Abstract
Glioblastoma multiforme (GBM) is the most common malignant primary brain tumor with poor prognosis, and currently effective therapeutic strategies are still limited. Although temozolomide (TMZ) is commonly used for GBM therapy and its mechanism was well characterized, while its side effects were required comprehensive investigation. In the present study, we revealed that TMZ-challenged GBM cells strongly suppressed pro-inflammatory cytokines expression in activated periphery blood mononuclear cells (PBMC), which depended on enhanced transcription of CD274 (encoding PD-L1), but not other immune checkpoints, such as CD276, HVEM and galectin-9. Moreover, abundance of membranous PD-L1 was also increased in TMZ-treated GBM cells. When PD-L1 expression was knocked down by short hairpin RNA (shRNA), inhibitory effect of TMZ-treated GBM cells on PBMC became weakened, suggesting that PD-L1 was crucial for immune inhibition capacity of TMZ-treated GBM cells. Additionally, actinomycin D reduced PD-L1 expression in GBM cells after TMZ challenge, indicating that PD-L1 induction occurred at transcriptional level. The immunoblotting results demonstrated that STAT3 signaling was involved in TMZ-mediated PD-L1 induction, and attenuated expression of PD-L1 was observed using STAT3 inhibitor VI or STAT3 shRNA. Finally, the animal study showed that combination of TMZ and PD-1 antibody therapy strongly inhibited tumor growth and achieved the improved survival rate of GBM mice. Accordingly, this study revealed the classical chemotherapy drug TMZ promoted GBM cells immune escape, even TMZ combine with PD-1 antibody treatment not further improve survival ratio of recurrent GBM patients compared with traditional therapy methods, while our animal study provided evidence that combination of TMZ and PD-1 antibody was a promising way to treat GBM, these contradictory results indicate improving the PD-1 antibody delivery efficiency can exert strong combinational therapy outcomes.
Keywords: GBM, temozolomide, PD-L1, immune escape, combinational therapy
Introduction
Glioblastoma multiforme (GBM) is the most common and aggressive primary brain tumor in adults. Patients with GBM have traditionally been thought to have terrible prognosis, despite multiple therapies such as surgical resection combined with radiotherapy and chemotherapy using temozolomide (TMZ). Due to growth feature of GBM cells, it is difficult to remove tumor tissue completely through surgery. Surgery concomitant radio- and chemotherapy increased median survival time of the patients to 12-15 months, while 5-year survival rate is still less than 5% [1]. Thus, how to improve the treatment outcome in GBM is one of the biggest therapeutic challenges of the modern medicine [2].
TMZ is an oral alkylating agent and regarded as the first-line chemotherapeutic drug to treat GBM after operation in this decade [3]. Anti-tumor effect of TMZ depends on its ability to alkylate/methylate DNA, which commonly occurs at N7 or O6 position of guanine residues [4]. This modification damages DNA and induces death of cancer cells. However, this type of DNA damage can be repaired by O6-alklguanine DNA alkyltransferase encoded by O-6-methylguanine-DNA methyltransferase (MGMT) gene [5]. Therefore, the MGMT expression level is highly correlated with the TMZ therapeutic efficacy on GBM. Yung et al. revealed that TMZ alone also suppressed tumor growth [6]. For improving the GBM patient survival time and the curative effect, physicians combines TMZ with radiotherapy, anti-angiogenesis therapy or immunotherapy to treat GBM, and TMZ concomitant radiotherapy is the most powerful strategy for GBM treatment until now [1,7-10].
In recent 5 years, several clinical studies showed that immunotherapy achieved good results in some cancers, like lung cancer, melanoma and prostate cancer [11-13]. Moreover, preclinical experimental study suggested that combination of classical chemotherapy with immunotherapy was a new encouraging way to combat GBM [14,15]. It is well known that chemotherapy can partially induce immune suppression like leucopenia and granulocytopenia, but remaining immune-related cells can exert their functions on tumor killing [16-18]. However, some chemotherapeutic drugs, such as anthracycline, doxorubicin and daunorubicin, are capable to induce activation markers on immune cells and immunogenic markers on tumor cells for further enhancing anti-tumor activity of immune cells [19,20]. Besides, small amount of chemotherapy agents exhibit immunoactivation effects, whereas some agents up-regulate immunosuppressive molecules in cancer cells or other cancer-surrounding cells for blocking the anti-tumor capacity of immune cells [21].
Ishikawa et al. showed that TMZ also has adverse effect as inducing leucopenia during GBM therapy, but whether TMZ can promote or impair immune evasion of GBM cells is not elucidated [22]. In order to have a better understanding of TMZ effects on tumor immune escape, we co-cultured periphery blood mononuclear cells (PBMC) with GBM cells or TMZ-treated GBM cells, and showed that TMZ-treated GBM cells had higher inhibition capacity on proinflammatory cytokines production of PBMC than control GBM cells. Knock-down of PD-L1 impaired TMZ-induced inhibition effect of GBM cells on PBMC, suggesting that PD-L1 was a key immune checkpoint involved in GBM cells immune evasion after TMZ challenge. Additionally, TMZ-mediated PD-L1 expression in GBM cells was depended on the activation of STAT3 signaling. Finally, combination therapy of TMZ and PD-1 antibody greatly shrank the tumor size and improved survival rate in GBM animal models. In general, these data illustrated that TMZ promotes immune escape of GBM cells via upregulating PD-L1, and combination of TMZ and PD-1 antibody was a promising strategy for GBM patients.
Materials and methods
Reagents, antibodies and immunoblot analysis
The Tubulin antibody (1:5000, T6074) and TMZ were obtained from Sigma-Aldrich (St. Louis, MO). STAT3 inhibitor VI was purchased from EMD Millipore (Billerica, MA). Antibodies against human PD-L1 (1:1000, 13684), STAT3 (1:1000, 9139), STAT3pY705 (1:1000, 9145), HIF-1α (1:1000, 72933) and c-Myc (1:1000, 13987) were purchased from Cell Signaling Technology (Danvers, MA). Cells were lyzed by RIPA lysis buffer (Beyotime, Beijing, China), and the extracts were clarified via centrifugation at 15000 g. Total protein concentration in the cell lysate was determined by BCA (Beyotime), and 50 μg protein was subjected to immunoblot analysis with corresponding antibodies. Each experiment was repeated at least 3 times.
Primers and qPCR
Total RNA isolation, reverse transcription (RT), and real-time PCR were performed as described the previous study [23]. The following primers were used for qPCR: human CD274 forward 5’-CTGCACTTTTAGGAGATTAGATC-3’ and reverse 5’-CTACACCAAGGCATAATAAGATG; murine CD274 forward 5’-GTGAAACCCTGAGTCTTATCC-3’ and reverse 5’-GACCATTCTGAGACAATTCC-3’; human β-actin forward 5’-CTCCATCCTGGCCTCGCTGT-3’ and reverse 5’-GCTGTCACCTTCACCGTTCC-3’; murine β-actin forward 5’-ATGGATGACGATATCGCTGCGC-3’ and reverse 5’-GCAGCACAGGGTGCTCCTCA-3; human IL-2 forward: 5’-ACTCACCAGGATGCTCACATT-3’ and reverse 5’-AGTCCCTGGGTCTTAAGTGA-3’; human IFN-γ forward 5’-CTGCCAGGACCCATATGTAA-3’ and reverse 5’-GAGACAATTTGGCTCTGCATT-3’; human CD276 forward 5’-AGCTGTGAGGAGGAGAATGCA-3’ and reverse; 5’-CTTGTCCATCATCTTCTTTGCTG-3’; human galectin-9 forward 5’-CCTTTTCTGGGACTATTCAAGG and reverse 5’-GAAGTGGAAGGCAATGTCATTTC-3’; human HVEM forward 5’-CAAAACCGACGTCTTGAGGCT-3’ and reverse 5’-CCTCCTTCACACGATAACCTG-3’.
Cell culture, treatment and shRNA
U87, U251, GL261 and luciferase-expressing GL261 (GL261-Luc) cells which were provided by BeNa culture collection (Beijing, China) were maintained in Dulbecco’s modified Eagle’s medium (DMEM, ThermoFisher Scientific, Grand Island, NY) or DMEM/F12 (ThermoFisher Scientific) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT). All the cell lines were tested for mycoplasma. Cells were plated at a density of 4*105 per 60 mm dish or 1*105 per well of 6-well plate for 24 h before treatment. The GBM cells were treated with different dosages of TMZ for various times as described in figure legends. Transfection was performed using lipofectamine 3000 reagent (ThermoFisher Scientific) according to the manufacture’s instruction. The following pGIPZ shRNAs (GE Dharmacon, Chicago, IL) were used: control shRNA oligonucleotide, GCTTCTAACACCGGAGGTCTT; human STAT3 shRNA oligonucleotide: TACCTAAGGCCATGAACTT; human PD-L1 shRNA oligonucleotide: TTGACTCCATCTTTCTTCA.
GL261-Luc mouse GBM model and animal experiment
One-month-old male C57BL/6 mice were intracranially injected 1*105 GL261-Luc cells. We divided the mice into 4 groups, 10 mice in each group. 100 mg of PD-1 antibody (RMP1-14, Bio X Cell) or a rat IgG (Bio X Cell) as a control was injected intratumorally on day 3, 6, 9, 12, 15 post GL261-Luc cell inoculation, and TMZ (800 μg in 100 μl PBS) or control PBS was injected intraperitoneally (i.p) on day 3, 7, 11, 15. All the mice were monitored for survival and tumor volume. Survival was determined as the number of days from tumor cell inoculation to the day that animals had to be euthanized due to following symptoms, such as hemiparesis, seizures, loss of weight more than 20%, cannot move and other heavy neurological deficits symptoms. The survival results were analyzed by using Graphpad 5 Prism 5.0 software. For tumor volume determination, 5 mice in each group were pick up randomly and anesthetized with isoflurane and i.p with 150 mg/kg D-luciferin in PBS, the bioluminescence imaging was conducted (IVIS 200, Xenogen; exposure time of 30 seconds, binning of 8, field of view of 15 cm, f/stop of 1, and no filter) after 10 minutes of injection. All of the mice were housed in the Wenzhou Medical University animal center (Wenzhou, China); All animal experiments were performed according to the guidelines and regulations of Zhejiang Medical Laboratory Animal Management Committee.
Generation of stable cell line, and co-culture experiments
The U87 and U251 were transfected with corresponding PGIPZ shRNA by using lipofectamine 3000 reagent for 48 hours, and the cells were selected with 1 μg/ml puromycin (InvivoGen, San Diego, CA). The blood was collected from healthy donors and stored at anticoagulant tube, the PBMC were isolated from blood by gradient density centrifugation on RosetteSepTM DM-L Density Medium (Stemcell Technologies, Cambridge, MA), and were activated by Anti-CD3/CD28 Dynabeads (ThermoFisher Scientific) according to the instruction for 12 h, meanwhile, the U87 and U251 were treated with or without TMZ for 24 h, then replace with fresh medium and co-cultured activated PBMC for 24 h at a ratio of 1:3 (cancer cell: PBMC). For neutralization experiment, the PD-L1 neutralization antibody (ThermoFisher Scientific, MIH1) or mouse IgG (ThermoFisher Scientific) were added into the cancer cell culture plates prior to adding PBMC. Finally, the supernatants were collected and subjected to enzyme-linked immune-sorbent assay (ELISA) for quantifying IFN-γ and IL-2 producytion (R&D System, Minnesota, USA) according to the instructions.
Flow cytometry analysis
The GBM cells were digested by 0.05% trypsin and washed in PBS for 3 times, then stained for cell surface PD-L1 on ice for 30 min in FACS blocking buffer (0.5% BSA and 2% FBS in PBS). PD-L1 PE-conjugated antibody (ThermoFisher Scientific, MIH1) or IgG1 PE-conjugated antibody (R&D System) were added to the cell suspension solution on ice for 30 min, then the cells were washed with FACS buffer (PBS contained 0.5% BSA) for 3 times and detected by a BD FACSAria III (BD Biosciences) cytometer. The results were analyzed by FlowJo V10.
Statistical analysis
Data in bar graphs represent the mean fold change relative to control groups with s.d. of three independent experiments. A 2 group comparison was conducted using a 2-sided, 2-sample Student’s t test. A simultaneous comparison of more than 2 groups was conducted using 1-way ANOVA through SPSS (Ver. 20, SPSS, Chicago, IL). A P value <0.05 was considered statistically significant.
Results
TMZ treated GBM cells exhibited higher inhibition capacity on pro-inflammatory cytokines production in activated PBMC than control cells
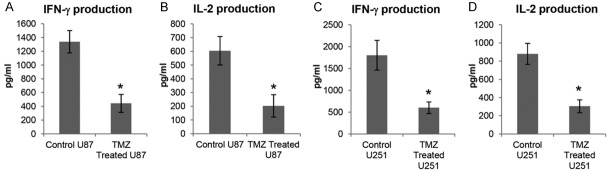
In order to determine whether TMZ can exacerbate the inhibition capacity of GBM on PBMC activation, we co-cultured the PBMC with GBM cells or TMZ treated GBM cells, then the pro-inflammatory cytokines IL-2 and IFN-γ in co-culture medium were detected in these two groups. As shown in Figure 1A, IFN-γ expression was significantly reduced in supernatant which collected from PBMC co-cultured with TMZ treated U87 cells compared to the supernatant collected from PBMC co-cultured with U87 cells. Furthermore, IL-2 production in the medium was also greatly inhibited when PBMC co-cultured with TMZ treated U87 cells (Figure 1B). In addition, IFN-γ and IL-2 had similar manners in co-culture medium when PBMC were co-cultured with TMZ treated U251 cells when compared to parallel group (Figure 1C and 1D).
Figure 1.
TMZ-treated GBM cells strongly impaired expression of pro-inflammatory cytokines. A, B. PBMC were preactivated with Anti-CD3/CD28 beads for 12 h, and co-cultured with U87 cells which were pretreated with or without 200 μM TMZ. Then the culture medium was collected and subjected to IFN-γ and IL-2 quantification by ELISA after 24 h co-incubation. C, D. The activated PBMC were co-cultured with U251 cells which were challenged with or without 50 μM TMZ. Then IFN-γ and IL-2 protein level in co-culture medium were measured by ELISA after 24 h co-incubation. *P < 0.05, on the basis of Student’s t test.
TMZ elevated mRNA levels of CD274, but not other immune checkpoints in GBM cells
To further uncover the underlying mechanism of the strengthened inhibition of GBM cells after TMZ challenge on pro-inflammatory cytokines expression in PBMC, we investigated a serial of immune checkpoints expression in U87 and U251 after TMZ stimulation. Results showed that CD274 (encoding PD-L1) mRNA was up-regulated in U87 after TMZ challenge, and CD274 mRNA level was increased gradually in a time-dependent manner (Figure 2A). However, mRNA levels of other immune checkpoints HVEM, galectin-9 and CD276 were not altered in U87 after TMZ treatment from 0-72 h (Figure 2B-D). Additionally, we also determined gene expression of the above immune checkpoints in U251 with or without TMZ treatment, and the same tendencies were observed (Figure 2E-H).
Figure 2.
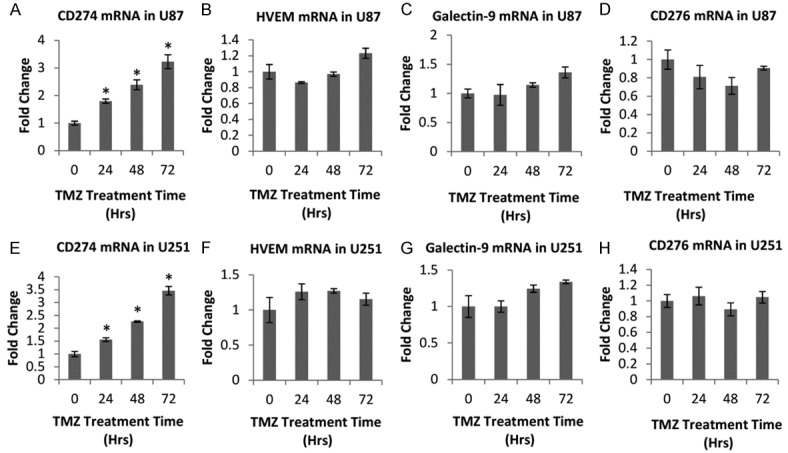
The expression levels of immune checkpoints in GBM cells after TMZ challenge. A-D. U87 cells treated with 200 μM TMZ for different times, were collected for measuring mRNA expression levels of CD274, HVEM, Galectin-9 and CD276 by realtime PCR. E-H. After treated with 50 μM TMZ for 0-72 h, U251 cells were collected for evaluating mRNA expression levels of CD274, HVEM, Galectin-9 and CD276 by realtime PCR. *P < 0.05, on the basis of Student’s t test.
The ratio of PD-L1 positive GBM cell is significantly increased after TMZ treatment
As PD-L1 expressed on the surface of tumor cells exerts immunosuppressive effects through binding to PD-1 on activated T cells, it is essential to assess the level of PD-L1 on cell surface or the percentage of PD-L1 positive cells with or without TMZ treatment. PD-L1 protein levels were evaluated in TMZ-stimulated U87 and U251 cells, showing that PD-L1 expression was increased along with time (Figure 3A and 3B). The membranous PD-L1 expression in U87 and U251 were also significantly increased after TMZ challenge which analyzed by flow cytometry (FCM, Figure 3C, 3D).
Figure 3.
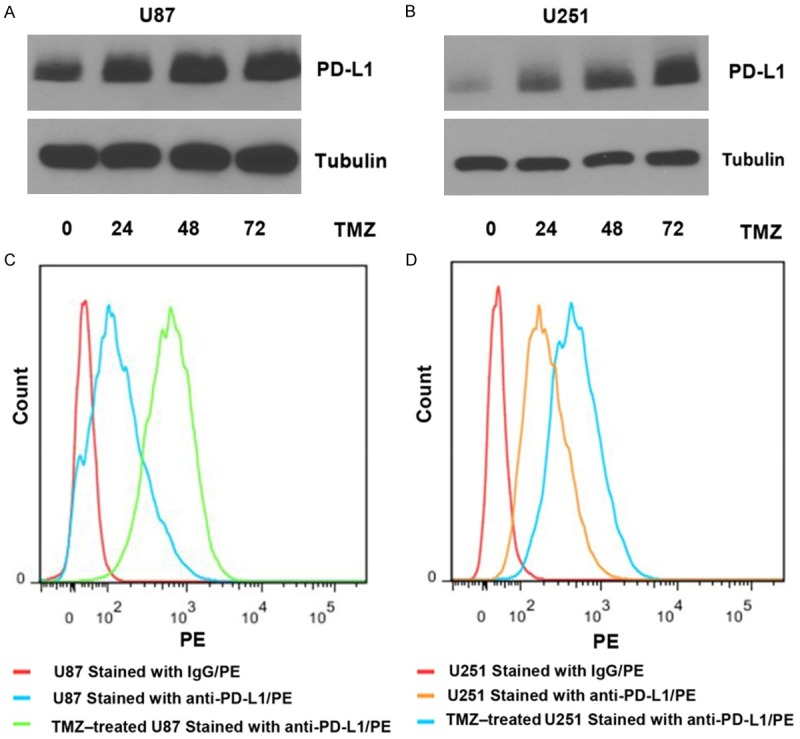
PD-L1 protein expression was dramatically increased in TMZ-treated GBM cells. A, B. U87 and U251 cells were treated with TMZ (200 μM for U87 and 50 μM for U251, similarly hereinafter) for 0-72 h, respectively. Then cells were lyzed by RIPA lysis buffer and immunoblotting analysis was performed with PD-L1 and Tubulin antibodies. C, D. U87 and U251 cells were treated with or without TMZ for 24 h, then the cells were digested and stained with PE-conjugated PD-L1 antibody and were analyzed using a flow cytometer. *P < 0.05, on the basis of Student’s t test.
Depletion of PD-L1 impaired enhanced inhibition of TMZ-treated GBM cells on activated PBMC
As shown in Figure S1A, CD274 mRNA levels in PD-L1 KO U87 were about 10% of that in control cells. Additionally, the PD-L1 depletion efficiency were also evaluated by FCM (Figure 4A). Depletion of PD-L1 greatly impaired inhibition ability of U87 cells on pro-inflammatory cytokines production in medium of PBMC and U87 co-culture system (Figure 4B, 4C). Despite TMZ strengthened inhibition activity of U87 cells, depletion of PD-L1 weakened the inhibitory effect of U87 cells on IFN-γ and IL-2 production in the supernatant of co-culture system (Figure 4B, 4C). Furthermore, the similar results were acquired when PBMC co-cultured with PD-L1 KO U251 after TMZ challenged (Figures 4D-F, S1B). In order to further confirm that upregulation of PD-L1 in TMZ treated GBM is a key factor for reduction of IL-2 and IFN-γ expression in co-culture medium, we added PD-L1 antibody into the co-culture medium, showed that neutralization of PD-L1 also weakened the inhibitory effect of U87 on pro-inflammatory cytokine production (Figure S1C, S1D).
Figure 4.
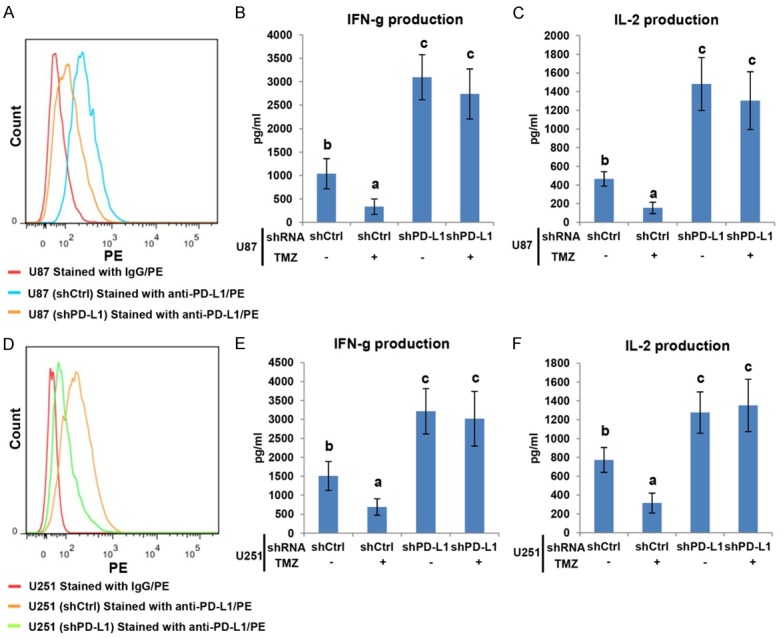
PD-L1 was essential for enhanced inhibition of TMZ-challenged GBM cells on activated PBMC. A. U87 cells were transfected with control shRNA or PD-L1 shRNA before collected for detecting PD-L1 expression. B, C. U87 cells which stable express shRNA or PD-L1 shRNA were pretreated with or without 200 μM TMZ, then co-cultured with pre-activated PBMC for 24 h. IFN-γ and IL-2 level in co-culture medium were analyzed using ELISA. D. U251 cells were transfected with control shRNA or PD-L1 shRNA before collected for detecting PD-L1 expression. E, F. U251 cells which stably express shRNA or PD-L1 shRNA were pretreated with or without 50 μM TMZ, then co-cultured with pre-activated PBMC for 24 h. IFN-γ and IL-2 in the supernatant were analyzed using ELISA. The asterisk or different alphabets denote a significant difference at P < 0.05.
STAT3 activation is vital for TMZ-induced PD-L1 expression in GBM cells
To explore whether TMZ-induced PD-L1 expression is regulated at transcriptional level or post-translational modification in cancer cells, we treated U87 and U251 with or without TMZ and actinomycin D. Figure 5A and 5B showed that actinomycin D attenuated induction effect of TMZ on PD-L1 expression in U87 and U251. Moreover, activation of transcription factors STAT3, HIF-1α and c-Myc could up-regulate CD274 (encoding PD-L1) mRNA level [24-26]. The immunoblotting results revealed that only STAT3pY705 (phosphorylation of tyrosine 705) was highly increased after TMZ treatment from 0.5 h-4 h (Figure 5C). To validate whether STAT3 was involved in TMZ-induced PD-L1 expression, our data showed that STAT3 inhibitor VI not only blocked phosphorylation of tyrosine 705 in STAT3, but also ameliorated induction of PD-L1 which triggered by TMZ (Figures 5D and S2A). What’s more, STAT3 shRNA could block TMZ-mediated PD-L1 induction in U87 cell (Figures 5E and S2C). Blockade of STAT3 signaling also attenuated TMZ-enhanced GBM inhibition capacity on pro-inflammatory cytokines production in the co-culture system (Figure S2B, S2C, S2E, S2F).
Figure 5.

STAT3 signaling is critical for TMZ-induced PD-L1 expression in GBM cells. A, B. U87 and U251 cells were pretreated with 1 μg/ml actinomycin D for 2 h and then challenged with or without TMZ for 24 h. The PD-L1 expression level in GBM cells were determined by FCM. C. U87 cells were treated with 200 μM TMZ for 0-48 h, followed by WB analysis using STAT3pY705, STAT3, HIF-1, c-Myc and Tubulin antibodies. D. U87 cells were pretreated with or without STAT3 inhibitor VI for 1 h and then treated with or without TMZ for 24 h, followed by FCM analysis using PE-conjugated PD-L1 antibody. E. U87 cells which stably express control shRNA (shctrl) or STAT3 shRNA (shSTAT3) were pretreated with or without TMZ for 24 h, followed by FCM quantification with PE-conjugated PD-L1 antibody.
Combination of TMZ with PD-L1 antibody in GL261 murine GBM models was superior to single therapy
We confirmed that TMZ could induce PD-L1 expression in mouse GBM cell line GL261 as well as in human GBM cell line (Figure S3). The GBM mice were divided into four groups, including control group, TMZ group, anti-PD-1 group, and TMZ plus anti-PD-1 group. The drug administration time were indicated at Figure 6A. TMZ or PD-1 antibody alone could suppress tumor growth in GBM mice model, furthermore, combinational therapy of TMZ and PD-L1 antibody had stronger inhibitory effect on tumor growth than control group or the other two single treatment groups (Figure 6B). In addition, survival curves also demonstrated that combination therapy using TMZ plus PD-1 antibody achieved the best survival proportion through all experimental observation process (Figure 6C).
Figure 6.
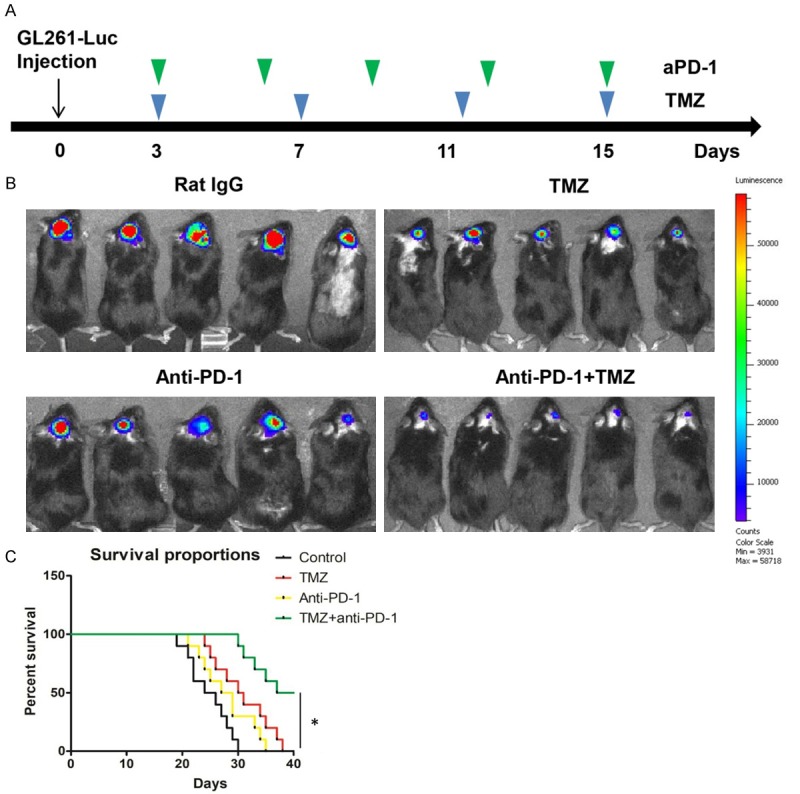
Combination of TMZ with PD-1 blockade therapy in GL261 murine GBM models was superior to single therapy. A. The 1*105 GL261-Luc cells were intracranially injected into C57BL/6 mice. Then the mice were treated with PD-1 antibody or TMZ as described above. B. The representative tumor growth was shown in vivo by bioluminescence imaging using IVIS 200 at 16 days after cell injection. C. The mouse survival times were recorded and visualized using Kaplan-Meier survival curve. The asterisk indicates a significant difference at P < 0.05.
Discussion
In recent years, immunotherapy has attracted considerable attention because of the efficient role played by anti-CTLA-4 and PD-1/PD-L1 blockade therapies for late-stage cancer patients, especially for melanoma and lung cancers patients [23-25]. Encouraged by the good performance of immunotherapy on other types of cancers, the neuro-oncologist also applied this strategy for recurrent GBM treatment. Unfortunately, the prognosis is not as good as scientists and physicians expected [2,26].
Although TMZ has been widely used as the first line chemotherapy drug for gliomas [27], the mechanisms by which TMZ affects characteristics or capacities of GBM cells remain elusive. In the present study, we revealed that suppression of pro-inflammatory cytokines IL-2 and IFN-γ expression in the medium which collected from PBMC co-cultured with TMZ treated U87 and U251 cells, indicating that TMZ exacerbated the immune evasion of GBM cells. These findings guided us to speculate that TMZ may give effect to the expression of immune checkpoints in GBM cell. To address this hypothesis, we investigated mRNA levels of several immune checkpoints in U87 and U251 cells, and found only CD274 expression level was remarkably enhanced after TMZ challenge, which was consistent with Derer et al. study that they showed TMZ induced murine PD-L1 expression in GBM cells [28], while whether TMZ could induce human GBM cells PD-L1 expression was not defined. What’s more, increase in membranous PD-L1 in GBM cells strengthened positive role of TMZ on immune evasion of GBM cells.
For further demonstrating the pivotal role of PD-L1 in immune escape of GBM cells with TMZ treatment, we knocked down PD-L1 expression by shRNA or neutralized PD-L1 by antibody in GBM cells. Depletion of PD-L1 in TMZ treated GBM cells, effectively rescued expression of pro-inflammatory cytokines IFN-γ and IL-2 in co-culture medium of PBMC and GBM, suggesting that PD-L1 functions as a key factor for inhibitory capacity of GBM cells after TMZ challenge on PBMC. Subsequently, we demonstrated that PD-L1 expression was induced by TMZ at transcriptional regulation, since actinomycin D could attenuate induction effect of TMZ on PD-L1 expression.
How to avoid immune evasion of GBM cells? Blocking PD-1/PD-L1 interaction with antibody or inhibiting the pathway involved in PD-L1 induction with inhibitor are effective strategy. But delivery of antibody into brain tumor is difficult due to the existence of blood-brain barrier [29], whereas small chemical inhibitors which can suppress PD-L1 expression in tumor cells may be the best option to counteract immune evasion, because lots of small chemical inhibitors are easier to cross blood-brain barrier. According to the other studies, CD274 which encoding PD-L1, is mainly regulated by STAT3, HIF-1α and c-Myc in several types of cancer cells [24-26], while our results revealed that only STAT3pY705 was increased after TMZ treatment in U87 cells. We proved that TMZ could trigger STAT3 activation, and STAT3 acted as a key transcription factor which promoted PD-L1 expression, indicating that STAT3 signaling was critical for PD-L1 induction after TMZ challenge. Despite of several experimental application small chemical inhibitors for STAT3 are available commercially, the inhibitors for the clinical use or even in late-stage clinical study are not available yet [30]. Accordingly, PD-1 antibody or PD-L1 antibody is the last option to block the TMZ-mediated immune inhibitory activity of GBM cells.
As we know, the combinational therapy is a popular and efficient way to fight with cancer. Combination of PD-1/PD-L1 blockade and TMZ can suppress tumor growth, but also can impair immune evasion of GBM cell after chemotherapy theoretically. The animal study showed that the combinational therapy is more effective than single therapy, both in inhibiting tumor growth and improving survival ratio.
Taken together, we demonstrated that TMZ contributed to GBM cells immune escape through upregulating PD-L1, which does not facilitate the treatment of GBM. In order to obtain good clinical curative effect, we proposed that combination of TMZ and PD-1 antibody was a promising way to treat GBM, which was strongly supported by our animal experiment. Nevertheless, injection of the PD-1 antibody intracranially as mentioned above, would increase the risk of brain infection, so developing an alternative approach to deliver antibody directly to brain tumor will be the main target of our further work.
Acknowledgements
This work was supported by grants from the Zhejiang Natural Science Foundation Grant (LQ19H160010) and Wenzhou Science & Technology Bureau Foundation Grant (Y20180074) to L.D, and from Education Department of Sichuan Province Foundation (17ZA0437), Luzhou City-Southwest Medical University Foundation (2015LZCYD-S01-8/15) and Southwest Medical University Foundation (2014ZD-004) to F.Y, and from Key Research Project of Traditional Chinese Medicine of Zhejiang Province of China (2019ZZ015) to Z.S.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Batash R, Asna N, Schaffer P, Francis N, Schaffer M. Glioblastoma multiforme, diagnosis and treatment; recent literature review. Curr Med Chem. 2017;24:3002–3009. doi: 10.2174/0929867324666170516123206. [DOI] [PubMed] [Google Scholar]
- 2.Filley AC, Henriquez M, Dey M. Recurrent glioma clinical trial, CheckMate-143: the game is not over yet. Oncotarget. 2017;8:91779–91794. doi: 10.18632/oncotarget.21586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spinelli GP, Miele E, Lo Russo G, Miscusi M, Codacci-Pisanelli G, Petrozza V, Papa A, Frati L, Della Rocca C, Gulino A, Tomao S. Chemotherapy and target therapy in the management of adult high-grade gliomas. Curr Cancer Drug Targets. 2012;12:1016–31. doi: 10.2174/156800912803251207. [DOI] [PubMed] [Google Scholar]
- 4.Hombach-Klonisch S, Mehrpour M, Shojaei S, Harlos C, Pitz M, Hamai A, Siemianowicz K, Likus W, Wiechec E, Toyota BD, Hoshyar R, Seyfoori A, Sepehri Z, Ande SR, Khadem F, Akbari M, Gorman AM, Samali A, Klonisch T, Ghavami S. Glioblastoma and chemoresistance to alkylating agents: involvement of apoptosis, autophagy, and unfolded protein response. Pharmacol Ther. 2018;184:13–41. doi: 10.1016/j.pharmthera.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 5.Vlachostergios PJ, Hatzidaki E, Befani CD, Liakos P, Papandreou CN. Bortezomib overcomes MGMT-related resistance of glioblastoma cell lines to temozolomide in a schedule-dependent manner. Invest New Drugs. 2013;31:1169–81. doi: 10.1007/s10637-013-9968-1. [DOI] [PubMed] [Google Scholar]
- 6.Yung WK, Prados MD, Yaya-Tur R, Rosenfeld SS, Brada M, Friedman HS, Albright R, Olson J, Chang SM, O’Neill AM, Friedman AH, Bruner J, Yue N, Dugan M, Zaknoen S, Levin VA. Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal Brain Tumor Group. J. Clin. Oncol. 1999;17:2762–71. doi: 10.1200/JCO.1999.17.9.2762. [DOI] [PubMed] [Google Scholar]
- 7.Li X, Huang R, Xu Z. Risk of adverse vascular events in newly diagnosed glioblastoma multiforme patients treated with bevacizumab: a systematic review and meta-analysis. Sci Rep. 2015;5:14698. doi: 10.1038/srep14698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Pan L, Sheng XF, Chen S, Dai JZ. Nimotuzumab, a humanized monoclonal antibody specific for the EGFR, in combination with temozolomide and radiation therapy for newly diagnosed glioblastoma multiforme: first results in Chinese patients. Asia Pac J Clin Oncol. 2016;12:e23–9. doi: 10.1111/ajco.12166. [DOI] [PubMed] [Google Scholar]
- 9.Hanif F, Muzaffar K, Perveen K, Malhi SM, Simjee ShU. Glioblastoma multiforme: a review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac J Cancer Prev. 2017;18:3–9. doi: 10.22034/APJCP.2017.18.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xue W, Du X, Wu H, Liu H, Xie T, Tong H, Chen X, Guo Y, Zhang W. Aberrant glioblastoma neovascularization patterns and their correlation with DCE-MRI-derived parameters following temozolomide and bevacizumab treatment. Sci Rep. 2017;7:13894. doi: 10.1038/s41598-017-14341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Domingues D, Turner A, Silva MD, Marques DS, Mellidez JC, Wannesson L, Mountzios G, de Mello RA. Immunotherapy and lung cancer: current developments and novel targeted therapies. Immunotherapy. 2014;6:1221–1235. doi: 10.2217/imt.14.82. [DOI] [PubMed] [Google Scholar]
- 12.Sanlorenzo M, Vujic I, Posch C, Dajee A, Yen A, Kim S, Ashworth M, Rosenblum MD, Algazi A, Osella-Abate S, Quaglino P, Daud A, Ortiz-Urda S. Melanoma immunotherapy. Cancer Biol Ther. 2014;15:665–74. doi: 10.4161/cbt.28555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simons JW. Prostate cancer immunotherapy: beyond immunity to curability. Cancer Immunol Res. 2014;2:1034–43. doi: 10.1158/2326-6066.CIR-14-0174. [DOI] [PubMed] [Google Scholar]
- 14.Dalgleish AG. Rationale for combining immunotherapy with chemotherapy. Immunotherapy. 2015;7:309–316. doi: 10.2217/imt.14.111. [DOI] [PubMed] [Google Scholar]
- 15.Ko EC, Raben D, Formenti SC. The integration of radiotherapy with immunotherapy for the treatment of non-small cell lung cancer. Clin Cancer Res. 2018;24:5792–5806. doi: 10.1158/1078-0432.CCR-17-3620. [DOI] [PubMed] [Google Scholar]
- 16.Juloori A, Koyfman SA, Geiger JL, Joshi NP, Woody NM, Burkey BB, Scharpf J, Lamarre EL, Prendes B, Adelstein DJ, Greskovich JF, Keller L. Definitive chemoradiation in locally advanced squamous cell carcinoma of the hypopharynx: long-term outcomes and toxicity. Anticancer Res. 2018;38:3543–3549. doi: 10.21873/anticanres.12626. [DOI] [PubMed] [Google Scholar]
- 17.Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med. 2016;14:73. doi: 10.1186/s12916-016-0623-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lazzari C, Karachaliou N, Bulotta A, Vigano M, Mirabile A, Brioschi E, Santarpia M, Gianni L, Rosell R, Gregorc V. Combination of immunotherapy with chemotherapy and radiotherapy in lung cancer: is this the beginning of the end for cancer? Ther Adv Med Oncol. 2018;10:1758835918762094. doi: 10.1177/1758835918762094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, Métivier D, Larochette N, van Endert P, Ciccosanti F, Piacentini M, Zitvogel L, Kroemer G. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi Y, Matsutani N, Nakayama T, Dejima H, Uehara H, Kawamura M. Immunological effect of local ablation combined with immunotherapy on solid malignancies. Chin J Cancer. 2017;36:49. doi: 10.1186/s40880-017-0216-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiao S, Xia W, Yamaguchi H, Wei Y, Chen MK, Hsu JM, Hsu JL, Yu WH, Du Y, Lee HH, Li CW, Chou CK, Lim SO, Chang SS, Litton J, Arun B, Hortobagyi GN, Hung MC. PARP inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin Cancer Res. 2017;23:3711–3720. doi: 10.1158/1078-0432.CCR-16-3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishikawa E, Yamamoto T, Sakamoto N, Nakai K, Akutsu H, Tsuboi K, Takano S, Matsumura A. Low peripheral lymphocyte count before focal radiotherapy plus concomitant temozolomide predicts severe lymphopenia during malignant glioma treatment. Neurol Med Chir (Tokyo) 2010;50:638–44. doi: 10.2176/nmc.50.638. [DOI] [PubMed] [Google Scholar]
- 23.Du L, Zhou H, Qin L, Wei H, Zhang A, Yang K, Wang X. Identification and functional evaluation of two STAT3 variants in grass carp: implication for the existence of specific alternative splicing of STAT3 gene in teleost. Dev Comp Immunol. 2017;76:326–333. doi: 10.1016/j.dci.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Marzec M, Zhang Q, Goradia A, Raghunath PN, Liu X, Paessler M, Wang HY, Wysocka M, Cheng M, Ruggeri BA, Wasik MA. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1) Proc Natl Acad Sci U S A. 2008;105:20852–7. doi: 10.1073/pnas.0810958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781–90. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casey SC, Tong L, Li Y, Do R, Walz S, Fitzgerald KN, Gouw AM, Baylot V, Gütgemann I, Eilers M, Felsher DW. MYC regulates the antitumor immune response through CD47 and PD-L1. Science. 2016;352:227–231. doi: 10.1126/science.aac9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stavrovskaya AA, Shushanov SS, Rybalkina EY. Problems of glioblastoma multiforme drug resistance. Biochemistry (Mosc) 2016;81:91–100. doi: 10.1134/S0006297916020036. [DOI] [PubMed] [Google Scholar]
- 28.Derer A, Spiljar M, Baumler M, Hecht M, Fietkau R, Frey B, Gaipl US. Chemoradiation increases PD-L1 expression in certain melanoma and glioblastoma cells. Front Immunol. 2016;7:610. doi: 10.3389/fimmu.2016.00610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix CC. CNS immune privilege: hiding in plain sight. Immunol Rev. 2006;213:48–65. doi: 10.1111/j.1600-065X.2006.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cafferkey C, Chau I. Novel STAT 3 inhibitors for treating gastric cancer. Expert Opin Investig Drugs. 2016;25:1023–31. doi: 10.1080/13543784.2016.1195807. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.