Abstract
Acute promyelocytic leukemia (APL) is a particularly aggressive subtype of acute myeloid leukemia (AML), with high rates of early death. It is important to examine how epidemiological characteristics, clinical and treatment factors, cytogenetic and genetic data affect survival and differ between APL and non-APL AML patients. We analyzed population data from the New York State Cancer Registry to characterize AML including APL incidence rates by demographics. APL incidence rates were higher among Hispanics than non-Hispanics [incidence rate ratio = 1.22; 95% confidence interval (CI) = 1.02–1.43]; and among foreign-born than USA-born persons. APL incidence rates increased more rapidly through 1995–2014 than non-APL AML; and its frequency increased faster among foreign-born persons. In a hospital cohort of 390 AML patients, the risk of death was significantly higher among APL patients with FLT3-internal tandem duplications than those without [hazard ratio (HR) = 11.74; 95% CI = 1.03–134.5]; and among APL patients with secondary versus de novo disease (HR = 17.32; 95% CI = 1.56–192.1). Among non-APL AML patients, risk of death was significantly associated with prior chemotherapy with antitubulin agents after adjusting for age, gender and ethnicity (adjusted HR = 3.30; 95% CI = 1.49–7.32); and separately with older age, unfavorable cytogenetics and complex karyotype. This study highlights FLT3-internal tandem duplications as a prognostic factor in APL and proposes consideration of prior antitubulin therapy as a prognostic factor in non-APL AML.
In this study, acute promyelocytic leukemia (APL) was more frequent among Hispanics and persons born outside the USA. Risk of death was increased among APL patients with FLT3-internal tandem duplications mutations and secondary leukemia, and among acute myeloid leukemia patients with prior chemotherapy with antitubulin agents.
Introduction
Acute myeloid leukemia (AML) is a hematological malignancy that arises from clonal proliferation of immature myeloid cells. Prognosis depends on a number of factors including age and disease biology; without treatment, AML is universally fatal (1). The American Cancer Society estimated 19 520 new cases and 10 670 deaths due to AML in the USA during 2018, and ~0.5% of the population will develop it during their lifetime (2). The AML age-adjusted incidence and mortality rates are 4.3 and 2.8 per 100 000 persons, respectively, and the 5-year survival rate is 27.4% (3). A number of factors have been associated with increased risk of AML in adults, including older age; antecedent hematological disease; and exposure to therapeutic and non-therapeutic ionizing radiation, chemicals such as benzene; pesticides; herbicides and certain chemotherapeutic drugs and agents (4). These exposures may result in acquired chromosomal abnormalities, which have been associated with lower survival in AML patients (5).
Acute promyelocytic leukemia (APL) is a rare but aggressive form of AML, comprising 5–15% of AML cases (6). Its incidence rate in the USA population was 0.32 per 100 000 persons during 2000–14 (7), representing a significant increase over time (0.11 per 100 000 persons in 1975–90) (8). Although APL had a poor prognosis historically, treatment regimens containing all-trans retinoic acid, anthracyclines, arsenic trioxide have achieved complete remission rates close to 90%, and cure rates of 80% (9). Five-year relative survival rates have increased over time from 18% in 1975–90 to 64% in 2000–08 (8). Despite the improvement in overall survival, APL is considered a medical emergency. Early death, within 1 month of diagnosis, occurs in an estimated 17.3% cases, most frequently attributed to severe intracranial or pulmonary hemorrhage (10).
APL is known to occur more frequently in patients of Hispanic or Latino origin compared with whites (11,12). It is important to fully study the epidemiological distribution and clinical characteristics of both APL and non-APL AML, and the presence of acquired cytogenetic abnormalities and genetic mutations associated with prognosis, to identify subpopulations with increased risk of disease and death and be able to personalize treatment options. A number of studies have characterized AML in terms of epidemiology, identified high-risk groups and suggested treatment strategies (13–15), but these do not necessarily differentiate between APL and non-APL AML patients. Population-based studies have examined incidence, risk factors and survival among patients with different AML types (16), but comparative clinical studies in the same patient cohort are sparse.
To address this gap, we conducted a retrospective study on both a population-based cancer registry and a hospital clinical registry, with the following objectives: (i) to characterize the incidence rates of AML and APL in New York State according to demographics; (ii) to describe a hospital-based clinical cohort of AML patients in terms of demographic characteristics, risk factors, cancer history, treatment history, cytogenetics and genetic mutations; stratified by APL status; (iii) to identify factors associated with survival among APL and non-APL AML patients.
Materials and methods
Data collection
Data on acute myeloid leukemia and patient characteristics were collected from two sources: a population-based cohort and a clinical (hospital-based) validation cohort.
Population-based cohort
New York State Cancer Registry (NYSCR) data were obtained from the publicly available website, New York State Public Access Cancer Epidemiology Data (NYSPACED) (17). The study sample consisted of NYSCR patients diagnosed with AML from 1995 to 2014, based on the variable Site recode ICD-O-3/WHO 2008. AML was defined as Site recode ICD-O-3/WHO 2008 ‘acute myeloid leukemia’ or ‘acute monocytic leukemia’; cases were further defined as ‘acute promyelocytic leukemia’ (APL) if ICD-O3 histology/ behavior code = 9866/3.
Clinical cohort
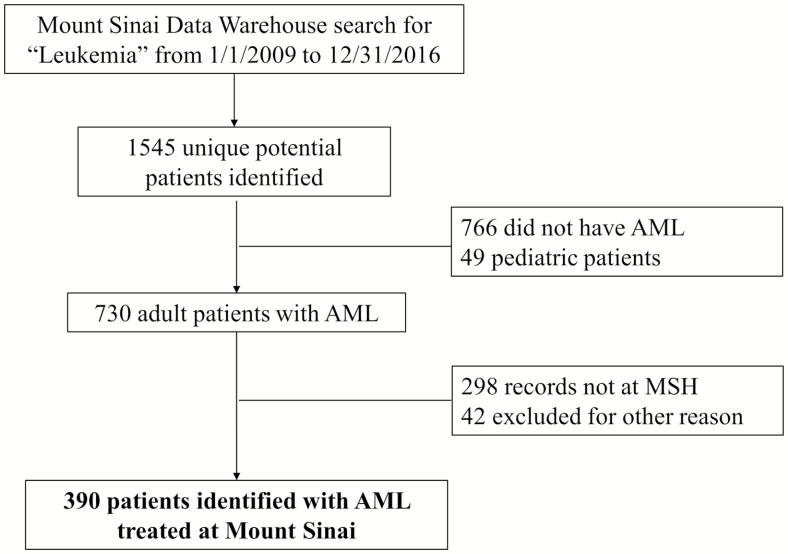
Patient data for the clinical cohort were extracted from the electronic medical record database of the Mount Sinai Health System, a tertiary care hospital in New York City. Patients were eligible for inclusion in the study if they were diagnosed with AML (including APL) and received care from a hematologist/oncologist at Mount Sinai Health System, from 1 January 2009 to 31 December 2016. The selection process for patients is outlined in Figure 1.
Figure 1.
Consort flow diagram for data collection (clinical cohort).
Data on demographic characteristics (age, gender, race, ethnicity and marital status), risk factors (alcohol and tobacco use) and cancer-related factors (AML type, history of solid tumor, hematological disorder and prior cancer therapy) were collected for 390 patients at diagnosis. AML was classified into ‘de novo’ AML and ‘secondary AML’ [secondary to myelodysplastic syndrome, myeloproliferative neoplasm or therapy related]. Cytogenetic information was available for 256 patients. A karyotype abnormality was defined as the presence of any structural or numerical chromosomal abnormality in two or more cells (three or more cells for monosomy) (18). They were further categorized as ‘favorable’ or ‘unfavorable’ (including intermediate I, intermediate II, adverse), according to European Leukemia Net (ELN) guidelines (19). Three or more different chromosomal abnormalities occurring in the same patient identified a ‘complex’ karyotype (20). If the patient’s genetic profile showed evidence of exposure to commonly known mutagens, they were considered to have an exposure signature present (21). Genetic profile data were available for 205 patients at diagnosis. Patients having at least one of the following gene mutations (ASXL1, FLT3, DNMT3A, RUNX1, TET2, TP53, PHF6) previously associated with risk of AML and/or poor prognosis (3) were classified as having a ‘deleterious’ mutation. This study was approved by the institutional review board at Mount Sinai Hospital (IRB Protocol Approval number IRB1701298). A waiver of informed consent was appropriate as data were retrospective and de-identified. All protected health information was anonymized and kept confidential.
Statistical analyses
Population-based cohort
Descriptive statistics, case frequencies and cancer incidence rates were presented according to demographic characteristics including gender, age group, race, ethnic origin and nativity. Stratum-specific, age-adjusted incidence rates per 100 000 persons for all AML cases and the subset of APL cases were calculated using the 2000 USA Census population as the standard (except for country of birth, due to lack of appropriate denominators in the NYSPACED data set). Age-adjusted incidence rates stratified according to the country of birth were calculated using age-specific USA population weights for the year 2005, the midpoint of the data collection period. Further, due to the large proportion of cases with unknown country of birth (11% AML cases, 25% APL cases), a sensitivity analysis was performed by incorporating cases with ‘unknown’ birthplace proportionately within the ‘USA-born’ and ‘foreign-born’ categories according to the original ratio of USA-born to foreign-born patients. Statistical analyses were conducted using SEER*Stat and SAS analytic software.
Clinical cohort
Demographic characteristics were described for the complete sample (N = 390). Characteristics for APL and non-APL patients were compared using the χ2 and Fisher’s exact tests. Risk of death was assessed using Cox proportional hazard regression for several potential risk factors including demographic characteristics, AML type, cytogenetic risk (ELN category), karyotype complexity, prior cancer therapy and presence of specific genetic mutations. Bivariate (unadjusted) regression for each risk factor was conducted, as well as a model adjusted for age, gender, ethnicity and prior cancer therapy. Statistical analyses were conducted using SAS analytic software, version 9.4 (SAS Institute, Cary, NC). Statistical significance was evaluated at α = 0.05.
Results
Population-based cohort
There were 58 664 patients with leukemia in the NYSCR; 17 120 of them had a diagnosis of AML, 1193 of which were APL. APL patients were significantly more likely to be non-white, Hispanic, foreign-born and were generally younger compared with non-APL patients (Table 1). The incidence rate of non-APL (per 100 000 persons) was lower among Hispanics compared with non-Hispanics [incidence rate ratio = 0.76; 95% confidence interval (CI) = 0.71–0.80], in contrast with APL, which was higher among Hispanics compared with non-Hispanics (incidence rate ratio = 1.22; 95% CI = 1.03–1.43; Table 2). Males had higher incidence rates of AML than females, and so did whites compared with blacks. Foreign-born patients had higher incidence rates of APL compared with USA-born patients (incidence rate ratio = 1.20), although its statistical significance could not be determined due to lack of individual denominator data (Table 2).
Table 1.
Distribution of demographic characteristics among AML cases in New York State, 1995–2014
| Distribution of AML patients | ||||
|---|---|---|---|---|
| AML (n = 17 120) | APL (n = 1193) | |||
| Characteristic | N (%) | N (%) | Non-APL (n = 15 927) | P valueb |
| Age group (years) | <0.0001* | |||
| <20 | 828 (4.8) | 87 (7.3) | 741 (4.7) | |
| 20–34 | 960 (5.6) | 189 (15.8) | 771 (4.8) | |
| 35–64 | 5570 (32.5) | 585 (49.0) | 4985 (31.3) | |
| 65+ | 9762 (57.0) | 332 (27.8) | 9430 (59.2) | |
| Gender | 0.069** | |||
| Male | 9159 (53.5) | 608 (51.0) | 8551 (53.7) | |
| Female | 7961 (46.5) | 585 (49.0) | 7376 (46.3) | |
| Race | <0.0001* | |||
| White | 14 498 (84.7) | 951 (79.7) | 13 547 (85.1) | |
| Black | 1783 (10.4) | 160 (13.4) | 1623 (10.2) | |
| Othera | 746 (4.4) | 69 (5.8) | 677 (4.3) | |
| Unknown/other | 93 (0.5) | 13 (1.1) | 80 (0.5) | |
| Ethnicity | <0.0001* | |||
| Non-Hispanic | 15 599 (91.1) | 1000 (83.8) | 14 599 (91.7) | |
| Hispanic | 1521 (8.9) | 193 (16.2) | 1328 (8.3) | |
| Birthplace | <0.0001* | |||
| USA-born | 12 253 (71.6) | 664 (55.7) | 11 589 (72.8) | |
| Foreign-born | 3000 (17.5) | 231 (19.4) | 2769 (17.4) | |
| Unknown | 1867 (10.9) | 298 (25.0) | 1569 (9.9) | |
aAmerican Indian/Alaskan Native, Asian/Pacific Islander.
bChi-square test comparing distribution of characteristics between APL and non-APL AML patients.
*P ≤ 0.001, **P ≤ 0.05.
Table 2.
Incidence rates of AML types according to demographic characteristics in NY State, 1995–2014
| All AML patients (n = 17 120) | APL (n = 1193) | Non-APL (n = 15 927) | ||||
|---|---|---|---|---|---|---|
| Characteristic | Incidence rate (95% CI) | Incidence rate ratio (95% CI) | Incidence rate (95% CI) | Incidence rate ratio (95% CI) | Incidence rate (95% CI) | Incidence rate ratio (95% CI) |
| Gendera | ||||||
| Male | 5.3 (5.2–5.4) | 1.0 (Ref) | 0.3 (0.3–0.4) | 1.0 (Ref) | 5.0 (4.9–5.1) | Ref |
| Female | 3.5 (3.5–3.6) | 0.67 (0.65–0.69) | 0.3 (0.3–0.3) | *0.85 (0.75–0.95) | 3.3 (3.2–3.3) | *0.65 (0.63–0.67) |
| Racea | ||||||
| White | 4.5 (4.4–4.6) | 1.0 (Ref) | 0.3 (0.3–0.3) | 1.0 (Ref) | 4.2 (4.1–4.3) | Ref |
| Black | 3.0 (2.9–3.1) | 0.66 (0.63–0.70) | 0.2 (0.2–0.3) | *0.78 (0.65–0.92) | 2.8 (2.6–2.9) | *0.66 (0.62–0.69) |
| Otherb | 3.5 (3.2–3.7) | 0.77 (0.71–0.82) | 0.3 (0.2–0.3) | 0.87 (0.68–1.10) | 3.2 (3.0–3.4) | *0.76 (0.71–0.82) |
| Ethnicitya | ||||||
| Non-Hispanic | 4.3 (4.3–4.4) | 1.0 (Ref) | 0.3 (0.3–0.3) | 1.0 (Ref) | 4.0 (4.0–4.1) | Ref |
| Hispanic | 3.4 (3.2–3.6) | 0.79 (0.75–0.83) | 0.4 (0.3–0.4) | *1.22 (1.02–1.43) | 3.1 (2.9–3.3) | *0.76 (0.71–0.80) |
| Birthplacec,d | ||||||
| USA-born | 4.2 (3.9–4.6) | 1.0 (Ref) | 0.2 (0.2–0.3) | 1.0 (Ref) | 4.0 (3.6–4.3) | Ref |
| Foreign-born | 3.5 (3.3–3.7) | 0.84 | 0.3 (0.2–0.4) | 1.18f | 3.2 (3.0–3.5) | 0.82f |
| Unknown | — | — | — | |||
| Birthplacec,e | ||||||
| USA-born | 4.7 (4.4–5.1) | 1.0 (Ref) | 0.3 (0.2–0.4) | 1.0 (Ref) | 4.4 (4.1–4.8) | Ref |
| Foreign-born | 4.0 (3.6–4.2) | 0.85 | 0.4 (0.3–0.5) | 1.20f | 3.6 (3.4–3.9) | 0.83f |
aAge-adjusted incidence rates per 100 000 using 19 age groups from the 2000 USA standard population.
bAmerican Indian/Alaskan Native, Asian/Pacific Islander.
cAge-adjusted annual average incidence rates, based on 2000 USA Census estimates for age distribution of USA and foreign-born subjects.
dData for ‘Unknown’ birth place excluded, denominators are either USA-born or foreign-born subjects.
eSensitivity analysis incorporating ‘Unknown’ birth place cases proportionately distributed within ‘USA-born’ and ‘Foreign-born’ categories.
fStandard errors for rate ratios not estimable due to aggregate denominator data.
*P ≤ 0.05.
The incidence rates of APL increased from 1995 to 2014 [average annual percentage change (AAPC) = 4.2; 95% CI = 2.4–6.0]; at a faster rate compared with non-APL AML (AAPC = 0.8; 95% CI = 0.2–1.4). Among foreign-born persons, the frequency of APL increased more per year (AAPC = 5.4; 95% CI = 2.8–8.0) than in USA-born persons (AAPC = 3.4; 95% CI = 1.8–5.1). Non-APL AML frequency also showed a greater increase in frequency among foreign-born persons (AAPC = 4.4; 95% CI = 3.3–5.6) than in USA-born persons (AAPC = 0.9; 95% CI = 0.3–1.5). The AAPC for APL incidence rate was 4.1 (95% CI = 2.7–5.5) among non-Hispanics but could not be estimated among Hispanics due to small number of cases. Annual APC for non-APL AML was not significantly different between Hispanic (1.6; 95% CI = 0.4–2.7) and non-Hispanic persons (0.8; 95% CI = 0.2–1.4).
Clinical cohort
The clinical cohort of 390 AML patients at Mount Sinai (APL = 31; non-APL = 343) had a mean age of 60 years and consisted of 52% males, 53% whites, 15% blacks and 32% other races, with roughly 20% of the sample of Hispanic ethnicity. The majority was married or partnered, reported not having used alcohol and tobacco, had de novo AML, and no history of solid tumor, hematologic disorder or prior cancer therapy (Table 3). Presence of genetic mutations, exposure signature and complex karyotype did not differ significantly between APL and non-APL patients, although the former had a non-significantly higher prevalence of FLT3-internal tandem duplications (ITD) compared with non-APL patients (39% versus 23%, P = 0.197; Table 3). APL patients were significantly more likely to be younger than non-APL patients (52% versus 24% below age 50 years, P = 0.006); had significantly lower prevalence of disease secondary to myelodysplastic syndrome/myeloproliferative neoplasm/therapy (10% versus 41%, P = 0.005) and of a history of prior hematologic disorder (10% versus 36%, P = 0.006) compared with patients without APL.
Table 3.
AML patient characteristics (clinical cohort)
| APLa | ||||
|---|---|---|---|---|
| AML (N = 390) | Yes (n1 = 31) | No (n2 = 343) | ||
| Characteristics | N (%) | N (%) | N (%) | P valueb |
| Demographics | ||||
| Age category, years (quartiles) | *0.006 | |||
| <50 | 95 (26.3) | 14 (51.9) | 81 (24.3) | |
| 50–62 | 91 (25.2) | 6 (22.2) | 85 (25.5) | |
| 63–72 | 90 (24.9) | 6 (22.2) | 84 (25.2) | |
| ≥73 | 85 (23.5) | 1 (3.7) | 84 (25.2) | |
| Gender | 0.925 | |||
| Male | 201 (51.5) | 174 (50.7) | 16 (51.6) | |
| Female | 189 (48.5) | 169 (49.3) | 15 (48.4) | |
| Race | 0.056 | |||
| Black | 54 (14.9) | 8 (27.6) | 44 (13.8) | |
| White | 192 (52.9) | 16 (55.2) | 166 (52.0) | |
| Otherc | 117 (32.2) | 5 (17.2) | 109 (34.2) | |
| Ethnicity | 0.589 | |||
| Non-Hispanic | 286 (80.3) | 23 (76.7) | 252 (80.8) | |
| Hispanic | 70 (19.7) | 7 (23.3) | 60 (19.2) | |
| Marital status at diagnosis | 0.454 | |||
| Married/domestic partner | 178 (45.7) | 156 (45.5) | 15 (48.4) | |
| Single | 94 (24.1) | 81 (23.6) | 10 (32.3) | |
| Divorced/separated/widowed | 59 (15.1) | 51 (14.9) | 4 (12.9) | |
| Unknown | 59 (15.1) | 55 (16.0) | 2 (6.5) | |
| Risk factors | ||||
| Alcohol use | 0.504 | |||
| Current | 64 (16.4) | 54 (15.7) | 6 (19.4) | |
| Former | 9 (2.3) | 8 (2.3) | 1 (3.2) | |
| Never | 273 (70) | 244 (71.1) | 19 (61.3) | |
| Unknown | 44 (11.3) | 37 (10.8) | 5 (16.1) | |
| Tobacco use | 0.575 | |||
| Current | 17 (4.4) | 16 (4.7) | 1 (3.2) | |
| Former | 64 (16.4) | 57 (16.6) | 3 (9.7) | |
| Never | 268 (68.7) | 236 (68.8) | 22 (71.0) | |
| Unknown | 41 (10.5) | 34 (9.9) | 5 (16.1) | |
| Cancer information | ||||
| AML | *0.005 | |||
| De novo | 236 (60.5) | 27 (87.1) | 198 (57.7) | |
| Secondary to myelodysplastic syndrome/myeloproliferative neoplasm/therapy | 148 (38) | 3 (9.7) | 140 (40.8) | |
| Unknown | 6 (1.5) | 1 (3.2) | 5 (1.5) | |
| History of prior solid tumor | 0.578 | |||
| Yes | 62 (17.0) | 4 (13.3) | 58 (17.3) | |
| No | 303 (83.0) | 26 (86.7) | 277 (82.7) | |
| History of prior heme disorder | *0.006 | |||
| Yes | 120 (33.06) | 3 (10.3) | 120 (35.6) | |
| No | 243 (66.94) | 26 (89.7) | 217 (64.4) | |
| Treatment history | ||||
| Prior cancer therapy | 0.238 | |||
| Yes | 94 (25.7) | 5 (16.7) | 89 (26.5) | |
| No | 272 (74.3) | 25 (83.3) | 247 (73.5) | |
| Prior radiotherapyd | 0.653 | |||
| Yes | 31 (32.0) | 2 (40.0) | 28 (31.5) | |
| No | 66 (68.0) | 3 (60.0) | 61 (68.5) | |
| Prior chemotherapyd | 0.333 | |||
| Yes | 75 (77.3) | 2 (40.0) | 69 (77.5) | |
| No | 22 (22.7) | 3 (60.0) | 20 (22.5) | |
| Prior radio and chemotherapyd | 1.000 | |||
| Yes | 16 (16.5) | 1 (20.0) | 14 (15.7) | |
| No | 81 (83.5) | 4 (80.0) | 75 (84.3) | |
| By type of chemotherapeutic agentd | ||||
| Alkylating agent | 0.082 | |||
| Yes | 22 (22.7) | 3 (60.0) | 19 (21.4) | |
| No | 75 (77.3) | 2 (40.0) | 70 (78.6) | |
| Topoisomerase II | *0.008 | |||
| Yes | 10 (10.3) | 3 (60.0) | 7 (7.9) | |
| No | 87 (89.7) | 2 (40.0) | 82 (92.1) | |
| Antimetabolite | 1.000 | |||
| Yes | 16 (16.5) | 1 (20.0) | 14 (15.7) | |
| No | 81 (83.5) | 4 (80.0) | 75 (84.3) | |
| Antitubulin | 0.471 | |||
| Yes | 11 (11.3) | 1 (20.0) | 10 (11.2) | |
| No | 86 (88.7) | 4 (80.0) | 79 (88.8) | |
| Other agent | 0.361 | |||
| Yes | 49 (50.5) | 1 (20.0) | 46 (51.7) | |
| No | 48 (49.5) | 4 (80.0) | 43 (48.3) | |
| Cytogeneticse | ||||
| Abnormal karyotype | N/Af | |||
| Yes | 170 (66.4) | 15 (100.0) | 152 (65.0) | |
| No | 86 (33.6) | 0 (0.0) | 82 (35.0) | |
| Cytogenetic risk (ELN category)g | — | |||
| Unfavorable | 101 (79.5) | N/A | 100 (84.0) | |
| Favorable | 26 (20.5) | N/A | 19 (16.0) | |
| Exposure signature | 0.178 | |||
| Yes | 46 (54.1) | 1 (20.0) | 44 (55.7) | |
| No | 39 (45.9) | 4 (80.0) | 35 (44.3) | |
| Complex karyotype | 0.093 | |||
| Yes | 38 (33.9) | 0 (0) | 37 (35.6) | |
| No | 74 (66.1) | 7 (100) | 67 (64.4) | |
| Genetic mutational profileh | ||||
| At least one genetic mutation | 0.385 | |||
| Yes | 122 (59.5) | 6 (46.2) | 111 (60.3) | |
| No | 83 (40.5) | 7 (53.8) | 73 (39.7) | |
| At least one deleterious mutation | 0.564 | |||
| Yes | 77 (37.6) | 6 (46.2) | 69 (37.5) | |
| No | 128 (62.4) | 7 (53.8) | 115 (62.5) | |
| Specific deleterious genetic mutations | ||||
| ASXL1 | 1.000 | |||
| Yes | 5 (2.5) | 0 (0.0) | 5 (2.7) | |
| No | 200 (97.5) | 13 (100) | 179 (97.3) | |
| FLT3 | 0.197 | |||
| Yes | 48 (23.4) | 5 (38.5) | 42 (22.8) | |
| No | 157 (76.6) | 8 (61.5) | 142 (77.2) | |
| DNMT3A | 1.000 | |||
| Yes | 17 (8.3) | 1 (7.7) | 15 (8.2) | |
| No | 188 (91.7) | 12 (92.3) | 169 (91.8) | |
| RUNX1 | 1.000 | |||
| Yes | 10 (4.9) | 0 (0.0) | 10 (5.4) | |
| No | 195 (95.1) | 13 (100) | 174 (94.6) | |
| TET2 | 0.604 | |||
| Yes | 15 (7.3) | 0 (0.0) | 15 (8.2) | |
| No | 190 (92.7) | 13 (100) | 169 (91.8) | |
| TP53 | 1.000 | |||
| Yes | 7 (3.4) | 0 (0.0) | 7 (3.8) | |
| No | 198 (96.6) | 13 (100) | 177 (96.2) | |
| PHF6 | 1.000 | |||
| Yes | 1 (0.5) | 0 (0.0) | 1 (0.5) | |
| No | 204 (99.5) | 13 (100) | 183 (99.5) | |
aHistological type information available for 374 patients.
b P value comparing characteristics between patients with and without APL.
cIncludes Asian, Pacific Islander, American Indian and other races.
dCalculated for patients who had history of any prior cancer therapy.
eCytogenetic information available for 256 patients.
fNot applicable because APL patients have an abnormal karyotype by definition.
gELN cytogenetic risk categories are not used for APL, hence marked N/A (not applicable).
hMutational profile information available for 205 patients.
*P ≤ 0.01.
Survival
In non-APL AML patients, the risk of death was significantly higher for patients who had received prior chemotherapy with antitubulins [hazard ratio (HR) = 2.57; 95% CI = 1.26–5.26] compared with those who had not (Table 4). Other significant factors included age ≥63 years (median age; HR = 1.85; 95% CI = 1.40–2.44), secondary AML (HR = 1.96; 95% CI = 1.49–2.58), unfavorable cytogenetic ELN risk category (HR = 2.93; 95% CI = 1.27–6.79), complex karyotype (HR = 2.56; 95% CI = 1.55–4.22) and history of any prior cancer therapy (HR = 2.00; 95% CI = 1.49–2.67). In an adjusted model, risk of death remained significantly higher among non-APL AML patients with prior chemotherapy with antitubulins [adjusted hazard ratio (AHR) = 3.30; 95% CI=1.49–7.32], those aged ≥63 years and older (AHR = 1.80; 95% CI = 1.34–2.41), patients with unfavorable cytogenetic ELN risk category (AHR = 4.10; 95% CI = 1.46–11.55) and complex karyotype (AHR = 2.33; 95% CI = 1.29–4.21). Among APL patients, those with secondary APL had significantly higher risk of death compared with those with de novo APL (HR = 17.32; 95% CI = 1.56–192.1) in unadjusted Cox regression (Table 4). Prior chemotherapy with antitubulins doubled the risk of death among APL patients, although this was not statistically significant. Other non-significant risk factors for death among APL patients included older age, non-white race and male.
Table 4.
Factors associated with risk of death among AML patients (clinical cohort)
| APL patients (n = 27) | non-APL AML patients (n = 331) | |||||||
|---|---|---|---|---|---|---|---|---|
| Hazard ratio (95% confidence interval)b | Hazard ratio (95% confidence interval)b | |||||||
| Characteristic | N (%)a | Median survival (days) | Unadjusted | Adjustedc | N (%)a | Median survival (days) | Unadjusted | Adjustedc |
| Age category (years) | ||||||||
| ≥63 | 7 (25.9) | 1588 | 5.60 (0.51–61.78) | 1.62 (0.14–18.31) | 167 (50.5) | 138 | *1.85 (1.40–2.44) | *1.80 (1.34–2.41) |
| <63 | 20 (74.1) | 808 | 1.0 (ref) | 1.0 (ref) | 164 (49.5) | 325 | 1.0 (ref) | 1.0 (ref) |
| Gender | ||||||||
| Male | 13 (48.2) | 766 | NE | NE | 169 (51.1) | 205 | 1.31 (0.99–1.71) | 1.26 (0.94–1.68) |
| Female | 14 (51.9) | 965 | 1.0 (ref) | 1.0 (ref) | 162 (48.9) | 270 | 1.0 (ref) | 1.0 (ref) |
| Race | ||||||||
| Non-white | 11 (44.0) | 1019 | 2.51 (0.23–27.72) | 2.56 (0.15–43.29) | 149 (48.2) | 249 | 0.84 (0.63–1.12) | 0.92 (0.68–1.24) |
| White | 14 (56.0) | 740 | 1.0 (ref) | 1.0 (ref) | 160 (51.8) | 243 | 1.0 (ref) | 1.0 (ref) |
| Ethnicity | ||||||||
| Hispanic | 6 (23.1) | 752 | NE | NE | 58 (19.2) | 250 | 0.87 (0.60–1.27) | 0.96 (0.65–1.42) |
| Non-Hispanic | 20 (76.9) | 838 | 1.0 (ref) | 1.0 (ref) | 244 (80.8) | 252 | 1.0 (ref) | 1.0 (ref) |
| Type of AML | ||||||||
| Secondary | 3 (11.1) | 198 | *17.32 (1.56–192.1) | NE | 133 (40.8) | 138 | *1.96 (1.49–2.58) | 1.32 (0.91–1.92) |
| De novo | 24 (88.9) | 880 | 1.0 (ref) | 1.0 (ref) | 193 (59.2) | 311 | 1.0 (ref) | 1.0 (ref) |
| ELN category | ||||||||
| Unfavorable | N/A | N/A | N/A | N/A | 99 (83.9) | 122 | *2.93 (1.27–6.79) | *4.10 (1.46–11.55) |
| Favorable | N/A | N/A | N/A | N/A | 19 (16.1) | 461 | 1.0 (ref) | 1.0 (ref) |
| Complex karyotype | ||||||||
| Yes | 0 (0.0) | — | NE | NE | 37 (35.6) | 67 | *2.56 (1.55–4.22) | *2.33 (1.29–4.21) |
| No | 6 (100) | — | 1.0 (ref) | 1.0 (ref) | 67 (64.4) | 192 | 1.0 (ref) | 1.0 (ref) |
| Prior cancer therapy | ||||||||
| Yes | 4 (15.4) | 156 | NE | NE | 85 (26.2) | 137 | *2.00 (1.49–2.67) | 1.04 (0.71–1.53) |
| No | 22 (84.6) | 965 | 1.0 (ref) | 1.0 (ref) | 239 (73.8) | 290 | 1.0 (ref) | 1.0 (ref) |
| Prior radiotherapyc | ||||||||
| Yes | 1 (25.0) | 1990 | NE | NE | 27 (31.8) | 171 | 0.86 (0.50–1.47) | 0.67 (0.37–1.22) |
| No | 3 (75.0) | 114 | 1.0 (ref) | 1.0 (ref) | 58 (68.2) | 131 | 1.0 (ref) | 1.0 (ref) |
| Prior chemotherapyc | ||||||||
| Yes | 3 (75.0) | 198 | NE | NE | 67 (78.8) | 125 | 1.16 (0.63–2.14) | 1.44 (0.72–2.87) |
| No | 1 (25.0) | 11 | 1.0 (ref) | 1.0 (ref) | 18 (21.2) | 185 | 1.0 (ref) | 1.0 (ref) |
| Prior radio and chemotherapyc | ||||||||
| Yes | 1 (25.0) | 1990 | NE | NE | 14 (16.5) | 114 | 1.22 (0.63–2.34) | 1.12 (0.57–2.12) |
| No | 3 (75.0) | 114 | 1.0 (ref) | 1.0 (ref) | 71 (83.5) | 158 | 1.0 (ref) | 1.0 (ref) |
| Chemotherapeutic agentc | ||||||||
| Alkylating agent | ||||||||
| Yes | 3 (75.0) | 198 | NE | NE | 19 (22.4) | 119 | 0.84 (0.44–1.58) | 0.90 (0.47–1.72) |
| No | 1 (25.0) | 11 | 1.0 (ref) | 1.0 (ref) | 66 (77.6) | 145 | 1.0 (ref) | 1.0 (ref) |
| Topoisomerase II | ||||||||
| Yes | 3 (75.0) | 198 | NE | NE | 7 (8.2) | 304 | 0.46 (0.14–1.48) | 0.54 (0.16–1.77) |
| No | 1 (25.0) | 11 | 1.0 (ref) | 1.0 (ref) | 78 (91.8) | 133 | 1.0 (ref) | 1.0 (ref) |
| Antimetabolite | ||||||||
| Yes | 1 (25.0) | 198 | 0.88 (0.08–10.26) | 0.45 (0.02–10.74) | 14 (16.5) | 146 | 0.79 (0.37–1.66) | 0.91 (0.42–1.96) |
| No | 3 (75.0) | 114 | 1.0 (ref) | 1.0 (ref) | 71 (83.5) | 137 | 1.0 (ref) | 1.0 (ref) |
| Antitubulin | ||||||||
| Yes | 1 (25.0) | 114 | 2.45 (0.15–39.72) | 2.21 (0.09–52.23) | 10 (11.8) | 24 | *2.57 (1.26–5.26) | *3.30 (1.49–7.32) |
| No | 3 (75.0) | 198 | 1.0 (ref) | 1.0 (ref) | 75 (88.2) | 166 | 1.0 (ref) | 1.0 (ref) |
| Other agent | ||||||||
| Yes | 1 (25.0) | 114 | 2.45 (0.15–39.72) | 2.21 (0.09–52.23) | 44 (51.8) | 139 | 1.23 (0.74–2.04) | 1.26 (0.74–2.17) |
| No | 3 (75.0) | 198 | 1.0 (ref) | 1.0 (ref) | 41 (48.2) | 137 | 1.0 (ref) | 1.0 (ref) |
| Specific genetic mutations | ||||||||
| ASXL1 | ||||||||
| Yes | 0 (0.0) | NE | NE | 5 (2.8) | 232 | 1.69 (0.69–4.11) | 1.73 (0.71–4.25) | |
| No | 11 (100) | 1.0 (ref) | 1.0 (ref) | 175 (97.2) | 243 | 1.0 (ref) | 1.0 (ref) | |
| FLT3 | ||||||||
| Yes | 5 (45.5) | 169 | *11.7 (1.03–134.5) | NE | 42 (23.3) | 317 | 0.77 (0.50–1.20) | 1.03 (0.65–1.61) |
| No | 6 (54.5) | 1041 | 1.0 (ref) | 1.0 (ref) | 138 (76.7) | 223 | 1.0 (ref) | 1.0 (ref) |
| DNMT3A | ||||||||
| Yes | 0 (0.0) | — | NE | NE | 15 (8.3) | 273 | 0.91 (0.43–1.94) | 1.17 (0.55–2.52) |
| No | 11 (100) | — | 1.0 (ref) | 1.0 (ref) | 165 (91.7) | 240 | 1.0 (ref) | 1.0 (ref) |
| RUNX1 | ||||||||
| Yes | 0 (0.0) | — | NE | NE | 10 (5.6) | 208 | 1.16 (0.42–3.18) | 1.51 (0.54–4.19) |
| No | 11 (100) | — | 1.0 (ref) | 1.0 (ref) | 170 (94.4) | 312 | 1.0 (ref) | 1.0 (ref) |
| TET2 | ||||||||
| Yes | 0 (0.0) | — | NE | NE | 15 (8.3) | 194 | 1.05 (0.52–2.14) | 1.09 (0.54–2.23) |
| No | 11 (100) | — | 1.0 (ref) | 1.0 (ref) | 165 (91.7) | 243 | 1.0 (ref) | 1.0 (ref)f |
| TP53 | ||||||||
| Yes | 0 (0.0) | — | NE | NE | 7 (3.9) | 196 | 1.65 (0.60–4.50) | 1.27 (0.46–3.54) |
| No | 11 (100) | — | 1.0 (ref) | 1.0 (ref) | 173 (96.11) | 311 | 1.0 (ref) | 1.0 (ref) |
| PHF6 | ||||||||
| Yes | 0 (0.0) | — | NE | NE | 1 (0.6) | 367 | 1.67 (0.23–12.05) | 1.24 (0.16–9.41) |
| No | 11 (100) | — | 1.0 (ref) | 1.0 (ref) | 179 (99.4) | 309 | 1.0 (ref) | 1.0 (ref) |
N/A: Not applicable since ELN category is not defined for APL patients.
NE: Not estimable due to small numbers in strata and/or too few or no events (deaths).
aNumber of patients for whom survival data are available.
bCox proportional hazards regression model.
cModel adjusted for age category, gender, ethnicity and prior cancer therapy. For specific types of cancer therapy, model adjusted for age, gender and ethnicity.
*P ≤ 0.05.
Discussion
We observed some key differences between AML patients with and without APL in the New York State population data. First, incidence rates of APL were significantly higher in Hispanics, but rates of non-APL AML were significantly lower compared with non-Hispanics. A population-based study (N = 709) found no difference in lifetime incidence rates of APL in Hispanics versus non-Hispanic whites; however, the study was limited to patients aged ≤44 (22). We report that when the effect of age is removed through statistical adjustment, Hispanic ethnicity is independently associated with APL rates and not with non-APL AML rates. We also observed that 75% non-APL AML cases were USA-born compared with only half of APL cases. Among Hispanics with APL, 20% were USA-born and 47% were foreign-born. Assuming that cases with ‘unknown’ country of birth had similar distribution of ‘USA-born’ and ‘Foreign-born’ as the population, the age-adjusted incidence rate of APL was 20% higher among foreign-born than USA-born persons. This finding has not been reported previously in this population. An estimated 24% USA immigrants do not possess legal documentation, making them less likely to report country of birth (23). Therefore, the true APL incidence rate ratio in foreign-born persons may be even higher than this study’s estimate. In addition, the greater increase in frequency of both AML types among foreign-born compared with USA-born persons suggests a possible role of country of origin in observed incidence trends. The effect of nativity on rates of different AML types has been studied previously, but this was limited to USA Hispanics (24). The proportion of AML cases that are APL is consistently higher in Latin America (Brazil: 28.2%; Mexico: 20%; Venezuela: 27.8%; Peru: 22%) compared with 10% in Northern European countries (UK, Scandinavia) (25). AML risk in foreign-born immigrants from non-Latino countries should be assessed, as this may be a proxy for early exposure to specific carcinogens.
Several known risk factors were associated with worse survival in our study, including older age, disease secondary to myelodysplastic syndrome, myeloproliferative neoplasm and therapy and prior cancer therapy. Older patients have a higher prevalence of several adverse prognostic factors, including poorer performance status, lower white blood cell counts and percentage of bone marrow blasts, higher multidrug resistance and less favorable cytogenetics (26). In this cohort, non-APL AML patients with unfavorable cytogenetics had a significantly higher risk of death than those with favorable cytogenetics, as did those with complex compared with non-complex karyotype. Higher prevalence of unfavorable cytogenetics and complex karyotype may explain poorer survival in secondary versus de novo AML patients (27). Unfavorable karyotype is the most important prognostic indicator for poor survival in AML, particularly in combination with complex karyotype (28).
Prior treatment for antecedent disease has been found to decrease response to treatment for subsequent AML, particularly in combination with adverse cytogenetics (29). Different chemotherapeutic agents can have varying effects on survival, e.g. the breakpoints induced by topoisomerase II inhibitors in PML and RARA genes can differ by the type of agent used (e.g. mitoxantrone, epirubicin and etoposide), potentially influencing prognosis (30). In this study, risk of death was increased among AML patients who had received prior chemotherapy with antitubulin agents (vincristine, vinblastine, vindesine, paclitaxel and docetaxel). Per standard clinical practice, antitubulin agents were mostly used in combination with other chemotherapeutic agents, although none affected survival to the same extent (31). Specific side effects of commonly used antitubulin agents may adversely affect survival in secondary AML. For example, vincristine has been associated with neurotoxicity, and paclitaxel with myelosuppression (32–34). Also, indications for vincristine use in adults include several cancers with inherently poor survival, including multiple myeloma, brain tumors and non-small cell lung cancer (32). To our knowledge, this association has not been reported previously.
Among patients with available genetic information, there was no difference in the prevalence of overall and deleterious mutations by APL status; although interestingly, 100% of all genetic mutations among APL patients were deleterious (6/6), compared with 62% (69/111) among non-APL patients. Among APL patients (n = 31), very few genetic mutations other than the pathogenic PML-RARA were detected; including FLT3-ITD (n = 5) and PHF6 (n = 1). FLT3-ITD was significantly more prevalent among APL compared with non-APL patients, consistent with three other studies that reported prevalences between 35% and 37% among APL patients (35–37). The prevalence of FLT3-ITD among all AML patients has been found to range from 16% to 25%, and our results fall within this range (37–39). FLT3-ITD has been established as a poor prognostic factor among all AML patients (40,41). In our cohort, the presence of FLT3-ITD significantly increased the risk of death and reduced overall survival time among APL but not non-APL patients. Almost half of the non-APL patients with FLT3-ITD also had a favorable mutation (NPM1), which may have mitigated the adverse effect of FLT3-ITD on survival (42). Among APL patients, however, none of the patients with FLT3-ITD had NPM1 mutations. In general, APL has a favorable prognosis following appropriate treatment (43). The effect of FLT3-ITD on APL survival has been mixed (43,44). APL patients with FLT3-ITD were found to have a higher relapse rate and poorer post-relapse survival than those with wild-type FLT3 (35,43). This mutation may represent an overall genetic instability, leading to accumulation of additional poor-prognosis mutations (35). Presence of FLT3-ITD has also been associated with leukocytosis, which constitutes a high-risk AML patient group (41,45,46). A pivotal phase 3 study confirmed that adding an FLT3 kinase inhibitor, midostaurin, to the chemotherapy regimen can improve survival duration in those with FLT3-ITD AML (47).
Our study has some notable limitations. The New York State Cancer Registry has country of birth information for cases only, therefore age-adjusted incidence rates for USA-born and foreign-born patients were manually calculated using a different standard population than those for other demographics; however, this calculation is expected to be reliable as it is also Census based. The variables for race and ethnicity are not mutually exclusive; and cytogenetic and survival data were not available from the NYSCR. Hospital data on cytogenetics and mutational profile were limited to the information available in the electronic medical records, in addition to missing information for some patients referred from other hospitals. Because of the limited number of mutations and survival data, certain associations could have been missed.
However, this is the first study to our knowledge to examine and compare data for APL and non-APL patients at both the population and patient-level. This study is novel in the comprehensiveness of risk factors assessed and is strengthened by the inclusion of a diverse population from New York, leading to adequate representation of racial and ethnic minorities, including both native and immigrant populations. It identified epidemiological as well as clinical factors that increase risk of disease and death among APL and non-APL AML patients. It is one of the first to report on incidence rates and trends by nativity. This study adds to the literature on the differences in cytogenetic and mutational profile of APL and non-APL AML patients and confirms the reliability of ethnicity, older age, secondary disease, and complex and unfavorable cytogenetics as predictors of AML incidence and mortality. It highlights the importance of FLT3-ITD as a prognostic factor in APL and proposes consideration of prior antitubulin therapy as a prognostic factor in non-APL AML and possibly in APL. Monitoring multiple risk factors can help develop effective, targeted treatment plans for high-risk subgroups of AML patients. Future studies should evaluate the long-term prognostic effect of prior cancer therapy and genetic mutations on a larger patient cohort, including high-risk subgroups. Population-based cancer registries should endeavor to collect complete data on country of origin. The effect of specific antitubulin agents on risk of subsequent cancers and overall and disease-free survival should be explored.
Funding
National Cancer Institute, National Institutes of Health (P30 CA196521).
Acknowledgments
G.R.K. and D.T. contributed equally to this article and are joint first authors. E.T. and J.M. conceptualized the study; G.R.K., D.T., V.N., A.C., J.C., G.L. and S.B. were responsible for data collection and curation; G.R.K. performed the statistical analyses; G.R.K. and D.T. wrote the original draft with critical input from E.T. and J. M. All authors reviewed the manuscript for important intellectual content and approved the final version.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- AAPC
average annual percentage change
- AML
acute myeloid leukemia
- APL
acute promyelocytic leukemia
- CI
confidence interval
- ELN
European Leukemia Net
- HR
hazard ratio
- ITD
internal tandem duplications
- NYSCR
New York State Cancer Registry
References
- 1. Short N.J., et al. (2018) Acute myeloid leukaemia. Lancet, 392, 593–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Cancer Society. (2018) Cancer Facts & Figures 2018.American Cancer Society, Atlanta, GA. [Google Scholar]
- 3. Tremblay D., et al. (2018) Implications of mutation profiling in myeloid malignancies-PART 1: myelodysplastic syndromes and acute myeloid leukemia. Oncology (Williston Park)., 32, e38–e44. [PubMed] [Google Scholar]
- 4. Deschler B., et al. (2006) Acute myeloid leukemia: epidemiology and etiology. Cancer, 107, 2099–2107. [DOI] [PubMed] [Google Scholar]
- 5. Ferrant A., et al. (1997) Karyotype in acute myeloblastic leukemia: prognostic significance for bone marrow transplantation in first remission: a European Group for Blood and Marrow Transplantation study. Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT). Blood, 90, 2931–2938. [PubMed] [Google Scholar]
- 6. Zelent A., et al. (2001) Translocations of the RARalpha gene in acute promyelocytic leukemia. Oncogene, 20, 7186–7203. [DOI] [PubMed] [Google Scholar]
- 7. Murthy G.S.G., et al. (2017) Recent trends in the incidence and outcomes of acute promyelocytic leukemia in United States. Blood, 130, 2578.29242205 [Google Scholar]
- 8. Chen Y., et al. (2012) Acute promyelocytic leukemia: a population-based study on incidence and survival in the United States, 1975-2008. Cancer, 118, 5811–5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coombs C.C., et al. (2015) Acute promyelocytic leukemia: where did we start, where are we now, and the future. Blood Cancer J., 5, e304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Breccia M., et al. (2010) Early hemorrhagic death before starting therapy in acute promyelocytic leukemia: association with high WBC count, late diagnosis and delayed treatment initiation. Haematologica, 95, 853–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Douer D., et al. (1996) High frequency of acute promyelocytic leukemia among Latinos with acute myeloid leukemia. Blood, 87, 308–313. [PubMed] [Google Scholar]
- 12. Douer D., et al. (2003) Acute promyelocytic leukaemia in patients originating in Latin America is associated with an increased frequency of the bcr1 subtype of the PML/RARalpha fusion gene. Br. J. Haematol., 122, 563–570. [DOI] [PubMed] [Google Scholar]
- 13. Estey E.H. (2014) Acute myeloid leukemia: 2014 update on risk-stratification and management. Am. J. Hematol., 89, 1063–1081. [DOI] [PubMed] [Google Scholar]
- 14. Oran B., et al. (2012) Survival for older patients with acute myeloid leukemia: a population-based study. Haematologica, 97, 1916–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Puumala S.E., et al. (2013) Epidemiology of childhood acute myeloid leukemia. Pediatr. Blood Cancer, 60, 728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Song X., et al. (2018) Incidence, survival, and risk factors for adults with acute myeloid leukemia not otherwise specified and acute myeloid leukemia with recurrent genetic abnormalities: analysis of the Surveillance, Epidemiology, and End Results (SEER) database, 2001-2013. Acta Haematol., 139, 115–127. [DOI] [PubMed] [Google Scholar]
- 17. New York State Cancer Registry. Cancer Incidence and Mortality in New York State, 1976–2015 http://www.health.ny.gov/statistics/cancer/registry/ (1 August 2018, date last accessed).
- 18. International Standing Committee on Human Cytogenomic Nomenclature (2016) ISCN: An International System for Human Cytogenomic Nomenclature (2016). Karger, Basel, Switzerland. [Google Scholar]
- 19. Döhner H., et al. ; European LeukemiaNet (2010) Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood, 115, 453–474. [DOI] [PubMed] [Google Scholar]
- 20. Mrózek K. (2008) Cytogenetic, molecular genetic, and clinical characteristics of acute myeloid leukemia with a complex karyotype. Semin. Oncol., 35, 365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Winters A.C., et al. (2017) MLL-rearranged leukemias—an update on science and clinical approaches. Front. Pediatr., 5, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Matasar M.J., et al. (2006) Incidence rates of acute promyelocytic leukemia among Hispanics, blacks, Asians, and non-Hispanic whites in the United States. Eur. J. Cancer Prev., 15, 367–370. [DOI] [PubMed] [Google Scholar]
- 23. Lopez G., et al. (2018) Key Findings about US immigrants. https://pewrsr.ch/2rdqfXK (1 August 2018, date last accessed). [Google Scholar]
- 24. Pollyea D.A., et al. (2014) Acute leukemia in adult Hispanic Americans: differences in incidence rates by nativity. JCPCR, 1. [Google Scholar]
- 25. Rego E.M., et al. (2011) Epidemiology and treatment of acute promyelocytic leukemia in latin america. Mediterr. J. Hematol. Infect. Dis., 3, e2011049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Appelbaum F.R., et al. (2006) Age and acute myeloid leukemia. Blood, 107, 3481–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Renaud L., et al. (2016) De novo and secondary acute myeloid leukemia, real world data on outcomes from the French Nord-Pas-De-Calais Picardie acute myeloid leukemia observatory. Blood, 128, 4013.27827828 [Google Scholar]
- 28. Orozco J.J., et al. (2012) Unfavorable, complex, and monosomal karyotypes: the most challenging forms of acute myeloid leukemia. Oncology (Williston Park)., 26, 706–712. [PubMed] [Google Scholar]
- 29. Boddu P., et al. (2017) Treated secondary acute myeloid leukemia: a distinct high-risk subset of AML with adverse prognosis. Blood Adv., 1, 1312–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mays A.N., et al. (2010) Evidence for direct involvement of epirubicin in the formation of chromosomal translocations in t(15;17) therapy-related acute promyelocytic leukemia. Blood, 115, 326–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sasaki K., et al. (2016) Outcome of patients with therapy-related acute myeloid leukemia with or without a history of myelodysplasia. Clin. Lymphoma. Myeloma Leuk., 16, 616–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gidding C.E., et al. (1999) Vincristine revisited. Crit. Rev. Oncol. Hematol., 29, 267–287. [DOI] [PubMed] [Google Scholar]
- 33. Quasthoff S., et al. (2002) Chemotherapy-induced peripheral neuropathy. J. Neurol., 249, 9–17. [DOI] [PubMed] [Google Scholar]
- 34. Scripture C.D., et al. (2006) Peripheral neuropathy induced by paclitaxel: recent insights and future perspectives. Curr. Neuropharmacol., 4, 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Callens C., et al. ; European APL Group (2005) Prognostic implication of FLT3 and Ras gene mutations in patients with acute promyelocytic leukemia (APL): a retrospective study from the European APL Group. Leukemia, 19, 1153–1160. [DOI] [PubMed] [Google Scholar]
- 36. Gaur G.C., et al. (2012) Analysis of mutational status, SNP rs16754, and expression levels of Wilms tumor 1 (WT1) gene in acute promyelocytic leukemia. Ann. Hematol., 91, 1855–1860. [DOI] [PubMed] [Google Scholar]
- 37. Levis M. (2013) FLT3 mutations in acute myeloid leukemia: what is the best approach in 2013? Hematology. Am. Soc. Hematol. Educ. Program, 2013, 220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Burnatt G., et al. (2017) Analysis of the presence of FLT3 gene mutation and association with prognostic factors in adult and pediatric acute leukemia patients. Braz. J. Pharm. Sci., 53, e16105. [Google Scholar]
- 39. Cuervo-Sierra J., et al. (2016) Prevalence and clinical significance of FLT3 mutation status in acute myeloid leukemia patients: a multicenter study. Arch. Med. Res., 47, 172–179. [DOI] [PubMed] [Google Scholar]
- 40. Kottaridis P.D., et al. (2001) The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood, 98, 1752–1759. [DOI] [PubMed] [Google Scholar]
- 41. Fröhling S., et al. ; AML Study Group Ulm. Acute myeloid leukemia (2002) Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood, 100, 4372–4380. [DOI] [PubMed] [Google Scholar]
- 42. Liu Y., et al. (2014) Prognostic significance of NPM1 mutations in acute myeloid leukemia: a meta-analysis. Mol. Clin. Oncol., 2, 275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Singh H., et al. (2010) Clinical outcome of patients with acute promyelocytic leukemia and FLT3 mutations. Am. J. Hematol., 85, 956–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Breccia M., et al. (2013) FLT3-ITD confers poor prognosis in patients with acute promyelocytic leukemia treated with AIDA protocols: long-term follow-up analysis. Haematologica, 98, e161–e163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pastore F., et al. (2014) The role of therapeutic leukapheresis in hyperleukocytotic AML. PLoS One, 9, e95062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Röllig C., et al. (2015) How I treat hyperleukocytosis in acute myeloid leukemia. Blood, 125, 3246–3252. [DOI] [PubMed] [Google Scholar]
- 47. Stone R.M., et al. (2017) Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N. Engl. J. Med., 377, 454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]