Abstract
Background/Aims
Carbohydrate antigen 125 (CA-125) is an emerging prognostic biomarker for heart failure. We aimed to test the long-term prognostic value of CA-125 in combination with N-terminal pro-brain natriuretic peptide (NT-proBNP) in patients with acute decompensated heart failure (ADHF).
Methods
This observational study included a total of 413 patients (64.1 ± 15.6 yearold, 214 men) with ADHF. All-cause mortality during the 2-year follow-up was investigated for the prognosis.
Results
During the follow-up (mean follow-up, 591 ± 233 days), 109 deaths (26.0%) were recorded. In the multivariable analysis model, CA-125 was an independent factor associated with all-cause mortality (log CA-125: hazard ratio, 1.23; 95% confidence interval, 1.02 to 1.48; p = 0.030) together with age, sex, New York Heart Association class, β-blocker, and NT-proBNP. The Kaplan-Meier survival analysis demonstrated that the group with both low marker levels showed the best 2-year survival (87.9%) followed by the group with low NT-proBNP and high CA-125 (76.1%), high NT-proBNP and low CA-125 (64.7%) and high NT-proBNP and high CA-125 levels (54.3%) (p < 0.001). Addition of CA-125 in combination with NT-proBNP and established risk factors further increased the predictive power for mortality in patients with ADHF.
Conclusions
CA-125 was an independent factor associated with all-cause mortality in patients with ADHF. Combination of CA-125 with NT-proBNP significantly improved the prediction of mortality in patients with ADHF.
Keywords: CA-125, Natriuretic peptides, Heart failure, Mortality
INTRODUCTION
Over the years a growing interest about the prognostic biologic markers detectable in heart failure has become evident. Several biological parameters, such as B-type natriuretic peptides (BNPs) [1-3], tumor necrosis factor α [4], and interleukin-6 [5] have evolved to be useful biomarkers in heart failure. Recently some tumor markers have been introduced in patients with heart failure. Among them carbohydrate antigen 125 (CA-125) has been investigated widely [6].
Traditionally, CA-125 has been used as a tumor marker for ovarian cancer. CA-125 is known to be elevated not only in gynecological malignancy but also in various non-gynecological diseases including liver disease, infectious disease, lung cancer, ascites by any cause, and Hodgkin’s lymphoma [7-11]. Recently, CA-125 is an emerging prognostic biomarker in patients with heart failure. Previous studies reported that there is a significant relationship between the serum levels of CA-125 and the clinical severity or prognosis of chronic heart failure [12-15]. Some data also showed the prognostic implication of CA-125 in acute decompensated heart failure (ADHF) [16,17].
BNP, a neurohormone synthesized in response to volume and pressure overloads in the cardiac ventricle, is an established and potent biomarker in both acute and chronic heart failure [18]. BNP is now widely used in diagnosing, estimating disease severity, and predicting the prognosis of heart failure [2,3,19]. There is limited data regarding whether CA-125 still has its own prognostic value when added to N-terminal pro-brain natriuretic peptide (NT-proBNP) associated with long-term mortality of patients with ADHF. We aimed to test the serum levels of NT-proBNP combined with CA-125 may be more valuable factor than only detecting NT-proBNP in patients with ADHF for the prediction of all-cause mortality in patients with ADHF.
METHODS
Study population
Among the patients with diagnosis of ADHF following current guideline [20] hospitalized in Kyungpook National University Hospital between January 2005 and July 2013, 482 patients who had tests for NT-proBNP or CA-125 at admission were retrospectively included. Diagnosis of ADHF was defined as the rapid onset or worsening of symptoms and/or signs of heart failure such as pulmonary crackles, peripheral edema and cardiomegaly. The patients were excluded before inclusion of the study if they had prior acute myocardial infarction in the previous 3 months, end stage renal or liver disease, pregnancy, malignancy, and age < 18 years (Supplementary Fig. 1). The diagnosis of heart failure was established on the basis of the current guidelines, symptoms, radiographic and echocardiographic findings, and biomarkers.
Data on the demographic characteristics, laboratory tests, medications, and prognosis were collected from the medical charts. Initial echocardiographic data were also collected from echocardiography reports and images from the database. Venous blood sampling for biomarkers was obtained on admission. Patients followed up for a period of 24 months after admission. The primary endpoint was all-cause mortality. Survival or death status was confirmed by reviewing the death certificates, telephone interviews, and data from the national health insurance system. The study protocol was approved by the Institutional Review Board of Kyungpook National University Hospital (KNUH 2016-09-013). Informed consent was waived because of the retrospective nature of the study.
Statistics
As baseline characteristics, categorical variables were presented as number with percentage, and the differences were compared using the chi-square test. The distribution of continuous variables was presented as mean ± SD or median (interquartile range) and comparison between them was conducted using the independent t test. Normality of the continuous variables was tested using the Shapiro-Wilk test. For the normality of both CA-125 and NT-proBNP, log transformed values were used in all analyses. The multivariate Cox regression analysis was used to determine the significant factors related to the 2-year mortality. A receiver operating characteristic (ROC) curve was used to identify the optimal cutoff points of the CA-125 levels and NT-proBNP levels to determine the potential relationship between CA-125 and 2-year mortality. Sequentially, patients were divided into four groups according to the cutoff levels of NT-proBNP and CA-125. The Kaplan-Meier survival analysis was performed on the basis of those four category groups. Four Cox-proportional hazard models were built to test the incremental prognostic value of CA-125 in addition to established risk factors and NT-proBNP level. Harrell’s C-statistics and likelihood ratio test were performed to assess the discriminative ability of each model. The models served to estimate the integrated discrimination improvement (IDI), net reclassification improvement (NRI) with a category-free option, and global-chi-square test among the models following the methodology of Pencina et al. [21,22] for the incremental predictive value. All analyses were 2-tailed, p values < 0.05 were considered statistically significant.
RESULTS
Baseline characteristics
Among the patients hospitalized in Kyungpook National University Hospital between January 2005 and July 2013, a total of 413 patients (64.1 ± 15.6 year-old, 214 men) have both NT-proBNP and CA-125 tests on admission. The study population included 103 patients (24.9%) with left ventricular ejection fraction > 40%. During follow-up (mean follow-up, 591 ± 233 days), a total of 109 deaths (26.4%) were recorded. Patients with mortality were more frequently men (60.6% vs. 48.6%, p = 0.044), older (age, 72.1 ± 13.6 years vs. 61.3 ± 15.3 years, p < 0.001), and had a lower body mass index (BMI) (21.8 ± 2.9 kg/m2 vs. 23.4 ± 4.2 kg/m2, p < 0.001), more frequent ischemic etiology (53.2% vs. 28.0%, p < 0.001), hypertension (56.0% vs. 43.1%, p < 0.028), diabetes (45.0% vs. 24.7%, p < 0.001), and New York Heart Association (NYHA) class III–IV dyspnea (93.6% vs. 68.1%, p < 0.001). Non-ischemic etiology was listed in Supplementary Table 1. β-Blocker and angiotensin-converting enzyme inhibitor (ACEI)/angiotensin receptor blocker (ARB) prescribed during admission were less frequently used in patient who died during the 2-year follow-up (β-blocker: 73.6% vs. 83.9%, p < 0.028; ACEI/ARB: 66.0% vs. 85.5%, p < 0.001). The serum sodium level was lower (137.7 ± 4.5 mmol/L vs. 139.0 ± 7.1 mmol/L, p < 0.031), and serum creatinine level was higher (1.5 ± 0.9 mg/dL vs. 1.1 ± 0.8 mg/dL, p < 0.001) in patients with mortality. Mean CA-125 and NT-proBNP levels were 83.5 ± 111.0 U/mL and 8,269.2 ± 10,544.1 pg/mL, respectively (Supplementary Fig. 2). Serum CA-125 and NT-proBNP levels on admission were higher in the patients with mortality (log CA-125: 4.2 ± 1.1 U/mL vs. 3.6 ± 1.2 U/mL, p < 0.001; log NT-proBNP: 8.9 ± 1.3 pg/mL vs. 8.0 ± 1.4 pg/mL, p < 0.001). The baseline characteristics of the patients are presented in Table 1.
Table 1.
Baseline characteristics of the patients
| Characteristic | All patients (n = 413) | Survivors (n = 304) | Event (death) (n = 109) | p value |
|---|---|---|---|---|
| Male sex | 214 (51.8) | 148 (48.7) | 66 (60.6) | 0.044 |
| Age | 67.0 (55.0–76.0) | 64.0 (51.0–73.0) | 74.0 (66.0–80.0) | < 0.001 |
| BMI, kg/m2 | 22.5 (20.3–25.0) | 22.9 (20.6–25.5) | 21.5 (20.0–23.7) | < 0.001 |
| Ischemic etiology | 143 (34.6) | 85 (28.0) | 58 (53.2) | < 0.001 |
| NYHA class | < 0.001 | |||
| I–II | 104 (25.2) | 97 (31.9) | 7 (6.4) | |
| III–IV | 309 (74.8) | 207 (68.1) | 102 (93.6) | |
| SBP, mmHg | 132.0 (111–155) | 133.0 (112.0–156.0) | 127.5 (106.3–153.0) | 0.395 |
| HR, bpm | 95.0 (80.0–112.0) | 94.0 (80.0–111.0) | 101.0 (79.3–115.8) | 0.103 |
| Smoking | 166 (40.2) | 120 (39.5) | 46 (42.2) | 0.701 |
| HTN | 192 (46.5) | 131 (43.1) | 61 (56.0) | 0.028 |
| DM | 124 (30.0) | 75 (24.7) | 49 (45.0) | < 0.001 |
| β-Blocker | 333 (80.6) | 255 (83.9) | 78 (73.6) | 0.028 |
| ACEI/ARB | 330 (79.9) | 260 (85.5) | 70 (66.0) | < 0.001 |
| Spironolactone | 179 (43.3) | 140 (46.1) | 39 (36.8) | 0.123 |
| Digoxin | 79 (19.1) | 54 (17.8) | 25 (23.6) | 0.244 |
| Sodium, mmol/L | 139.0 (146.0–142.0) | 140.0 (137.0–142.0) | 138.0 (135.0–141.0) | 0.031 |
| Potassium, mmol/L | 4.20 (3.7–4.5) | 4.0 (3.7–4.5) | 4.2 (3.8–4.7) | 0.097 |
| Creatinine, mg/dL | 1.0 (0.8–1.4) | 0.95 (0.8–1.2) | 1.2 (0.9–1.9) | < 0.001 |
| LVEF, % | 26.0 (18.0–40.0) | 26.0 (18.0–41.0) | 26.0 (19.0–36.0) | 0.475 |
| NT-proBNP, pg/mL | 4,336.0 (1,663.5–10,775.5) | 3,528.0 (1,347–8,067.5) | 9,166.0 (3,087.0–18,465.0) | < 0.001 |
| CA-125, U/mL | 38.3 (15.8–112.0) | 29.6 (13.9–94.2) | 80.7 (27.1–139.3) | < 0.001 |
Values are presented as number (%) or median (interquartile range).
BMI, body mass index; NYHA, New York Heart Association; SBP, systolic blood pressure; HR, heart rate; HTN, hypertension; DM, diabetes mellitus; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-brain natriuretic peptide; CA-125, carbohydrate antigen 125.
Prognostic value of serum CA-125 in combination with NT-proBNP
The ROC curve analysis of CA-125 and NT-proBNP predicting the 2-year mortality is shown in Fig. 1. Areas under the curves were 0.635 and 0.666 for CA-125 and NT-proBNP, respectively. A baseline level of CA-125 > 54.5 U/mL could predict 2-year mortality with 58.6% sensitivity and 61.8% specificity; a baseline level of NT-proBNP > 5,269 pg/mL could the predict 2-year mortality with 58.6% sensitivity and 68.5% specificity (Fig. 2).
Figure 1.
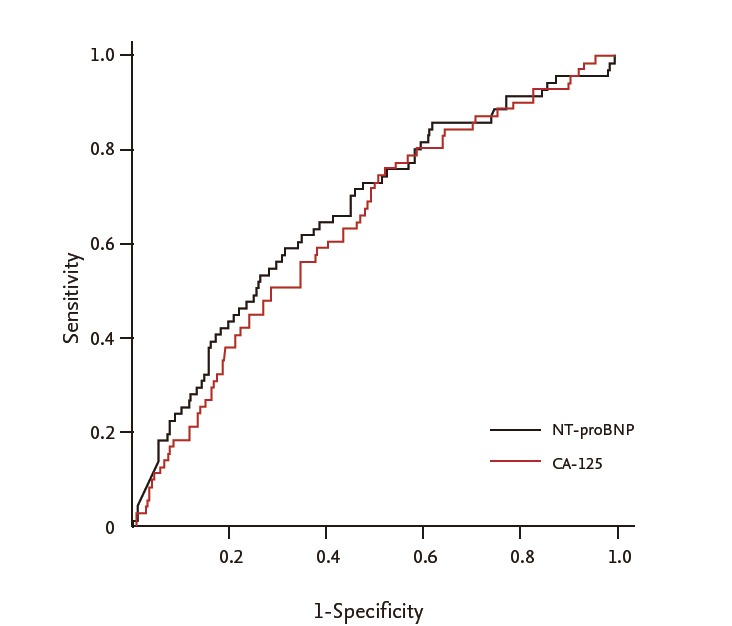
Receiver-operating curve analysis of N-terminal pro-brain natriuretic peptide (NT-proBNP) and carbohydrate antigen 125 (CA-125) in predicting the 2-year mortality. Areas under the curves of CA-125 and NT-proBNP in predicting the 2-year mortality were 0.635 and 0.666, respectively.
Figure 2.

Kaplan-Meier survival analysis of the groups according to the levels of carbohydrate antigen 125 (CA-125) and N-terminal pro-brain natriuretic peptide (NT-proBNP) (cutoff values of 54.5 U/mL and 5,269 pg/mL for CA-125 and NT-proBNP levels, respectively). The 2-year survival rate of the group with both low NT-proBNP and CA-125 levels was 87.9%, followed by 76.1% of the group with low NT-proBNP and high CA-125, 64.7% of the group with high NT-proBNP and low CA-125, and 54.3% of the group with both high NT-proBNP and CA-125 levels.
In the multivariate analyses using the Cox proportional hazard model for the 2-year mortality, both high CA-125 and NT-proBNP levels were independently associated with an increased 2-year mortality (CA-125: hazard ratio [HR], 1.230; 95% confidence interval [CI], 1.021 to 1.482; p = 0.030) (NT-proBNP: HR, 1.220; 95% CI, 1.006 to 1.480; p = 0.044) together with age, sex, NYHA class, and β-blocker use (Table 2).
Table 2.
Multivariate analyses using Cox proportional hazard model for 2-year mortality
| Variable | HR | 95% CI | p value |
|---|---|---|---|
| Age | 1.033 | 1.015–1.050 | < 0.001 |
| Male sex | 0.478 | 0.309–0.741 | 0.001 |
| BMI, kg/m2 | 0.962 | 0.898–1.031 | 0.275 |
| Ischemic etiology | 1.191 | 0.770–1.031 | 0.275 |
| NYHA class III–IV | 5.784 | 2.298–14.555 | < 0.001 |
| HTN | 1.304 | 0.818–2.078 | 0.265 |
| DM | 1.588 | 1.036–2.435 | 0.034 |
| β-Blocker | 0.526 | 0.327–0.845 | 0.008 |
| ACEI/ARB | 0.768 | 0.484–1.218 | 0.620 |
| Serum sodium, mmol/L | 1.000 | 0.964–1.038 | 0.998 |
| Serum creatinine, mg/dL | 1.096 | 0.895–1.341 | 0.376 |
| Ln NT-proBNP, pg/mL | 1.220 | 1.006–1.480 | 0.044 |
| Ln CA-125, U/mL | 1.230 | 1.021–1.482 | 0.030 |
HR, hazard ratio; CI, confidence interval; BMI, body mass index; NYHA, New York Heart Association; HTN, hypertension; DM, diabetes mellitus; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; Ln, natural logarithm; NT-proBNP, N-terminal pro-brain natriuretic peptide; CA-125, carbohydrate antigen 125.
We divided the study cohort into four groups according to the cutoff values of CA-125 and NT-proBNP described above: those with low NT-proBNP and low CA-125 (n = 173), low NT-proBNP and high CA-125 (n = 67), high NT-proBNP and low CA-125 (n = 68), and high NT-proBNP and high CA-125 (n = 105). The Kaplan-Meier survival analysis demonstrated that the group with both low marker levels showed best the 2-year survival (87.9%) followed by the group with low NT-proBNP and high CA-125 (76.1%), high NT-proBNP and low CA-125 (64.7%), and both high NT-proBNP and CA-125 levels (54.3%) (p < 0.001) (Fig. 2).
To investigate the incremental predictive value of CA-125 in addition to the established risk factors, we built four models: (1) baseline model including the established risk factors (age, sex, BMI, ischemic etiology, NYHA class, hypertension, diabetes, β-blocker use, ACEI/ARB use, and serum sodium and creatinine levels); (2) baseline model + NT-proBNP; (3) baseline model + CA-125; and (4) baseline model + NT-proBNP + CA-125. The global chi-square of model 1 was 130.2. The addition of serum NT-proBNP and/or CA-125 to the established risk factors increased the global chi-square and provided incremental information for predicting mortality; after adding the NT-proBNP, the global chi-square increased to 138.2 (p < 0.005); combination of CA-125 to the model comprising the established risk factors and NT-proBNP showed additional improvement of the prognostic power for the 2-year mortality (global chi-square from 1,382 to 142.9, p = 0.030) (Fig. 3).
Figure 3.
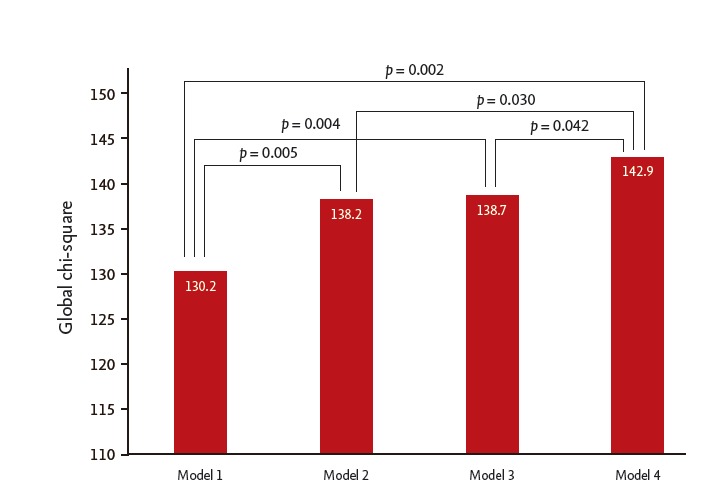
Comparison among the models including the established risk factors, carbohydrate antigen 125 (CA-125) and N-terminal pro-brain natriuretic peptide (NT-proBNP) in predicting the 2-year mortality. Model 1: established risk factors of age, sex, body mass index, ischemic etiology, New York Heart Association class, hypertension, diabetes, β-blocker use, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker use, inotropics use, serum sodium level, and serum creatinine level; Model 2: Model 1 + NT-proBNP; Model 3: Model 1 + CA-125; Model 4: Model 1 + NT-proBNP + CA-125.
Discrimination analysis of the four models described above is presented in Table 3. Addition of CA-125 alone to the baseline model yielded a category-free NRI (0.456, p < 0.001) and IDI (0.025, p = 0.004), improving the discriminative power of the baseline model; adding NT-proBNP alone to baseline model did not yield significant IDI (category-free NRI 0.262, p = 0.017; IDI 0.012, p = 0.061).
Table 3.
Discrimination analysis of the multivariate model in predicting 2-year mortality
| Models | Harrell’s C index | NRI | p value | IDI | p value |
|---|---|---|---|---|---|
| Model 1a | 0.804 | Reference | Reference | ||
| Model 2b | 0.805 | 0.262 | 0.017 | 0.012 | 0.061 |
| Model 3c | 0.818 | 0.453 | < 0.001 | 0.021 | 0.010 |
| Model 4d | 0.816 | 0.456 | < 0.001 | 0.025 | 0.004 |
NRI, net reclassification improvement; IDI, integrated discrimination improvement.
Model 1: established risk factors including age, sex, body mass index, ischemic etiology, New York Heart Association class, hypertension, diabetes, β-blocker use, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker use, serum sodium level, and serum creatinine level.
Model 2: Model 1 + N-terminal pro-brain natriuretic peptide (NT-proBNP).
Model 3: Model 1 + carbohydrate antigen 125 (CA-125).
Model 4: Model 1 + NT-proBNP + CA-125.
DISCUSSION
In the present study, both high serum NT-proBNP and CA-125 levels in the patients with ADHF were independent factors associated with the 2-year all-cause mortality. Our study divided the study cohort into four groups according to CA-125 and NT-proBNP: those with low NT-proBNP and low CA-125 showed best the 2-year survival and high NT-proBNP and high CA-125 showed worst result. We also found the prognostic value of these two biomarkers in addition to the established subgroups of patients with ADHF for death. Our result shows the advantage of CA-125 addition to well-established biomarker of NT-proBNP for a better risk stratification. Simultaneous measurement of NT-proBNP and CA-125 can help estimate the predictive ability for death more accurately than only detecting NT-proBNP in patients with ADHF.
CA-125 is a glycoprotein produced by the serous epithelium, and has been known as a tumor marker used for diagnosing ovarian cancer. Recently, the significance of CA-125 emerged in heart failure being associated with clinical status, hemodynamic abnormalities, and echocardiographic parameters. It has been broadly investigated in various types of heart failure such as valvular heart disease, atrial fibrillation, and heart failure with preserved ejection fraction [23-26]. Although the mechanism for the association between heart failure and elevated serum CA-125 level is obscure, CA-125 levels are closely related to serosal fluid accumulation and positively correlated with various inflammatory cytokines in patients with heart failure. It is hypothesized that mechanical stress, such as fluid overload, and inflammatory stimuli may initiate CA-125 synthesis in the mesothelial cells located on the surfaces of the pleura, pericardium, and peritoneum [27]. The serum level of CA-125 was higher in patients with advanced NYHA class and also associated with invasive or non-invasive findings, such as the right atrial pressure and pulmonary artery wedge pressure by cardiac catheterization or right ventricular systolic pressure by echocardiography. After medical therapy optimization in patients with chronic heart failure, CA-125 level decreased compared with the levels on the day of admission [12,13]. Additionally, CA-125 could be used as a prognostic factor in patients with acute and chronic heart failure [12-17]. CA-125 had a similar accuracy in predicting major adverse events or death alone compared with serum NT-proBNP, which is a traditional and potent prognostic biomarker of mortality and morbidity in patients with ADHF [28].
NT-proBNP is a well-established biomarker in various heart diseases as well as heart failure for its correlation with clinical status, various laboratory findings, echocardiographic parameters, and short-term and longterm prognosis [18,29-31]. NT-proBNP is a neurohormone secreted by the ventricles of the heart in response to volume overload or pressure overload, which has a different mechanism from the secretion of CA-125. Our data presented that there is a synergic improvement in predicting long-term prognosis of patients with ADHF when the biomarkers of the two different mechanism are combined. Simultaneous application of both biomarkers of CA-125 and NT-proBNP can help estimate the prognosis of patients with ADHF more accurately in the clinical practice.
Some limitations of the present study should be considered. First, this is a relatively small-sized retrospective study conducted in a single center. Results of the present study needs to be validated in larger trials. Second, although we included all patients available during the index period, there may be some selection bias in study population. For instance, the rate of ischemic etiology is quite low considering the real-world prevalence of ischemic heart failure. Third, outcome data are only limited to all-cause mortality. We could not include data on the cause of death, or re-hospitalization for ADHF.
In conclusion, we identified that CA-125 was an independent prognostic marker in patients with ADHF. Combination of CA-125 with NT-proBNP significantly improved the prediction of mortality in patients with AHDF, indicating that simultaneous application of both biomarkers can help estimate the accurate prognosis of those patients.
KEY MESSAGE
1. Carbohydrate antigen 125 (CA-125) is an emerging prognostic biomarker in patients with heart failure.
2. Simultaneous measurement of N-terminal probrain natriuretic peptide (NT-proBNP) and CA-125 can help estimate the predictive ability for death more accurately than only detecting NT-proBNP in patients with acute decompensated heart failure (ADHF).
3. The addition of serum NT-proBNP and CA-125 to the established risk factors provided incremental information for predicting mortality in patients with ADHF.
Footnotes
No potential conflict of interest relevant to this article was reported.
Supplementary Materials
Non-ischemic etiology of the study patients according to survivor status
Study design. NT-proBNP, N-terminal pro-brain natriuretic peptide; CA-125, carbohydrate antigen 125; ADHF, acute decompensated heart failure.
(A) Box plots showing N-terminal pro-brain natriuretic peptide (NT-proBNP) levels (median; interquartile range [IQR], pg/mL) according to survivor status. (B) Box plots showing carbohydrate antigen 125 (CA-125) levels (median; IQR, U/mL) according to survivor status.
REFERENCES
- 1.Whitcomb BW, Schisterman EF, Klebanoff MA, Baumgarten M, Luo X, Chegini N. Circulating levels of cytokines during pregnancy: thrombopoietin is elevated in miscarriage. Fertil Steril. 2008;89:1795–1802. doi: 10.1016/j.fertnstert.2007.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161–167. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 3.Anand IS, Fisher LD, Chiang YT, et al. Changes in brain natriuretic peptide and norepinephrine over time and mortality and morbidity in the Valsartan Heart Failure Trial (Val-HeFT) Circulation. 2003;107:1278–1283. doi: 10.1161/01.cir.0000054164.99881.00. [DOI] [PubMed] [Google Scholar]
- 4.Baumgarten G, Knuefermann P, Mann DL. Cytokines as emerging targets in the treatment of heart failure. Trends Cardiovasc Med. 2000;10:216–223. doi: 10.1016/s1050-1738(00)00063-3. [DOI] [PubMed] [Google Scholar]
- 5.Tsutamoto T, Hisanaga T, Wada A, et al. Interleukin-6 spillover in the peripheral circulation increases with the severity of heart failure, and the high plasma level of interleukin-6 is an important prognostic predictor in patients with congestive heart failure. J Am Coll Cardiol. 1998;31:391–398. doi: 10.1016/s0735-1097(97)00494-4. [DOI] [PubMed] [Google Scholar]
- 6.Vizzardi E, D'Aloia A, Curnis A, Dei Cas L. Carbohydrate antigen 125: a new biomarker in heart failure. Cardiol Rev. 2013;21:23–26. doi: 10.1097/CRD.0b013e318265f58f. [DOI] [PubMed] [Google Scholar]
- 7.Crespo Valades E, Malmierca Corral M. Elevated CA 125 in chronic liver disease with ascites. Gastroenterol Hepatol. 2004;27:558. doi: 10.1016/s0210-5705(03)70525-1. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez J, De Quiros B, Telenti M, et al. CA125 serum levels in tubercolosis patients. Int J Biol Markers. 1995;10:180–181. doi: 10.1177/172460089501000309. [DOI] [PubMed] [Google Scholar]
- 9.Lindgren J, Kuusela P, Hellstrom PE, Pettersson T, Klockars M. The ovarian cancer associated antigen CA 125 in patients with pleural effusions. Eur J Cancer Clin Oncol. 1988;24:737–739. doi: 10.1016/0277-5379(88)90308-2. [DOI] [PubMed] [Google Scholar]
- 10.Zuckerman E, Lanir A, Sabo E, et al. Cancer antigen 125: a sensitive marker of ascites in patients with liver cirrhosis. Am J Gastroenterol. 1999;94:1613–1618. doi: 10.1111/j.1572-0241.1999.01152.x. [DOI] [PubMed] [Google Scholar]
- 11.Mezger J, Wilmanns W, Lamerz R. Elevated serum CA 125 levels in patients with benign ascitic or pleural effusions. Tumour Biol. 1988;9:47–52. doi: 10.1159/000217544. [DOI] [PubMed] [Google Scholar]
- 12.Kouris NT, Zacharos ID, Kontogianni DD, et al. The significance of CA125 levels in patients with chronic congestive heart failure. Correlation with clinical and echocardiographic parameters. Eur J Heart Fail. 2005;7:199–203. doi: 10.1016/j.ejheart.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 13.D'Aloia A, Faggiano P, Aurigemma G, et al. Serum levels of carbohydrate antigen 125 in patients with chronic heart failure: relation to clinical severity, hemodynamic and Doppler echocardiographic abnormalities, and shortterm prognosis. J Am Coll Cardiol. 2003;41:1805–1811. doi: 10.1016/s0735-1097(03)00311-5. [DOI] [PubMed] [Google Scholar]
- 14.Varol E, Ozaydin M, Dogan A, Kosar F. Tumour marker levels in patients with chronic heart failure. Eur J Heart Fail. 2005;7:840–843. doi: 10.1016/j.ejheart.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Yilmaz MB, Zorlu A, Tandogan I. Plasma CA-125 level is related to both sides of the heart: a retrospective analysis. Int J Cardiol. 2011;149:80–82. doi: 10.1016/j.ijcard.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Kouris NT, Kontogianni DD, Papoulia EP, et al. Clinical and prognostic value of elevated CA125 levels in patients with congestive heart failure. Hellenic J Cardiol. 2006;47:269–274. [PubMed] [Google Scholar]
- 17.Mansour IN, Napan S, Tarek Alahdab M, Stamos TD. Carbohydrate antigen 125 predicts long-term mortality in African American patients with acute decompensated heart failure. Congest Heart Fail. 2010;16:15–20. doi: 10.1111/j.1751-7133.2009.00110.x. [DOI] [PubMed] [Google Scholar]
- 18.Kantar M, Levent E, Cetingul N, et al. Plasma natriuretic peptides levels and echocardiographic findings in late subclinical anthracycline toxicity. Pediatr Hematol Oncol. 2008;25:723–733. doi: 10.1080/08880010802435393. [DOI] [PubMed] [Google Scholar]
- 19.Koglin J, Pehlivanli S, Schwaiblmair M, Vogeser M, Cremer P, vonScheidt W. Role of brain natriuretic peptide in risk stratification of patients with congestive heart failure. J Am Coll Cardiol. 2001;38:1934–1941. doi: 10.1016/s0735-1097(01)01672-2. [DOI] [PubMed] [Google Scholar]
- 20.Nunez J, Sanchis J, Bodi V, et al. Improvement in risk stratification with the combination of the tumour marker antigen carbohydrate 125 and brain natriuretic peptide in patients with acute heart failure. Eur Heart J. 2010;31:1752–1763. doi: 10.1093/eurheartj/ehq142. [DOI] [PubMed] [Google Scholar]
- 21.Pencina MJ, D'Agostino RB Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pencina MJ, D'Agostino RB Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 23.Hung CL, Hung TC, Liu CC, et al. Relation of carbohydrate antigen-125 to left atrial remodeling and its prognostic usefulness in patients with heart failure and preserved left ventricular ejection fraction in women. Am J Cardiol. 2012;110:993–1000. doi: 10.1016/j.amjcard.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 24.Karaca O, Guler GB, Guler E, et al. Serum carbohydrate antigen 125 levels in nonischemic dilated cardiomyopathy: a useful biomarker for prognosis and functional mitral regurgitation. Congest Heart Fail. 2012;18:144–150. doi: 10.1111/j.1751-7133.2011.00260.x. [DOI] [PubMed] [Google Scholar]
- 25.De Gennaro L, Brunetti ND, Montrone D, De Rosa F, Cuculo A, Di Biase M. Inflammatory activation and carbohydrate antigen-125 levels in subjects with atrial fibrillation. Eur J Clin Invest. 2012;42:371–375. doi: 10.1111/j.1365-2362.2011.02592.x. [DOI] [PubMed] [Google Scholar]
- 26.Antonini-Canterin F, Popescu BA, Popescu AC, et al. Heart failure in patients with aortic stenosis: clinical and prognostic significance of carbohydrate antigen 125 and brain natriuretic peptide measurement. Int J Cardiol. 2008;128:406–412. doi: 10.1016/j.ijcard.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 27.Huang F, Chen J, Liu Y, Zhang K, Wang J, Huang H. New mechanism of elevated CA125 in heart failure: the mechanical stress and inflammatory stimuli initiate CA125 synthesis. Med Hypotheses. 2012;79:381–383. doi: 10.1016/j.mehy.2012.05.042. [DOI] [PubMed] [Google Scholar]
- 28.Ordu S, Ozhan H, Alemdar R, et al. Carbohydrate antigen-125 and N-terminal pro-brain natriuretic peptide levels: compared in heart-failure prognostication. Tex Heart Inst J. 2012;39:30–35. [PMC free article] [PubMed] [Google Scholar]
- 29.Bhalla V, Willis S, Maisel AS. B-type natriuretic peptide: the level and the drug. Partners in the diagnosis of congestive heart failure. Congest Heart Fail. 2004;10(1 Suppl 1):3–27. doi: 10.1111/j.1527-5299.2004.03310.x. [DOI] [PubMed] [Google Scholar]
- 30.Atisha D, Bhalla MA, Morrison LK, et al. A prospective study in search of an optimal B-natriuretic peptide level screen patients for cardiac dysfunction. Am Heart J. 2004;148:518–523. doi: 10.1016/j.ahj.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 31.Huang B, Shen J, Li L, Huang Y, Luo S. Effect of B-type natriuretic peptide level on long-term outcome in patients with end-stage heart failure. Am J Cardiol. 2016;118:383–388. doi: 10.1016/j.amjcard.2016.05.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Non-ischemic etiology of the study patients according to survivor status
Study design. NT-proBNP, N-terminal pro-brain natriuretic peptide; CA-125, carbohydrate antigen 125; ADHF, acute decompensated heart failure.
(A) Box plots showing N-terminal pro-brain natriuretic peptide (NT-proBNP) levels (median; interquartile range [IQR], pg/mL) according to survivor status. (B) Box plots showing carbohydrate antigen 125 (CA-125) levels (median; IQR, U/mL) according to survivor status.


