half-life of 1–2 min, only a small portion of secreted GLP-1 is thought to reach target organs. Therefore, in addition to binding to GLP-1R at the target organs, GLP-1 effect is also mediated through activation of afferent neural pathways initiating in the gut or hepatic portal veins.22,23
Glycemic-reducing effects of GLP-1 in patients with type 2 diabetes led to development of a new class of drugs utilizing GLP-1 signaling to treat diabetes. Using strategies to resist the degrading effects of DPP-4 on GLP-1 has produced two categories of GLP-1 based drugs: DPP-4 inhibitors, resulting in higher concentrations of native GLP-1; and GLP-1RAs, modified so as to have longer half-lives than native GLP-1. In general, compared to DPP-4 inhibitors, GLP-1RAs are more effective in reducing HbA1c (0.4%–1.6% vs. 0.4%–0.8%; Table 1). Findings from the clinical trials have also revealed that administration of GLP-1RAs induces weight loss in addition to glucose improvement (Table 1). While the glycemic-reducing effects of GLP-1RAs are attributed mainly to endocrine actions of these compounds at the level of pancreatic islets, their effect on energy homeostasis likely involves central GLP-1 signaling.
Table 1.
The Effect of Available Dipeptidyl Peptidase-4 Inhibitors and Glucagon-Like Peptide-1 Receptor Agonists on Glycemic Control and Weight Loss
| Medication | Dose | Change in HbA1c (%) | Change in body weight (kg) | Change in body weight (%) |
|---|---|---|---|---|
| DPP-4 inhibitors | ||||
| Sitagliptin90 | 100 mg | −0.8 | −1.5 | n/a |
| Vildagliptin91 | 50 mg | −0.4 | −0.2 | n/a |
| Saxagliptin92 | 5 mg | −0.5 | n/a | n/a |
| Linagliptin93 | 5 mg | −0.4 | n/a | n/a |
| Alogliptin94 | 25 mg | −0.6 | n/a | n/a |
| GLP-1RAs | ||||
| Exenatide95 | 10 μg twice daily | −0.9 | −2.9 | 3.4 |
| Exenatide extended release96 | 2 mg once weekly | −1.6 | −2.3 | 2.4 |
| Albiglutide74 | 50 mg once weekly | −0.9 | −0.4 to −0.9 | n/a |
| Dulaglutide97 | 1.5 mgonce weekly | −0.8 | −2.3 | 2.5 |
| Liraglutide98 | 1.8 mg once daily | −1.1 | −2.5 | 2.7 |
| Liraglutide99 | 3.0 mg once dailyb | −0.35a | −6.5a | −6.1a |
| Lixisenatide100 | 20 μg once daily | −0.8 | −0.6 | n/a |
| Semaglutide101 | 1 mg once weekly | −1.6 | −4.7 | 5.0 |
| n/a | Intarcia 650102 | −1.5 | −4.0 | n/a |
Data are reported as mean. The source of the data is from the product label unless otherwise indicated. Percentage change in body weight is calculated for products reporting a baseline weight measurement in the product label. Product data are for monotherapy.
Data are from the largest clinical trial with the given product.
Approved dose for weight loss management.
DPP-4, dipeptidyl peptidase-4; GLP-1RA, glucagon-like peptide-1 receptor agonist; n/a, data not available.
Physiological Effects of GLP-1 Focusing on Energy Balance
Energy homeostasis is governed by a complex interplay between nutrients, neural signaling, adipokines, and gut peptides, whose actions are mediated by binding directly to neuronal receptors in the CNS or PNS. Both enteric and autonomic nervous systems are involved in carrying afferent signals initiated by nutrient or GI hormones from the gut to the CNS via vagal or spinal pathways, as well as efferent signals from the CNS back to the gut.9
Central nervous system
Rodent studies
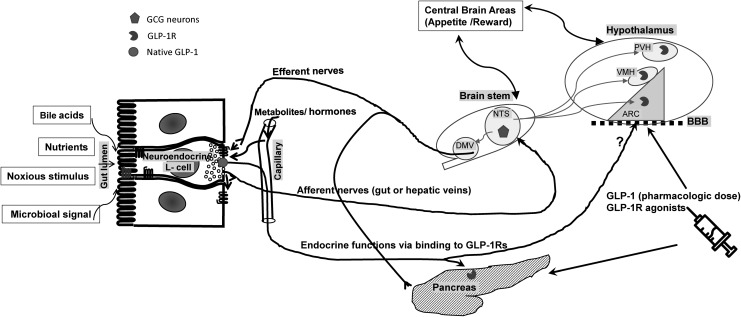
One of the key brain regions involved in energy intake and balance is the arcuate nucleus (ARC) of hypothalamus (Fig. 1).24 The ARC is situated near the median eminence of the hypothalamus, which is thought to lie partially outside of the blood–brain barrier in humans.25 This positioning is potentially advantageous in that it allows ready access for peripheral hormones that regulate food intake without having to first penetrate the blood–brain barrier. Similarly, the area postrema, another important brain area involved in energy regulation and juxtaposed to the NTS in the brainstem,7 also possesses an incomplete blood–brain barrier.26 Hence, meal-related circulating gut hormones can readily cross from the bloodstream and bind directly to their receptors in this region of the brainstem.
FIG. 1.
The role of GLP-1 in energy balance within the complex gut–brain interplay. Gut-derived GLP-1 is secreted from intestinal L-cells into circulation as a result of nutrient ingestion as well as other stimuli, such as bile acids, microbiota, and noxious stimuli. Physiologic effects of endogenous gut-derived GLP-1 is mediated by its binding to GLP-1Rs (pancreatic islet cells) or stimulating vagal nervous system, transmitting signals to the CNS (afferent nerves) and from the CNS back to the peripheral organs (efferent nerves). GLP-1Rs are found within some brain regions, particularly the ARC, PVH, and the VMH. Endogenous GLP-1 produced by GCG neurons of NTS of the medulla affects food intake by its binding to the hypothalamic GLP-1Rs. The GCG neurons receive information from peripheral organ through afferent nerves as well as areas in the brain and project back to the hypothalamus as well as the vagal efferent neurons in the DMV and the intermediolateral cell column of spinal cord. Anorexic effects of GLP-1 (above physiologic concentrations) or GLP-1R agonists are likely mediated through binding directly to hypothalamic GLP-1Rs after crossing the blood–brain barrier (BBB). ARC, arcuate nucleus; CNS, central nervous system; DMV, dorsal motor vagal nucleus; GCG, preproglucagon; GLP-1R, glucagon-like peptide-1 receptor; NTS, nucleus of the solitary tract; PVH, paraventricular nuclei of the hypothalamus; VMH, ventromedial nuclei of the hypothalamus.
GLP-1Rs are found within many of these brain regions, including the arcuate, paraventricular, and the ventromedial nuclei of the hypothalamus.27–30 In addition to direct receptor binding and neuronal activation at these sites, peripherally secreted GLP-1 also targets GPCRs on the vagus nerve, which transmit signals to the NTS via vagal afferents.31 Centrally, GLP-1 is secreted by the GCG neurons of NTS and directly activates GLP-1Rs found throughout the brain.32 Absence of GLP-1R on GCG neurons suggests that the function of these neurons is not solely regulated by GLP-1 itself.33 From their location in the hindbrain, GCG neurons receive information via afferent neurons from the periphery as well as the CNS (including the hypothalamus), and, in turn, project reciprocally up to the hypothalamus and amygdala as well as to vagal efferent neurons in the dorsal motor nucleus of the vagus and to the intermediolateral cell column of spinal cord.34 Both nutrient and noxious stimuli,35,36 as well as gastric distension,37 have been shown to activate these neurons.
The role of GCG neuronal regulation in energy homeostasis circuitry remains largely unclear. Reduction in GCG expression in the NTS using viral knockdown of GCG expression38 has been shown to induce weight gain and increase adiposity, mirroring the effects of chronic blockade of CNS GLP-1R using intracerebroventricular infusion of exendin-9-39.39 In a recent study, selective activation of GCG neurons using designer receptors exclusively activated by designer drugs acutely reduced food intake in rats on a standard or high-fat diet.40 However, chronic activation of GCG neurons using this method led to weight loss only in mice on high-fat diet with no effect on animals on standard diet.40 Activation of GCG neurons in this study had no influence on the known GLP-1 actions in regulating glucose metabolism or stress response (malaise, hypothalamic-pituitary-adrenal axis activation, or sympathetic nerve activation).40
Although both peripherally and centrally administered GLP-1 affects appetite, the differential effects of endogenous GLP-1 secreted from the gut versus CNS in regulation of energy intake are poorly understood. Given the rapid degradation of GLP-1 by DPP-4, peripheral vagal activation rather than direct CNS effect likely mediates the feeding effect of postprandial gut-derived GLP.34 Moreover, the effects of endogenous gut- and brain-derived GLP-1 on energy balance seem to be independent of each other since CNS administration of physiologic concentrations of GLP-1 in rodent models has been shown to lower calorie consumption and induce weight loss, whereas peripheral GLP-1 infusion has no effect on energy food intake.41
In contrast to endogenous GLP-1 at physiologic concentrations, peripherally administered GLP-1RAs that avoid DPP-4 degradation have been shown to lower food intake mainly through direct action on CNS GLP-1R, as concurrent administration of a central GLP-1R antagonist eliminates the anorectic effect of the peripheral GLP-1RA.28 Using a labeled GLP-1RA (liraglutide), it was also demonstrated that this compound binds exclusively to neurons within the ARC and other areas in the hypothalamus rather than to peripheral locations such as the vagus nerve.30 Furthermore, selective deletion of neuronal GLP-1Rs eliminates both the uptake of liraglutide, a GLP-1RA, into the brain30 and its ability to affect appetite and weight loss,42 indicative of its central effect.
Human studies
The effect of exogenous infusion of GLP-1-(7-36) on energy consumption in humans is consistent with those in animal model studies, in which ad libitum food intake in both lean and obese individuals is decreased dose-dependently43 when circulatory GLP-1 is increased to levels above postprandial physiological concentrations.44 In an early GLP-1RA study in humans, 5 weeks of once-daily treatment with liraglutide 1.8 or 3.0 mg in 49 nondiabetic individuals with obesity resulted in ∼15% lower energy intake accompanied by a ∼2 kg weight loss.45
Over the last decade, functional magnetic resonance imaging (fMRI) has been used to measure neural activity in various brain areas for the study of mediators of food responses in humans. In lean healthy subjects, blocking GLP-1R alters neural activities in central brain regions responsive to oral food stimuli.46 In individuals with obesity, with or without type 2 diabetes, appetite- and reward-related areas of the brain that have shown increased brain response to food pictures compared to lean controls47,48 are reduced during infusion of GLP-1RA.47 The specificity of these effects was demonstrated by prevention of these neural responses to visual and oral food cues when a GLP-1RA was administered during coinfusion of a GLP-1R blocker.47,48
Weight loss accompanying GLP-1 infusion in humans has also been attributed to drug-induced malaise or food aversion. However, clinical trials using GLP-1RAs have shown that nausea, if experienced at all, is generally short-lived and typically resolves within the first few weeks of treatment, yet, the weight loss from these compounds is durable, and the frequency and duration of nausea is not related to the amount of weight loss achieved.49 Furthermore, in SCALE Obesity and Prediabetes, a large clinical trial (n = 3731) in which liraglutide 3.0 mg was administered daily, there were no significant differences in weight loss between individuals experiencing at least one episode of nausea or vomiting (n = 301) and those reporting no episodes of nausea or vomiting during the 56 weeks on treatment.50 These findings indicate that the weight loss observed during GLP-1RA treatment cannot be fully accounted for by the occurrence of GI adverse events.
Altogether, studies in animal models and humans confirm an important role for central GLP-1 signaling in the regulation of appetite control and energy balance.
Gut
The bidirectional communication of the gut–brain axis involves the modulation of gut functions that may affect energy intake and expenditure through changes in gut motility or gut microbiota.9
Gut motility
While endogenous GLP-1 has been shown to have trivial effects on gastric emptying (GE),51–53 pharmacological doses of GLP-1 or GLP-1RAs have shown notable reductions in the rate of food emptying from the stomach into the gut.54,55 The mechanisms underpinning this reduction or delay in GE are complex. It is likely that the effects on gastric motility are mediated by GLP-1-related activation of the vagal nerve as well as direct actions56 through GLP-1Rs found along the GI tract, such as enteric neurons of the small and large intestine.14,57
Interestingly, short-acting GLP-1RAs appear to reduce GE to a greater extent than long-acting GLP-1RAs.58 Although underlying mechanisms for differential effects on GE are yet to be fully elucidated, it is plausible that GE effect is subject to tachyphylaxis/desensitization during steady, elevated concentrations of long-acting GLP-1RAs compared with peaks and troughs of drug concentrations of short-acting GLP-RAs.58
Whether the delay in GE comprises part of the weight-loss mechanism of action of GLP-1RAs is debated. Using scintigraphy54 (the gold standard technique for measuring GE), liraglutide has been shown to be associated with significantly delayed GE of solids after 5 and 16 weeks of treatment compared with placebo. GE at week 5 was significantly associated with the degree of weight loss at 16 weeks. However, despite evidence of tachyphylaxis to drug-induced deceleration of GE after 16 weeks, weight loss effects of treatment remained unchanged. Therefore, the early changes in GE may also contribute to liraglutide-induced weight loss.
Notably, delayed GE does not seem to be linked to one of the most common side effects of GLP-1RAs, nausea.59 GE measured using scintigraphy did not differ among those individuals taking liraglutide who reported nausea and those who did not.54 Similarly, in a study of 16 individuals treated with exenatide, no significant differences in the rates of GE, determined using scintigraphy, were observed between individuals who experienced GI adverse events (e.g., nausea) and those who did not.60
Intestinal flora
Long thought to be inactive commensal organisms, microbiota has been implicated in alteration in glucose metabolism and energy homeostasis within their host based on studies in animal. Enteric microbiota are observed throughout the GI tract, and although the profile differs between individuals, the relative abundance of differing species is similar in healthy subjects.9 Given the large diversity displayed in gut flora, defining what is “normal composition” for human gut microbiota is difficult.61 However, individuals with obesity display less microbial diversity than those of normal weight.62
The differences in bacterial diversity between individuals who are lean and those with obesity are thought to result in alterations to microbiota-generated metabolites that, in turn, affect energy homeostasis through the regulation of peptide secretion, including GLP-1.62,63 Mice bred to exhibit an absence of gut flora demonstrate a reduced expression of a number of GPCRs thought to mediate gut peptide release from enteroendocrine cells, which is thought to promote increased energy intake in these animals.64 Furthermore, in mice, the imbalance of gut microbiota that occurs in obesity reduces the expression of GLP-1Rs.65 In humans, evidence suggests that prebiotic treatment increases microbiome diversity, which in turn may modulate levels of GLP-166 and promote the feeling of satiety.67 Given the close anatomical proximity, gut microbiota could potentially alter the nutrient-sensing capacity of the enteroendocrine cells and subsequent gut peptide release.68 However, 7-day treatment with antibiotics had no effect on glucose tolerance despite increasing bacterial diversity in nondiabetic obese individuals diagnosed with either impaired fasting glucose or impaired glucose tolerance.69
Interestingly, and with implications for the development of future obesity treatments, the gut microbiota profile in rats appears to change following treatment with liraglutide and after bariatric surgery to reflect the profile of lean subjects.70,71 It has been suggested that the GLP-1RAs that influence gut transit time and the rate of GE could modify microbiota diversity due to alterations nutrient composition.71 Taking these data into consideration, while it is possible that microbiota may play a role in the gut–brain axis promoting improved energy balance, well-designed studies in human are lacking.
GLP-1-Based Therapeutic Intervention for Treatment of Obesity
Although GLP-1RAs were originally developed to treat diabetes, the additional role of GLP-1 in energy balance broadens the spectrum of uses for its beneficial effects, including for weight management in people with obesity. This is evidenced by the fact that all GLP-1RAs approved to treat diabetes have been shown to result in some degree of weight loss (Table 1), with one currently approved for chronic weight management in both the United States and Europe.72
The weight-loss efficacy of these compounds differs, likely due to variations in chemical modifications that alter their pharmacokinetic parameters or pharmacodynamic properties to increase their half-lives compared with native GLP-1.73 For example, the relatively large molecule albiglutide74 may activate GLP-1R in the hypothalamus to a lesser extent than smaller molecules such as liraglutide and exenatide. This may be the result of a hindered passage through fenestrated capillaries as the compounds enter the brain through circumventricular organs,75 leading to a smaller weight loss response when compared with smaller molecules that are able to activate central GLP-1R to a greater extent.
Weight-loss procedures such as Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG) have gained popularity in recent years, given their effectiveness in the treatment of obesity and its comorbidities.76–78 Patients following RYGB, and to a lesser extent following SG, experience changes to a number of appetite hormones, including an 10-fold increase in meal-induced GLP-1 secretion,79–81 likely due to rapid nutrient emptying from the stomach pouch to the distal small intestine. Given the known anorectic effect of GLP-1, the secretion of this peptide in response to nutrient ingestion was one of the first gut-hormones proposed to explain the robust and sustainable weight loss following RYGB.
However, the benefit of these procedures on weight regulation is unlikely to be explained by alteration in just one factor, as rerouted GI surgeries leads to a myriad of alterations in gut function, meal-induced hormone secretion, and changes in microbiome. For example, GLP-1R knockout mice do not appear to show any difference in weight loss after gastric bypass or SG compared to wild animals.82–84 On the contrary, a recent study in humans using fMRI to measure CNS activation in response to food consumption in women with obesity showed significant reductions in activity of insular cortex (key structure controlling emotion involved with the pleasure experienced from palatable food) from presurgery to 4 weeks after RYGB, corresponding with large increases in meal-stimulated GLP-1 levels.85 Importantly, they demonstrated a reversal of these changes by coadministration of a GLP-1R antagonist, hinting toward a beneficial contribution of this peptide in energy regulation after RYGB.85 While these data are only suggestive of the role GLP-1 in mediating weight loss after RYGB, the discrepancy between animal model and human responses points out the necessity of well-designed studies in humans to address this question. Interestingly, the broad changes in different gut-hormonal profiles following bariatric surgeries that favor sustained weight loss81,86,87 have encouraged strategies to replicate these effects through production of hybrid peptides and coformulations. Examples of these hybrid peptides are GLP-1/glucagon88 or GLP-1/GIP89 coagonists, which have shown promise in their effectiveness to improve appetite and diabetes control.
Conclusion
Energy homeostasis is regulated by a complex interaction of multiple organ systems, including the gut–brain axis. Disruption of this complex system is associated with the development of obesity and diabetes. GLP-1, a 30-amino acid peptide predominantly secreted by neuroendocrine L-cells in the gut, is thought to be a key regulator of this homeostatic axis. Although the effect of gut-derived GLP-1 at physiologic concentrations on energy balance seems to be mediated by action on peripheral vagal afferents, recently developed GLP-1RAs appear to have significant weight-loss effects predominantly through direct central activation of GLP-1Rs.
The role that GLP-1 plays in the gut–brain axis offers rich clinical potential not only for improved glucose metabolism but also to improve appetite control and adiposity. Weight-loss efficacy differs among GLP-1RAs, in part, due to differing pharmacokinetic and pharmacodynamic properties. Although GLP-1RAs are mainly approved for the management of type 2 diabetes, liraglutide (3 mg) is recently approved for weight management in patients with obesity, and several are in early clinical trials.
Acknowledgments
The authors are grateful to Jamie Cozens, MSc, of Watermeadow Medical, UK (an Ashfield company), for writing assistance in the development of this article. This assistance was funded by Novo Nordisk, which also had a role in the review of the article for scientific accuracy.
Authors' Contributions
Dr. M.S. and J.Q.P. planned out the first draft in detail, both have commented extensively on subsequent drafts, and both have edited the final draft for submission.
Author Disclosure Statement
M.S. receives grant funding from NIH (DK083554 and DK105379); J.Q.P. receives compensation as an advisor for Novo Nordisk and receives grant funding from the NIH.
References
- 1. Fothergill E, Guo J, Howard L, et al. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity (Silver Spring) 2016;24:1612–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med 2011;365:1597–1604 [DOI] [PubMed] [Google Scholar]
- 3. Schwartz MW, Seeley RJ, Zeltser LM, et al. Obesity pathogenesis: An endocrine society scientific statement. Endocr Rev 2017;38:267–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hill JO, Wyatt HR, Peters JC. Energy balance and obesity. Circulation 2012;126:126–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bray GA, Heisel WE, Afshin A, et al. The science of obesity management: An endocrine society scientific statement. Endocr Rev 2018;39:79–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Badman MK, Flier JS. The gut and energy balance: Visceral allies in the obesity wars. Science 2005;307:1909–1914 [DOI] [PubMed] [Google Scholar]
- 7. Matafome P, Eickhoff H, Letra L, et al. Neuroendocrinology of adipose tissue and gut-brain axis. Adv Neurobiol 2017;19:49–70 [DOI] [PubMed] [Google Scholar]
- 8. Yi CX, Tschop MH. Brain-gut-adipose-tissue communication pathways at a glance. Dis Model Mech 2012;5:583–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carabotti M, Scirocco A, Maselli MA, et al. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 2015;28:203–209 [PMC free article] [PubMed] [Google Scholar]
- 10. Pavlow I. The Work of the Digestive Glands. London: C. Griffin; 1902 [Google Scholar]
- 11. Bayliss WM, Starling EH. The mechanism of pancreatic secretion. J Physiol 1902;28:325–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reimann F, Tolhurst G, Gribble FM. G-protein-coupled receptors in intestinal chemosensation. Cell Metab 2012;15:421–431 [DOI] [PubMed] [Google Scholar]
- 13. Suzuki K, Simpson KA, Minnion JS, et al. The role of gut hormones and the hypothalamus in appetite regulation. Endocr J 2010;57:359–372 [DOI] [PubMed] [Google Scholar]
- 14. Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007;132:2131–2157 [DOI] [PubMed] [Google Scholar]
- 15. Mojsov S, Heinrich G, Wilson IB, et al. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. J Biol Chem 1986;261:11880–11889 [PubMed] [Google Scholar]
- 16. Drucker DJ, Asa S. Glucagon gene expression in vertebrate brain. J Biol Chem 1988;263:13475–13478 [PubMed] [Google Scholar]
- 17. Sandoval DA, D'Alessio DA. Physiology of proglucagon peptides: Role of glucagon and GLP-1 in health and disease. Physiol Rev 2015;95:513–548 [DOI] [PubMed] [Google Scholar]
- 18. Trapp S, Richards JE. The gut hormone glucagon-like peptide-1 produced in brain: Is this physiologically relevant? Curr Opin Pharmacol 2013;13:964–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vrang N, Larsen PJ. Preproglucagon derived peptides GLP-1, GLP-2 and oxyntomodulin in the CNS: Role of peripherally secreted and centrally produced peptides. Prog Neurobiol 2010;92:442–462 [DOI] [PubMed] [Google Scholar]
- 20. Runge S, Thogersen H, Madsen K, et al. Crystal structure of the ligand-bound glucagon-like peptide-1 receptor extracellular domain. J Biol Chem 2008;283:11340–11347 [DOI] [PubMed] [Google Scholar]
- 21. Singh AK. Dipeptidyl peptidase-4 inhibitors: Novel mechanism of actions. Indian J Endocrinol Metab 2014;18:753–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. D'Alessio D. Is GLP-1 a hormone: Whether and When? J Diabetes Investig 2016;7 Suppl 1:50–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vahl TP, Tauchi M, Durler TS, et al. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology 2007;148:4965–4973 [DOI] [PubMed] [Google Scholar]
- 24. Valassi E, Scacchi M, Cavagnini F. Neuroendocrine control of food intake. Nutr Metab Cardiovasc Dis 2008;18:158–168 [DOI] [PubMed] [Google Scholar]
- 25. Simpson KA, Martin NM, Bloom SR. Hypothalamic regulation of food intake and clinical therapeutic applications. Arq Bras Endocrinol Metabol 2009;53:120–128 [DOI] [PubMed] [Google Scholar]
- 26. Seo S, Ju S, Chung H, et al. Acute effects of glucagon-like peptide-1 on hypothalamic neuropeptide and AMP activated kinase expression in fasted rats. Endocr J 2008;55:867–874 [DOI] [PubMed] [Google Scholar]
- 27. Farr OM, Sofopoulos M, Tsoukas MA, et al. GLP-1 receptors exist in the parietal cortex, hypothalamus and medulla of human brains and the GLP-1 analogue liraglutide alters brain activity related to highly desirable food cues in individuals with diabetes: A crossover, randomised, placebo-controlled trial. Diabetologia 2016;59:954–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kanoski SE, Fortin SM, Arnold M, et al. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology 2011;152:3103–3112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pannacciulli N, Le DS, Salbe AD, et al. Postprandial glucagon-like peptide-1 (GLP-1) response is positively associated with changes in neuronal activity of brain areas implicated in satiety and food intake regulation in humans. Neuroimage 2007;35:511–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Secher A, Jelsing J, Baquero AF, et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest 2014;124:4473–4488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Holmes GM, Browning KN, Tong M. Qualls-Creekmore E, Travagli RA, Vagally mediated effects of glucagon-like peptide 1: In vitro and in vivo gastric actions. J Physiol 2009;587:4749–4759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol 1999;403:261–280 [DOI] [PubMed] [Google Scholar]
- 33. Hisadome K, Reimann F, Gribble FM, et al. Leptin directly depolarizes preproglucagon neurons in the nucleus tractus solitarius: Electrical properties of glucagon-like Peptide 1 neurons. Diabetes 2010;59:1890–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab 2012;16:296–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gaykema RP, Daniels TE, Shapiro NJ, et al. Immune challenge and satiety-related activation of both distinct and overlapping neuronal populations in the brainstem indicate parallel pathways for viscerosensory signaling. Brain Res 2009;1294:61–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rinaman L. A functional role for central glucagon-like peptide-1 receptors in lithium chloride-induced anorexia. Am J Physiol 1999;277:R1537–R1540 [DOI] [PubMed] [Google Scholar]
- 37. Vrang N, Phifer CB, Corkern MM, et al. Gastric distension induces c-Fos in medullary GLP-1/2-containing neurons. Am J Physiol Regul Integr Comp Physiol 2003;285:R470–R478 [DOI] [PubMed] [Google Scholar]
- 38. Barrera JG, Jones KR, Herman JP, et al. Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide-1 loss of function. J Neurosci 2011;31:3904–3913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Meeran K, O'Shea D, Edwards CM, et al. Repeated intracerebroventricular administration of glucagon-like peptide-1-(7-36) amide or exendin-(9-39) alters body weight in the rat. Endocrinology 1999;140:244–250 [DOI] [PubMed] [Google Scholar]
- 40. Gaykema RP, Newmyer BA, Ottolini M, et al. Activation of murine pre-proglucagon-producing neurons reduces food intake and body weight. J Clin Invest 2017;127:1031–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Navarro M, Rodriquez de Fonseca F, Alvarez E, et al. Colocalization of glucagon-like peptide-1 (GLP-1) receptors, glucose transporter GLUT-2, and glucokinase mRNAs in rat hypothalamic cells: Evidence for a role of GLP-1 receptor agonists as an inhibitory signal for food and water intake. J Neurochem 1996;67:1982–1991 [DOI] [PubMed] [Google Scholar]
- 42. Sisley S, Gutierrez-Aguilar R, Scott M, et al. Neuronal GLP1R mediates liraglutide's anorectic but not glucose-lowering effect. J Clin Invest 2014;124:2456–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Verdich C, Flint A, Gutzwiller JP, et al. A meta-analysis of the effect of glucagon-like peptide-1 (7–36) amide on ad libitum energy intake in humans. J Clin Endocrinol Metab 2001;86:4382–4389 [DOI] [PubMed] [Google Scholar]
- 44. Flint A, Raben A, Ersboll AK, et al. The effect of physiological levels of glucagon-like peptide-1 on appetite, gastric emptying, energy and substrate metabolism in obesity. Int J Obes Relat Metab Disord 2001;25:781–792 [DOI] [PubMed] [Google Scholar]
- 45. van Can J, Sloth B, Jensen CB, et al. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes (Lond) 2014;38:784–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ten Kulve JS, Veltman DJ, van Bloemendaal L, et al. Endogenous GLP1 and GLP1 analogue alter CNS responses to palatable food consumption. J Endocrinol 2016;229:1–12 [DOI] [PubMed] [Google Scholar]
- 47. van Bloemendaal L IJ.zerman RG, Ten Kulve JS, et al. GLP-1 receptor activation modulates appetite- and reward-related brain areas in humans. Diabetes 2014;63:4186–4196 [DOI] [PubMed] [Google Scholar]
- 48. ten Kulve JS, Veltman DJ, van Bloemendaal L, et al. Endogenous GLP-1 mediates postprandial reductions in activation in central reward and satiety areas in patients with type 2 diabetes. Diabetologia 2015;58:2688–2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lean ME, Carraro R, Finer N, et al. Tolerability of nausea and vomiting and associations with weight loss in a randomized trial of liraglutide in obese, non-diabetic adults. Int J Obes (Lond) 2014;38:689–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lean M, Le Roux C, Fujioka K, et al. The imapct of gastrointestinal adverse events on weight loss with liraglutide 3.0 mg as adjunct to a diet and exercise program. Endocr Pract 2015;21(Suppl. 2):118–119 [Google Scholar]
- 51. Salehi M, Aulinger B, Prigeon RL, et al. Effect of endogenous GLP-1 on insulin secretion in type 2 diabetes. Diabetes 2010;59:1330–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stevens JE, Horowitz M, Deacon CF, et al. The effects of sitagliptin on gastric emptying in healthy humans—A randomised, controlled study. Aliment Pharmacol Ther 2012;36:379–390 [DOI] [PubMed] [Google Scholar]
- 53. Vella A, Bock G, Giesler PD, et al. Effects of dipeptidyl peptidase-4 inhibition on gastrointestinal function, meal appearance, and glucose metabolism in type 2 diabetes. Diabetes 2007;56:1475–1480 [DOI] [PubMed] [Google Scholar]
- 54. Halawi H, Khemani D, Eckert D, et al. Effects of liraglutide on weight, satiation, and gastric functions in obesity: A randomised, placebo-controlled pilot trial. Lancet Gastroenterol Hepatol 2017;2:890–899 [DOI] [PubMed] [Google Scholar]
- 55. Horowitz M, Flint A, Jones KL, et al. Effect of the once-daily human GLP-1 analogue liraglutide on appetite, energy intake, energy expenditure and gastric emptying in type 2 diabetes. Diabetes Res Clin Pract 2012;97:258–266 [DOI] [PubMed] [Google Scholar]
- 56. Amato A, Baldassano S, Liotta R, et al. Exogenous glucagon-like peptide 1 reduces contractions in human colon circular muscle. J Endocrinol 2014;221:29–37 [DOI] [PubMed] [Google Scholar]
- 57. Amato A, Cinci L, Rotondo A, et al. Peripheral motor action of glucagon-like peptide-1 through enteric neuronal receptors. Neurogastroenterol Motil 2010;22:664–e203 [DOI] [PubMed] [Google Scholar]
- 58. Uccellatore A, Genovese S, Dicembrini I, et al. Comparison review of short-acting and long-acting glucagon-like peptide-1 receptor agonists. Diabetes Ther 2015;6:239–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kashyap P, Farrugia G. Oxidative stress: Key player in gastrointestinal complications of diabetes. Neurogastroenterol Motil 2011;23:111–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Linnebjerg H, Park S, Kothare PA, et al. Effect of exenatide on gastric emptying and relationship to postprandial glycemia in type 2 diabetes. Regul Pept 2008;151:123–129 [DOI] [PubMed] [Google Scholar]
- 61. Aguirre M, Bussolo de Souza C, Venema K. The gut microbiota from lean and obese subjects contribute differently to the fermentation of arabinogalactan and inulin. PLoS One 2016;11:e0159236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. de Clercq NC, Frissen MN, Groen AK, et al. Gut microbiota and the gut-brain axis: New insights in the pathophysiology of metabolic syndrome. Psychosom Med 2017;79:874–879 [DOI] [PubMed] [Google Scholar]
- 63. Zadeh-Tahmasebi M, Duca FA, Rasmussen BA, et al. Activation of short and long chain fatty acid sensing machinery in the ileum lowers glucose production in vivo. J Biol Chem 2016;291:8816–8824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Duca FA, Swartz TD, Sakar Y, et al. Increased oral detection, but decreased intestinal signaling for fats in mice lacking gut microbiota. PLoS One 2012;7:e39748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Grasset E, Puel A, Charpentier J, et al. A specific gut microbiota dysbiosis of type 2 diabetic mice induces GLP-1 resistance through an enteric NO-dependent and gut-brain axis mechanism. Cell Metab 2017;26:278. [DOI] [PubMed] [Google Scholar]
- 66. Everard A, Cani PD. Diabetes, obesity and gut microbiota. Best Pract Res Clin Gastroenterol 2013;27:73–83 [DOI] [PubMed] [Google Scholar]
- 67. Cani PD, Joly E, Horsmans Y, et al. Oligofructose promotes satiety in healthy human: A pilot study. Eur J Clin Nutr 2006;60:567–572 [DOI] [PubMed] [Google Scholar]
- 68. Duca FA, Lam TK. Gut microbiota, nutrient sensing and energy balance. Diabetes Obes Metab 2014;16 Suppl 1:68–76 [DOI] [PubMed] [Google Scholar]
- 69. Reijnders D, Goossens GH, Hermes GDA, et al. Short-term microbiota manipulation and forearm substrate metabolism in obese men: A randomized, double-blind, placebo-controlled trial. Obes Facts 2018;11:318–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Magouliotis DE, Tasiopoulou VS, Sioka E, et al. Impact of bariatric surgery on metabolic and gut microbiota profile: A systematic review and meta-analysis. Obes Surg 2017;27:1345–1357 [DOI] [PubMed] [Google Scholar]
- 71. Wang L, Li P, Tang Z, et al. Structural modulation of the gut microbiota and the relationship with body weight: Compared evaluation of liraglutide and saxagliptin treatment. Sci Rep 2016;6:33251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kumar RB, Aronne LJ. Pharmacologic Treatment of Obesity. South Dartmouth, MA: Endotext; 2000 [Google Scholar]
- 73. Runge S, Wulff BS, Madsen K, et al. Different domains of the glucagon and glucagon-like peptide-1 receptors provide the critical determinants of ligand selectivity. Br J Pharmacol 2003;138:787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. GlaxoSmithKline. TANZEUM (albiglutide) for injection, for subcutaneous use. 2014; Accessed at www.accessdata.fda.gov/drugsatfda_docs/label/2015/125431s009lbl.pdf on July 10, 2018
- 75. Madsbad S. Review of head-to-head comparisons of glucagon-like peptide-1 receptor agonists. Diabetes Obes Metab 2016;18:317–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Courcoulas AP, King WC, Belle SH, et al. Seven-year weight trajectories and health outcomes in the longitudinal assessment of bariatric surgery (LABS) study. JAMA Surg 2018;153:427–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Adams TD, Davidson LE, Litwin SE, et al. Weight and metabolic outcomes 12 years after gastric bypass. N Engl J Med 2017;377:1143–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med 2017;376:641–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Salehi M, Gastaldelli A, D'Alessio DA. Altered islet function and insulin clearance cause hyperinsulinemia in gastric bypass patients with symptoms of postprandial hypoglycemia. J Clin Endocrinol Metab 2014;99:2008–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Peterli R, Wolnerhanssen B, Peters T, et al. Improvement in glucose metabolism after bariatric surgery: Comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: A prospective randomized trial. Ann Surg 2009;250:234–241 [DOI] [PubMed] [Google Scholar]
- 81. Purnell JQ, Johnson GS, Wahed AS, et al. Prospective evaluation of insulin and incretin dynamics in obese adults with and without diabetes for 2 years after Roux-en-Y gastric bypass. Diabetologia 2018;61:1142–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ye J, Hao Z, Mumphrey MB, et al. GLP-1 receptor signaling is not required for reduced body weight after RYGB in rodents. Am J Physiol Regul Integr Comp Physiol 2014;306:R352–R362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wilson-Perez HE, Chambers AP, Ryan KK, et al. Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like Peptide 1 receptor deficiency. Diabetes 2013;62:2380–2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mokadem M, Zechner JF, Margolskee RF, et al. Effects of Roux-en-Y gastric bypass on energy and glucose homeostasis are preserved in two mouse models of functional glucagon-like peptide-1 deficiency. Mol Metab 2014;3:191–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ten Kulve JS, Veltman DJ, Gerdes VEA, et al. Elevated postoperative endogenous GLP-1 levels mediate effects of Roux-en-Y gastric bypass on neural responsivity to food cues. Diabetes Care 2017;40:1522–1529 [DOI] [PubMed] [Google Scholar]
- 86. Yousseif A, Emmanuel J, Karra E, et al. Differential effects of laparoscopic sleeve gastrectomy and laparoscopic gastric bypass on appetite, circulating acyl-ghrelin, peptide YY3–YY36 and active GLP-1 levels in non-diabetic humans. Obes Surg 2014;24:241–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. le Roux CW, Aylwin SJ, Batterham RL, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg 2006;243:108–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Cegla J, Troke RC, Jones B, et al. Coinfusion of low-dose GLP-1 and glucagon in man results in a reduction in food intake. Diabetes 2014;63:3711–3720 [DOI] [PubMed] [Google Scholar]
- 89. Frias JP, Bastyr EJ, 3rd, Vignati L, et al. The sustained effects of a dual GIP/GLP-1 receptor agonist, NNC0090-2746, in patients with type 2 diabetes. Cell Metab 2017;26:343–352.e2 [DOI] [PubMed] [Google Scholar]
- 90. Merck & Co Inc., JANUVIA® (sitagliptin) Tablets 2011. Accessed at www.accessdata.fda.gov/drugsatfda_docs/label/2012/021995s019lbl.pdf on March 13, 2018
- 91. Novartis Europharm Limited., Galvus 50 mg tablets: Summary of Product Characteristics. 2008. Accessed at www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000771/WC500020327.pdf on March 13, 2018
- 92. AstraZeneca. ONGLYZA (saxagliptin) tablets. 2009. Accessed at www.accessdata.fda.gov/drugsatfda_docs/label/2009/022350lbl.pdf on March 13, 2018
- 93. Boehringer Ingelheim International GmbH. Tradjenta™ (linagliptin) tablets. 2011. Accessed at www.accessdata.fda.gov/drugsatfda_docs/label/2011/201280lbl.pdf on March 13, 2018
- 94. Takeda Pharmaceuticals. NESINA (alogliptin) tablets, for oral use. 2016. Accessed at www.accessdata.fda.gov/drugsatfda_docs/label/2016/022271s005lbl.pdf on March 13, 2018
- 95. Amylin Pharmaceuticals Inc. BYETTA® (exenatide) Injection. 2009. Accessed at www.accessdata.fda.gov/drugsatfda_docs/label/2009/021773s9s11s18s22s25lbl.pdf on March 13, 2018
- 96. Amylin Pharmaceuticals Inc. BYDUREON™ (exenatide extended-release for injectable suspension). 2012. Accessed at https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/022200s026lbl.pdf#page=31
- 97. Eli Lilly and Company. TRULICITY (dulaglutide) injection, for subcutaneous use. 2017. Accessed at www.medicines.org.uk/emc/medicine/29747 on March 13, 2018
- 98. Novo Nordisk. Victoza® (liraglutide [rDNA origin] injection), solution for subcutaneous use. 2010. Accessed at www.accessdata.fda.gov/drugsatfda_docs/label/2010/022341lbl.pdf
- 99. le Roux CW, Astrup A, Fujioka K, et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: A randomised, double-blind trial. Lancet 2017;389:1399–1409 [DOI] [PubMed] [Google Scholar]
- 100. Sanofi-Aventis. Lyxumia solution for injection. 2013. Accessed at www.medicines.org.uk/emc/files/pil.2965.pdf on March 13, 2018
- 101. Novo Nordisk. OZEMPIC (semaglutide) injection, for subcutaneous use. 2017. Accessed at www.accessdata.fda.gov/drugsatfda_docs/label/2017/209637lbl.pdf on March 13, 2018
- 102. Intarcia Therapeutics Inc. Intarcia Presents FREEDOM-2 Trial Results in Type 2 Diabetes Demonstrating Clinically Meaningful Superiority and Sustained Glucose Control and Weight Reduction for ITCA 650 vs. Januvia®: Oral Presentation at ADA 76th Scientific Sessions. 2016. Accessed at www.intarcia.com/media/press-releases/2016-june-12-freedom-2-trial-results-in-type-2-diabetes.html