Abstract
In attempts to delay tumor progression after surgery or minimally invasive local treatments, multidisciplinary strategies have been broadly studied in patients with hepatocellular carcinoma (HCC). The objective of this present study was to evaluate the efficacy of autologous transplantations of cytokine-induced killer (CIK) cells as an adjuvant therapy for patients with HCC. A total of 264 patients with HCC were enrolled in this retrospective study. Of these patients, 165 received either CIK cell therapy alone or as adjuvant therapy to surgery, transcatheter arterial chemoembolization (TACE), or TACE-based comprehensive treatments (CT). The remaining 99 patients received only surgery or TACE. Kaplan-Meier analysis and the Chi-squared test were used to analyze the overall survival (OS), progression-free survival (PFS), and clinical characteristics of the patients in the different treatment subgroups. Kaplan-Meier analysis suggested that patients in the Surgery+CIK group had a significantly improved OS compared with those in the other three groups (P < 0.001). Furthermore, patients who developed a fever after the CIK cell treatments manifested a likely better OS (P = 0.028). Subgroup analysis indicated that patients in the Surgery+CIK group likely had an improved PFS but a similar OS compared with the patients in the Surgery-alone group (P = 0.055 for PFS, and P = 0.746 for OS). Further subgroup analysis showed that the OS in both the TACE+CIK and CT+CIK groups was prolonged significantly compared with that in the TACE-alone group (P = 0.015 and P = 0.018, respectively). However, similar OS was observed between the TACE+CIK and CT+CIK groups (P = 0.686). Autologous transplantation of CIK cells as an adjuvant therapy was associated with better survival for patients with HCC, especially for those who had also undergone TACE. A fever reaction might be a potential event for assessing the curative effect of the CIK treatment.
Keywords: Hepatocellular carcinoma, cytokine-induced killer cell, immunotherapy, transcatheter arterial chemoembolization, prognosis
Introduction
The long-term prognosis of hepatocellular carcinoma (HCC) is still unsatisfactory to date owing to the high recurrence and mortality rates either after curative operations or palliative therapy [1,2]. To help patients with HCC achieve better long-term survival, multiple strategies have been explored, including the combination of local therapies, adjustment of antiviral agent usage, and application of advanced image technologies [3-7]. However, whether or not patients with HCC will benefit from adjuvant approaches after liver resection and minimally invasive local therapies, including chemotherapy, sorafenib, and immunotherapy, is still a matter of controversy [8-10].
Cytokine-induced killer (CIK) cells (hereafter CIKs), comprising CD3+CD56+ cells, CD3-CD56+ natural killer (NK) cells, and CD3+CD56- cytotoxic T cells, are mainly T effector memory CD8+ T cells that have acquired NK-like cytotoxicity in ex vivo culture [11]. CIKs can be easily expanded according to a 14-day culture protocol using three main reagents; namely, anti-CD3 OKT antibody, interferon gamma (IFNγ), and interleukin 2 (IL2) [12]. Previous preclinical experiments have shown that CIKs killed HCC cells in vitro [13], and were located within the tumor mass in vivo and induced no severe side effects after their sequential transplantation [14,15]. CIKs were designed to ameliorate the immunosuppressive microenvironment in tumors and consequently presented certain efficacies in several malignancies [16-20]. Autologous transplantation of CIKs was considered as an important adjuvant treatment for HCC therapy to prolong the survival of cancer patients. For instance, these patients would receive CIK therapy after a standard treatment, including surgical resection, transcatheter arterial chemoembolization (TACE), and TACE-based comprehensive treatments (CT). Moreover, CIK therapy was also the only effective way to improve the prognosis of patients with advanced tumors, who were not able to tolerate the standard treatments owing to their worse physical status. Recently, CIKs were extensively studied in patients with hepatitis B virus (HBV)-related HCC, which remains the main causal factor among patients with this type of cancer [21,22]. However, it is still unclear which types of patients with HCC will benefit most from sequential CIK treatments.
On the basis of such information, we retrospectively studied the efficacy of adjuvant CIK treatments in patients with HCC who had undergone surgical resection or minimally invasive treatments of TACE or CT. Our aims were to predict the potential clinical benefits derived from CIKs and to indicate an experience of CIK treatment in HCC.
Patients and methods
Study design and participants
From October 2013 to March 2017, a total of 264 patients with HCC were investigated at The Third Affiliated Hospital of Sun Yat-san University, Guangzhou, China. Patients were included in the study if they met the following criteria: (1) was 18-75 years old; (2) had a confirmed diagnosis of HCC by pathologic examination, or at least two concordant imaging studies (computed tomography and/or magnetic resonance imaging) showing both early enhancement and delayed decreased enhancement, in accordance with the American Association for the Study of Liver Disease Practice Guideline for Management of HCC; (3) had not undergone liver transplantation; and (4) had an Eastern Cooperative Oncology Group (ECOG) performance status score of less than 2.
The exclusion criteria were as follows: the presence of (1) an immune deficiency or autoimmune diseases; (2) other types of malignancies; (3) severe dysfunction of the heart, lung, or other organs; (4) an active infection, except for viral hepatitis; and (5) a severe allergic disorder.
All patients with hepatocellular carcinoma underwent treatment including surgery, TACE and TACE-based comprehensive treatment like radiofrequency or target therapy combined with TACE were strictly following the criteria of NCCN guidelines. However, According to the recommendations of the NCCN Guide: “NCCN believes that the best management of any patient with cancer is in a clinical trial. Participation in clinical trials is especially encouraged”. The patients had a free choice to the adoptive immune cell therapy CIK, as an adjuvant treatment. A lot of previously reported researches demonstrated that CIK combined with TACE, resection and other adjuvant treatment have brought survival benefits to patients, based on these evidences, CIK therapy is recommended for patients with surgery, TACE, and combined therapy, as well as patients with advanced HCC (who cannot tolerate any treatment) in our center. The patients were free to choose CIK according to their own willingness. The patients were divided into two groups based on undergoing CIK or not. We then further divided the two groups into subgroups according to the mode of basic treatment, and analyzed the baseline data of each subgroup and found that there was no significant statistical difference between these two groups. Such as surgical plus CIK and single surgery (Table 5) TACE +CIK, CT+CIK and TACE alone (Table 7). Therefore, further statistical analysis is based on this basis.
Table 5.
Baseline characteristics of patients in Surgery with and without CIK groups
| Characteristics | With CIK | Without CIK | P-value |
|---|---|---|---|
| Age (yrs) | 53.31+11.79 (54) | 49.82+11.19 (54) | 0.142 |
| Gender | 0.854 | ||
| Male | 41 (91.1%) | 45 (90%) | |
| Female | 4 (8.9%) | 5 (10%) | |
| Tumor size | 53.18+30.15 | 47.51+29.79 | 0.360 |
| Vascular invasion | 0.058 | ||
| Positive | 18 (40%) | 11 (22%) | |
| Negative | 27 (60%) | 39 (78%) | |
| AFP Levels | 105.76+359.29 | 1069.53+4737.82 | 0.158 |
| HBV infection | 0.716 | ||
| Positive | 39 (86.7%) | 42 (84%) | |
| Negative | 6 (13.3%) | 8 (16%) | |
| Liver cirrhosis | 0.436 | ||
| Positive | 30 (66.7%) | 37 (74%) | |
| Negative | 15 (33.3%) | 13 (26%) | |
| Tumor number | 0.716 | ||
| > 1 | 6 (13.3%) | 8 (16%) | |
| 1 | 39 (86.7%) | 42 (84%) | |
| TNM stage | 0.143 | ||
| I+II | 23 (51.1%) | 33 (66%) | |
| III+IV | 22 (48.9%) | 17 (34%) | |
| Child-pugh classification grade | 1.000 | ||
| A | 39 (86.7%) | 44 (88%) | |
| B | 6 (13.3%) | 6 (12%) | |
| C | 0 (0%) | 0 (0%) | |
| ALT | 44.69+ 28.14 | 54.24+41.97 | 0.192 |
| ALB | 41.15+3.62 | 40.65+4.42 | 0.548 |
| TBIL | 25.05+71.67 | 19.31+9.99 | 0.601 |
| PT | 14.18+1.87 | 14.27+1.47 | 0.807 |
Table 7.
Baseline characteristics of patients in CT with CIK, TACE with CIK and TACE groups
| Characteristics | TACE With CIK | CT with CIK | TACE without CIK | P-value |
|---|---|---|---|---|
| Age (yrs) | 54.38+11.40 | 54+12.13 | 57.92+12.12 | 0.142 |
| Gender | 0.663 | |||
| Male | 48 (92.3%) | 54 (93.1%) | 57 (96.6%) | |
| Female | 4 (7.7%) | 4 (6.9%) | 2 (3.4%) | |
| Tumor size | 75.83+35.95 | 72.21+34.72 | 75.26+28.67 | 0.894 |
| Vascular invasion | 0.124 | |||
| Positive | 17 (32.7%) | 19 (32.8%) | 27 (45.8%) | |
| Negative | 35 (67.3%) | 39 (67.2%) | 32 (54.2%) | |
| AFP Levels | 400.78+551.94 | 1248.35+4790.08 | 561.41+560.82 | 0.246 |
| HBV infection | 0.700 | |||
| Positive | 47 (90.4%) | 55 (94.8%) | 56 (94.9%) | |
| Negative | 4 (7.7%) | 2 (5.2%) | 3 (5.1%) | |
| Liver cirrhosis | 0.324 | |||
| Positive | 44 (84.6%) | 46 (79.3%) | 43 (72.9%) | |
| Negative | 8 (15.4%) | 12 (20.7%) | 16 (27.1%) | |
| Tumor number | 0.015 | |||
| > 1 | 19 (36.5%) | 10 (17.2%) | 24 (40.7%) | |
| 1 | 33 (63.5%) | 48 (82.8%) | 35 (59.3%) | |
| Tumor stage | 0.763 | |||
| I+II | 17 (32.7%) | 19 (32.8%) | 16 (27.1%) | |
| III+IV | 35 (67.3%) | 39 (67.2%) | 43 (72.9%) | |
| Child-Pugh Classification Grade | 0.021* | |||
| A | 28 (53.8%) | 35 (60.3%) | 46 (78%) | |
| B and C | 24 (46.2%) | 23 (39.7%) | 13 (22%) | |
| ALT | 46.88+ 30.53 | 60.24+43.41 | 49.98+28.29 | 0.255 |
| ALB | 37.21+4.64 | 38.24+4.08 | 38.49+5.36 | 0.336 |
| TBIL | 25.10+18.88 | 20.97+17.96 | 18.4+9.69 | 0.090** |
| PT | 14.85+2.47 | 14.04+2.01 | 13.94+1.24 | 0.033*** |
(TACE with CIK VS. TACE without CIK, P = 0.008; CT with CIK VS. TACE without CIK P = 0.045);
(TACE without CIK VS. CT with CIK, P = 0.029);
(TACE with CIK VS. TACE without CIK, P = 0.004; TACE with CIK VS. CT with CIK, P = 0.034).
The enrolled patients who had received surgery, TACE, or CT together with adjuvant autologous transplantation of CIKs were designated as the Surgery+CIK, TACE+CIK, and CT+CIK groups, respectively. TACE and TACE-based comprehensive treatments (CT) based on the conventional therapy, and CT were defined as the combined TACE with chemothepeutic agents or targeted drugs (such as cisplatin or sorafenib). Patients who were treated with only CIK therapy were designated as the CIK group. Control patients who had received only surgery or TACE alone were designated as the Surgery and TACE groups, respectively.
This study was approved by the ethics committee of The Third Affiliated Hospital of Sun Yat-san University. All study procedures conformed to the norms of the Declaration of Helsinki. Written informed consent was obtained from each patient according to institutional guidelines.
Autologous CIK preparation and transplantation
Peripheral blood mononuclear cells (PBMCs) were isolated from 30 mL of peripheral blood collected from individual patients, and the CIKs used for adoptive cell therapy were prepared at the Good Manufacturing Practice (GMP) Production Facility of Biotherapy Center in the Third Affiliated Hospital of Sun Yat-sen University. In brief, the isolated PBMCs were cultured with anti-CD3 OKT antibody, INFγ, and IL2 at 37°C for 14 days according to a modified CIK preparation protocol [23]. The patients received a 250-mL intravenous administration of the CIK product in 60 min without any premedication and were then observed for at least 30 min after the treatment either as an inpatient or outpatient. The recommended frequency of CIK treatments for the patients was every 2 weeks for the first 4 treatments, every 4 weeks for the second 4 treatments, and then every 3 months for the following maintenance treatments. A reasonable delay of a maximum of 1 week was acceptable if the CIK agent was not prepared on time owing to the cell status of the respective patients.
Follow-up
The enrolled patients in the different treatments groups were routinely followed up every 3 months, including for clinical evaluation, liver function tests, serum alpha-fetoprotein (AFP) determination, ultrasound examination, and dynamic computed tomography scanning. The follow-up period was calculated from the fifth day of receiving surgical resection or TACE or CT to either the death of the patient or to the end of this study on March 30, 2017. All patients received a final assessment at the end of the study.
End points of adjuvant treatment
The primary end point of this study was overall survival (OS). The secondary end point was progression-free survival (PFS). OS was defined as the time from the treatments to death or to the date of the last follow-up for censored patients. PFS was defined as the time from curative resection to either local or distant recurrence or death (any cause). For patients who underwent TACE or CT, PFS was calculated from the time when no viable tumor existed after treatments to cancer progression or death (any cause). Reactions to CIK treatment were defined as side effects that manifested during the therapy, including pyrexia, flu-like symptoms, digestive reactions, and deterioration of liver function.
Statistical analysis
Clinical data of the enrolled patients comprised patient demographics, disease characteristics, details of treatments, and CIK therapy-related reactions. Baseline characteristics were reported as the median for continuous variables and as numbers with percentages for categorical variables. The Chi-squared test, Fisher’s exact test, and Mann-Whitney U test were used in performing univariate analyses. Multivariate analyses using the Cox proportional hazards model were performed for determining independent significance by backward elimination of the insignificant baseline characteristics and explanatory variables. The OS and PFS data were analyzed using the Kaplan-Meier method and compared using the log-rank test. All values quoted were two-sided, and a p value of less than 0.05 was considered as statistical significance. The statistical analyses were conducted using SPSS v.20.0 (SPSS, Inc., Chicago, IL, USA).
Results
Outcomes for patients who received CIK treatment
Baseline characteristics
A total of 165 patients treated with CIKs were included in the final analysis. They comprised 10, 45, 52, and 58 patients in the CIK, Surgery+CIK, TACE+CIK, and CT+CIK groups, respectively, among all of which CIK treatment was performed an average of 9.7 times. The baseline characteristics of these 4 groups are shown in Table 1. The major primary liver disease was hepatitis B. Of these 165 patients, 128 (77.6%) were pathologically confirmed with liver cirrhosis.
Table 1.
Clinical characteristics in total of patients treated with CIKs
| Characteristics | Total patients | CIK | Surgery+CIK | TACE+CIK | CT+CIK | P-value |
|---|---|---|---|---|---|---|
| Age (yrs) | 54.33+12.01 | 59.56+15.44 | 53.31+11.79 | 54.38+11.40 | 54+12.13 | 0.276 |
| Gender | ||||||
| Male | 151 (91.5%) | 8 (80%) | 41 (91.1%) | 48 (92.3%) | 54 (93.1%) | 0.499 |
| Female | 14 (8.5%) | 2 (20%) | 4 (8.9%) | 4 (7.7%) | 4 (6.9%) | |
| HBV infection | 0.204 | |||||
| Positive | 151 (91.5%) | 8 (80%) | 39 (86.7%) | 48 (92.3%) | 56 (94.8%) | |
| Negative | 14 (8.5%) | 2 (20%) | 6 (13.3%) | 4 (7.7%) | 2 (5.2%) | |
| Liver cirrhosis | 0.201 | |||||
| Positive | 128 (77.6%) | 8 (80%) | 30 (66.7%) | 44 (84.6%) | 46 (79.3%) | |
| Negative | 37 (22.4%) | 2 (20%) | 15 (33.3%) | 8 (15.4%) | 12 (20.7%) | |
| Tumor number | 0.027 | |||||
| > 1 | 38 (23%) | 3 (30%) | 6 (13.3%) | 19 (36.5%) | 10 (17.2%) | |
| 1 | 127 (77%) | 7 (70%) | 39 (86.7%) | 33 (63.5%) | 48 (82.8%) | |
| TNM stage | 0.125 | |||||
| I+II | 61 (37%) | 2 (20%) | 23 (51.1%) | 17 (32.7%) | 19 (32.8%) | |
| III+IV | 104 (63%) | 8 (80%) | 22 (48.9%) | 35 (67.3%) | 39 (67.2%) | |
| Vascular invasion | 0.018 | |||||
| Positive | 62 (37.6%) | 8 (80%) | 18 (40%) | 17 (32.7%) | 19 (32.8%) | |
| Negative | 103 (62.4%) | 2 (20%) | 27 (60%) | 35 (67.3%) | 39 (67.2%) | |
| Child-Pugh | < 0.001 | |||||
| A | 103 (62.4%) | 1 (10%) | 39 (86.7%) | 28 (53.8%) | 35 (60.3%) | |
| B | 55 (33.3%) | 7 (70%) | 6 (13.3%) | 21 (40.4%) | 21 (36.2%) | |
| C | 7 (4.3%) | 2 (20%) | 0 (0%) | 3 (5.8%) | 2 (3.5%) | |
| Tumor size | 69.90+37.10 | 98.56+62.43 | 53.18+30.15 | 75.83+35.95 | 72.21+34.72 | 0.001 |
| AFP Levels | 619.39+2848.86 | 607+524.44 | 105.76+359.29 | 400.78+551.94 | 1248.35+4790.08 | 0.217 |
HBV, Hepatitis B virus; TNM stage, Tumor-Node-Metastasis stage.
At the time of primary anticancer treatment, 127 (77%) of the 165 patients were diagnosed with 1 tumor nodule within the liver, whereas 38 (23%) patients had more than 1 nodule. Of these 165 patients, 104 (63%) were at TNM Stage 3 and 4, whereas 61 (37%) patients were at Stage 1 and 2. The AFP level was elevated in all the patients but with considerable standard deviations. No statistical significance was presented among these four groups with regard to these factors above.
On the other hand, the tumor number, vascular invasion, Child-Pugh Classification Grade, and tumor size were significantly different among the 4 groups. For instance, patients in the TACE+CIK and CT+CIK groups had greater tumor numbers than those of the other 2 groups. Patients treated with CIKs alone had a higher incidence of vascular invasion than those in the Surgery+CIK, TACE+CIK, and CT+CIK groups. Patients in the Surgery+CIK group had significantly smaller tumor sizes than those of the other 3 groups. Moreover, patients in the CIK group had the highest proportion of Child-Pugh Classification Grade B and C tumors, whereas the patients in the Surgery+CIK group had the lowest proportion of such tumors among the 4 groups. Furthermore, the probabilities of Child-Pugh Classification Grade B and C tumors in the TACE+CIK and CT+CIK groups were similar.
Survival data
The survival data revealed that the patients treated with surgery with adjuvant CIK therapy had a significantly increased OS. In contrast, the patients treated with CIKs alone demonstrated the shortest survival period, whereas there were no great differences in OS between the TACE+CIK and CT+CIK groups (log-rank P < 0.001; Figure 1A). As shown by the baseline characteristics, patients in the Surgery+CIK group had a smaller tumor size, whereas those treated with CIKs alone had a higher incidence of vascular invasion, which may be the reasons for the differences in OS among the 4 groups.
Figure 1.
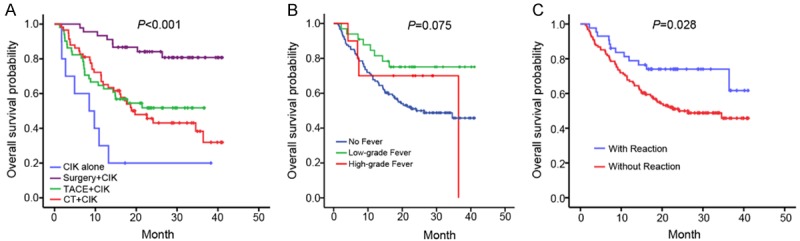
Comparison of the overall survival of patients in the different treatment subgroups by Kaplan-Meier analysis. A. The overall survival times among the Surgery+CIK, TACE+CIK, CT+CIK, and CIK groups were significantly different (P ≤ 0.001). B. The overall survival times among no fever, low-grade fever and high grade fever groups. C. Reaction-positive patients had a more favorable overall survival than the reaction-negative ones (P = 0.028).
The adverse events (AEs) that occurred in the CIK-treated individuals are indicated in Table 2. Among these, fever was the most common AE, with 26.1% of the patients experiencing low-grade (ranging from 37°C to 38°C) and high-grade (higher than 38°C) fevers. However, no severe AEs related to CIK treatment were observed in this study. we divided the patients who underwent CIK into three groups including the group without fever, with low-grade fever and with high-grade fever. Then the overall survival (OS) were analyzed by Kaplan-Meier and the results showed that there was no significant difference among the three groups (P = 0.075), which indicated that there was no relationship between the degree of fever and the outcome (Figure 1B). Those patients who manifested fevers were further divided into the reaction-positive group (fever) and the patients who did not manifest fevers were allocated to the reaction-negative group (no fever). The number of patients in both reaction groups was balanced among the Surgery+CIK, TACE+CIK, and CT+CIK groups, and there was no significant difference among the 4 treatment groups (Table 2). Interestingly, the OS was significantly improved for the reaction-positive patients (log-rank P = 0.028; Figure 1C). A multivariate Cox proportional hazards analysis was performed for all the CIK-treated individuals. Of the baseline characteristics included into the analysis, the tumor size, vascular invasion, Child-Pugh Classification Grade, and reaction were correlated with OS (Table 3), which suggested that these 4 parameters were independent factors for prognosis in the cohorts. The relationships between the reaction groups and the clinical characteristics were further analyzed, with results indicating no correlation between the reaction and any other characteristic (Table 4).
Table 2.
Frequency of Reactions in total of patients treated with CIKs
| Team | Reactions | Low-grade Fever | High-grade Fever | P-value |
|---|---|---|---|---|
| Total | 43/165 (26.7%) | 33 | 10 | 0.457** |
| CIK | 2/10 (20%) | 2 | 0 | |
| Surgery | 13/32 (40.6%) | 12 | 1 | |
| TACE | 17/35 (48.6%) | 11 | 6 | |
| CT | 12/46 (28.9%) | 9 | 3 |
Fischer’s accurate test, multiple comparisons.
Table 3.
Multivariate Cox proportional-hazards analysis in total patients
| Variable | OS | ||
|---|---|---|---|
|
| |||
| HR | 95% CI | P value | |
| Tumor size (< 5 cm vs. ≥ 5 cm) | 2.045 | 1.204-3.474 | 0.003 |
| Vascular invasion (Negative vs. Positive) | 1.796 | 1.115-2.894 | 0.006 |
| Child-Pugh Classification Grade (A vs. B and C) | 1.620 | 1.001-2.620 | < 0.001 |
| Reaction (Negative vs. Positive) | 0.491 | 0.268-0.899 | 0.006 |
Table 4.
Reactions related to clinical features in patients with HCC
| Characteristics | Reaction | P value | |
|---|---|---|---|
|
| |||
| Negative (n = 121) | Positive (n = 44) | ||
| Age | |||
| < 50 | 54 | 17 | 0.594 |
| ≥ 50 | 67 | 27 | |
| Gender | |||
| Male | 112 | 39 | 0.527 |
| Female | 9 | 5 | |
| Tumor size | |||
| < 5 cm | 44 | 17 | 0.856 |
| ≥ 5 cm | 77 | 27 | |
| Tumor multiplicity | |||
| Single | 94 | 33 | 0.835 |
| Multiple | 27 | 11 | |
| HBV infection | |||
| Negative | 10 | 4 | 1.000 |
| Positive | 110 | 40 | |
| Liver cirrhosis | |||
| Negative | 30 | 7 | 0.293 |
| Positive | 91 | 37 | |
| Histological grade | |||
| Poor and moderate | 4 | 12 | 1.000 |
| Well | 28 | 80 | |
| Vascular invasion | |||
| Negative | 80 | 26 | 0.464 |
| Positive | 41 | 18 | |
| TNM stage | |||
| I+II | 45 | 16 | 1.000 |
| III+IV | 76 | 28 | |
| Serum AFP | |||
| < 400 ug/L | 14 | 54 | 0.155 |
| ≥ 400 ug/L | 18 | 38 | |
| Child-Pugh Classification Grade | |||
| A | 22 | 61 | 0.832 |
| B and C | 10 | 31 | |
Comparison of outcomes for patients treated with or without CIKs
As a control experiment, we also studied patients treated by surgery, TACE, or CT only for comparison with the patients who received adjuvant CIKs. For patients who had primarily received a liver resection, the OS and PFS were compared between the CIK-treated and non-CIK-treated groups. As shown in Table 5, the baseline characteristics were comparable between these two groups. Nevertheless, it seemed that more patients in the CIK group had severe advanced vascular invasion, albeit without statistical significance (P = 0.058). No remarkable differences in OS were observed between the CIK and non-CIK groups (log-rank P = 0.746; Figure 2A). However, a slight improvement of PFS was observed in the CIK group (log-rank P = 0.055; Figure 2B). Further analysis of the 3-year (36 months) OS and PFS revealed that the 3-year OS was similar between these two groups (log-rank P = 0.650; Figure 2C), whereas the 3-year PFS was significantly improved in the CIK group (log-rank P = 0.033; Figure 2D).
Figure 2.
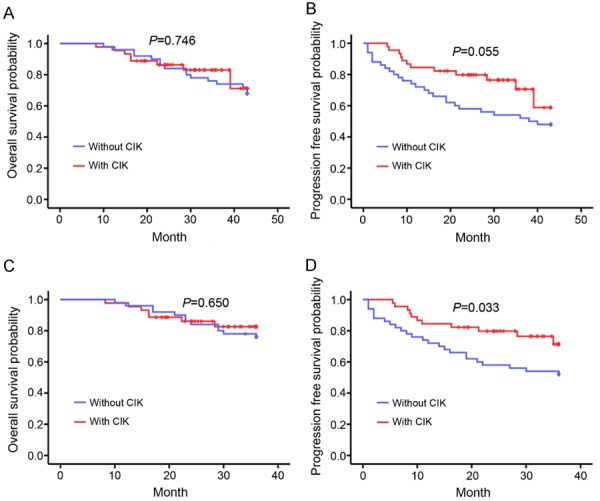
Comparison of the overall survival and progression-free survival of patients with HCC treated with Surgery with or without CIK by Kaplan-Meier analysis. Patients treated with Surgery with and without CIK had similar overall survival (A, P = 0.746) and progression-free survival times (B, P = 0.055). (C) The patients in the Surgery and Surgery+CIK groups had similar 3-year survival times (P = 0.650). (D) The patients treated with CIKs after Surgery had a longer 3-year progression-free survival time than those who received Surgery only (P = 0.033).
The multivariate Cox proportional hazards analysis was tested in the surgery group regardless of CIK treatment status. For determination of the PFS, the tumor size, vascular invasion, Child-Pugh Classification Grade, and CIK therapy were examined instead. As shown in Table 6, only CIK treatment was an independent predictive factor for a higher PFS for all patients (hazards ratio (HR) = 0.382, 95% confidence interval (CI) = 0.182-0.802, P = 0.011).
Table 6.
Multivariate Cox proportional-hazards analysis in the patients with surgery
| Variable | PFS | ||
|---|---|---|---|
|
| |||
| HR | 95% CI | P value | |
| Tumor size (< 5 cm vs. ≥ 5 cm) | 1.293 | 0.644-2.594 | 0.470 |
| Vascular invasion (Negative vs. Positive) | 2.009 | 0.971-4.155 | 0.060 |
| Child-Pugh Classification Grade (A vs. B and C) | 1.205 | 0.450-3.225 | 0.711 |
| CIK (With vs. Without) | 0.382 | 0.182-0.802 | 0.011 |
The patients primarily treated with TACE or CT plus adjuvant CIKs were also compared with control patients who received TACE alone. The baseline characteristics were comparable among the TACE+CIK, CT+CIK, and TACE groups except for tumor number, Child-Pugh Classification Grade, total bilirubin (TBIL), and prothrombin time (PT) (Table 7). Of the 58 patients in the CT+CIK group, 48 (82.8%) were diagnosed with 1 tumor in the liver, whereas the same was diagnosed in 33 (63.5%) of 52 patients and 35 (59.3%) of 59 patients in the TACE+CIK and TACE-alone groups, respectively (P = 0.015). The patients treated with CIK (TACE+CIK and CT+CIK groups) had poorer liver function (high probability of Child-Pugh Classification Grade B and C) than those in the TACE group (P = 0.008 and P = 0.045, respectively). The average TBIL level of patients in the TACE group was significantly lower than that in the CT+CIK group of patients (P = 0.029). Patients in the TACE+CIK group had a remarkably longer PT than those in the CT+CIK and TACE groups (P = 0.024 and P = 0.004, respectively).
Survival data suggested that the OS for the TACE group was the lowest among these 3 groups (log-rank P = 0.013; Figure 3A). Two-two comparisons were performed to better analyze the survival data. As shown in Figure 3B, a significant improvement of OS was observed in the TACE+CIK group compared with that in the TACE group (log-rank P = 0.015). Similar OS results were obtained between the CT+CIK and TACE groups (log-rank P = 0.018; Figure 3C). However, patients in the TACE+CIK and CT+CIK groups showed similar OS times (log-rank P = 0.686; Figure 3D). To further analyze the beneficial roles of CIKs in TACE, we integrated the TACE+CIK and TACE subgroups as a group and then performed multivariate Cox proportional hazards analysis of the tumor size, vascular invasion, ascites, and CIK therapy in the new cohort (Table 8). The results showed that CIK therapy was the single and independent risk factor for the OS of patients who had undergone minimally invasive local treatments (HR = 0.557, 95% CI = 0.327-0.949, P = 0.031).
Figure 3.
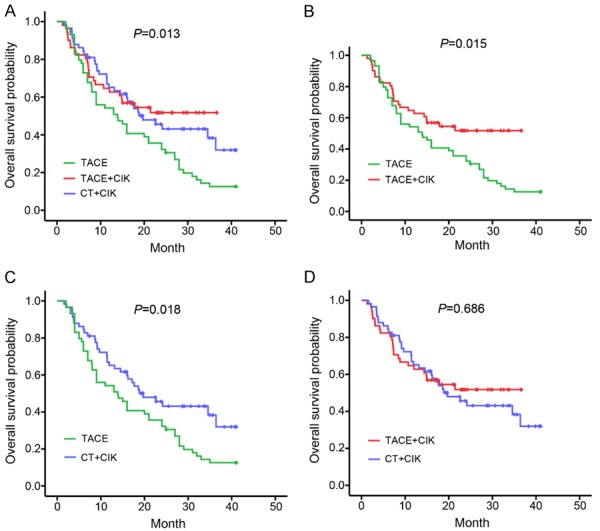
Comparison of the overall survival of patients in the TACE, TACE+CIK, and CT+CIK groups by Kaplan-Meier analysis. A. The overall survival was significantly different among the TACE, TACE+CIK, and CT+CIK groups (P = 0.013). B. Patients in the TACE+CIK group had a better overall survival than those in the TACE group (P = 0.015). C. Patients in the CT+CIK group had a more favorable overall survival than those in the TACE group (P = 0.018). D. The patients in the TACE+CIK and CT+CIK groups had similar overall survival times (P = 0.686).
Table 8.
Multivariate Cox proportional-hazards analysis in the patients with TACE
| Variable | OS | ||
|---|---|---|---|
|
| |||
| HR | 95% CI | P value | |
| Tumor size (< 5 cm vs. ≥ 5 cm) | 1.409 | 0.819-2.425 | 0.215 |
| Vascular invasion (Negative vs. Positive) | 1.165 | 0.718-1.889 | 0.537 |
| Child-Pugh Classification Grade (A vs. B and C) | 1.554 | 0.950-2.542 | 0.079 |
| CIK (With vs. Without) | 0.557 | 0.327-0.949 | 0.031 |
Discussion
Reversing the tumorous microenvironment from an immune-suppressive pattern into an immune-activating one would be a promising curative treatment for HCC [24,25]. As a treatment with tremendous potential, adoptive cell therapy involves several types of lymphocytes, including tumor-infiltrating lymphocytes, CIKs, T cells, and their genetically modified transformation. CIKs have been tested in various solid tumors and hematological neoplasms ever since Ingo GH Schmidt-Wolf and his colleagues first reported the clinical application of these cells [26,27]. The benefits of different combinations of conventional therapies with CIKs were extensively studied among patients with HCC in China. In the present study, CIKs proved to be an effective adjuvant treatment by improving the OS for patients who had primarily undergone TACE or CT for HCC, and by improving the PFS for those who had primarily undergone surgery.
We found that the best improvement of OS was for the patients in the Surgery+CIK group, and to a lesser extent for those in the TACE+CIK and CT+CIK groups. Moreover, the patients treated with CIKs alone had the worst OS among these 4 treatment groups. However, the baseline characteristics showed that the average tumor size in the Surgery+CIK group was much smaller than that in the other 3 groups. Besides this, only 10 patients were included in the group treated with CIKs alone, and 8 of them were at TNM Stage 3 and 4. The above imbalances might partially explain the difference in the improvements of OS among the groups of this study. To our surprise, when all CIK-treated patients were divided into reaction-positive and reaction-negative groups, patients in the reaction-positive group showed a significance of OS improvement compared with those in the reaction-negative group. This implied that a reaction to CIK therapy might reflect an advantageous prognosis for these patients, and provides a potential approach to easily evaluate the efficacy of CIKs for patients with HCC.
As is already known, CIKs were found to have an adjuvant role in cancer therapy, as reported by many research groups including our team, ever since they were used by Schmidt-Wolf to treat malignances. However, not all patients benefited from CIK therapy. Therefore, there was an urgent need to select the appropriate patient population in order to make cancer immunotherapies more precise. In this present study, the OS was significantly improved in the reaction-positive patients (fever), thus potentially providing a convenient method to assess the therapeutic effect of CIKs by surveying the fever reaction in patients. Nevertheless, the difference was not significant enough to distinguish between reaction-positive and reaction-negative effects in the 4 subgroups. The reason for this negative result could be the limited number of patients in the subgroups, and thus further studies with greater case numbers remain to be carried out. The fever might be a common immunoreaction that was induced by inflammatory cytokines released in the immune response [28,29]. Thus, further study on whether the fever was caused through an inflammatory reaction induced by CIKs attacking the tumor cells should be performed. Additionally, deeper research regarding the quantitative analysis of biological factors, such as serum biomarkers, would provide a more precise means for individualized treatment.
Furthermore, we compared the OS of patients who received surgery, TACE, or CT plus CIK treatments with that of patients who received these interventions without CIK treatments. First, among the surgery-receiving patients, both the 3-year and total OS were similar between the Surgery+CIK and Surgery groups. However, a tendency of improved total PFS and a significant improvement of the 3-year PFS were observed in the Surgery+CIK group. Accordingly, sequential CIK treatments after liver resection would retard the progression of the tumor and ameliorate the prognosis for patients with HCC. In our further analysis, we found that the OS of surgery-receiving patients was related to many clinical aspects, such as the tumor size, vascular invasion, ascites, and reaction to CIKs. Our previous studies also suggested that the OS of patients with HCC was significantly influenced by subsequent treatments, which made it difficult to improve the OS by CIKs [30]. However, the PFS was related to vascular invasion and CIKs instead, which suggested that CIKs could delay the disease progression at a promising level.
Second, among the patients who primarily underwent TACE or CT, significant improvements of OS were found in both the TACE+CIK and CT+CIK groups compared with the TACE group. Because no remarkable differences were presented between the TACE+CIK and CT+CIK groups thus far, it suggested that TACE-based comprehensive therapies might not bring about much more advantages for patients who had undergone minimally invasive treatments. Therefore, taking the patient’s quality of life into account, TACE+CIK would be a better choice than CT+CIK for patients who have undergone minimally invasive therapies. As shown by the multivariate Cox analysis, CIKs was the only independent prognostic factor for OS in patients who had undergone TACE (TACE and TACE+CIK). This obvious advantage of CIKs might be associated with an increase in the tumor immunogenicity and the unmasking of tumor-specific antigens after transarterial embolization [31,32]. However, the exact mechanisms are still unconfirmed to date.
Our present study had some limitations. First, as a retrospective study, the selection bias in determining whether the patients could receive CIKs or not was hardly avoided. Some clinical elements (e.g., tumor cell differentiation) and economic considerations during the treatments might have facilitated the CIK usage in a certain subset of patients, which could make them intrinsically different from those who did not receive the CIKs. Second, although we compared important clinical characteristics between the groups, some measured and other unmeasured factors would inevitably affect the outcomes of this study to some extent. For example, the heterogeneity of the TACE procedure might affect the OS of patients. Last but not least, although multiple control patients were eligible for the study, the chosen patient could be a study confounder despite the comparable outcomes observed. Prospective and randomized controlled trials are required to resolve this problem. To improve the cytotoxicity and clinical efficacy of CIKs, more efforts should be made to optimize the various aspects of targeted CIK therapy and combination of CIKs with antiangiogenic drugs or oncolytic viruses [33].
In conclusion, adjuvant CIK therapy is a promising clinical approach for improving the PFS and OS of patients who primarily receive surgery and TACE for HCC, respectively.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 81402509 to Tian-Tian Wang, No. 81402426 to Chang-Chang Jia, No. 81372374 to Qi Zhang, 81670601, No. 81372374 to Xiang-Yuan Wu, No. 81600505 to Yang Li, No. 31701116 to Shu-Juan Xie, and No. 81572726). Natural Science Foundation of Guangdong Province (No. 82000-18823955 to Dong-Bo Qiu, No. 2015B020226004 to Qi Zhang).
Disclosure of conflict of interest
None.
References
- 1.Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63:844–55. doi: 10.1136/gutjnl-2013-306627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin. 2012;62:394–9. doi: 10.3322/caac.21161. [DOI] [PubMed] [Google Scholar]
- 3.Granito A, Bolondi L. Non-transplant therapies for patients with hepatocellular carcinoma and Child-Pugh-Turcotte class B cirrhosis. Lancet Oncol. 2017;18:e101–e112. doi: 10.1016/S1470-2045(16)30569-1. [DOI] [PubMed] [Google Scholar]
- 4.Jung KS, Kim SU, Song K, Park JY, Kim DY, Ahn SH, Kim BK, Han KH. Validation of hepatitis B virus-related hepatocellular carcinoma prediction models in the era of antiviral therapy. Hepatology. 2015;62:1757–66. doi: 10.1002/hep.28115. [DOI] [PubMed] [Google Scholar]
- 5.Chen K, Chen G, Wang H, Li H, Xiao J, Duan X, He J, He K, Xiang G. Increased survival in hepatocellular carcinoma with iodine-125 implantation plus radiofrequency ablation: a prospective randomized controlled trial. J Hepatol. 2014;61:1304–11. doi: 10.1016/j.jhep.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 6.Papatheodoridis GV, Chan HL, Hansen BE, Janssen HL, Lampertico P. Risk of hepatocellular carcinoma in chronic hepatitis B: assessment and modification with current antiviral therapy. J Hepatol. 2015;62:956–67. doi: 10.1016/j.jhep.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Yamashita T, Kitao A, Matsui O, Hayashi T, Nio K, Kondo M, Ohno N, Miyati T, Okada H, Yamashita T, Mizukoshi E, Honda M, Nakanuma Y, Takamura H, Ohta T, Nakamoto Y, Yamamoto M, Takayama T, Arii S, Wang X, Kaneko S. Gd-EOB-DTPA-enhanced magnetic resonance imaging and alpha-fetoprotein predict prognosis of early-stage hepatocellular carcinoma. Hepatology. 2014;60:1674–85. doi: 10.1002/hep.27093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, Cai J, Poon RT, Han KH, Tak WY, Lee HC, Song T, Roayaie S, Bolondi L, Lee KS, Makuuchi M, Souza F, Berre MA, Meinhardt G, Llovet JM STORM investigators. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344–54. doi: 10.1016/S1470-2045(15)00198-9. [DOI] [PubMed] [Google Scholar]
- 9.Murawski M, Weeda VB, Maibach R, Morland B, Roebuck DJ, Zimmerman A, Casanova M, Perilongo G, Laithier V, Kebudi R, Scopinaro MJ, Shun A, Brichard B, de Camargo B, Childs M, Aronson DC, Czauderna P. Hepatocellular carcinoma in children: does modified platinum- and doxorubicin-based chemotherapy increase tumor resectability and change outcome? Lessons learned from the SIOPEL 2 and 3 studies. J. Clin. Oncol. 2016;34:1050–6. doi: 10.1200/JCO.2014.60.2250. [DOI] [PubMed] [Google Scholar]
- 10.Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12:681–700. doi: 10.1038/nrgastro.2015.173. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt-Wolf IG, Negrin RS, Kiem HP, Blume KG, Weissman IL. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J Exp Med. 1991;174:139–49. doi: 10.1084/jem.174.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ochoa AC, Gromo G, Alter BJ, Sondel PM, Bach FH. Long-term growth of lymphokine-activated killer (LAK) cells: role of anti-CD3, beta-IL 1, interferon-gamma and -beta. J Immunol. 1987;138:2728–33. [PubMed] [Google Scholar]
- 13.Konomi Y, Sekine T, Takayama T, Fuji M, Tanaka T. Cytotoxic activity of CD4+ T cells against autologous tumor cells. Jpn J Cancer Res. 1995;86:854–60. doi: 10.1111/j.1349-7006.1995.tb03096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takayama T, Makuuchi M, Sekine T, Terui S, Shiraiwa H, Kosuge T, Yamazaki S, Hasegawa H, Suzuki K, Yamagata M, et al. Distribution and therapeutic effect of intraarterially transferred tumor-infiltrating lymphocytes in hepatic malignancies. A preliminary report. Cancer. 1991;68:2391–6. doi: 10.1002/1097-0142(19911201)68:11<2391::aid-cncr2820681110>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Takayama T, Sekine T, Kondo Y, Kakizoe T, Makuuchi M. Adjuvant adoptive immunotherapy against hepatocellular carcinoma. Hepatology. 1998;28:1436–7. doi: 10.1002/hep.510280539. [DOI] [PubMed] [Google Scholar]
- 16.Li R, Wang C, Liu L, Du C, Cao S, Yu J, Wang SE, Hao X, Ren X, Li H. Autologous cytokine-induced killer cell immunotherapy in lung cancer: a phase II clinical study. Cancer Immunol Immunother. 2012;61:2125–33. doi: 10.1007/s00262-012-1260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu L, Zhang W, Qi X, Li H, Yu J, Wei S, Hao X, Ren X. Randomized study of autologous cytokine-induced killer cell immunotherapy in metastatic renal carcinoma. Clin Cancer Res. 2012;18:1751–9. doi: 10.1158/1078-0432.CCR-11-2442. [DOI] [PubMed] [Google Scholar]
- 18.Shi L, Zhou Q, Wu J, Ji M, Li G, Jiang J, Wu C. Efficacy of adjuvant immunotherapy with cytokine-induced killer cells in patients with locally advanced gastric cancer. Cancer Immunol Immunother. 2012;61:2251–9. doi: 10.1007/s00262-012-1289-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linn YC, Niam M, Chu S, Choong A, Yong HX, Heng KK, Hwang W, Loh Y, Goh YT, Suck G, Chan M, Koh M. The anti-tumour activity of allogeneic cytokine-induced killer cells in patients who relapse after allogeneic transplant for haematological malignancies. Bone Marrow Transplant. 2012;47:957–66. doi: 10.1038/bmt.2011.202. [DOI] [PubMed] [Google Scholar]
- 20.Rambaldi A, Biagi E, Bonini C, Biondi A, Introna M. Cell-based strategies to manage leukemia relapse: efficacy and feasibility of immunotherapy approaches. Leukemia. 2015;29:1–10. doi: 10.1038/leu.2014.189. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Liu A, Bo W, Feng X, Hu Y, Tian L, Zhang H, Tang X. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma patients after curative resection, a systematic review and meta-analysis. Dig Liver Dis. 2016;48:1275–82. doi: 10.1016/j.dld.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, Gwak GY, Kim KM, Kim YJ, Lee JW, Yoon JH. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148:1383–91.e6. doi: 10.1053/j.gastro.2015.02.055. [DOI] [PubMed] [Google Scholar]
- 23.Anderson PM, Bach FH, Ochoa AC. Augmentation of cell number and LAK activity in peripheral blood mononuclear cells activated with anti-CD3 and interleukin-2. Preliminary results in children with acute lymphocytic leukemia and neuroblastoma. Cancer Immunol Immunother. 1988;27:82–8. doi: 10.1007/BF00205763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Umemoto Y, Okano S, Matsumoto Y, Nakagawara H, Matono R, Yoshiya S, Yamashita Y, Yoshizumi T, Ikegami T, Soejima Y, Harada M, Aishima S, Oda Y, Shirabe K, Maehara Y. Prognostic impact of programmed cell death 1 ligand 1 expression in human leukocyte antigen class I-positive hepatocellular carcinoma after curative hepatectomy. J Gastroenterol. 2015;50:65–75. doi: 10.1007/s00535-014-0933-3. [DOI] [PubMed] [Google Scholar]
- 25.Calderaro J, Rousseau B, Amaddeo G, Mercey M, Charpy C, Costentin C, Luciani A, Zafrani ES, Laurent A, Azoulay D, Lafdil F, Pawlotsky JM. Programmed death ligand 1 expression in hepatocellular carcinoma: relationship with clinical and pathological features. Hepatology. 2016;64:2038–46. doi: 10.1002/hep.28710. [DOI] [PubMed] [Google Scholar]
- 26.Sangiolo D. Cytokine induced killer cells as promising immunotherapy for solid tumors. J Cancer. 2011;2:363–8. doi: 10.7150/jca.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giraudo L, Gammaitoni L, Cangemi M, Rotolo R, Aglietta M, Sangiolo D. Cytokine-induced killer cells as immunotherapy for solid tumors: current evidence and perspectives. Immunotherapy. 2015;7:999–1010. doi: 10.2217/imt.15.61. [DOI] [PubMed] [Google Scholar]
- 28.Hayakawa K, Ramasamy B, Chandrasekar PH. Fever of unknown origin: an evidence-based review. Am J Med Sci. 2012;344:307–16. doi: 10.1097/MAJ.0b013e31824ae504. [DOI] [PubMed] [Google Scholar]
- 29.Wong JJ, Au AY, Gao D, Pinello N, Kwok CT, Thoeng A, Lau KA, Gordon JE, Schmitz U, Feng Y, Nguyen TV, Middleton R, Bailey CG, Holst J, Rasko JE, Ritchie W. RBM3 regulates temperature sensitive miR-142-5p and miR-143 (thermomiRs), which target immune genes and control fever. Nucleic Acids Res. 2016;44:2888–97. doi: 10.1093/nar/gkw041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong YF, Chen ZH, Ma XK, Li X, Wu DH, Chen J, Dong M, Wei L, Wang TT, Ruan DY, Lin ZX, Wen JY, Lin Q, Jia CC, Wu XY. Comparison of five models for end-stage liver disease in predicting the survival rate of patients with advanced hepatocellular carcinoma. Tumour Biol. 2016;37:5265–73. doi: 10.1007/s13277-015-4366-2. [DOI] [PubMed] [Google Scholar]
- 31.Ali MY, Grimm CF, Ritter M, Mohr L, Allgaier HP, Weth R, Bocher WO, Endrulat K, Blum HE, Geissler M. Activation of dendritic cells by local ablation of hepatocellular carcinoma. J Hepatol. 2005;43:817–22. doi: 10.1016/j.jhep.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 32.Dromi SA, Walsh MP, Herby S, Traughber B, Xie J, Sharma KV, Sekhar KP, Luk A, Liewehr DJ, Dreher MR, Fry TJ, Wood BJ. Radiofrequency ablation induces antigen-presenting cell infiltration and amplification of weak tumor-induced immunity. Radiology. 2009;251:58–66. doi: 10.1148/radiol.2511072175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jakel CE, Schmidt-Wolf IG. An update on new adoptive immunotherapy strategies for solid tumors with cytokine-induced killer cells. Expert Opin Biol Ther. 2014;14:905–16. doi: 10.1517/14712598.2014.900537. [DOI] [PubMed] [Google Scholar]


