Abstract
Objectives
To provide a clinical laboratory perspective on the Verifying Accurate Leading-edge IVCT Development Act (VALID) discussion draft. This potential legislative effort, if enacted, would overhaul the regulatory oversight of in vitro diagnostics (IVDs) in the United States and create a single system for regulation of conventional IVDs and laboratory-developed tests (LDTs).
Methods
A concise literature-based review of LDT regulation is presented followed by a discussion of key concerns pertinent to clinical laboratories that should be considered in future IVD regulatory reform efforts.
Results
Key issues identified include the importance of fostering innovation, preserving patient safety, protecting the practice of laboratory medicine, and minimizing undue regulatory burden. Clinical laboratories are not equivalent to manufacturing facilities and would therefore encounter challenges in implementing device-centric regulatory oversight models.
Conclusions
It is imperative that a clinical laboratory perspective on LDTs is understood and incorporated prior to advancement of future legislative proposals.
Keywords: Laboratory-developed test, In vitro clinical test, In vitro diagnostics, Regulations
Laboratory-Developed Test Regulation in the United States
Laboratory-developed tests (LDTs, previously known as “home brew” tests) have been described by the US Food and Drug Administration (FDA) as “an [in vitro diagnostic] IVD that is intended for clinical use and designed, manufactured and used within a single laboratory.” 1 Regulatory authority over medical devices “introduced into interstate commerce for commercial distribution” is granted to the FDA under the Medical Device Amendments (MDA) of 1976, which specifically includes in vitro reagents under the definition of devices.2 The FDA also has oversight over in vitro diagnostic (IVD) products under promulgated regulations.3 The concept of LDTs, however, was not specifically discussed during Congressional hearings leading to the passage of the MDA, nor does the MDA specifically describe how LDTs should be addressed.2,4 Sixteen years after the enactment of the MDA, the FDA first noted in a draft compliance policy guide that LDTs were subject to medical device regulatory requirements.5 For several decades, however, the FDA followed a general practice of dividing IVDs into two broad categories, those that are commercially distributed (eg, manufactured reagents and equipment that are sold to clinical laboratories for subsequent use) and LDTs, which are developed and operated within a single clinical laboratory facility.6 The FDA has maintained that it has the authority to regulate LDTs under the MDA, but it has followed a policy of enforcement discretion (eg, not exercising regulatory oversight) as a “matter of general practice.” 1
A separate effort to modernize clinical laboratory regulations in the 1980s culminated in the Clinical Laboratory Improvement Amendments of 1988 (CLIA ’88; or CLIA). As mentioned in written testimony prior to CLIA’s enactment, it was generally believed at this time that “labs that use their own techniques and reagents need no approval” from the FDA.7 While the CLIA statute does not specifically address LDTs, subsequent regulations promulgated by the Centers for Medicare and Medicaid Services (CMS) established a standard regarding performance specifications addressing “test system[s] not subject to FDA clearance or approval (including methods developed in-house…).” 8 Development of this standard was likely a practical response to community uncertainty regarding how such testing should be addressed and regulated under CLIA. Since this time, many clinical laboratories have developed and implemented LDTs in their own operations in accordance with these requirements.
In July 2010, however, the FDA announced that it intended to shift from its policy of enforcement discretion regarding LDTs to exercising regulatory oversight at a future date.9 The FDA’s decision was driven by many factors described in this announcement, including growth of LDTs in the marketplace, development of LDTs in commercial operations instead of hospital laboratories, shifting business models where LDTs had a more favorable pathway to market than FDA cleared or approved tests, increasing complexity of LDTs, and lack of pre- and postmarket regulatory requirements for LDTs that are used to assess safety and efficacy of IVDs in the commercially distributed pathway.9-11 Draft guidance documents regarding a proposed framework for LDT regulatory oversight and corresponding notification and medical device reporting requirements were subsequently released in October 2014.1,12 Among the more contentious aspects of the FDA’s approach was to pursue regulatory oversight through the guidance process, and not through notice and comment rulemaking required for new regulations under the Administrative Procedures Act.
Community reaction to the draft guidance was both strong and diverse, and it highlighted different perspectives from clinical laboratories, IVD manufacturers, patient-care organizations, and professional associations as they worked to understand the potential impact of proposed regulations on laboratory operations, innovation, marketplace competition, and patient safety.13 The FDA reiterated its argument for LDT regulatory oversight by releasing a document outlining case studies of problematic LDTs immediately prior to a Congressional hearing focused on diagnostic test regulation in 2015.14
Shortly after the 2016 presidential election the FDA suspended its plans to implement the draft guidance.15,16 Subsequently, FDA leadership publicly affirmed that a legislative (eg, congressional) solution would be the preferred mechanism for future IVD regulatory reform.17 Since 2017, there have been two discussion drafts circulated by congressional offices regarding potential changes in how IVDs could be regulated in the future. The Diagnostic Accuracy and Innovation Act (DAIA) was released for public comments in 2017.18 A different regulatory approach was subsequently released by the FDA in August 2018, in response to an original request for technical assistance regarding DAIA.19 Congressional representatives subsequently released the Verifying Accurate Leading-edge IVCT Development Act (VALID) for public comment in late 2018.20 VALID presents a more device-centric model than DAIA. Both discussion drafts have adopted the term “in vitro clinical test” (IVCT) to describe all IVDs (eg, regardless of whether they were previously considered commercially distributed assays versus LDTs).
LDTs in the Clinical Laboratory Setting
Development and Operation
Why do clinical laboratories develop LDTs? Clinical laboratories generally develop and implement LDTs to meet unmet analytical and/or clinical care needs.21 Most commonly, an FDA cleared or approved assay is not commercially available, is not compatible with instrumentation owned by the laboratory, or does not meet the performance goals desired for clinical care in a particular setting. In most small, medium, and large hospital laboratories in the United States, it is cost-prohibitive to maintain a wide array of LDTs when existing FDA cleared or approved options are commercially available. Due to logistical, contractual, and reagent-pricing concerns, clinical laboratories may consolidate around a very small number of instrument vendors to offer the widest affordable assortment of FDA cleared or approved assays appropriate for their patient population and anticipated test mix. Most clinical laboratories therefore have a limited assortment of analytical instruments (eg, analyzers) available for testing and upon which LDTs could be implemented.
LDTs have played a critical role at the forefront of diagnostic innovation, particularly in academic and university clinical laboratories. For example, LDTs based on molecular diagnostics are routinely used in diagnosis of malignancy, in the identification of mutations that suggest additional therapeutic options, in the characterization of genetic mutations found in inheritable diseases, and in the diagnosis and treatment of infectious diseases.22-24 In many of these cases, no FDA cleared or approved options exist. In other cases, modification of FDA-approved molecular assays may allow for more clinical practice variety.25 As other examples, mass spectrometry methods for the measurement of hormones, drugs, and proteins may offer analytical advantages over conventional immunoassay-based approaches, although FDA clearance or approval of mass spectrometry-based assays is often not pursued by instrument manufacturers.26
LDTs are frequently developed in academic clinical laboratories and in reference (eg, referral or “send-out”) laboratories. In reference laboratories, requests for otherwise esoteric tests and/or rare disorders can be relatively common, as specimens are received from clinics and hospital facilities extending over wider geographic areas or networks. Given the high costs of obtaining premarket approval, as well as the limited financial incentive for IVD manufacturers to develop esoteric tests or tests for rare diseases, these laboratories address unmet clinical needs through the development of LDTs that are performed in a single laboratory location. Depending on the size of the laboratory, it is reasonable to assume that hundreds of different LDTs may be a part of a single reference laboratory’s test menu to meet client laboratory clinical needs.
It should also be noted that as experts in clinical laboratory testing operations, clinical pathologists, doctoral-level clinical laboratory scientists, and laboratory personnel become aware of the strengths and limitations of different assays and testing platforms. This awareness comes from direct experience with assay and instrument operation, as well as peer-to-peer information sharing within the clinical laboratory community, scientific literature, and national and international conferences. This experience may prompt the desire for test modifications or LDT developments where needed. Clinical laboratorians may currently pursue test modifications or LDT development in the United States under CLIA regulations.8
Regulatory Oversight Across Different Settings
A general concept of recent legislative and regulatory proposals has been to regulate activities associated with IVDs in an identical manner, regardless of where the activity is conducted. While ideal in principle, this model can also be interpreted as a top-down view of regulatory structure. In other words, LDT design might be regulated by the FDA, whereas LDT operation would still be regulated under CLIA. A bottom-up view from the clinical laboratories could interpret this as duplicative regulatory oversight, as now both FDA and CMS would oversee interconnected activities in clinical laboratories that perform LDTs.
It is important to highlight that the FDA has publicly asserted that clinical laboratories act as manufacturers with regards to LDTs.5 Current federal regulations regarding FDA medical device quality system requirements (QSRs) define a manufacturer as “any person who designs, manufactures, fabricates, assembles, or processes a finished device. Manufacturer includes but is not limited to those who perform the functions of contract sterilization, installation, relabeling, remanufacturing, repacking, or specification development, and initial distributors of foreign entities performing these functions.” 27 Defining manufacturing can be a challenge across industries, and it is further complicated in high-tech areas where associated services often generate more value than the physical components.28
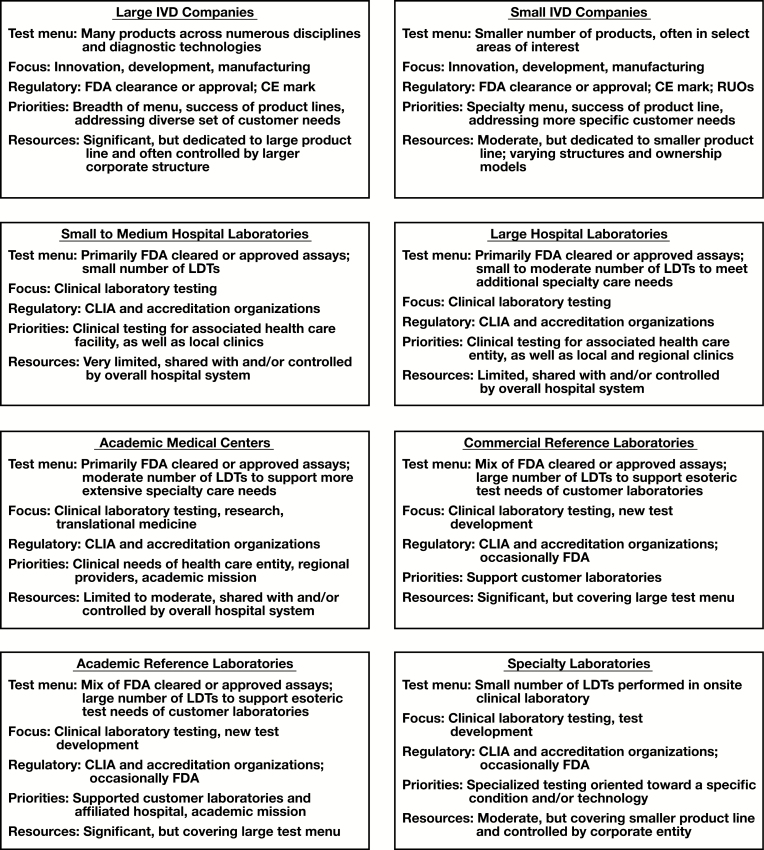
Presently, it is likely that few clinical laboratorians would self-identify their operations as manufacturing facilities, even if they develop and/or operate LDTs. Clinical laboratories operate under CLIA in a culture focused on assays, protocols, and procedures, and more importantly the application of these for clinical care. For many clinical laboratories, the validation and operational practices related to LDTs may also be closely aligned in both performance and documentation intended for CLIA-centric regulatory oversight. For example, the personnel who contribute to LDT development may also participate in the performance of such clinical testing once the test is “live” on a laboratory test menu. This is in sharp contrast to manufacturing industries where production is removed from operation. Examples of the diverse priorities, goals, and resources in different settings across the IVD community are presented in Figure 1.
Figure 1 .
Differences in clinical laboratory and in vitro diagnostic (IVD) organization types. Examples are general and do not represent the diversity and complexity of all activities and priorities in each setting. Areas of shared importance across all groups (eg, quality, improving patient care) are not included. CE, European Conformity; CLIA, Clinical Laboratory Improvement Amendments; FDA, Food and Drug Administration; LDT, laboratory-developed test; RUO, research use only.
Ensuring Patient Safety
Safety and Effectiveness in an Environment of Analytical and Clinical Validity
FDA oversight of medical device safety and effectiveness is contained in MDA statute language.2 Clinical laboratory tests are used by licensed practitioners in making health care decisions with their patients, by providing information that is then used to influence medical, surgical, dietary, or other potential interventions. Some diagnostic testing is used for informational or educational purposes only and may not be linked to any specific intervention. In either context, the concepts of analytical validity (eg, does the test perform as intended) and clinical validity (eg, does the test result relate to the presence, absence, and/or risk of a disease or condition) are predominant concerns. A test that is not analytically or clinically valid would justifiably be considered unsafe, as its use could lead to adverse or unintended outcomes. Ultimate effectiveness, however, depends on use of the information in patient management and the associated medical interventions. Effectiveness is therefore a more difficult concept to translate directly to device-centric LDT oversight models.
The Risk of Overregulation
While appropriate regulatory review can help to ensure analytical and clinical validity, excessive regulation carries additional costs—not just financial, but also in hindering innovation and in missed opportunity for making accessible newer, more robust, or cost-effective diagnostic technologies. The European CE marking system,29 for example, has enabled the commercial offering of diagnostic and medical technologies outside the United States that have not been submitted to the FDA for clearance or approval by their respective manufacturers, primarily due to cost, timing, and regulatory requirements associated with device approval in the United States.29-31 It is important that legislators and regulators consider that patient care can be adversely impacted not just by unsafe devices but also by lack of availability of diagnostic technologies that could assist patients with existing medical needs. A goal of regulatory efforts should therefore be to identify an optimal balance to both protect patient safety, but also not deny access to valuable laboratory services due to cost, process, and/or timing issues associated with IVD submissions and reviews.
Impact of VALID on Clinical Laboratories
Financial, Personnel, and Resources Repercussions
Would clinical laboratories have the resources to support ongoing LDT development and operation under the FDA regulatory framework proposed in VALID? Many clinical laboratories operate as cost centers for health care facilities and systems. The industry is facing significant financial cuts brought by the roll-out of the recent CMS Clinical Laboratory Fee Schedule in response to Protecting Access to Medicare Act requirements,32 a recent 20% across-the-board increase in CLIA certificate clinical laboratory user fees,33 and anticipated increases in costs associated with proposed changes to proficiency testing regulations from CMS.34
While precise IVCT submission fees were not specified in the VALID discussion draft, current FDA fees under the Medical Device User Fee Amendments (MDUFA)35 are likely beyond the financial capacity of both small and medium clinical laboratories (which run a small variety of LDTs) as well as larger clinical laboratories (which run a larger variety of LDTs). It seems reasonable to speculate that submissions that require higher degrees of regulatory review (eg, “high-risk” tests) may be subject to higher submission fees, more analogous to current premarket approval versus 510k submissions.35 Substantive financial analyses on the market impact of user and submission fees are therefore essential prior to advancing legislative proposals, to determine whether clinical laboratories can financially sustain LDT development and operations under any new regulatory framework. This would also be consistent with existing MDA statute requirements regarding the records and reports on devices, which prohibit the agency from imposing requirements that create an undue regulatory burden, balancing cost with the obvious need to protect the public health [SEC. 519 (a)].2
User and submission fees, however, are just one component of resources that would be necessary to comply with the proposed VALID discussion draft framework. QSRs related to manufacturing (eg, FDA 21 Code of Federal Regulations [CFR] Part 820 and International Organization for Standardization [ISO] 13485:2016) are relatively unfamiliar standards in the broader clinical laboratory community, which has yet to widely implement medical laboratory-specific international standards associated with ISO 15189 accreditation in the United States.36 Indeed, implementation of manufacturing-oriented QSRs (even in a proposed limited form) would likely face several obstacles if applied across clinical laboratories. QSR implementation in clinical laboratories would require training of quality specialists who are familiar with not only applicable manufacturing-oriented principles, but also clinical laboratory operations. It is widely acknowledged, however, that the clinical laboratory community faces a critical laboratory workforce shortage.37 QSR staffing could not be filled entirely from the laboratories’ existing technical workforce without further exacerbating the critical shortage in technical personnel.
There are also aspects of manufacturing-oriented QSRs for which a clinical laboratory may simply not have direct control. For example, purchasing and supplier activities at an organizational level (eg, hospital system) may be outside a clinical laboratory’s direct oversight. Prompting an entire health care system to adopt manufacturing-oriented purchasing and supplier controls—just to maintain a small number of clinically important LDTs—could be an uphill battle in many settings, and therefore prompt the discontinuation of future LDT development and/or operation.
As proposals have not specifically outlined anticipated user and submission fees, and as there has been no substantive assessment of the financial implication of increased quality structure and personnel requirements, the magnitude of impact on the clinical laboratory community is difficult to fully evaluate. Additional financial costs associated with developing a robust IVCT regulatory infrastructure that could review submissions in a timely manner, including potential outsourcing to accredited bodies that might assist in these regulatory reviews, would further add to the system-level impact of VALID. Also difficult to assess is the implication of system-wide regulatory changes on subsequent payer reimbursement determinations, which may currently differ between FDA cleared or approved assays versus LDTs. Whether changes in regulatory oversight (ie, considering all tests as IVCTs) would be associated with increased or decreased overall expenditures is an area in need of further analysis and financial modeling.
Test Modifications and Level of Regulatory Review
The level of regulatory scrutiny that might be applied to test modifications is also critically important for clinical laboratory settings. For example, package inserts (PIs) from FDA cleared or approved assays typically include reference intervals derived from vendor-conducted studies and/or based on the scientific literature. CLIA regulations require verification that reference intervals for FDA cleared or approved test systems are “appropriate for the laboratory’s patient population,” as well that reference intervals are established for modified FDA cleared or approved test systems.8 While currently a practice under CLIA oversight, modification of reference intervals from PI predefined limits could be considered a potential change in intended use (ie, result reporting) under a strict interpretation of the VALID discussion draft.
Another example of a common potential test modification involves the subjectivity associated with specimen type acceptability for FDA cleared or approved assays. For example, does a specimen type described in a PI as “heparin plasma” mean that both sodium heparin and lithium heparin (common anticoagulants) are acceptable for testing? Does “serum” mean that any type of serum (eg, “off the clot” vs serum separator tube) is acceptable for testing? In the current CLIA framework, the laboratory director is ultimately responsible for ensuring that clinical testing is conducted appropriately and in addressing and/or validating acceptability in such situations. In future regulatory proposals, would all such decisions be considered modifications subject to FDA review, because they affect labeling and notification elements? Would a laboratory still have operational flexibility to follow laboratory director-approved guidance without risking additional FDA oversight?
Specimen stability is another common example of a potential test modification that could introduce additional oversight risk. While PIs from FDA cleared or approved assays may list specimen stability limits, these time limits often reflect the maximum duration of stability actually tested (eg, verified) for a given condition by the manufacturer, rather than a time point at which instability begins to occur. Should the extension (or shortening) of PI-defined stability limits based on laboratory-conducted studies need to be considered a test modification subject to FDA oversight, or could CLIA-centric processes provide a more optimal mechanism for regulation of minor test modifications?
Regulatory notification and/or submission requirements for minor test modifications could inadvertently deter laboratories from conducting their own internal studies to better characterize assay performance, as such studies would introduce an increased risk of regulatory scrutiny if any modifications were made. Clinical laboratories currently perform an essential role by conducting such studies, which also include detection of potential interferences and analytical limitations not considered during original FDA submissions. Future regulatory proposals should incorporate appropriate flexibility, so that minor modifications can be left to the discretion of the laboratory director based on the clinical needs of the respective patient population and setting.
Precertification as an Approach to Decreasing Regulatory Burden
An interesting aspect of the VALID discussion draft has been the proposal for a precertification program for clinical test developers. Precertification could allow eligible developers to market certain IVCTs without a full premarket submission. If widely implemented, precertification might alleviate the increased number of IVCT submissions that the FDA would otherwise be required to review. The initial discussion draft of VALID, however, suggested that precertification should not apply to several categories of tests, including first-of-a-kind and high-risk IVCTs [Sec.587D (b)(2)(B)]. Clinical laboratories with diverse test menus may struggle to benefit from precertification programs that do not apply to a substantial number of LDTs performed within a facility, given that LDTs are frequently performed for novel and high-complexity scenarios. A more widely applicable precertification program, however, might reduce the potential regulatory burden of future oversight proposals and therefore would benefit from further discussion between regulators, legislators, and community stakeholders.
Examining Other Models of Risk-Based Review
It is also important to consider New York State Department of Health (NYSDOH) requirements when evaluating potential precertification and LDT regulatory models, as there are some parallels to the example of New York submission “exemptions.” Laboratories that perform clinical testing on specimens from New York patients are required to have a clinical laboratory permit from the NYSDOH and follow standards set forth by the Clinical Laboratory Evaluation Program (CLEP).38 Risk-based review and approval by CLEP is required for LDTs prior to their use in patient testing, and this review is conducted according to a revised policy implemented in 2016.39 However, laboratories that have received approval for a “sampling of tests that utilize a methodology that is common across analytes/targets” can request an exemption from future comprehensive submissions for LDTs with that methodology.40 The request includes requirements related to standardized validation protocols as well as ongoing quality monitoring. LDT additions under an exemption are still subject to risk-based review. A currently enacted model therefore exists outside the FDA for LTD submission and review, with a carve-out for exemptions when a laboratory has previously demonstrated proficiency with assay validation and operation using that methodology. A more extensive evaluation of the NYDOH LDT review program could be helpful to better understand the financial and administrative impact on regulatory agencies if more wide-ranging regulatory oversight was considered at a federal level.
Such an analysis could also be informative in understanding how real-world risk-based stratification methods distribute tests across different levels of regulatory review. LDT regulatory proposals, for example, have shown varying reliability and validity when applying stratification criteria across a representative set of assays.41 Mechanisms for establishing levels of regulatory review should therefore be thoroughly evaluated—in collaboration with the broader stakeholder communities—prior to their advancement in any future regulatory and/or legislative proposals.
Protecting the Practice of Laboratory Medicine
Patient care contributions resulting from the practice of laboratory medicine are underrecognized in modern health care, where clinical laboratory testing is often viewed as a service and rarely considered peer-to-peer medical engagement. This problem is multifactorial and deeply rooted in our current regulatory structure and clinical practices. Ongoing efforts such as the advancement of diagnostic management teams can help to improve this dynamic and reduce diagnostic errors by leveraging expertise from the clinical laboratory.42
The practice of laboratory medicine is also inadequately acknowledged in current regulatory systems. CLIA-mandated high-complexity laboratory directorship qualifications can be satisfied by physicians (eg, most commonly pathologists) and PhD-trained laboratory scientists (42 CFR 493.1443), whereas master’s and bachelor’s degree holders may also meet directorship qualifications for moderate complexity testing (42 CFR 493.1405). While laboratory directorship is not described as a medical activity in CLIA regulations (which align medical consultation with a role of “clinical consultant”), clinical testing scenarios that are challenging and frequently subjective are common occurrences and rely on the judgement and experience of laboratory directors, clinical consultants, supervisors, and laboratory professionals for their effective resolution. While the MDA statute discusses professional practice in the context of exemption of licensed practitioners from requirements for FDA reporting and device customization, it does not specifically address a role of laboratory medicine.2 VALID describes the practice of medicine in relation to the “authority of a health care practitioner to prescribe or administer any legally marketed in vitro clinical test for any condition or disease within a legitimate health care practitioner-patient relationship” [Sec. 587A (a)(3)(A)], but it also does not specifically acknowledge or protect the practice of laboratory medicine.
As described in the American Board of Pathology entry on the American Board of Medical Specialties website, board certified pathologists—as licensed medical professionals—contribute to “diagnosis, prognosis and treatment through knowledge gained by the laboratory application of the biologic, chemical and physical sciences.” 43 Pathology is a fundamental practice of medicine. Compelling arguments have been put forth advocating differing positions on whether laboratory testing services are (or are not) considered practice of medicine, and therefore how they may be impacted by medical device regulatory reform.44,45 Without increased advocacy regarding the value of laboratory medicine in test development, implementation, and operation, it is possible that future IVD proposals may diminish the ability of clinical pathologists and laboratory scientists to use their professional judgement and expertise in providing clinically optimal testing services for their patients.
Fostering Meaningful Partnerships With Patient Advocacy Organizations
Patient care is a fundamental priority for the clinical laboratory community. As clinical laboratory testing is often conducted in physical settings removed from the delivery of direct-facing patient care activities, a patient-to-laboratory line of communication is uncommon in many laboratories and practice environments. Patient care and advocacy organizations also have a clear focus on the quality of laboratory services provided to their patient populations and membership. As such, a stronger relationship with the clinical laboratory community would be productive in both informing laboratory professionals on the priorities of patient-centric needs, as well as for informing patient organizations on the depth and diversity of testing that is currently being supported by LDTs across disease states. Forums to develop and support these potential relationships would be tremendously helpful to all involved.
Conclusions
Device-based and/or manufacturing-centric IVD regulatory proposals would be challenging to implement in most clinical laboratory settings. It is important to identify concepts that have worked well in clinical laboratories when developing future regulatory proposals, as these ideas could be used to strengthen future regulatory efforts across differing operational environments. Commonsense principles that could be considered in future IVD regulatory proposals are presented in Table 1 to inform further discussion. Principles for diagnostic reform have also been advanced by organizations such as the American Clinical Laboratory Association.46
Table 1 .
Principles for Future Regulatory Proposals
| Patient care | Patient care involves not just appropriate ordering, operation, and interpretation of laboratory tests, but also a market and regulatory climate that supports test development, innovation, and availability, particularly in testing for rare disorders that may not benefit from typical market-based incentives. |
| Quality | Quality of test results is fundamentally important to both in vitro diagnostics (IVD) manufacturers and clinical laboratories. Prioritize systems that focus attention on analytical and clinical performance. |
| Clarity | Regulations should be clear and understandable to the clinical laboratory community. Regulations that are too manufacturer centric will not integrate well into clinical laboratory operations. |
| Practice of medicine | Regulatory proposals should not hinder the practice of laboratory medicine by licensed and qualified practitioners, a critically important component underlying test development, modification, and operation in many clinical laboratory settings. |
| Transparency | Promulgated rules in regards to laboratory-developed test oversight should follow notice-and-comment rulemaking and not be subject to change without substantive input from stakeholder communities. Analyses of financial impact should be shared with the public. |
| Innovation | Regulations should promote the unique contributions of IVD manufacturers and clinical laboratories in regards to test innovation. Excessive regulations can harm innovation and diminish future test development. |
| Efficiency | Regulations should be efficient and designed for a least-burdensome impact. Limit documentation and notification requirements to essential elements. Promote systems and processes that are streamlined and that foster innovation. |
| Affordability | Regulatory structures that are not affordable for involved stakeholders cannot reasonably be maintained over the long term. |
One aspect of CLIA that has worked well has been the process of accreditation, with laboratories being able to maintain a renewable certificate of accreditation to continue operations under well-defined regulatory limits and periodic inspections (42 CFR 493.61). All clinical laboratories must meet these requirements to perform clinical testing on patient specimens in the United States. It is interesting to note that a full precertification program—at least conceptually—is analogous to accreditation. If developed and administered appropriately, could a robust precertification concept for IVCTs be used to address patient safety concerns without creating an undue burden on existing laboratory operations? Alternatively, could a more detailed “LDT checklist” for clinical laboratories under CLIA certification accomplish similar objectives to current proposals but at a lower regulatory cost? Questions such as these should be thoroughly investigated with the stakeholder community before legislative proposals are advanced.
There does not currently appear to be any administrative or legislative momentum toward active consideration of a CLIA-centric model of enhanced LDT oversight. If an FDA-centric model is ultimately advanced, extensive grandfathering, wide-spanning precertification, restricted QSRs tailored to existing CLIA quality requirements, minimal or waived user and submission fees, as well as streamlined documentation, registration, and notification requirements would be essential to envisioning a solution that works for even a portion of clinical laboratory community. Even with these considerations, it is possible that many clinical laboratories would abandon LDT offerings due to lack of sufficient resources and inability to comply with additional regulatory requirements. This would be a tragic outcome, as it would decrease testing options for patients and hinder innovation throughout the field of laboratory diagnostics. It is therefore essential that legislative proposals fully reflect the needs, complexity, and resources involved in LDT development and operation in a clinical laboratory environment.
Acknowledgement
The author thanks Dr Sherrie L. Perkins (University of Utah/ARUP Laboratories), Dr Curtis A. Hanson (Mayo Clinic Laboratories), and Jonathan Carr, JD (ARUP Laboratories) for thoughtful comments on a draft of this article.
References
- 1. Center for Devices and Radiological Health. Framework for Regulatory Oversight of Laboratory Developed Tests (LDTs). Guidance for Industry, Food and Drug Administration Staff, and Clinical Laboratories. Draft. Rockville, MD: Food and Drug Administration. Center for Devices and Radiological Health; 2014. [Google Scholar]
- 2.Medical Device Amendments of 1976. (PL 94-295, May 28, 1976) United States Statutes at Large, 90 (1976) pp. 539-583.
- 3.21 CFR §167 (1973).
- 4. Genzen JR, Mohlman JS, Lynch JL, et al. Laboratory-developed tests: A legislative and regulatory review. Clin Chem. 2017;63:1575-1584. [DOI] [PubMed] [Google Scholar]
- 5. Center for Devices and Radiological Health. Commercialization of Unapproved In Vitro Diagnostic Devices Labeled for Research and Investigation (Draft Compliance Policy Guide). Rockville, MD: Food and Drug Administration; 1992. [Google Scholar]
- 6. Gutierrez A.Oversight of laboratory developed tests. APHL Annual Meeting 2015. https://www.aphl.org/conferences/proceedings/Documents/2015/Annual-Meeting/70Gutierrez.pdf. Accessed January 3, 2019. [Google Scholar]
- 7. Dickerman HW. U.S. Senate, Committee on Governmental Affairs, Subcommittee on Oversight of Government Management. Health Care Financing Administration’s Management of Medical Laboratories, Written Testimony, March 23, 1988 (Serial No. 100–765). Washington: Government Printing Office; 1988:235. [Google Scholar]
- 8.68 Fed. Reg. 3703 (January 24, 2003); 68 Fed. Reg. 50724 (August 22, 2003); Codified at 42 CFR §493.1253 (2003).
- 9. Food and Drug Administration. FDA to host public meeting on oversight of laboratory-developed tests. FDA news release. June 16, 2010. [Google Scholar]
- 10. Food and Drug Administration. Oversight of laboratory developed tests. Public meeting. Request for comments. June 17, 2010. 75Federal Register. 34463. [Google Scholar]
- 11. Harper C. Presentation at the Public Meeting on Oversight of Laboratory Developed Tests. Food and Drug Administration. Transcript. Washington, DC: Neal R. Gross & Co; July 19, 2010:37-47. [Google Scholar]
- 12. Center for Devices and Radiological Health. FDA Notification and Medical Device Reporting for Laboratory Developed Tests (LDTs). Guidance for Industry, Food and Drug Administration Staff, and Clinical Laboratories. Draft. Rockville, MD: Food and Drug Administration; 2014. [Google Scholar]
- 13. Center for Devices and Radiological Health. Public Workshop on Laboratory Developed Tests. Bethesda, MD: Food and Drug Administration; 2015. [Google Scholar]
- 14. Office of Public Health Strategy and Analysis, Office of the Commissioner. The Public Health Evidence for FDA Oversight of Laboratory Developed Tests: 20 Case Studies. Bethesda, MD: Food and Drug Administration; 2015. [Google Scholar]
- 15. Burton TM. FDA backs off plans to issue rules governing lab-developed tests. Wall Street Journal. November 18, 2016. [Google Scholar]
- 16. Food and Drug Administration. Discussion paper on laboratory developed tests. January 13, 2017. https://www.fda.gov/downloads/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/LaboratoryDevelopedTests/UCM536965.pdf. Accessed January 3, 2019. [Google Scholar]
- 17. Gottlieb S.Remarks at the American Clinical Laboratory Association Annual Meeting, March 6, 2018. https://www.fda.gov/NewsEvents/Speeches/ucm599551.htm. Accessed December 31, 2018. [Google Scholar]
- 18.Press release. Dr. Bucshon, DeGette release draft of the Diagnostic Accuracy and Innovation Act (DAIA). March 21, 2017. https://bucshon.house.gov/news/documentsingle.aspx?DocumentID=337. Accessed December 30, 2018.
- 19. Hyman, Phelps and McNamara. FDA’s IVD TA: It’s not just technical assistance. FDA law blog. http://www.fdalawblog.net/2018/08/fdas-ivd-ta-its-not-just-technical-assistance/. Accessed January 4, 2019. [Google Scholar]
- 20. DeGette D.DeGette, colleagues release draft legislation to modernize FDA regulation of diagnostic tests. Press release. December 6, 2018. https://degette.house.gov/media-center/press-releases/degette-colleagues-release-draft-legislation-to-modernize-fda-regulation. Accessed December 30, 2018. [Google Scholar]
- 21. Snozek CLH. FDA-cleared versus laboratory developed tests: why start from scratch when kits are available? J Appl Lab Med. 2017;2:130-131. [DOI] [PubMed] [Google Scholar]
- 22. Joseph L, Cankovic M, Caughron S, et al. The spectrum of clinical utilities in molecular pathology testing procedures for inherited conditions and cancer: a report of the Association for Molecular Pathology. J Mol Diagn. 2016;18:605-619. [DOI] [PubMed] [Google Scholar]
- 23. Caliendo AM, Couturier MR, Ginocchio CC, et al. ; Infectious Diseases Society of America; the American Society for Microbiology; and the Pan-American Society for Clinical Virology Maintaining life-saving testing for patients with infectious diseases: Infectious Diseases Society of America, American Society for Microbiology, and Pan American Society for Clinical Virology recommendations on the regulation of laboratory-developed tests. Clin Infect Dis. 2016;63:151-154. [DOI] [PubMed] [Google Scholar]
- 24. Kaul KL, Sabatini LM, Tsongalis GJ, et al. The case for laboratory developed procedures: quality and positive impact on patient care. Acad Pathol. 2017;4:2374289517708309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim AS, Bartley AN, Bridge JA, et al. Comparison of laboratory-developed tests and FDA-approved assays for BRAF, EGFR, and KRAS testing. JAMA Oncol. 2018;4:838-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lathrop JT, Jeffery DA, Shea YR, et al. US Food and Drug Administration perspectives on clinical mass spectrometry. Clin Chem. 2016;62:41-47. [DOI] [PubMed] [Google Scholar]
- 27.21 CFR §8203.
- 28. Levinson M.What is manufacturing? Why does the definition matter? February 6, 2017. Congressional Research Service. 7-5700. R44755. https://www.nist.gov/sites/default/files/documents/2017/02/08/r44755.pdf. Accessed April 19, 2019. [Google Scholar]
- 29. Van Norman GA. Drugs and devices: comparison of European and U.S. approval processes. JACC Basic Transl Sci. 2016;1:399-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Van Norman GA. Drugs, devices, and the FDA: part 2: an overview of approval processes: FDA approval of medical devices. JACC Basic Transl Sci. 2016;1:277-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Emergo. How long it takes the US FDA to clear medical devices via the 510(k) process: an examination of 15,000 medical device applications cleared by the US Food and Drug Administration between 2012 and 2016. March, 2017. https://www.emergogroup.com/sites/default/files/emergo-fda-510k-data-analysis-2017.pdf. Accessed April 21, 2019. [Google Scholar]
- 32. Centers for Medicare and Medicaid Services. Information regarding the final CY 2018 private payor rate-based clinical laboratory fee schedule (CLFS) Payment rates. November 17, 2017. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Downloads/CY2018-CLFS-HCPCS-Median-Calculations.pdf. Accessed December 31, 2018. [Google Scholar]
- 33. Centers for Medicare and Medicaid Services. Medicare Program: Clinical Laboratory Improvement Amendments (CLIA) of 1988 Fees. CMS-3356-NC. December 28, 2018. https://www.cms.gov/newsroom/fact-sheets/medicare-program-clinical-laboratory-improvement-amendments-clia-1988-fees-cms-3356-nc. Accessed December 31, 2018. [Google Scholar]
- 34.84 Fed. Reg. 23 (February 4, 2019), p. 1536.
- 35. Food and Drug Administration. FY 2019 MDUFA User Fees. https://www.fda.gov/ForIndustry/UserFees/MedicalDeviceUserFee/ucm615142.htm. Accessed December 31, 2018. [Google Scholar]
- 36. Schneider F, Maurer C, Friedberg RC. International organization for standardization (ISO) 15189. Ann Lab Med. 2017;37:365-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. American Society for Clinical Laboratory Science. Addressing the clinical laboratory workforce shortage. https://www.ascls.org/position-papers/321-laboratory-workforce/440-addressing-the-clinical-laboratory-workforce-shortage. Accessed December 31, 2018. [Google Scholar]
- 38. Wadsworth Center, New York State Department of Health. Laboratory standards. Clinical laboratory evaluation program. https://www.wadsworth.org/regulatory/clep/clinical-labs/laboratory-standards. Accessed February 26, 2019. [Google Scholar]
- 39. Wadsworth Center, New York State Department of Health. NYSDOH policy for risk-based evaluation of laboratory developed tests (LDT). https://www.wadsworth.org/sites/default/files/WebDoc/1118127246/Risk-based_LDT_Policy_10142016.pdf. Accessed January 18, 2019. [Google Scholar]
- 40. Wadsworth Center, New York State Department of Health. Test approval. Clinical laboratory evaluation program. https://www.wadsworth.org/regulatory/clep/clinical-labs/obtain-permit/test-approval#Making%20a%20submission. Accessed January 18, 2019. [Google Scholar]
- 41. Mohlman JS, Genzen JR, Weiss RL, et al. Reliability and validity of proposed risk stratification methods for laboratory developed tests. Lab Med. 2019;50:194-201. [DOI] [PubMed] [Google Scholar]
- 42. Verna R, Velazquez AB, Laposata M. Reducing diagnostic errors worldwide through diagnostic management teams. Ann Lab Med. 2019;39:121-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. American Board of Medical Specialties. American Board of Pathology. Pathology. https://www.abms.org/member-boards/contact-an-abms-member-board/american-board-of-pathology/. Accessed December 31, 2018. [Google Scholar]
- 44. Clement PD, Tribe LH. Counsel to the American Clinical Laboratory Association Laboratory testing services, and the practice of medicine, cannot be regulated as medical devices. January 6, 2015. https://www.acla.com/wp-content/uploads/2015/01/Tribe-Clement-White-Paper-1-6-15.pdf. Accessed January 3, 2019. [Google Scholar]
- 45. Sherrin JJ, Holley DE. Counsel to the American Association of Bioanalysts Laboratory testing services are not the practice of medicine: a response to Paul D. Clement and Laurence H. Tribe. http://www.aab.org/images/aab/pdf/2015/LDTs%20Not%20the%20Practice%20of%20Medicine_Final_sherrin_holley.pdf. Accessed January 3, 2019. [Google Scholar]
- 46. American Clinical Laboratory Association. Key principles for diagnostic reform. May, 2017. https://www.acla.com/wp-content/uploads/2017/05/Key-Principles-of-Diagnostic-Reform.pdf. Accessed April 20, 2019. [Google Scholar]