Abstract
Systemic chemotherapy is the standard treatment modality for stage IV lung adenocarcinoma patients with EGFR wild-type or unknown mutation status. Recent years, there is increasing evidence showed that selected patients with stage IV disease could benefit from aggressive thoracic radiotherapy. Either pemetrexed or docetaxel, combined with cisplatin, can be used for patients with stage IV lung adenocarcinoma. However, no prospective trials have confirmed that Pem-Cis was superior to Doc-Cis in lung adenocarcinoma. In this randomized phase 2 trial, we evaluated survival outcomes, and toxicity of Pemetrexed-Cisplatin (arm A) or Docetaxel-Cisplatin (arm B) with concurrent IMRT to the primary tumor for stage IV lung adenocarcinoma patients with EGFR wild-type or unknown mutation status. Totally, 101 patients were randomly assigned (50 in arm A and 51 in arm B). Using an intention-to-treat analysis, one-year survival rates were 72.0% and 52.9%, respectively (P=0.020). Progression-free survival was also significantly improved in the arm A (median, 12.6 v 7.5 months, P=0.013). The incidence and severity of acute pneumonitis and esophagitis was similar between two arms. Although more of grade 3 or 4 anemia and thrombocytopenia in arm A, and higher rates grade 3 or 4 neutropenia, and leukopenia were observed in arm B. Pem-Cis first-line chemotherapy with concurrent radiation therapy for stage IV lung adenocarcinoma patients with EGFR wild-type or unknown mutation status represents a potential treatment option with acceptable toxicity and high overall survival rates.
Keywords: Stage IV, lung adenocarcinoma, concurrent chemoradiotherapy, pemetrexed, docetaxel, phase II study
Introduction
Approximately 60% of patients who have been newly diagnosed with non-small cell lung cancer (NSCLC) have distant metastases [1]. Platinum-based chemotherapy typically produces response rates of approximately 30% and median survival times of 8 to 10 months, and different chemotherapy regimens have had similar efficacy [2,3]. The efficacy of chemotherapy in NSCLC might have reached a plateau [4]. At present, adenocarcinoma has become the most common histologic type in NSCLC, accounted for approximately 90% in women and 50% in men [5].
In current clinical practice, epidermal growth factor receptor gene (EGFR) tyrosine kinase inhibitors (TKIs) are used first for the treatment of lung adenocarcinomas with EGFR sensitizing mutation. However, the prevalence of EGFR mutations in adenocarcinoma is 10% of Western and up to 50% of Asian patients [6]. Although, antibodies against programmed death protein 1 (PD-1), such as pembrolizumab monotherapy, can be used as first-line therapy to patients with metastatic NSCLC without sensitising EGFR or ALK alterations with PD-L1 TPS of 1% or greater [7]. However, the cost of pembrolizumab is high, and many patients cannot afford pembrolizumab treatment. The China National Health Insurance does not reimburse the expenditure associated with this drug. Pembrolizumab is not likely to be cost-effective in the treatment of PD-L1 positive, NSCLC patients [8,9]. Thus, platinum-based chemotherapy has been also most commonly used to treat lung adenocarcinoma patients with negative or unknown EGFR mutations.
For patients with metastatic NSCLC disease, clinicians tend to attach more importance to systemic therapy to control the metastatic lesions than to local treatment to control the primary tumor. However, the status of primary tumor was associated with OS. Higginson et al. [10] reported that patients with advanced NSCLC and bulky central disease, bronchial/vascular compression, and/or pulmonary symptoms had worse OS. Primary tumor volume was also the main contributors to OS [11,12]. Recent years, there is increasing evidence showed that selected patients with stage IV disease could benefit from aggressive thoracic radiotherapy [11,13-16]. For patients with advanced stages of EGFR-mutant lung adenocarcinomas, Yen et al. also demonstrated the survival benefits of combining thoracic RT (45 Gy at least) and EGFR TKI [17]. Docetaxel plus cisplatin (Doc-Cis) chemotherapy with concurrent thoracic radiation to the primary tumor has produced favorable survival outcomes with acceptable toxicity in our previous prospective studies and in other retrospective studies [11,18,19]. According to a randomized study comparing the efficacy of pemetrexed plus cisplatin (Pem-Cis) with Doc-Cis in patients with nonsquamous NSCLC, Pem-Cis showed a similar response and survival outcomes compared with Doc-Cis [20]. However, no prospective trials have directly compared the efficacy and toxicity of concurrent use of thoracic radiation with either Pem-Cis or Doc-Cis for patients stage IV lung adenocarcinoma. Compared with three-dimensional conformal radiation therapy (3D-CRT) for NSCLC, intensity-modulated radiation therapy (IMRT) was associated with lower rates of severe pneumonitis and cardiac doses, and the routine use of IMRT was recommended [21]. Thus, we conducted this randomized phase 2 trial to test survival outcomes, and toxicity of Pem-Cis or Doc-Cis plus concurrent thoracic IMRT for stage IV lung adenocarcinoma (Chinese Clinical Trial Registry ChiCTR-TRC-13004184).
Material and methods
Patients selection
Patients fulfilled all of the following criteria have been treated using a prospective institutional protocol at our cancer centre. The inclusion criteria were as follows: (1) histologically or cytologically confirmed lung adenocarcinoma; (2) newly diagnosed stage IV disease [22] (staged according to the 7th edition of the staging system); (3) no previous anticancer treatment; (4) 18 to 75 years of age; (5) a Karnofsky performance status (KPS) score ≥70; (6) no contraindications to radiation therapy or chemotherapy; (7) metastatic disease limited to 3 organs; (8) presumed ability to tolerate to received at least two chemotherapy cycles; (9) EGFR mutation status was unknown or wild-type; and (10) patients were eligible for randomization only if radiation plan satisfied normal tissue constraints with tumor dose at least 60 Gy. Key exclusion criteria were (1) a history of thoracic surgery; (2) pregnancy or lactation at the time of enrollment; (3) previous malignancy or other concomitant malignant disease; and (4) having pleural effusion and pericardial effusion; (5) having activating EGFR mutations. This study was reviewed by the ethical review boards in China (Ethics Committee of Guizhou Cancer Hospital, GuiYang, China), and informed consent was obtained from all patients.
Pretreatment evaluations
All patients underwent fiberoptic bronchoscopy and contrast-enhanced computed tomography (CT) of the chest to evaluate the extent of the primary tumor and regional lymph node status. All patients also underwent bone scintigraphy, contrast-enhanced CT of the abdominal region, and magnetic resonance imaging (MRI) of the head to detect distant metastases. Positron emission tomography (PET) scan was optional and not required. Positive findings of skeleton on positron emission tomography (PET) or bone scintigraphy required other additional radiologic confirmation (eg, MRI or CT of bone). Pretreatment evaluations were to be completed within 2 weeks before treatment was begun.
Treatment protocol
We designed a randomized prospective phase 2 study to compare Pem-Cis (arm A) or Doc-Cis (arm B) combined with concurrent thoracic IMRT. Patients were randomized (1:1) to arm A: pemetrexed 500 mg/m2 administered intravenously on day 1 followed by cisplatin 75 mg/m2 intravenously on day 2, or to arm B: docetaxel 65 mg/m2 administered intravenously on day 1 followed by cisplatin 75 mg/m2 on day 2. The drug regimens were administered every 3 weeks, up to a maximum of six cycles or until drug discontinuation because of progressive disease, unacceptable toxicity, or any other reason. No maintenance therapy was given in both arms.
The same radiation therapy protocol was used to the two arms. Radiation to primary tumor was implemented by IMRT techniques. The gross tumor volume (GTV) included the thoracic primary tumor plus positive lymph nodes (>1 cm on short axis, or 18F-FDG standard uptake value ≥2.5 on PET/CT) and was outlined on the treatment planning CT scan. The clinical target volume (CTV) was defined as the GTV plus a 0.6-cm margin; the planning target volume (PTV) was defined as the CTV plus another margin of 0.5 to 1.0 cm. The percentage of total lung volume receiving ≥20 Gy (V20) was to be kept at ≤32%, the maximum point dose for the spinal cord to ≤50 Gy, and the mean esophageal dose to ≤35 Gy for all individual treatment plans. Patients were eligible for study only if radiation plan satisfied normal tissue constraints with tumor dose at least 60 Gy. Patients received late-course accelerated hyperfractionated radiation therapy (LCAHRT) to the primary tumor as follows. The first course of radiation therapy was given in 2.0-Gy fractions, 5 days per week, to a total dose of 40 Gy; LCAHRT was then delivered in twice-daily fractions of 1.50 Gy each, separated by 6 to 8 hours per day. The prescribed dose to the PTV was to be 60-70 Gy. Radiation therapy was given concurrently with the chemotherapy, beginning within 1 week after beginning the first course of chemotherapy. The treatment team decided whether to deliver radiation to metastatic sites.
Evaluation of treatment-related toxicity and response
Treatment-related acute toxicity was scored with National Cancer Institute’s Common Terminology Criteria for Adverse Events version 3.0. During the course of treatment, routine blood tests were performed at least once per week, and routine test results of blood, liver, and renal function and electrocardiography were evaluated before chemotherapy. Symptoms suggestive of pneumonitis or esophagitis were evaluated with chest radiography or CT examination and barium meal radiography. The treatment response, including complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD), were evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) system [23].
The protocol specified that patients should be assessed for treatment response after every two cycles of chemotherapy. At 1 month after completion of treatment, patients underwent CT scanning of the chest and abdominal region and MRI of the head to assess tumor response. These tests were then repeated every 3 months for 2 years and every 6 months thereafter. Bone scintigraphy was done every 6 months for 2 years and every 12 months thereafter.
Statistical methods
The primary objective was 1-year overall survival (OS) rate. Secondary objectives were to evaluate progression free survival (PFS), and toxicity of these two regimens. The sample size was calculated with the 2-sided significance level of 0.05 and 80% statistical power using a 2-sample log-rank test. On the basis of our previous multicenter phase 2 study [19], we assessed the patients randomly assigned to arm B to yield a 50% 1-year survival rate. According to the JMDB trial, 1-year OS rate of the Pem-Cis chemotherapy was nearly 50% [24]; we predicted the patients assigned to arm A to have a 70% 1-year survival rate.
The Statistical Package for Social Sciences, version 13.0 (SPSS, Chicago, IL, USA) was used for statistical analysis. Intergroup comparisons were performed using the Mann-Whitney U test for continuous variables and Pearson’s χ2 test for categorical variables. OS was measured from the date of random assignment to the date of death as a result of any cause. PFS was measured from the date of random assignment to the first date of documented objective progression of disease or of death as a result of any cause. The Kaplan-Meier method was used to calculate the OS and PFS. The log-rank test was used to compare the survival curves. Multivariate Cox regression analysis was used to test independent significant prognostic factors for OS. All factors with P value ≤0.10 in univariate analysis were further tested in the multivariate analysis. All statistical tests were two-sided, and a P value <0.05 was considered as being statistically significant. The intent-to-treat (ITT) population included all randomized patients regardless of whether they received treatment. Patients who received at least 2 chemotherapy cycles and a thoracic radiation dose of at least 60 Gy were a per-protocol (PP) population.
Results
Patient characteristics
From January 2011 to October 2015, 102 patients were enrolled in the study. One patient concomitant with plasmacytoma were considered ineligible after review. Therefore, 101 patients were included in the analysis based on the intention-to-treat principle, 50 patients in arm A, and, 51 patients in arm B. Of the 101 patients, 79 (78.2%) completed treatment in accordance with the protocol (i.e., received at least 2 chemotherapy cycles and a thoracic radiation dose of at least 60 Gy). Of the 22 patients who did not complete treatment, 9 patients refused for personal reasons (none of whom had grade 3 or worse toxicity or disease progression, 4 in arm A and 5 in arm B), 9 for grade 4 hematologic toxicity (6 in arm A and 3 in arm B), 2 for gastrointestinal toxicity (1 in arm A and 1 in arm B), and 2 for new metastases (1 in each of the two arms). Thus the per-protocol analysis included only those 79 patients. Overall, a total of 12 patients (6 in each of the two arms) had EGFR gene aberration tests at the initial diagnosis. The clinical characteristics of the patients were well balanced between both treatment arms (Table 1).
Table 1.
Baseline patient and disease characteristics for randomly assigned patients
| Characteristic | ITT Set (n=101) | PP Set (n=79) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Arm A (n=50) | Arm B (n=51) | P value | Arm A (n=38) | Arm B (n=41) | P value | |
| Gender | ||||||
| Male | 30 (60.0%) | 31 (60.8%) | 21 (55.3%) | 24 (58.5%) | ||
| Famale | 20 (40.0%) | 20 (39.2%) | 0.936 | 17 (44.7%) | 17 (41.5%) | 0.769 |
| Age (years) | ||||||
| <60 years | 34 (68.0%) | 31 (60.8%) | 26 (68.4%) | 25 (61.0%) | ||
| ≥60 years | 16 (32.0%) | 20 (39.2%) | 0.138 | 12 (31.6%) | 16 (39.0%) | 0.214 |
| KPS | ||||||
| <80 | 3 (6.0%) | 1 (2.0%) | 2 (5.3%) | 1 (2.4%) | ||
| ≥80 | 47 (94.0%) | 50 (98.0%) | 0.298 | 36 (94.7%) | 40 (97.6%) | 0.512 |
| T stage | ||||||
| T1-2 | 11 (22.0%) | 19 (37.3%) | 9 (23.7%) | 16 (39.0%) | ||
| T3-4 | 39 (78.0%) | 32 (62.7%) | 0.093 | 29 (76.3%) | 25 (61.0%) | 0.716 |
| N stage | ||||||
| N0-1 | 3 (6.0%) | 9 (17.6%) | 3 (7.9%) | 8 (19.6%) | ||
| N2-3 | 47 (94.0%) | 42 (82.4%) | 0.133 | 35 (92.1%) | 33 (80.4%) | 0.136 |
| No. of chemotherapy cycle | ||||||
| <4 | 24 (48.0%) | 21 (41.1%) | 16 (42.1%) | 15 (36.6%) | ||
| ≥4 | 26 (52.0%) | 30 (58.9%) | 0.476 | 22 (57.9%) | 26 (63.4%) | 0.616 |
| EGFR mutation status | ||||||
| Unknown | 44 (88.0%) | 45 (88.2%) | 32 (84.2%) | 37 (90.2%) | ||
| Wild-type | 6 (12.0%) | 6 (11.8%) | 0.971 | 6 (15.8%) | 4 (9.8%) | 0.420 |
| PET-CT examination | ||||||
| Yes | 43 (86.0%) | 45 (88.2%) | 32 (84.2%) | 36 (87.8%) | ||
| No | 7 (14.0%) | 6 (11.8%) | 0.737 | 6 (15.8%) | 5 (12.2%) | 0.645 |
| Metastatic disease status | ||||||
| Single organ | 26 (52.0%) | 19 (37.3%) | 0.136 | 20 (52.6%) | 18 (43.9%) | 0.438 |
| Bone | 6 (12.0%) | 5 (9.8%) | 6 (15.8%) | 4 (9.8%) | ||
| Brain | 5 (10.0%) | 6 (11.8%) | 4 (10.5%) | 6 (14.6%) | ||
| Lung | 8 (16.0%) | 5 (9.8%) | 7 (18.4%) | 6 (14.6%) | ||
| Other | 7 (14.0%) | 3 (5.9%) | 3 (7.9%) | 2 (4.9%) | ||
| Two or three organs | 24 (48.0%) | 32 (62.7%) | 18 (47.4%) | 23 (56.1%) | ||
| Bone | 17 (34.0%) | 24 (47.1%) | 11 (28.9%) | 17 (41.5%) | ||
| Brain | 10 (20.0%) | 13 (25.5%) | 7 (18.4%) | 9 (22.0%) | ||
| Lung | 17 (24.0%) | 15 (29.4%) | 11 (28.9%) | 11 (26.8%) | ||
| Liver | 4 (8.0%) | 3 (5.9%) | 3 (7.9%) | 3 (7.3%) | ||
| Adrenal | 6 (12.0%) | 6 (11.8%) | 4 (10.5%) | 3 (7.3%) | ||
| Distant lymph nodes | 16 (32.0%) | 13 (25.5%) | 8 (21.1%) | 11 (26.8%) | ||
| Radiation to all metastases | ||||||
| Yes | 21 (42.0%) | 20 (39.2%) | 15 (39.5%) | 18 (43.9%) | ||
| No | 29 (58.0%) | 31 (60.8%) | 0.404 | 23 (60.5%) | 23 (56.1%) | 0.690 |
| Gross tumor volume (cm3), Range (Median) | 244.8 (76.9-862.3) | 198.6 (71.2-630.0) | 0.193 | 242.6 (76.9-862.3) | 198.3 (71.2-630.0) | 0.372 |
| Mean lung dose (cGy), Range (Median) | 2017 (1164-2472) | 1994 (1273-2498) | 0.708 | 2084 (1164-2472) | 2033 (1325-2498) | 0.458 |
| V20 of all lung (%), Range (Median) | 31 (21-33) | 31 (19-33) | 0.506 | 32 (21-33) | 31 (19-33) | 0.586 |
| Mean esophagus dose (cGy), Range (Median) | 3233 (365-5365) | 3052 (1401-4938) | 0.869 | 3179 (1176-5366) | 3375 (1401-4938) | 0.822 |
| V60 of esophagus (%), Range (Median) | 17 (0-68) | 21 (0-58) | 0.705 | 25 (0-68) | 23 (0-58) | 0.657 |
| Prescribed dose | ||||||
| <60 Gy | 6 (11.8%) | 12 (24.0%) | ||||
| ≥60 Gy | 45 (88.2%) | 38 (76.0%) | 0.108 | |||
ITT: intent-to treat; PP: per-protocol.
Response and survival
In arm A, 4.0% (2/50) had a complete response, 64.0% (31/50) had a partial response, 24.0% (12/50 had stable disease, and 8.0% (4/50) had progressive disease; corresponding rates in arm B were 3.9% (2/51), 68.6% (35/51), 23.5% (12/51), and 3.9% (2/51). The treatment response rate was not statistically different between the two arms (χ2=0.250, P=0.617).
The last follow-up was in March 2017. The median survival time (MST), and 1-, 2-, and 3-year OS rates were 19.6 (95% CI, 13.9-25.3) months, and 72.0%, 28.0%, and 16.0%, respectively for patients in arm A; whereas the MST was 12.1 (95% CI, 10.7-13.5) months, and 52.9%, 17.6%, and 13.7%, respectively for patients in arm B. OS in arm A was significantly longer than in arm B (χ2=3.886, P=0.049) (Figure 1A). The median PFS was 5.1 months longer in arm A than in arm B, and this increase was statistically significant (median, 12.6 v 7.5 months, χ2=4.126, P=0.042; Figure 1B). After progression, a total of 30 patients received second-line therapy. In arm A, eleven patients received second-line chemotherapy, and 3 patients received second-line EGFR-TKI. In arm B, twelve patients received second-line chemotherapy, and 4 patients received second-line EGFR-TKI. Totally, only 7 patients were treated with EGFR-TKI, the OS rates at 1, 2, and 3 years for those patients were 57.1%, 42.9%, and 28.6%, respectively. No survival differences were noted between patients treated with and those not treated with EGFR-TKI (χ2=2.374, P=0.078). Univariate analysis showed that gender (χ2=4.435, P=0.035), and the number of distant metastatic organs (χ2=3.884, P=0.049) were associated with OS. Platelet count (χ2=3.043, P=0.081), hemoglobin (Hb) level (χ2=3.395, P=0.065), KPS score (χ2=3.102, P=0.078) were marginally associated with OS. White blood cell (WBC) count (χ2=0.698, P=0.403), radiation therapy to metastatic sites (χ2=0.133, P=0.715), age (χ2=0.375, P=0.540), T-stage (χ2=0.001, P=0.997), N-stage (χ2=0.001, P=0.995), and primary tumor volume (χ2=0.395, P=0.530) did not affect OS.
Figure 1.
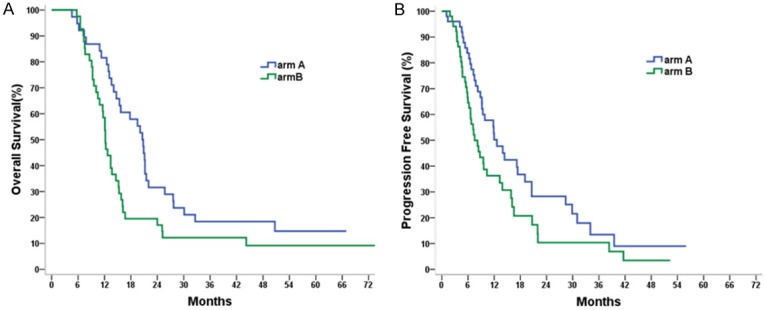
A. Comparison of OS in the intent-to treat population between two arms. B. Comparison of PFS in the intent-to treat population between two arms.
For the 79 patients in the per-protocol analysis, the MST, and 1-, 2-, and 3-year OS rates were 20.6 (95% CI, 18.5-22.7) months, and 81.6%, 31.1%, and 15.6%, respectively for arm A; whereas the MST were 12.2 (95% CI, 11.2-13.2) months, and 58.5%, 17.1%, and 12.2%, respectively for arm B. The difference between the two arms was statistically significant (χ2=5.419, P=0.020). Compared with arm B, arm A have a trend to improve PFS for patients (median, 12.0 v 8.7 months; χ2=2.679, P=0.102). Multivariate analysis showed that receiving Pem-Cis chemotherapy, woman, and HB level ≥135 g/L independently predicted better OS (Table 2).
Table 2.
Multivariate analysis of factors for the prediction of overall survival
| Variable | ITT Set | PP Set | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| HR | 95.0% confidence interval | P value | HR | 95.0% confidence interval | P value | |||
|
|
|
|||||||
| Lower | Upper | Lower | Upper | |||||
| Sex (female vs. male) | 0.563 | 0.345 | 0.918 | 0.021 | 0.510 | 0.296 | 0.878 | 0.015 |
| KPS score (>80 vs. ≤80) | 0.842 | 0.535 | 1.325 | 0.457 | 0.808 | 0.487 | 1.341 | 0.410 |
| Regimens (Pem-Cis vs. Doc-Cis) | 0.907 | 0.829 | 0.992 | 0.032 | 0.866 | 0.781 | 0.959 | 0.006 |
| No. of metastatic organs (2-3 vs. 1) | 1.194 | 0.752 | 1.896 | 0.453 | 1.159 | 0.681 | 1.971 | 0.587 |
| Hb level (>135 vs. ≤135 g/L) | 0.487 | 0.300 | 0.790 | 0.004 | 0.507 | 0.296 | 0.866 | 0.013 |
| Platelet count (>235 vs. ≤235×109/L) | 1.311 | 0.830 | 2.071 | 0.246 | 1.591 | 0.941 | 2.690 | 0.083 |
Treatment complications
There was no any Grade 5 toxicity in both treatment arms, and hematologic toxicity was the most common and severe complication. The difference of treatment toxicity between the two arms was mainly in hematologic toxicity. The incidence of grade 3 or 4 leukocytes and neutropenia were significantly greater in the arm B. Whereas, Grade 3 to 4 thrombocytopenia and anemia were significantly greater in the arm A. No Grade 3 to Grade 5 radiation pneumonitis was observed in both treatment arms, Grade 2 radiation pneumonitis was low in both treatment arms (7.8% v 8.0%, P=0.625). Rates of severe (grade 3) acute radiation esophagitis were similar between two arms (12.0% in arm A v 7.8% in arm B, P=0.484). The toxicity profiles for both treatment arms are presented in Table 3.
Table 3.
Incidence of acute toxicity, n (%)
| Adverse effects | Arm A (n=51) | Arm B (n=50) | P value |
|---|---|---|---|
| Leukocytes | |||
| Grade 0-2 | 19 (37.2%) | 26 (52.0%) | |
| Grade 3-4 | 32 (62.8%) | 24 (48.0%) | 0.098 |
| Leukopenia | |||
| Grade 0-2 | 18 (35.3%) | 33 (66.0%) | |
| Grade 3-4 | 33 (64.7%) | 17 (34.0%) | 0.002 |
| Anemia | |||
| Grade 0-2 | 45 (90.0%) | 35 (62.0%) | |
| Grade 3-4 | 6 (10.0%) | 15 (30.0%) | 0.024 |
| Thrombocytopenia | |||
| Grade 0-2 | 44 (86.2%) | 30 (60.0%) | |
| Grade 3-4 | 7 (13.8%) | 20 (40.0%) | 0.003 |
| Pneumonitis | |||
| Grade 0-2 | 47 (92.2%) | 44 (88.0%) | |
| Grade 3 | 4 (7.8%) | 6 (12.0%) | 0.484 |
| Esophagitis | |||
| Grade 0-1 | 47 (92.2%) | 46 (92.0%) | |
| Grade 2 | 4 (7.8%) | 4 (8.0%) | 0.625 |
| Gastrointestinal | |||
| Grade 0-2 | 45 (88.2%) | 47 (94.0%) | |
| Grade 3 | 6 (11.8%) | 3 (6.0%) | 0.309 |
Discussion
The results from previous studies showed that chemotherapy given concurrently with radiation to the primary tumor produced satisfactory outcomes for selected patients with stage IV NSCLC [11,13-15,18,19,25]. In the present trail, we evaluated survival outcomes, and toxicity of Doc-Cis or Pem-Cis with concurrent IMRT to the primary tumor for stage IV lung adenocarcinoma patients with unknown EGFR mutation status or EGFR wild type.
This trail showed that the Pem-Cis and Doc-Cis regimens resulted in similar response rates. However, the OS and PFS with the Pem-Cis regimen was longer than those in the Doc-Cis regime, the 1-year OS rates were 72.0% and 52.9%, respectively. Both regimes given concurrently with IMRT to the primary tumor achieved better survival times than chemotherapy alone for advanced NSCLC on the basis of historical data [3,26]. ECOG 1594 showed that Doc-Cis regimens result in survival rates of 31% at one year and MST of 7.4 months [3]. The survival rates of 52.9% at one year in current study are similar to results from our previous prospective studies, in which concurrent chemotherapy and thoracic radiotherapy was also given for patients with stage IV NSCLC [18,19]. Previous publications showed that Pem-Cis chemotherapy produces 1-year survival rate of approximately 50% [27,28]. In the present study, 1-year survival rate was 72% in the Pem-Cis arm. Superior survival in the present study may be attributable to our additional use of aggressive irradiation to the primary tumor. Zhang et al. retrospectively analyzed 41 advanced NSCLC patients were treated with pemetrexed plus cisplatin as the first-line chemotherapy combined with concurrent thoracic radiotherapy and revealed that the 1-, 2-, and 3-year overall survival rates were 87.5%, 67.1%, and 43.4%, respectively [29].
The JMDB trial showed that combination chemotherapy with pemetrexed plus cisplatin was superior to gemcitabine plus cisplatin in terms of efficacy and toxicity [24]. We searched the PubMed data base and found only one randomized phase III study which conducted by Park et al., have directly assessed the efficacy of Pem-Cis with Doc-Cis in chemotherapy-naive nonsquamous NSCLC patients [20]. The randomized phase III study by Park et al. [20]. revealed that the survival outcomes were similar between Pem-Cis and Doc-Cis. Whereas, patients survival outcomes in Pem-Cis arm was improved significantly compared with Doc-Cis arm in the present trail, and, both arms achieved the prespecified criteria of a 1-year survival rate, 70% and 50%, respectively. This may have been due in part to the following reasons. Firstly, radiation therapy to the primary tumor with concurrent chemotherapy were used in our study. This treatment modality have been identified as improving survival outcomes stage IV NSCLC. Meanwhile, as the radiotherapy involved, it may be suggested to re-evaluate the value and efficacy of the chemotherapy. Secondly, lung adenocarcinoma patients with EGFR mutations receiving pemetrexed have a better response rate and longer PFS than those with wild type EGFR [30]. In the study of Park et al. [20], only patients with wild-type EGFR gene were included. However, most patients enrolled in the present study with unknown EGFR mutation status. Furthermore, Park et al. enrolled only 27.8% of the initially planned target subjects. Thus, they could not reach a statistically meaningful conclusion.
For patients with advanced NSCLC without EGFR or ALK mutations and a PD-L1 tumor proportion score of 50% or greater, pembrolizumab has been recommended as first-line treatment [31]. Compared with chemotherapy alone, the addition of pembrolizumab to pemetrexed and a platinum-based drug produce favorable OS and PFS in patients with metastatic nonsquamous NSCLC without EGFR or ALK mutations [32]. Radiation can induce effects beyond the radiation treatment fields and produce systemic response to local radiation with anti-PD-1 therapy [33,34]. The value of thoracic radiation in combination with immunotherapy for patients with advanced NSCLC needs to be further investigated.
Another issue with the use of concurrent chemoradiation for advanced NSCLC relates to its potential toxicity; and, acute radiation pneumonitis, esophagitis, and hematologic toxicity were the most common and severe complication. The incidence of acute radiation pneumonitis and esophagitis were similar between two arms in the present trail. No patients experienced grade 3 to 5 radiation pneumonitis, and grade 2 events were observed in less than 10% of patients in both arms. Rates of radiation pneumonitis and esophagitis in our study were not increased compared with studies of concurrent chemoradiation therapy for locally advanced NSCLC in the PROCLAIM and KCSG-LU05-04 trials [35,36]. Hematologic toxicities in Doc-Cis arm were as expected and similar to that from other trials [3,37,38]. Compared with PROCLAIM and JMDB trials, more severe hematologic toxicities were observed in Pem-Cis arm in the present trail. Severe hematologic complications might have developed because of the use of combined treatment modalities and the differences of patient populations in the present trail, as stage IV NSCLC patients who included in JMDB trial were treated with chemotherapy alone, and the patients in the PROCLAIM trial had stage III NSCLC. Furthermore, the different ethnicity in these studies might have developed different severe complications. Only approximately 18% and 14% of patients were of Asian origin in PROCLAIM and JMDB trials, respectively. In the present trail, we found that Pem-Cis arm had lower rates of grade 3 or 4 neutropenia and leukopenia, and higher rates of anemia and thrombocytopenia compared with Doc-Cis arm. Rodrigues et al. [39] and Socinski et al. [40] also reported that pemetrexed had a significantly lower incidence of grade 3 or 4 neutropenia and leukopenia but a higher rate of anemia and thrombocytopenia compared with docetaxel. We acknowledge several limitations to the current study. First, the main limitation is that EGFR mutation testing was done in only 12% patients. Before 2017, insurance did not cover EGFR-TKI. Therefore, many patients cannot afford anti-EGFR mutation-positive lung adenocarcinoma treatment. Thus, EGFR mutation testing is not usually recommended in many patients who can not afford EGFR-TKI. Cheng et al. reported that EGFR testing rate was 42.54% in Northern China in 2014 and was significantly related to city level (first-tier cities vs. new first-tier cities vs. second-tier cities vs. third-tier and above cities : 69.04% vs. 38.08% vs. 34.05% vs. 14.11%, P<0.001) [41]. Second, pemetrexed maintenance treatment is not used in current study. Maintenance treatment was not given because of maintenance treatment with pemetrexed was not the standard of care when we designed this protocol, and close observation is also another option for patients not progressing on first-line chemotherapy.
Conclusions
This trial demonstrated an increase of nearly 20% of 1-year survival rate for Pem-Cis with concurrent radiation therapy compared with Doc-Cis plus concurrent concurrent radiation therapy. The incidence and severity of acute pneumonitis and esophagitis was similar between Pem-Cis and Doc-Cis arms. Although more grade 3 or 4 neutropenia, and leukopenia were observed in the Doc-Cis arm, and higher rates of grade 3 or 4 anemia and thrombocytopenia in the Pem-Cis arm. The toxicity was tolerable in both arms. Pem-Cis first-line chemotherapy with concurrent radiation therapy for patients with EGFR mutation unknown or negative stage IV lung adenocarcinoma represents a potential treatment option with acceptable toxicity and high overall survival rates. The results of this study require confirmation in a subsequent phase 3 study.
Acknowledgements
This work was supported by Guizhou Provincial Education Office, China [grant number KY (2016)032]; and Science and Technology Office of Guizhou Province, China (LG 2012-062, and SZ 2014-3021). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Scagliotti GV, De Marinis F, Rinaldi M, Crino L, Gridelli C, Ricci S, Matano E, Boni C, Marangolo M, Failla G, Altavilla G, Adamo V, Ceribelli A, Clerici M, Di Costanzo F, Frontini L, Tonato M. Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J. Clin. Oncol. 2002;20:4285–4291. doi: 10.1200/JCO.2002.02.068. [DOI] [PubMed] [Google Scholar]
- 3.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 4.Morgensztern D, Waqar S, Subramanian J, Gao F, Govindan R. Improving survival for stage IV non-small cell lung cancer: a surveillance, epidemiology, and end results survey from 1990 to 2005. J Thorac Oncol. 2009;4:1524–1529. doi: 10.1097/JTO.0b013e3181ba3634. [DOI] [PubMed] [Google Scholar]
- 5.Sakurai H, Asamura H, Goya T, Eguchi K, Nakanishi Y, Sawabata N, Okumura M, Miyaoka E, Fujii Y. Survival differences by gender for resected non-small cell lung cancer: a retrospective analysis of 12,509 cases in a Japanese lung cancer registry study. J Thorac Oncol. 2010;5:1594–1601. doi: 10.1097/JTO.0b013e3181f1923b. [DOI] [PubMed] [Google Scholar]
- 6.Hirsch FR, Bunn PA Jr. EGFR testing in lung cancer is ready for prime time. Lancet Oncol. 2009;10:432–433. doi: 10.1016/S1470-2045(09)70110-X. [DOI] [PubMed] [Google Scholar]
- 7.Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G Jr, Srimuninnimit V, Laktionov KK, Bondarenko I, Kubota K, Lubiniecki GM, Zhang J, Kush D, Lopes G. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 8.Hu X, Hay JW. First-line pembrolizumab in PD-L1 positive non-small-cell lung cancer: a cost-effectiveness analysis from the UK health care perspective. Lung Cancer. 2018;123:166–171. doi: 10.1016/j.lungcan.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Liao W, Huang J, Hutton D, Li Q. Cost-effectiveness analysis of first-line pembrolizumab treatment for PD-L1 positive, non-small cell lung cancer in China. J Med Econ. 2019;22:344–349. doi: 10.1080/13696998.2019.1570221. [DOI] [PubMed] [Google Scholar]
- 10.Higginson DS, Chen RC, Tracton G, Morris DE, Halle J, Rosenman JG, Stefanescu M, Pham E, Socinski MA, Marks LB. The impact of local and regional disease extent on overall survival in patients with advanced stage IIIB/IV non-small cell lung carcinoma. Int J Radiat Oncol Biol Phys. 2012;84:e385–392. doi: 10.1016/j.ijrobp.2012.04.045. [DOI] [PubMed] [Google Scholar]
- 11.Lopez Guerra JL, Gomez D, Zhuang Y, Hong DS, Heymach JV, Swisher SG, Lin SH, Komaki R, Cox JD, Liao Z. Prognostic impact of radiation therapy to the primary tumor in patients with non-small cell lung cancer and oligometastasis at diagnosis. Int J Radiat Oncol Biol Phys. 2012;84:e61–e67. doi: 10.1016/j.ijrobp.2012.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su S, Hu Y, Ouyang W, Ma Z, Li Q, Li H, Wang Y, Wang X, Li T, Li J, Chen M, Lu Y, Bai Y, He Z, Lu B. Might radiation therapy in addition to chemotherapy improve overall survival of patients with non-oligometastatic Stage IV non-small cell lung cancer?: Secondary analysis of two prospective studies. BMC Cancer. 2016;16:908. doi: 10.1186/s12885-016-2952-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arrieta O, Villarreal-Garza C, Zamora J, Blake-Cerda M, de la Mata MD, Zavala DG, Muniz-Hernandez S, de la Garza J. Long-term survival in patients with non-small cell lung cancer and synchronous brain metastasis treated with whole-brain radiotherapy and thoracic chemoradiation. Radiat Oncol. 2011;6:166. doi: 10.1186/1748-717X-6-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashworth A, Rodrigues G, Boldt G, Palma D. Is there an oligometastatic state in non-small cell lung cancer? A systematic review of the literature. Lung Cancer. 2013;82:197–203. doi: 10.1016/j.lungcan.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 15.Parikh RB, Cronin AM, Kozono DE, Oxnard GR, Mak RH, Jackman DM, Lo PC, Baldini EH, Johnson BE, Chen AB. Definitive primary therapy in patients presenting with oligometastatic non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2014;89:880–887. doi: 10.1016/j.ijrobp.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Conibear J, Chia B, Ngai Y, Bates AT, Counsell N, Patel R, Eaton D, Faivre-Finn C, Fenwick J, Forster M, Hanna GG, Harden S, Mayles P, Moinuddin S, Landau D. Study protocol for the SARON trial: a multicentre, randomised controlled phase III trial comparing the addition of stereotactic ablative radiotherapy and radical radiotherapy with standard chemotherapy alone for oligometastatic non-small cell lung cancer. BMJ Open. 2018;8:e020690. doi: 10.1136/bmjopen-2017-020690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yen YC, Hsu HL, Chang JH, Lin WC, Chang YC, Chang CL, Chow JM, Yuan KS, Wu ATH, Wu SY. Efficacy of thoracic radiotherapy in patients with stage IIIB-IV epidermal growth factor receptor-mutant lung adenocarcinomas who received and responded to tyrosine kinase inhibitor treatment. Radiother Oncol. 2018;129:52–60. doi: 10.1016/j.radonc.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Su SF, Hu YX, Ouyang WW, Lu B, Ma Z, Li QS, Li HQ, Geng YC. Overall survival and toxicities regarding thoracic three-dimensional radiotherapy with concurrent chemotherapy for stage IV non-small cell lung cancer: results of a prospective single-center study. BMC Cancer. 2013;13:474. doi: 10.1186/1471-2407-13-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Su S, Li T, Lu B, Wang X, Li J, Chen M, Lu Y, Bai Y, Hu Y, Ouyang W, Ma Z, Li Q, Li H, Wang Y. Three-dimensional radiation therapy to the primary tumor with concurrent chemotherapy in patients with stage iv non-small cell lung cancer: results of a multicenter phase 2 study from PPRA-RTOG, China. Int J Radiat Oncol Biol Phys. 2015;93:769–777. doi: 10.1016/j.ijrobp.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Park CK, Oh IJ, Kim KS, Choi YD, Jang TW, Kim YS, Lee KH, Shin KC, Jung CY, Yang SH, Ryu JS, Jang SH, Yoo SS, Yong SJ, Lee KY, In KH, Lee MK, Kim YC. Randomized phase III study of docetaxel plus cisplatin versus pemetrexed plus cisplatin as first-line treatment of nonsquamous non-small-cell lung cancer: a TRAIL trial. Clin Lung Cancer. 2017;18:e289–e296. doi: 10.1016/j.cllc.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Chun SG, Hu C, Choy H, Komaki RU, Timmerman RD, Schild SE, Bogart JA, Dobelbower MC, Bosch W, Galvin JM, Kavadi VS, Narayan S, Iyengar P, Robinson CG, Wynn RB, Raben A, Augspurger ME, MacRae RM, Paulus R, Bradley JD. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: a secondary analysis of the NRG oncology RTOG 0617 randomized clinical trial. J. Clin. Oncol. 2017;35:56–62. doi: 10.1200/JCO.2016.69.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest. 2009;136:260–271. doi: 10.1378/chest.08-0978. [DOI] [PubMed] [Google Scholar]
- 23.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 24.Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U, Digumarti R, Zukin M, Lee JS, Mellemgaard A, Park K, Patil S, Rolski J, Goksel T, de Marinis F, Simms L, Sugarman KP, Gandara D. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J. Clin. Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 25.Koshy M, Malik R, Mahmood U, Sher DJ. Comparative effectiveness of aggressive locoregional therapy in metastatic lung cancer: associations between high-dose thoracic radiation therapy and/or chemoradiation therapy and survival in a large population-based cohort. Int J Radiat Oncol Biol Phys. 2015;93:S68. doi: 10.1016/j.prro.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Paz-Ares L, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, Molinier O, Sahoo TP, Laack E, Reck M, Corral J, Melemed S, John W, Chouaki N, Zimmermann AH, Visseren-Grul C, Gridelli C. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2012;13:247–255. doi: 10.1016/S1470-2045(12)70063-3. [DOI] [PubMed] [Google Scholar]
- 27.Novello S, Scagliotti G, de Castro G Jr, Kiyik M, Kowalyszyn R, Deppermann KM, Arriola E, Bosquee L, Novosiadly RD, Nguyen TS, Forest A, Tang S, Kambhampati SRP, Cosaert J, Reck M. An open-label, multicenter, randomized, phase II study of cisplatin and pemetrexed with or without cixutumumab (IMC-A12) as a first-line therapy in patients with advanced nonsquamous non-small cell lung cancer. J Thorac Oncol. 2017;12:383–389. doi: 10.1016/j.jtho.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 28.Schuette WH, Groschel A, Sebastian M, Andreas S, Muller T, Schneller F, Guetz S, Eschbach C, Bohnet S, Leschinger MI, Reck M. A randomized phase II study of pemetrexed in combination with cisplatin or carboplatin as first-line therapy for patients with locally advanced or metastatic non-small-cell lung cancer. Clin Lung Cancer. 2013;14:215–223. doi: 10.1016/j.cllc.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Q, Cai XW, Zhu ZF, Yu W, Liu Q, Feng W, Xue MC, Fu XL. Full-dose pemetrexed plus cisplatin combined with concurrent thoracic radiotherapy for previously untreated advanced nonsquamous non-small cell lung cancer. Anticancer Drugs. 2015;26:456–463. doi: 10.1097/CAD.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 30.Wu SG, Yang CH, Yu CJ, Lee JH, Hsu YC, Chang YL, Shih JY, Yang PC. Good response to pemetrexed in patients of lung adenocarcinoma with epidermal growth factor receptor (EGFR) mutations. Lung Cancer. 2011;72:333–339. doi: 10.1016/j.lungcan.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 31.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 32.Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, Cheng SY, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Hui R, Garon EB, Boyer M, Rubio-Viqueira B, Novello S, Kurata T, Gray JE, Vida J, Wei Z, Yang J, Raftopoulos H, Pietanza MC, Garassino MC KEYNOTE-189 Investigators. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 33.Bernstein MB, Krishnan S, Hodge JW, Chang JY. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nat Rev Clin Oncol. 2016;13:516–524. doi: 10.1038/nrclinonc.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan Z, Fromm A, Ahmed KA, Grass GD, Yang GQ, Oliver DE, Dilling TJ, Antonia SJ, Perez BA. Radiotherapy rescue of a nivolumab-refractory immune response in a patient with PD-L1-negative metastatic squamous cell carcinoma of the lung. J Thorac Oncol. 2017;12:e135–e136. doi: 10.1016/j.jtho.2017.04.029. [DOI] [PubMed] [Google Scholar]
- 35.Senan S, Brade A, Wang LH, Vansteenkiste J, Dakhil S, Biesma B, Martinez Aguillo M, Aerts J, Govindan R, Rubio-Viqueira B, Lewanski C, Gandara D, Choy H, Mok T, Hossain A, Iscoe N, Treat J, Koustenis A, San Antonio B, Chouaki N, Vokes E. PROCLAIM: randomized phase III trial of pemetrexed-cisplatin or etoposide-cisplatin plus thoracic radiation therapy followed by consolidation chemotherapy in locally advanced nonsquamous non-small-cell lung cancer. J. Clin. Oncol. 2016;34:953–962. doi: 10.1200/JCO.2015.64.8824. [DOI] [PubMed] [Google Scholar]
- 36.Ahn JS, Ahn YC, Kim JH, Lee CG, Cho EK, Lee KC, Chen M, Kim DW, Kim HK, Min YJ, Kang JH, Choi JH, Kim SW, Zhu G, Wu YL, Kim SR, Lee KH, Song HS, Choi YL, Sun JM, Jung SH, Ahn MJ, Park K. Multinational randomized phase III trial with or without consolidation chemotherapy using docetaxel and cisplatin after concurrent chemoradiation in inoperable stage III non-small-cell lung cancer: KCSG-LU05-04. J. Clin. Oncol. 2015;33:2660–2666. doi: 10.1200/JCO.2014.60.0130. [DOI] [PubMed] [Google Scholar]
- 37.Kiura K, Ueoka H, Segawa Y, Tabata M, Kamei H, Takigawa N, Hiraki S, Watanabe Y, Bessho A, Eguchi K, Okimoto N, Harita S, Takemoto M, Hiraki Y, Harada M, Tanimoto M. Phase I/II study of docetaxel and cisplatin with concurrent thoracic radiation therapy for locally advanced non-small-cell lung cancer. Br J Cancer. 2003;89:795–802. doi: 10.1038/sj.bjc.6601217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Segawa Y, Kiura K, Takigawa N, Kamei H, Harita S, Hiraki S, Watanabe Y, Sugimoto K, Shibayama T, Yonei T, Ueoka H, Takemoto M, Kanazawa S, Takata I, Nogami N, Hotta K, Hiraki A, Tabata M, Matsuo K, Tanimoto M. Phase III trial comparing docetaxel and cisplatin combination chemotherapy with mitomycin, vindesine, and cisplatin combination chemotherapy with concurrent thoracic radiotherapy in locally advanced non-small-cell lung cancer: OLCSG 0007. J. Clin. Oncol. 2010;28:3299–3306. doi: 10.1200/JCO.2009.24.7577. [DOI] [PubMed] [Google Scholar]
- 39.Rodrigues-Pereira J, Kim JH, Magallanes M, Lee DH, Wang J, Ganju V, Martinez-Barrera L, Barraclough H, van Kooten M, Orlando M. A randomized phase 3 trial comparing pemetrexed/carboplatin and docetaxel/carboplatin as first-line treatment for advanced, nonsquamous non-small cell lung cancer. J Thorac Oncol. 2011;6:1907–1914. doi: 10.1097/JTO.0b013e318226b5fa. [DOI] [PubMed] [Google Scholar]
- 40.Socinski MA, Raju RN, Stinchcombe T, Kocs DM, Couch LS, Barrera D, Rousey SR, Choksi JK, Jotte R, Patt DA, Periman PO, Schlossberg HR, Weissman CH, Wang Y, Asmar L, Pritchard S, Bromund J, Peng G, Treat J, Obasaju CK. Randomized, phase II trial of pemetrexed and carboplatin with or without enzastaurin versus docetaxel and carboplatin as first-line treatment of patients with stage IIIB/IV non-small cell lung cancer. J Thorac Oncol. 2010;5:1963–1969. doi: 10.1097/JTO.0b013e3181fd42eb. [DOI] [PubMed] [Google Scholar]
- 41.Cheng Y, Wang Y, Zhao J, Liu Y, Gao H, Ma K, Zhang S, Xin H, Liu J, Han C, Zhu Z, Wang Y, Chen J, Wen F, Li J, Jie Z, Zheng Z, Dai Z, Piao H. A multicenter, non-interventional study on real world EGFR testing and in patients with IIIB/IV NSCLC in Northern China. J Thorac Oncol. 2017;12:S2208. [Google Scholar]


