Abstract
Chemoradiotherapy in inoperable non-small cell lung cancer (NSCLC) is standard, but accompanied by undesirable adverse effects such as radiation pneumonitis. Polyene phosphatidylcholine (PPC) is a hepatoprotective agent and can be used as nutritional adjuvant to chemotherapy. We aimed to investigate influence of PPC on tumor radiosensitivity as well as radiation therapy related injury in healthy tissues. Thus, a retrospective analysis was carried out in 133 NSCLC patients to assess impact of daily PPC administration on radiation pneumonitis. PPC effects on radiation related tissue injury were additionally investigated in mice receiving total body irradiation. Influence of PPC on tumor radiosensitivity was further evaluated using tumor xenografted mice, lewis lung carcinoma (LLC) and A549 cell lines. Uni- and multivariate analyses suggested that daily PPC intake is significantly associated with reduced risk in developing symptomatic radiation pneumonitis in NSCLC patients. In comparison to patients without PPC supplementation, patients who received PPC benefited from a slower decline in lung function post radiotherapy. Total body irradiation in mice further confirmed that PPC administration protected against radiation induced fatal tissue damage and this protective effect was directly linked to increased cellular antioxidant defense. Radiation resulted in significant growth inhibition of cultured LLC and A549 cells as well as of LLC xenografted tumors, however, this was not affected by PPC treatment. In conclusion, PPC protects against radiation induced injury of healthy tissues and thus may serve as meaningful adjuvant for radiotherapy in NSCLC as well for other cancer entities to dampen adverse effects.
Keywords: PPC, NSCLC, radiotherapy, radiation pneumonitis
Introduction
Radiation therapy is a major treatment modality for non-small cell lung cancer (NSCLC) and often used in curative, palliative, and prophylactic treatment regimens. Mechanistically, ionizing radiation can damage DNA [1] and thus prevents tumor cell reproduction. However, the lung is a radiosensitive organ and delivering radiation to tumors may also damage healthy tissues. Radiation induced lung injury encompasses acute (< 6 months, early phase) radiation pneumonitis (RP) and chronic (> 6 months, late phase) radiation induced lung fibrosis [2,3]. RP is one of the most challenging complications following radiation therapy in NSCLC. The incidence and severity of RP is depending on multiple factors, including patient, disease and treatment related factors. As examples, a few meta-analyses have suggested age > 65 years [4], poor pulmonary function tests (decreased FEV1 and poor DLCO) [5], tumors in mid and lower lobes of lung [5], high V20 (the volume of normal lung receiving ≥ 20 Gy) [4] and higher mean lung dose (MLD) [6] being associated with increased risk of developing RP.
The well described adverse effect of ionizing radiation affecting adjacent and even distant tissues is caused by oxidative stress [7-12], produced by irradiated cells and spreading to non-targeted cells through intercellular communication mechanisms [9-11,13]. Ionizing radiation continuously generates free oxygen radicals, resulting in production and accumulation of reactive oxygen species (ROS), which play a causative role in death of healthy cells [14]. Under normal cellular conditions, accumulation of ROS is buffered by an antioxidant defense system consisting of both non-enzymatic factors such as bilirubin, glutathione (GSH), vitamins A, C, and E, and antioxidant enzymes such as superoxide dismutase (SOD), catalase, and glutathione peroxidase (GSH-Px) [15]. If the antioxidant defense mechanism fails to control ROS accumulation, increased ROS can lead to oxidative conversion of polyunsaturated fatty acids into several toxic and reactive aldehyde metabolites such as malondialdehyde (MDA), one of the end products of lipid peroxidation, and finally result in extensive damage of DNA, proteins, and plasma/organelle membranes in cells [16], subsequently culminating in execution of cell death.
Polyene phosphatidylcholine (PPC), also referred to as phosphatidylcholine, is a non-toxic phospholipid, enriched in polyunsaturated fatty acids, which serves as resource for biomembranes and was shown to increase membrane function and integrity [17]. PPC has demonstrated hepatoprotective functions via reducing cellular stress and inflammation, and thus is widely used in clinic for treatment of hepatopathies [18]. In cancer therapy, phosphatidylcholine can be supplied as a hepatoprotective nutritional supplement for chemotherapy as hepatoxicity is a frequent side effect of platinum drugs. Since combined chemoradiotherapy is the standard management for locally advanced NSCLC, a significant number of patients may use PPC or its derivatives as dietary adjuvant during treatment. Although our previous studies have demonstrated PPC can increase tumor cell sensitivity to platinum-based chemotherapeutic drugs [19,20], it is unknown whether phosphatidylcholine supplementation has any influence on radiation therapy.
The present study determined the impact of PPC administration on radiation therapy in NSCLC patients. Our findings suggest a radioprotective effect of PPC on normal lung tissues. The tissue protective effect of PPC was further confirmed in mice receiving total body irradiation. Mechanistically, PPC may prevent cell death via modulating tissue activities of antioxidant enzymes. In addition, we demonstrate that PPC has no measurable negative influence on radiotherapeutic efficacy using both in vitro and lung cancer xenograft mouse models.
Methods
Patients
A total of 133 NSCLC patients were included in this retrospective study. All patients received radiation therapy (10 Gy/week for 5-6.3 weeks) in the Affiliated Hospital of Qingdao University from 2013 to 2016. Among them were 119 patients who had received chemotherapy in addition and therefore, PPC was administrated to a fraction as hepatoprotective nutritional supplement. The patients were included in the study according to the following enrollment conditions: 1) NSCLC confirmed by histopathology, 2) total radiation dose ≥ 50 Gy, 3) patients either received complete course of PPC treatment (3 × 456 mg per day over the radiotherapy period) or without PPC treatment, 4) patient follow-up for at least six months. Exclusions criteria were: 1) patients that previously received radiation therapy, 2) dosimetry data was missing, 3) lack of CT or PET reports within one year of radiotherapy completion, 4) severe heart disease or other lung disease. Enrolled patients were checked every three months. Two radiologists independently diagnosed for radiation pneumonitis according to Common Toxicity Criteria for Adverse Events (Version 4.0) [21]. Grading criteria were as follows: Grade 1, asymptomatic, radiographic findings only; Grade 2, symptomatic, but not interfering with activities of daily living; Grade 3, symptomatic and interfering with activities of daily living, oxygen supply indicated; Grade 4, life threatening, ventilator support is indicated; Grade 5, death.
In addition, pulmonary function was evaluated from 86 of 133 Patients who had a complete report of pulmonary function tests (PFT, at least FEV1 and DLCO) at baseline and during the follow-up period (up to 18 months).
All studies involving human participants were conducted in accordance with ethical standards and the Declaration of Helsinki and according to national and international guidelines. The study was approved and supervised by the Ethics Committee of the Affiliated Hospital of Qingdao University. Informed consent was obtained from all individual patients included in the study.
Animals
Male C57BL/6 mice were purchased from Shanghai Laboratory Animal Center, CAS (SLACCAS). On arrival, mice were housed in an environment controlled for temperature (25°C), humidity, and light (12 h light/dark cycle). Standard laboratory chow and water were provided ad libitum to all animals. Mice were acclimated for 2 to 3 weeks prior to irradiation and xenograft studies. All animal experiments were performed with prior approval by the Ethics Committee of the Affiliated Hospital of Qingdao University.
Irradiation and PPC treatment
At the age of 8-10 weeks, mice were randomly divided into 3 groups (n = 27 per group): control group (Ctrl), vehicle irradiation group (6 Gy), and PPC + irradiation treatment group (6 Gy + PPC). Mice from 6 Gy and 6 Gy + PPC group were whole-body exposed to a single dose of 6 Gy gamma radiation using a varian 23EX Electron linear accelerator at 6 MV X-rays, and survival was monitored for up to 30 days or as indicated. Ctrl group animals were sham-irradiated. In the PPC treatment group, mice were intragastrically administered 30 mg/kg/day PPC (Sanofi, Paris, France) as previously described [18] for up to 31 days or as indicated. First PPC treatment was carried out 24 h before receiving irradiation. Ctrl and 6 Gy groups received the same volume of saline (placebo). All mice were sacrificed at the end of the study (on day 30 post-irradiation or at indicated time points). Blood samples were obtained from each animal and tissue samples were collected for histological studies and immunofluorescence.
Histology and H&E staining
Tissue samples of lung, liver and kidney were fixed in 4% paraformaldehyde and subsequently embedded in paraffin. 4 µm thick slides were prepared for hematoxylin and eosin (H&E) staining. Briefly, the slides were deparaffinized in xylene and rehydrated in a series of ethanol with gradually decreasing concentration into deionized water. Slides were then incubated with hematoxylin for 3 seconds, rinsed with water, and followed by an eosin staining for up to 45 seconds. After dehydration with a series of ethanol into xylene, slides were mounted with Permount mounting medium, and subsequently analyzed under light microscopy.
In situ apoptosis assay
In situ apoptosis was determined using the Terminal deoxynucleotidyl transferase (Tdt)-mediated dUTP nick-end labeling (TUNEL, Roche Diagnostics) method according to the manufacturer’s instructions. Briefly, 4 µm sections of cryopreserved tissues were prepared and mounted on 3-aminopropyltriethoxysilane (APES) - treated glass slides (Sigma Aldrich, US). Slides were then fixed in 4% paraformaldehyde for 30 min, rinsed with PBS, and followed by 2 min incubation in permeabilisation solution (1% Triton-X100 in 1% sodium citrate) on ice. After rinsing with PBS, tissue samples were incubated with TUNEL reaction mixture for 60 min at 37°C in the dark. DNase I-treated sections with labelling solution (TdT enzyme omitted) or complete TUNEL reaction solution were used as negative and positive controls, respectively. Apoptotic cells were evaluated and counted using a fluorescence microscope. 10 high power fields (HPF, 400 ×) were randomly selected from each slide and results were presented as mean percentage (%) of TUNEL positive cells per high power field.
Biochemical analyses
Serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT) and creatinine were determined by routine colorimetric methods from commercial diagnosis kits (Jiancheng Bioengineering Inc., Nanjing, China).
Peripheral hematopoietic evaluation
Some mice (n = 5 per group) were sacrificed on day 1, day 7 and day 14 post-irradiation for bone marrow cell isolation and spleen weight comparison. To obtain bone marrow cells (BMCs), the femur from the hind leg was removed and the ends of the bone were cut bluntly. The bone marrow cavity was flushed with 2 ml PBS using a sterile syringe with 22-gauge needle. BM cell suspension was collected, washed and pelleted by centrifugation for 8 min at 250 g. Cells were resuspended in PBS and samples were taken for cell counting. Results were expressed as number of bone marrow cells × 106/femur.
Blood was also collected on day 7 for peripheral complete blood cell analyses. Briefly, mice were sacrificed by CO2 asphyxiation followed by cardiac puncture under sterile conditions. Blood was collected in Eppendorf tubes containing 20 U heparin and kept at ambient temperature until counting.
Antioxidant enzyme activities
Liver and spleen samples were collected on day 7 post-irradiation and homogenized in the ratio 1:10 (w/v) with ice-cold Tris buffer (10 mM, pH 7.4). The homogenate was centrifuged at 12,000 g at 4°C for 20 min and supernatants were taken and assayed as previously described [20] for methylenedioxyamphetamine (MDA) level, superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activities according to the manufacturer’s instructions (Jiancheng Bioengineering Inc., Nanjing, China). Changes in absorbance were determined using a spectrophotometer and results were calculated as nmol/mg protein for MDA, and U/mg protein for SOD and GSH-Px activity.
Xenograft model
To establish the tumor model, 10-week-old C57BL/6 mice were inoculated subcutaneously into the right abdomen with 2 × 106 murine Lewis lung carcinoma (LLC) cells suspended in 200 μL sterile phosphate buffered saline (PBS). Tumor growth was monitored by measuring tumor width (W) and length (L) using calipers. The tumor volume (V) was calculated using the fomula: V = 0.52 (ellipsoid) × L × W2. When tumor volume had reached approximately 150 mm3, mice were randomized into three groups (n = 5 per group): Tumor control group (T-Ctrl), Tumor irradiation group (T-6 Gy) and PPC treatment group (T-6 Gy + PPC). The tumor region was irradiated with a single dose of 6 Gy and mice were administered 30 mg/kg/day PPC or placebo (saline). At the end of the experiment, all mice were sacrificed by CO2 asphyxiation and tumors were excised, weighted, and preserved for further analyses.
Cell culture and treatment
Murine LLC and human A549 cell lines were purchased from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences. LLC cell line was cultured in RPMI 1640 medium (FisherScientific, US), supplemented with penicillin (100 units/ml), streptomycin (100 units/ml) and 10% FBS. A549 cells were maintained in Dulbecco’s modified eagle medium (DMEM, Invitrogen, US) containing 100 units/ml penicillin, 100 units/ml streptomycin and 10% FBS. Cells were incubated at 37°C in a humidified atmosphere containing 5% CO2.
During the irradiation procedure, cells were cultured in a T75 flask, containing medium at a height of 1.5-cm depth from the cell surface and cell confluence was ~80%. The distance from the radiation source to the cell surface was 100 cm. For irradiation, cells were exposed to a single dose of 2 Gy with a radiation area of 10 × 15 cm. Cells were kept for 24 h after irradiation, and subsequently were exposed to PPC treatment and analyzed for viability and proliferation.
Proliferation assay
Cell proliferation was determined by cell counting kit-8 (CCK-8 kit, Sigma-Aldrich) according to the manufacturer’s instructions. Briefly, 5,000 cells per well were seeded into 96-well plates with complete culture medium and incubated for 24 h. The cells were then treated with or without different concentrations (0-20 µM) of PPC for 72 h or as indicated. Before measurement, 10 µL CCK-8 was added to each well and the plates were incubated for 3 h at 37°C. Cell proliferation and viability were subsequently analyzed by measuring absorbance at 450 nm using a microplate reader. Each treatment was measured in triplicates and means were calculated from at least three experiments.
Statistical analyses
Data analysis was performed using SPSS 24.0 and Graphpad Prism 6.0 software. The log-rank test was applied for survival comparision and One-way ANOVA (more than 2 group comparisons) or Student’s t tests (two group comparison) were used to calculate significance in other experiments. Data were presented as mean ± SEM and calculated from three independent experiments, or as indicated. A P < 0.05 was considered statistically significant.
Results
PPC protects patients from radiation pneumonitis
The clinical characteristics of patients are shown in Table 1. A total of 133 patients with NSCLC were enrolled in the study, including 90 males and 43 females with a median age of 57 years. After receiving radiotherapy, 44 patients (33.1%) developed grade 2 or higher radiation pneumonitis (Table 1). Univariate Cox proportional hazard analysis revealed a significant association of lung injury with radiation dosimetric factors (MLD or V20; Table 2). Notably, patients receiving a complete course of PPC treatment were associated with a significantly lower risk to develop symptomatic radiation pneumonitis (27.6%) than those patients without PPC supplemention (43.5%; Tables 1 and 2). When using no-PPC group as reference, the HR of the PPC treatment group was 0.551 (95% CI 0.304 to 0.998; P = 0.049) for ≥ grade 2 radiation pneumonitis. This effect was virtually unchanged after adjustment for other clinical variables by multivariate analyses (PPC treatment group: HR = 0.531, 95% CI 0.283 to 0.995; P = 0.048), which further suggested PPC may play a radioprotective role in these patients.
Table 1.
Patient characteristics and radiation pneumonitis incidence after radiotherapy
| Patient Characteristics | No. of patients (%) | No. of Radiation Pneumonitis (%) | ||
|---|---|---|---|---|
|
| ||||
| Grade < 2 (%) | Grade ≥ 2 (%) | |||
| Sex | Male | 90 (67.7) | 58 (64.4) | 32 (35.6) |
| Female | 43 (32.3) | 31 (72.1) | 12 (27.9) | |
| Age | < 57 | 68 (51.1) | 43 (63.2) | 25 (36.8) |
| ≥ 57 | 65 (48.9) | 46 (70.8) | 19 (29.2) | |
| Stage | I-II | 22 (16.5) | 15 (68.2) | 7 (31.8) |
| III-IV | 111 (83.5) | 74 (66.7) | 37 (33.3) | |
| Smoking | No | 57 (42.9) | 39 (68.4) | 18 (31.6) |
| Yes | 76 (57.1) | 50 (65.8) | 26 (34.2) | |
| Chemotherapy | No | 14 (10.5) | 11 (78.6) | 3 (21.4) |
| Yes | 119 (89.5) | 78 (65.6) | 41 (34.4) | |
| Radiation Dose | < 5600 | 68 (51.1) | 44 (64.7) | 24 (35.3) |
| ≥ 5600 | 65 (48.9) | 45 (69.2) | 20 (30.8) | |
| MLD | < 15 | 67 (50.4) | 52 (77.6) | 15 (22.4) |
| ≥ 15 | 66 (49.6) | 37 (56.1) | 29 (43.9) | |
| V20 | < 25% | 64 (48.1) | 50 (78.1) | 14 (21.9) |
| ≥ 25% | 69 (51.9) | 39 (56.5) | 30 (43.5) | |
| PPC | No | 46 (34.6) | 26 (56.5) | 20 (43.5) |
| Yes | 87 (65.4) | 63 (72.4) | 24 (27.6) | |
Abbreviations: MLD, mean lung dose; V20, volume of normal lung receiving ≥ 20 Gy; radiation dose in cGy.
Table 2.
The influence of PPC on radiation pneumonitis in NSCLC patients
| Patient Characteristics | No. of patients | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| HR | 95% CI | P | HR | 95% CI | P | |||
| Sex | Male | 90 | 1 | 1 | ||||
| Female | 43 | 0.743 | 0.383-1.443 | 0.381 | 0.644 | 0.321-1.291 | 0.215 | |
| Age | < 57 | 68 | 1 | 1 | ||||
| ≥ 57 | 65 | 0.733 | 0.404-1.331 | 0.307 | 0.729 | 0.398-1.337 | 0.307 | |
| Stage | I-II | 22 | 1 | 1 | ||||
| III-IV | 111 | 1.063 | 0.474-2.386 | 0.881 | 1.090 | 0.475-2.504 | 0.839 | |
| Smoking | No | 57 | 1 | 1 | ||||
| Yes | 76 | 1.114 | 0.611-2.033 | 0.724 | 1.374 | 0.728-2.593 | 0.327 | |
| Chemotherapy | No | 14 | 1 | 1 | ||||
| Yes | 119 | 1.706 | 0.528-5.510 | 0.372 | 1.803 | 0.548-5.939 | 0.332 | |
| Radiation Dose | < 5600 | 68 | 1 | 1 | ||||
| ≥ 5600 | 65 | 0.818 | 0.452-1.482 | 0.508 | 0.852 | 0.456-1.592 | 0.616 | |
| MLD | < 15 | 67 | 1 | 1 | ||||
| ≥ 15 | 66 | 2.230 | 1.195-4.161 | 0.012 | 2.374 | 1.266-4.451 | 0.007 | |
| V20 | < 25% | 64 | 1 | 1 | ||||
| ≥ 25% | 69 | 2.334 | 1.237-4.403 | 0.009 | 2.365 | 1.231-4.544 | 0.010 | |
| PPC | No | 46 | 1 | 1 | ||||
| Yes | 87 | 0.551 | 0.304-0.998 | 0.049 | 0.531 | 0.283-0.995 | 0.048 | |
Abbreviations: HR, hazard ratio; CI, confidence interval; MLD, mean lung dose; V20, volume of normal lung receiving ≥ 20 Gy; radiation dose in cGy. Multivariate analyses were adjusted for all factors listed in the table. Either MLD or V20 was used in multivariate analyses.
To confirm this finding, we further analyzed changes in PFT results from 86 patients (43 patients per group at start) to compare PPC treatment group vs patients without PPC. In both patient groups, FEV1 and DLCO declined after radiotherapy, however, this effect was less pronounced upon PPC administration (Figure 1).
Figure 1.
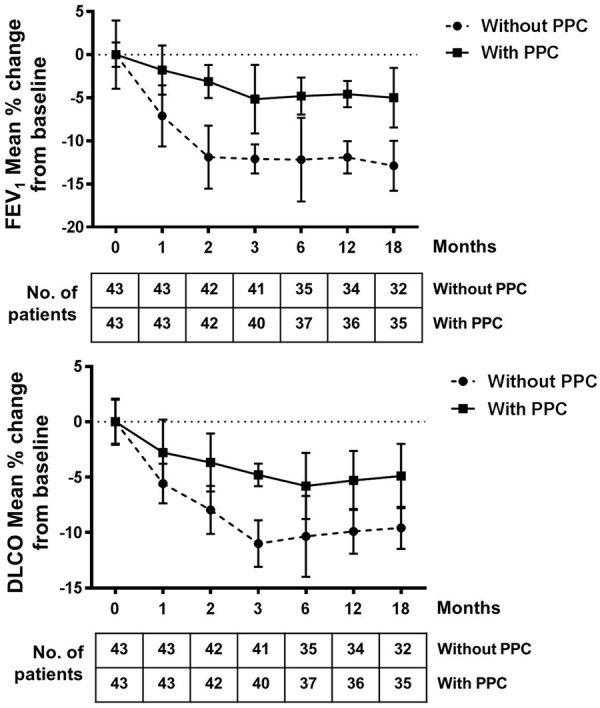
Pulmonary function tests (PFT) of 86 NSCLC patients. Decline of FEV1 and DLCO values post radiotherapy are shown. Data are expressed as % of change from patients’ baseline value (before receiving radiation). Numbers of patients included in each analysis time point are indicated.
PPC protects against irradiation-mediated tissue damage
To confirm the findings in NSCLC patients, a protective effect of PPC was investigated in mice that received total body γ-irradiation with a single dose of 6 Gy. Survival in different treatment groups was subsequently monitored for up to 30 days. The median survival time in vehicle irradiation group (6 Gy) was 18 days and irradiation resulted in loss of 75% of mice on day 30. Administration of PPC significantly increased survival rate of irradiated mice, showing a median survial of 25 days. Irradiation induced mortality on day 30 reduced to 50% (Figure 2A).
Figure 2.
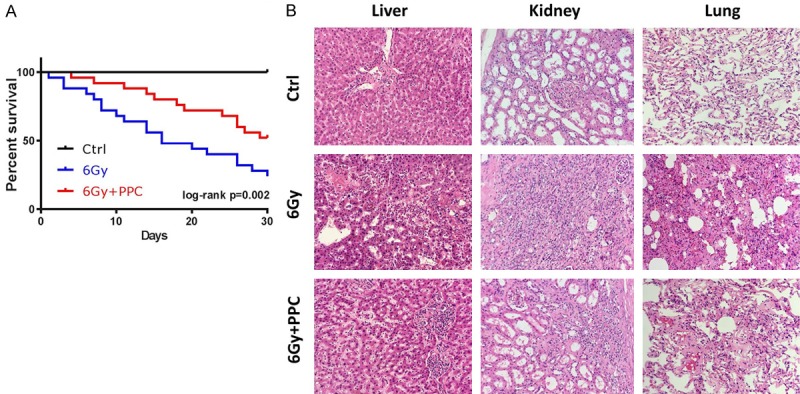
Whole body irradiation in mice. A. Kaplan-Meier plot of mice that received a total body irradiation with a single dose of 6 Gy in presence or absence of PPC treatment; survival was monitored up to 30 days. Log-rank test was carried out between 6 Gy and 6 Gy + PPC groups (P = 0.002). Control mice received sham irradiation. n = 12 mice per group. B. Representative H&E staining in liver, kidney and lung tissues on day 30. Shown images are at 100 × magnification.
Histological analysis also confirmed the protective role of PPC (Figure 2B). In vehicle irradiated mice (6 Gy group), H&E staining detected central vein occlusion in the liver. Infiltration of inflammatory cells and loss of the normal architecture of the hepatic lobules was also observed. In kidney, irradiation induced injury manifested in renal tubular epithelial cell necrosis, tubular obstruction and interstitial inflammatory cell infiltration (Figure 2B, 6 Gy). In lung, after exposure to irradiation, parts of the alveoli lost their original structure, the alveolar wall became thicker and inflammatory cells accumulated in the alveoli (Figure 2B, 6 Gy). Irradiation induced tissue injury was less severe in the PPC treated mice (Figure 2B, 6 Gy + PPC).
Tissue damage was further evaluated via detection of cell apoptosis using TUNEL staining (Figure 3A). In control mice, the baseline of TUNEL labelled parenchymal cells in all tested tissues (liver, lung and kidney) was very low. A striking increase in the number of TUNEL positive cells post radiation was observed in the 6 Gy group. In comparison, apoptosis was significantly less detectable in irradiated mice receiving PPC (6 Gy + PPC).
Figure 3.
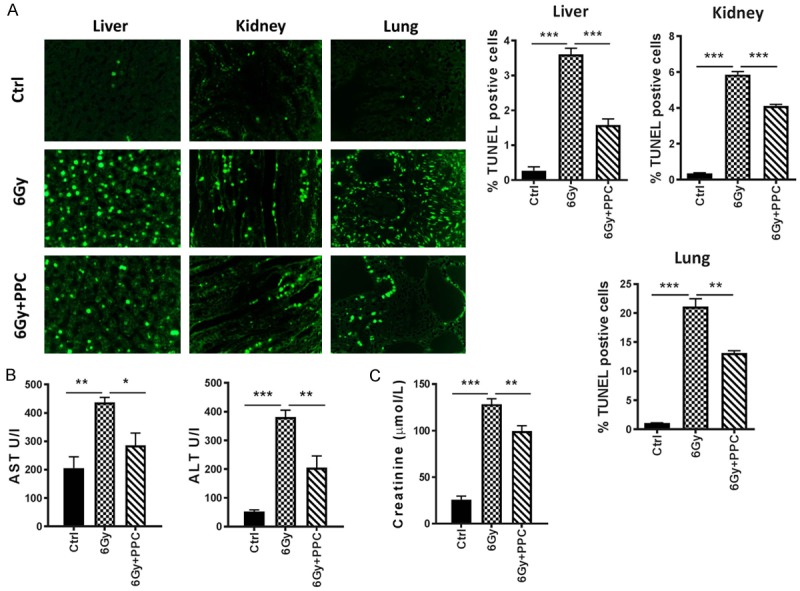
Analyses of tissue damage upon irradiation of mice. A. Representative TUNEL stainings in liver, kidney and lung tissue on day 30. Quantitative analysis is presented as mean percentage of TUNEL positive cells per high power field. n = 3 mice per group. B. Liver injury-related parameters of mice. Serum ALT, AST were measured (n = 5 mice per group). C. Renal function test. Blood creatinine is shown (n = 5 mice per group). *P < 0.05; **P < 0.01; ***P < 0.001.
Moreover, biochemical analyses revealed dramatic elevation of AST and ALT values in the 6 Gy group compared to control, indicating impaired liver function by irradiation (Figure 3B). PPC treatment led to less liver damage, as indicated by significantly reduced levels of liver enzymes in comparison to the 6 Gy group. Changes in blood creatinine were also measured to test for renal function (Figure 3C). Elevated creatinine levels in the 6 Gy group vs control group (128.5 μmol/L vs 25.9 μmol/L) suggested malfunction of kidney induced by irradiation. Again, PPC administration reduced damage, with a creatinine level (99.6 μmol/L) significantly lower than that detected in the 6 Gy group.
Taken together, these data suggest a strong radioprotective effect of PPC on healthy tissues.
Radioprotective effects of PPC on the hematopoietic system
Femur bone marrow cells (BMCs) were collected and counted on day 1, day 7 and day 14 after irradiation. Reduction of BMC count was already evident on day 1 and the strongest influence on the hematopoietic system by irradiation was detected on day 7 in the 6 Gy group. In comparison with the vehicle irradiated animals, the mice receiving PPC had a significantly higher BMC count after irradiation (Figure 4A). Spleen weight was also analyzed (Figure 4B). In both 6 Gy and 6 Gy + PPC groups, spleen weight was reduced by > 50% on the first day after irradiation with no significant difference between the two groups. Yet, weight recovery was detected in 6 Gy + PPC mice at later time points (day 7 and 14), indicating that PPC might also accelerate healing of injury.
Figure 4.
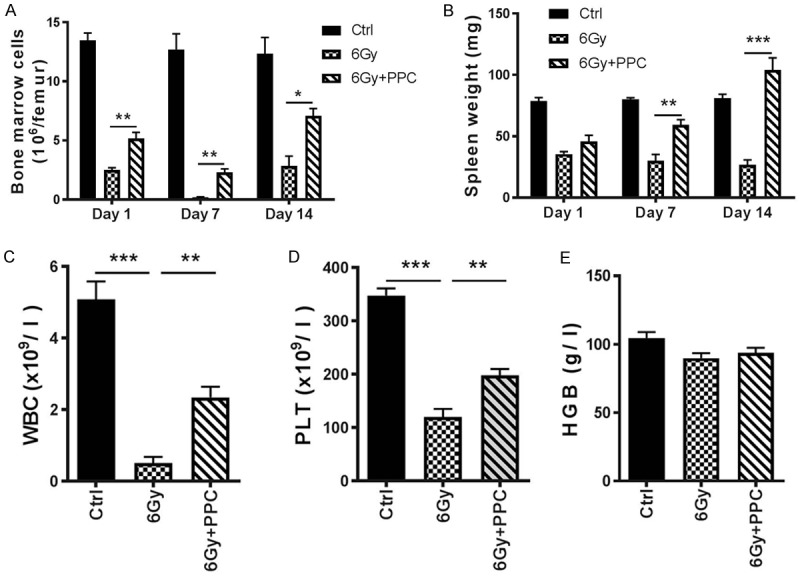
Radiation influence on the hematopoietic system. (A) Femur bone marrow cell count and (B) spleen weight was measured on day 1, day 7 and day 14 after irradiation; n = 5 mice per group. (C-E) Peripheral blood cell count in mice on day 7 post-irradiation; n = 5 mice per group. WBC: white blood cells; PLT: platelets; HGB: hemoglobin. *P < 0.05; **P < 0.01; ***P < 0.001.
On day 7 post irradiation, peripheral blood cells were counted. Pronounced reduction in numbers of white blood cells (WBC) and platelets (PLT) was observed upon irradiation (Figure 4C and 4D), however a significantly less reduction was found in 6 Gy + PPC mice compared to vehicle irradiated mice (6 Gy). In both groups, irradiation had no measureable effect on hemoglobin concentration (Figure 4E).
PPC modulates antioxidant defense mechanism
To investigate whether the radioprotective effect of PPC was associated with increased antioxidant defense in tissues, changes in antioxidant enzyme activities and extent of lipid peroxidation were measured in liver and spleen tissues on day 7 post total body irradiation. As shown in Table 3, activities of SOD and GSH-Px, two major antioxidant enzymes, decreased upon irradiation in both organs (6 Gy group). In contrast, no obvious changes were observed in 6 Gy + PPC group compared to control mice, suggesting PPC is triggering an antioxidant defense mechanism in response to radiation injury. In line with these findings, whole body irradiation resulted in increased reactive aldehyde metabolite MDA level, which is an end product of lipid peroxidation. This effect was abolished by administration of PPC (Table 3).
Table 3.
The effect of PPC on splenic and hepatic antioxidant activities of irradiated mice
| MDA | SOD activity | GSH-Px activity | ||
|---|---|---|---|---|
| nmol/mg protein | U/mg protein | U/mg protein | ||
| Spleen | Control | 1.29 ± 0.32 | 59.43 ± 4.44 | 23.66 ± 1.06 |
| 6 Gy | 2.93 ± 0.11 | 39.10 ± 5.89 | 22.73 ± 3.46 | |
| 6 Gy + PPC | 1.83 ± 0.34* | 58.68 ± 2.13* | 24.14 ± 1.89 | |
| Liver | Control | 0.65 ± 0.07 | 979.28 ± 79.76 | 23.49 ± 1.23 |
| 6 Gy | 2.01 ± 0.35 | 721.56 ± 43.08 | 19.46 ± 2.10 | |
| 6 Gy + PPC | 0.73 ± 0.22* | 1064.57 ± 99.45* | 23.01 ± 5.75 |
Mice were sacrificed 7 days after radiation. Spleens and livers were collected and homogenized. The antioxidant activity was determined spectrophotometrically using diagnostic reagent kits. Values are expressed as means ± SEM, n = 5;
P < 0.05 (6 Gy + PPC vs 6 Gy).
PPC does not affect radiotherapeutic efficacy
Since the antioxidant defense system was found to be modulated by PPC in the total body irradiation model, we subsequently examined whether the cellular protective effect of PPC had potential influence on radiotherapeutic efficacy in cancer. Thus, the LLC xenograft mouse model (LLC mice) was established. After reaching a volume of 150 mm3, tumors were exposed to a single dose irradiation of 6 Gy and PPC (or placebo) was administered starting one day prior irradiation, as outlined in Figure 5A. Tumor volume was measured every day for 10 days post radiation treatment (Figure 5B). As seen in Figure 5C, tumor growth was successfully controlled by irradiation compared to control group (T-Ctrl). PPC application had no measurable influence on the radiotherapeutic effect on tumor growth. On day 10 post-irradiation, tumors were excised and size and weight were compared among different groups. No difference in tumor weight was found between placebo (T-6 Gy) and PPC (T-6 Gy + PPC) treatment groups, although irradiation per se led to smaller tumors, as expected (Figure 5C).
Figure 5.
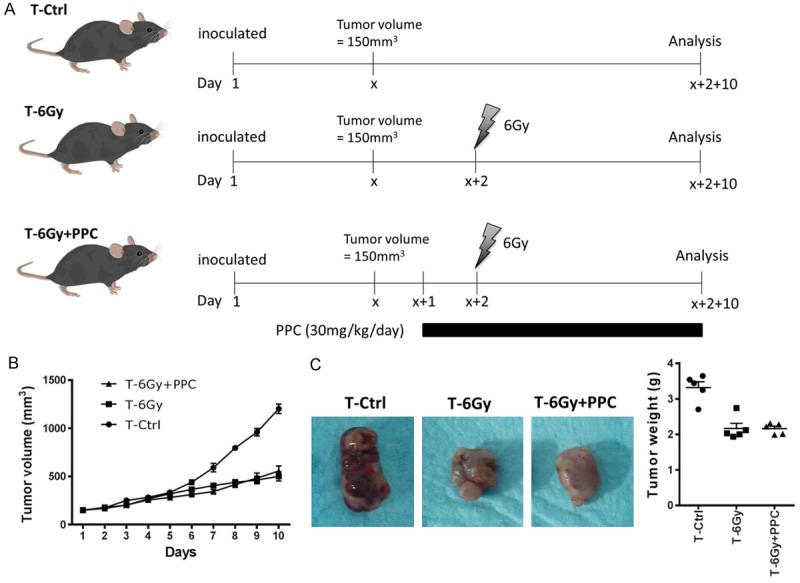
Effect of PPC on radiation therapy in lung cancer. A. Scheme of the study design of xenografted mice receiving radiation therapy and PPC treatment. C57BL/6 mice were inoculated subcutaneously with 2 × 106 LLC cells and received tumor irradiation of a single dose of 6 Gy after tumor volume had reached ~150 mm3. In T-6 Gy + PPC group, 30 mg/kg/day PPC was administered to mice starting one day prior irradiation. T-6 Gy group received placebo (saline) instead of PPC. Control mice received sham irradiation treatment. B. Tumor volume was measured daily. C. Comparison of tumor size and weight on day 10 post-irradiation.
To consolidate these findings, the effect of PPC on tumor cell radiosensitivity was also investigated in vitro with two lung cancer cell lines, A549 and LLC. The response to different concentrations of PPC treatment was measured first using CCK-8 assay. Cell viability was monitored (PPC 0 µM = 100% as reference) for up to 72 h and a dosage dependent effect of PPC on cell growth was not detected (Figure 6A and 6B) except that low concentrations (up to 3.2 µM) of PPC slightly increased cancer cell survival in both cell lines (Figure 6A and 6B). However, upon irradiation, PPC treatment at 3.2 µM had no impact on cell proliferation up to 96 h (Figure 6C and 6D), confirming that PPC had no direct influence on radiation efficacy.
Figure 6.
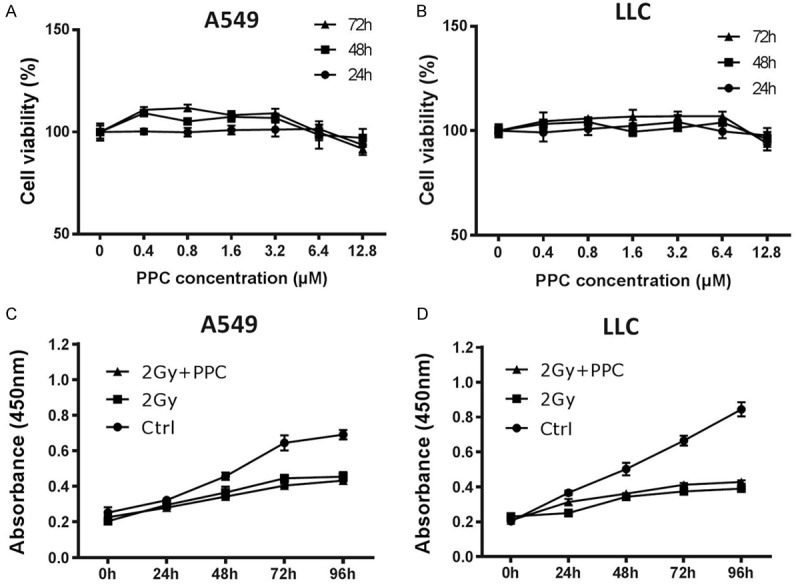
Effect of PPC on irradiated cells in vitro. (A) A549 and (B) LLC cells were treated with different dosages of PPC and cell viability (growth) was measured using CCK-8 assay. (C) A549 and (D) LLC cells were exposed to a single irradiation of 2 Gy. Cells were treated with or without PPC for up to 96 hours. Cell proliferation was measured using CCK-8 assay. No statistically relevant differences in growth between 2 Gy and 2 Gy + PPC groups were identified.
Discussion
Radiation therapy plays an important role in treatment of inoperable NSCLC and many other locally advanced cancers, where a combined radiochemotherapy often serves as standard treatment regimen. PPC is an essential phospholipid in cell membranes [22]. As a beneficial nutrient for liver health, PPC is often suggested as dietary supplement to dampen chemotherapy caused liver toxicity. Since PPC is generally well tolerated and has no known contraindications, side effects, or interactions with other medications, no randomized clinical trial has ever studied its beneficial effect in cancer therapy. Our previous studies demonstrated a potential benefit of PPC to increase tumor cell sensitivity to platinum based chemotherapeutic drugs [19,20], however, its potential role in reducing radiation induced tissue toxicity and possible influence on radiotherapeutic efficacy has never been elucidated to date. To bridge this gap, the present study investigated the impact of PPC on radiation induced toxicity in NSCLC patients and subsequently validated the protective effect of PPC on radiation induced tissue injury in animal models. In addition, we showed that PPC supplementation does not affect radiation sensitivity of tumor cells, thus providing strong evidence for its beneficial use as adjuvant in cancer therapy.
Radiation induced toxicity has long been considered a treatment limiting factor for patients as radiation not only damages tumor cells, but also generates oxidative stress in normal tissues. Here, in our retrospective study with 133 NSCLC patients, the potential risk factors associated with acute RP were investigated. As previously reported, we found that symptomatic RP (grade ≥ 2) was strongly dependent on dosimetric factors (MLD, V20) in radiation therapy [4,6]. However a significant correlation of RP with poor baseline pulmonary function tests (FEV1 and DLCO) was not observed in our patients collective (data not shown). Similar observations were also described previously in Wang et al. [23], indicating poor PFT may not be an independent risk factor for RP. In both univariate and multivariate analyses, daily PPC intake significantly correlated with reduced risk to develop RP. The radioprotective function of PPC was subsequently confirmed by improved FEV1 and DLCO compared to patients without PPC treatment.
Following the promising findings in NSCLC patients, we subsequently used the whole body irradiation mouse model to explore the influence of PPC on radiation induced toxicity. Noteworthy, the radioprotective effect of PPC was not only seen in lung, but also observed in other organs, such as liver, kidney, spleen as well as in the hematopoietic system. This observation indicates that PPC itself may serve as an active antioxidant in preventing lipid peroxidation in response to radiation injury. Indeed, previous studies in alcoholic liver disease suggested PPC can dampen alcohol-induced hepatic oxidative stress by restoring s-adenosylmethionine [24]. Interestingly, the antioxidative effect of PPC seems to be disease context dependent, as another study reported that PPC does not protect against iron induced oxidative stress in liver [25]. In the present work, we evaluated the antioxidative effect of PPC by measuring activities of two central antioxidant enzymes in liver and spleen: SOD and GSH-Px. Both enzymes are considered first line defense antioxidants. SOD converts superoxide into hydrogen peroxide (H2O2) and molecular oxygen (O2), while GSH-Px catalyzes breakdown of H2O2 into water and reduction of lipid peroxides to their corresponding alcohols [26]. The radiation resulting decrease in tissue activities of both enzymes was no longer detectable by PPC treatment, confirming the effective antioxidative role of PPC. In accordance with these findings, the radiation triggered massive increase of tissue MDA, the end product of lipid peroxidation, was also inhibited by PPC administration.
Noteworthy, although PPC protected healthy tissues against radiation induced oxidative stress, no negative influence on radiation efficacy in tumor cells was observed. Radiation mediated inhibition of tumor growth was not affected by PPC treatment as shown in both, the xenograft mouse model and tumor cell cultures, indicating radiation induced direct DNA damage cannot be corrected by PPC. However, as also in human tumor entities, radiation induced oxidative stress may influence tumor cell death, future studies are warranted to mechanistically investigate PPC function in healthy and transformed cells in context of radiation triggered oxidative stress.
To summarize, the present study reports on a clinically highly relevant observation that PPC potently antagonizes radiotherapy mediated damage of healthy tissues via stimulating cellular antioxidant defense mechanisms. Our retrospective study thus suggests PPC as adjuvant in NSCLC patients receiving radiation therapy. Future studies are needed to consolidate our findings in NSCLC and also to test for beneficial PPC effects in other cancer entities where radiation is applied.
Acknowledgements
We acknowledge Ruyong Yao, the director of the Central Laboratory of the Affiliated Hospital of Qingdao University, for his helpful support on this study. The study was supported by the Natural Science Foundation of Shandong Province (NO:ZR2017PH048) and Clinical Medicine + X Project of Qingdao University (NO: 2018044).
Disclosure of conflict of interest
None.
Abbreviations
- ALT
alanine transaminase
- AST
aspartate transaminase
- BMCs
bone marrow cells
- DLCO
diffusing capacity of the lung for carbon monoxide
- FEV1
forced expiratory volume in one second
- GSH-Px
glutathione peroxidase
- HGB
hemoglobin
- LLC
Lewis lung carcinoma
- MDA
malondialdehyde
- MLD
mean lung dose
- NSCLC
non-small cell lung cancer
- PFT
pulmonary function test
- PLT
platelets
- PPC
polyene phosphatidylcholine
- ROS
reactive oxygen species
- RP
radiation pneumonitis
- SOD
superoxide dismutase
- V20
the volume of normal lung receiving ≥ 20 Gy
- WBC
white blood cells
References
- 1.Hall S, Rudrawar S, Zunk M, Bernaitis N, Arora D, McDermott CM, Anoopkumar-Dukie S. Protection against radiotherapy-induced toxicity. Antioxidants (Basel) 2016;5 doi: 10.3390/antiox5030022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giridhar P, Mallick S, Rath GK, Julka PK. Radiation induced lung injury: prediction, assessment and management. Asian Pac J Cancer Prev. 2015;16:2613–2617. doi: 10.7314/apjcp.2015.16.7.2613. [DOI] [PubMed] [Google Scholar]
- 3.Huang Y, Zhang W, Yu F, Gao F. The cellular and molecular mechanism of radiation-induced lung injury. Med Sci Monit. 2017;23:3446–3450. doi: 10.12659/MSM.902353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palma DA, Senan S, Tsujino K, Barriger RB, Rengan R, Moreno M, Bradley JD, Kim TH, Ramella S, Marks LB, De Petris L, Stitt L, Rodrigues G. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2013;85:444–450. doi: 10.1016/j.ijrobp.2012.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogelius IR, Bentzen SM. A literature-based meta-analysis of clinical risk factors for development of radiation induced pneumonitis. Acta Oncol. 2012;51:975–983. doi: 10.3109/0284186X.2012.718093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsujino K, Hirota S, Endo M, Obayashi K, Kotani Y, Satouchi M, Kado T, Takada Y. Predictive value of dose-volume histogram parameters for predicting radiation pneumonitis after concurrent chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys. 2003;55:110–115. doi: 10.1016/s0360-3016(02)03807-5. [DOI] [PubMed] [Google Scholar]
- 7.Spitz DR, Azzam EI, Li JJ, Gius D. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: a unifying concept in stress response biology. Cancer Metastasis Rev. 2004;23:311–322. doi: 10.1023/B:CANC.0000031769.14728.bc. [DOI] [PubMed] [Google Scholar]
- 8.Tamminga J, Kovalchuk O. Role of DNA damage and epigenetic DNA methylation changes in radiation-induced genomic instability and bystander effects in germline in vivo. Curr Mol Pharmacol. 2011;4:115–125. doi: 10.2174/1874467211104020115. [DOI] [PubMed] [Google Scholar]
- 9.Azzam EI, de Toledo SM, Little JB. Oxidative metabolism, gap junctions and the ionizing radiation-induced bystander effect. Oncogene. 2003;22:7050–7057. doi: 10.1038/sj.onc.1206961. [DOI] [PubMed] [Google Scholar]
- 10.Hei TK, Zhou H, Chai Y, Ponnaiya B, Ivanov VN. Radiation induced non-targeted response: mechanism and potential clinical implications. Curr Mol Pharmacol. 2011;4:96–105. doi: 10.2174/1874467211104020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mothersill C, Seymour CB. Radiation-induced bystander effects--implications for cancer. Nat Rev Cancer. 2004;4:158–164. doi: 10.1038/nrc1277. [DOI] [PubMed] [Google Scholar]
- 12.Azzam EI, Jay-Gerin JP, Pain D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012;327:48–60. doi: 10.1016/j.canlet.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prise KM, O’Sullivan JM. Radiation-induced bystander signalling in cancer therapy. Nat Rev Cancer. 2009;9:351–360. doi: 10.1038/nrc2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prise KM, Schettino G, Folkard M, Held KD. New insights on cell death from radiation exposure. Lancet Oncol. 2005;6:520–528. doi: 10.1016/S1470-2045(05)70246-1. [DOI] [PubMed] [Google Scholar]
- 15.Artur Y, Herbeth B, Guemouri L, Lecomte E, Jeandel C, Siest G. Age-related variations of enzymatic defenses against free radicals and peroxides. EXS. 1992;62:359–367. doi: 10.1007/978-3-0348-7460-1_36. [DOI] [PubMed] [Google Scholar]
- 16.Bansal A, Simon MC. Glutathione metabolism in cancer progression and treatment resistance. J Cell Biol. 2018;217:2291–2298. doi: 10.1083/jcb.201804161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan W, Hao WT, Xu HW, Qin SP, Li XY, Liu XM, Sun FF, Li H, Tang RX, Zheng KY. Polyene Phosphatidylcholine inhibited the inflammatory response in LPS-stimulated macrophages and ameliorated the adjuvant-induced rat arthritis. Am J Transl Res. 2017;9:4206–4216. [PMC free article] [PubMed] [Google Scholar]
- 18.Okiyama W, Tanaka N, Nakajima T, Tanaka E, Kiyosawa K, Gonzalez FJ, Aoyama T. Polyenephosphatidylcholine prevents alcoholic liver disease in PPARalpha-null mice through attenuation of increases in oxidative stress. J Hepatol. 2009;50:1236–1246. doi: 10.1016/j.jhep.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang H, Song H, Yuan R, Zhang X, Yu H, Zhao Y, Jiang T. Polyene phosphatidylcholine overcomes oxaliplatin resistance in human gastric cancer BGC823 cells. Biochem Biophys Res Commun. 2018;497:108–114. doi: 10.1016/j.bbrc.2018.02.033. [DOI] [PubMed] [Google Scholar]
- 20.Jiang T, Zhang H, Liu X, Song H, Yao R, Li J, Zhao Y. Effect of oxaliplatin combined with polyenephosphatidylcholine on the proliferation of human gastric cancer SGC-7901 cells. Oncol Lett. 2016;12:4538–4546. doi: 10.3892/ol.2016.5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bledsoe TJ, Nath SK, Decker RH. Radiation pneumonitis. Clin Chest Med. 2017;38:201–208. doi: 10.1016/j.ccm.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Gundermann KJ, Gundermann S, Drozdzik M, Mohan Prasad VG. Essential phospholipids in fatty liver: a scientific update. Clin Exp Gastroenterol. 2016;9:105–117. doi: 10.2147/CEG.S96362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Cao J, Yuan S, Ji W, Arenberg D, Dai J, Stanton P, Tatro D, Ten Haken RK, Wang L, Kong FM. Poor baseline pulmonary function may not increase the risk of radiation-induced lung toxicity. Int J Radiat Oncol Biol Phys. 2013;85:798–804. doi: 10.1016/j.ijrobp.2012.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aleynik SI, Lieber CS. Polyenylphosphatidylcholine corrects the alcohol-induced hepatic oxidative stress by restoring s-adenosylmethionine. Alcohol Alcohol. 2003;38:208–212. doi: 10.1093/alcalc/agg066. [DOI] [PubMed] [Google Scholar]
- 25.Aleynik SI, Leo MA, Aleynik MK, Lieber CS. Polyenylphosphatidylcholine protects against alcohol but not iron-induced oxidative stress in the liver. Alcohol Clin Exp Res. 2000;24:196–206. [PubMed] [Google Scholar]
- 26.Ray G, Husain SA. Oxidants, antioxidants and carcinogenesis. Indian J Exp Biol. 2002;40:1213–1232. [PubMed] [Google Scholar]


