As high as 63% of pregnant women who were still smoking cigarettes after entering prenatal care quit smoking successfully through our multicomponent behavioral intervention.
Keywords: Smoking cessation, Incentives, Contingency management, Multicomponent, Pregnancy, Multiple baseline
Abstract
Given serious consequences of maternal smoking, we aimed to develop and test a multicomponent behavioral intervention to enhance smoking cessation during pregnancy. In this nonconcurrent, multiple-baseline intervention pilot study, 48 daily smoking pregnant women (mean 13.7 weeks of gestation) were recruited from Buffalo, NY, USA. Upon completion of the repeated baseline smoking monitoring (up to 3 weeks), 30 continuous smokers received a contingent financial incentive-based intervention with three additional components (education and counseling, monitoring and feedback, and family support). After the quit date, participants met with counselors (~1 hr/visit) daily for 2 weeks and twice a week for another 6 weeks. Twenty-one out of 30 participants quit smoking completely (verified by urine cotinine) after receiving the intervention, and the other nine nonquitters decreased smoking substantially. The estimated smoking cessation rate was 70.0% (21/30) at the second week of the intervention, and 63.3% (19/30) at the conclusion of the 8-week intervention assuming the dropouts as smoking. In interrupted time series analysis, the mean daily number of cigarettes smoked among quitters decreased by 6.52, 5.34, and 4.67 among early, delayed, and late intervention groups, respectively. Quitters’ mean urine cotinine level maintained stably high before the intervention but decreased rapidly to the nonsmoking range once the intervention was initiated. Most participants (85.7%) reported meeting or exceeding expectations, and 100% would recommend the program to others. This pilot multicomponent intervention was feasible and acceptable to most participants, resulting in a high smoking cessation rate among pregnant smokers who were unlikely to quit spontaneously.
Implications
Summary: Our pilot multicomponent intervention can achieve a relatively high smoking cessation rate (63%) among pregnant smokers who were unlikely to quit spontaneously.
Practice: It was feasible and acceptable to integrate stage-tailored education and counseling, monitoring and feedback, and family support into an incentive-based intervention to further enhance maternal smoking abstinence during pregnancy.
Policy: Our effective multicomponent intervention may help to reduce the widening disparities in smoking cessation by socioeconomic status and to decrease the prevalence of smoking in the population.
Research: Frequent secondhand smoke exposure and popular co-use of marijuana among this socioeconomically disadvantaged population may merit a refinement of the intervention to address them in future research.
INTRODUCTION
About 23% of U.S. women smoke before pregnancy [1]. Pregnancy is a unique opportunity and “teachable moment” for smokers to quit, as their high intrinsic motivation is often enhanced by support from family members and prenatal care providers [2]. Indeed, about 54% of smoking women report abstinence during pregnancy [1]. Women who quit or reduce smoking typically do so shortly (e.g., in the first few days or weeks) after learning of their pregnancy [3]. Maternal smoking during pregnancy is a well-known risk factor for multiple adverse outcomes, including low birth weight, preterm birth, delayed neurodevelopment, and obesity in offspring [4–7]. Therefore, effective interventions are needed to enhance smoking cessation rates during pregnancy.
Various behavioral, pharmaceutical, and economic approaches have been tested to promote smoking cessation during pregnancy [8]. Among these approaches, a financial incentive-based intervention seems to be the most effective, with a 24% pooled rate of successful quitting compared with 6% by other approaches [8]. This approach is based on decades of research on using incentives and contingency management to motivate behavior change [9]. Pregnant smokers earn financial incentives such as cash, vouchers, discounts, and/or baby-care items, if they can successfully quit smoking verified by biochemical methods such as urine or saliva cotinine and breath carbon monoxide (CO) tests. Particularly, investigators at the University of Vermont developed and tested an effective and reliable intervention—voucher-based reinforcement therapy (VBRT)—to promote smoking cessation among pregnant women. Combining data across three controlled trials (n = 166), VBRT was associated with significant improvements in smoking cessation compared with enhanced usual care (34% vs. 7% at the end of pregnancy) [10, 11]. But there is still substantial room to further enhance the abstinence rate, as almost two thirds of women treated with VBRT fail to achieve abstinence.
One way to potentially improve incentive-based smoking cessation rates is combining it with other evidence-based interventions such as counseling, self-monitoring, and family support. In addition to the potential to enhance smoking cessation initiation, these components may help to maintain smoking abstinence induced by contingent incentives as they promote the opportunity to access additional and longer-term naturalistic sources of reinforcement for smoking abstinence, behavioral change skills, and a supportive environment [12, 13]. Another way to potentially enhance smoking cessation rates is to use higher value incentives, given the seemingly dose–response relationship between the value of incentive and the rate of successful substance use abstinence [14].
Therefore, we aimed to develop and test a multicomponent behavioral intervention that integrated stage-tailored education and counseling, smoking monitoring and feedback, and family support into our existing incentive-based intervention with an increased incentive value, to further enhance cessation rates during pregnancy [15]. We chose these additional strategies because they have demonstrated efficacy in previous research with pregnant women [8]. We expected this multicomponent intervention to achieve a higher smoking cessation rate (>60%) during pregnancy in this pilot study, compared with our prior cessation rate (34%) by the intervention using contingent financial incentives only.
METHODS
Participants
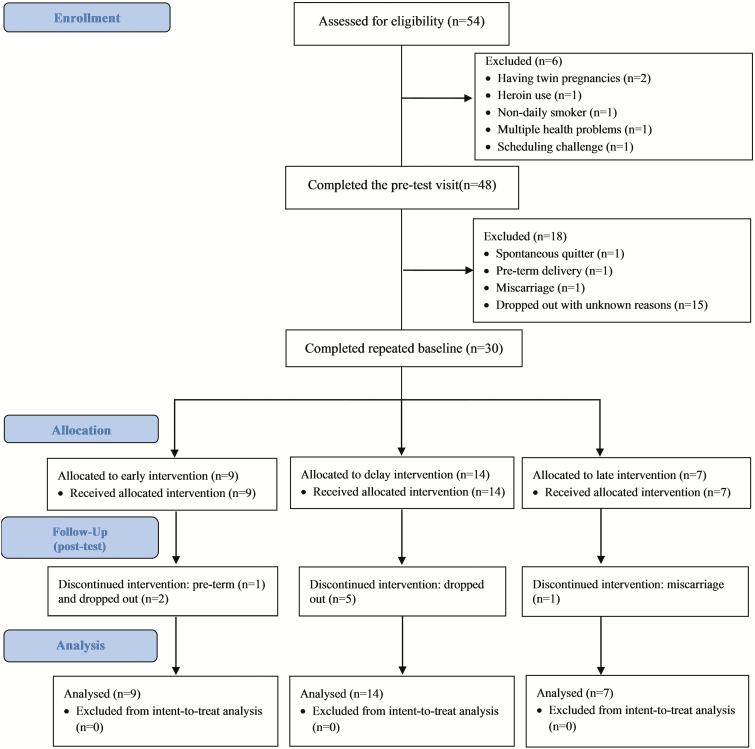
In this multiple-baseline intervention pilot study, we recruited potential participants from local prenatal care clinics and communities in the Greater Buffalo area, NY, USA. In the screening survey, the status of cigarette smoking during pregnancy was asked using a multiple-choice question shown previously to enhance accurate reporting among pregnant women [16]. Adult participants who reported smoking at least one cigarette every day in the past 7 days were invited for a lab screening visit to verify their smoking status with urine cotinine, a metabolite of nicotine with longer half-life than nicotine (17 hr vs. 2 hr, respectively) [17, 18]. Those participants with a urine cotinine level of ≥100 ng/mL were classified as current smokers and invited to participate in this study. Pregnancy, urine temperature, and urine validity tests were used to ensure the validity of the sample (preventing cheating or “gaming”) [19]. Exclusion criteria included self-reported current heavy alcohol use, current use of illicit drugs (except for marijuana), maternal age <18 or ≥40 years, uncontrolled mental health disorders such as major depression and bipolar disorder, having a multiple pregnancy, and non-English speakers. Between July 1, 2015 and November 30, 2016, 54 interested women completed the screening visit, 48 met study criteria and completed the pretest visit, and 30 finished the scheduled repeated baseline visits and received the intervention (Fig. 1). These 30 participants were included in our intent-to-treat analysis. All participants signed consent form, and this study was approved by the institutional review board.
Fig 1 |.
Study design and sample flow diagram.
Study procedures
We used a nonconcurrent, multiple-baseline (across participants) design [20] to isolate intervention effects from the impact of attention and time with participants serving as their own controls. In this design, the intervention is introduced in a staggering fashion across participants who are enrolled at different time points [21]. The outcome is measured repeatedly within the participant to assess time trend and intervention effect. This powerful, participant-saving design holds high promise for personalized intervention [21, 22] and has been successfully used in previous smoking cessation studies [23, 24]. It can also help to accurately estimate the extent to which the outcome change is attributed to the intervention versus to the preintervention change process [25]. Guided by this design, we did not initiate the smoking cessation intervention immediately after enrollment (Supplemental Table 1). Instead, a varying repeated baseline period was used to track participants’ smoking patterns until a relatively stable smoking level (±25% number of cigarettes/day) was achieved. Specifically, after the pretest visit, participants were sequentially assigned into one of three groups with different durations of the repeated baseline: early intervention (about 1 week), delayed intervention (about 2 weeks), or late intervention (about 3 weeks). After the assigned baseline period was completed and a fairly stable smoking level was achieved, all participants received the same multicomponent behavioral intervention. One spontaneous quitter during repeated baseline was removed from the study. The intervention was 8 weeks long. Participants completed the post-test visit at the conclusion of the intervention.
Intervention
Intervention schedule
At the initial intervention visit, participants chose a quit date within the next 14 days and signed a smoke-free pledge and a quitting contract. A total of 22 subsequent intervention visits were scheduled after the quit date. Pregnant women were supposed to meet with a smoking cessation counselor daily (Monday to Friday) for 2 weeks and then twice a week with at least a 2-day interval between visits for another 6 weeks. The average length of each intervention visit was about 1 hr.
Intervention components
Our behavioral intervention consisted of four integrated components: stage-tailored education and counseling, smoking monitoring and feedback, contingent financial incentives, and family support. Specifically, participants received a series of educational materials that were tailored to fit their stage of smoking cessation. At the initial intervention visit, the counselor and the participant reviewed a self-help booklet entitled “Need Help Putting Out That Cigarette?” distributed by American College of Obstetrics and Gynecology [26]. They discussed benefits of quitting smoking, reasons for smoking, smoking situations, alternatives to smoking, plans for smoking cessation, setting a quit date, and social support. Then, participants subsequently read our edited “Participant Manual for Quitting Smoking in Pregnancy” [27]. Once participants stopped smoking, they read a 10-booklet set entitled “Forever Free for Baby and Me” to prevent smoking relapse [28]. Lastly, participants read an educational book entitled “Your Guide to Breastfeeding” to further prevent postpartum smoking relapse [29], given the strong evidence that breastfeeding is likely to protect against smoking relapse [30–32]. All educational materials were written at the fifth- to sixth-grade reading level. Participants took a quiz for each reading assignment and received $5 reward for passing it (two attempts). Right after the quiz, the counselor reviewed answer keys with the participant together, probed reasoning for her chosen answers, and corrected misunderstandings.
At each intervention visit, the counselor first conducted a timeline follow-back interview to assess the participant’s smoking frequency since the last visit [33]. Next the counselor reviewed the participant’s recent quitting process, praised her for smoking abstinence or reduction, rewarded successful achievement, encouraged further quitting efforts, examined details of smoking craving, analyzed any slips or relapses, adjusted quitting strategies if necessary, and discussed seeking support from others. Motivational interviewing techniques were used throughout counseling sessions [34]. Urine cotinine test was conducted with the fast-testing NicAlert strip (Nymox Pharmaceutical Corporation, Montreal, Quebec, Canada ), which reflects tobacco exposure in the past several days [17, 18]. If the urine cotinine level was within the nonsmoking range (i.e., below Level 3, equivalent to <100 ng/mL), the participant received an incentive that began at $25/visit and escalated by $5/visit for each consecutive nonsmoking result of urine cotinine until reaching the maximum incentive of $50/visit. A positive urine cotinine test result would reset the incentive value back to the original $25 value, but two consecutive negative test results could restore the value to the pre-reset level.
In addition, we asked the participant to nominate one influential family member (whenever possible) to support her to quit smoking [13]. The family supporter could be a nonsmoker, ex-smoker, or current smoker. He or she received a package of reading materials with rewarded quizzes including our patient manual, peer support pamphlet, and the booklet number 2 for partner support from “Forever Free for Baby and Me” [28]. The family supporter also received a 5-hr training led by a smoking cessation counselor to learn positive support skills in addition to gaining knowledge on benefits of maternal smoking cessation and avoiding secondhand smoke exposure during pregnancy [35]. The family supporter worked with the pregnant woman to create a smoke-free home environment. The family supporter received equivalent to 10% of the financial incentives that the pregnant woman was earning at that time. Smoking family supporters were asked to track their own smoking frequency and were also referred to New York State Smokers’ Quitline [36] if they were motivated to quit or reduce smoking as well.
Fidelity
The principal investigator (PI: X.W.) and his research staff with backgrounds in psychology, behavioral sciences, social work, biomedical science, and other relevant fields implemented the intervention. Research staff completed a 2-week training led by the PI on how to implement counseling for smoking cessation during pregnancy. Careful evaluation was conducted via a trial run to ensure new counselors were qualified. All study visits were audio-recorded for quality assurance.
Outcome measures
Participants used a modified calendar to record the number of cigarettes that they smoked everyday throughout the study period. Based on their self-recorded daily numbers of cigarettes smoked, we classified smoking abstinence status in the past 7 days (7-day point-prevalence) prior to the two outcome assessment visits at the end of the second week of intervention and the post-test, respectively [11, 15].
At each study visit, participants provided a fresh urine sample for monitoring the concentration of urine cotinine with the fast-testing NicAlert strip. This onsite semiquantitative test yielded cotinine results within 15–30 min, and its readings ranged from Level 0 to 6: Level 0 (0–10 ng/mL of cotinine, no or minimal tobacco exposure), Level 1 (11–30 ng/mL, low secondhand smoke exposure), Level 2 (31–99 ng/mL, moderate secondhand smoke exposure), Level 3 (100–200 ng/mL, heavy secondhand smoke exposure or light smoking), Level 4 (201–500 ng/mL, light smoking), Level 5 (501–1,000 ng/mL, moderate smoking), and Level 6 (1,001+ ng/mL, heavy smoking) [37]. Smoking abstinence was confirmed if the urine cotinine test result was within the nonsmoking range (i.e., below Level 3), a cut-off point with high agreement (sensitivity = 94.0%, specificity = 97.0%) with gas chromatography classification [38].
Statistical analysis
A small sample size is usually needed for a multiple-baseline design like ours, with a minimal of one participant for each group. In order to estimate a relatively reliable smoking cessation rate, however, we decided to enroll 30 participants in total to receive intervention. A multiple interrupted time series approach [39] was used to analyze the group-level trajectories of smoking outcomes in three steps. In Step 1, we calculated the mean daily number of cigarettes smoked and the mean level of urine cotinine on the days of study visits for the early, delayed, and late intervention groups, respectively.
In Step 2, we fit autoregressive integrated moving average (ARIMA) models [40] with SAS PROCEDURE ARIMA to estimate the trajectories of mean daily numbers of cigarettes and mean urine cotinine. There are three key terms in an ARIMA model: p is the order of the autoregressive part, d is the order of the differencing, and q is the order of the moving-average process. An adequate ARIMA model can yield a residualized time series of a smoking outcome that is stationary, that is, not increasing or decreasing over time. We used the lowest Schwarz’s Bayesian criterion to choose the best-fit ARIMA model among various candidate models with different combinations of p, d, and q.
In Step 3, we used CROSSCORR function to add the variable of the start of daily intervention visits (i.e., right after the quit date) into the best-fit ARIMA model to examine the effect of our multicomponent intervention on a smoking outcome trajectory. In the ARIMA model, a significant negative coefficient (p < .05) for the variable of the start of daily intervention visits was interpreted as a significant reduction in the mean value of a smoking outcome after the intervention, that is, interrupted time series. If a significant reduction in a smoking outcome right after the intervention was consistently observed across all three groups with multiple baseline, there was strong evidence to support the causal effect of intervention, that is, smoking reduction or cessation was attributed to intervention rather than other time-varying noise factors such as time (progress of pregnancy) and participation attention [39].
RESULTS
Sample characteristics
Among the 30 study participants who received the intervention (Table 1), their mean age was 28.3 years; 30.0% were non-Hispanic Whites and 60.0% were non-Hispanic African Americans; 40.0% had high school or lower education level; 26.7% were employed; and 53.3% had annual household income <$12,000; 36.7% were married; mean gestational age was 14.8 weeks; 36.7% were heavy smokers (≥10 cigarettes/day); 46.7% had used marijuana during this pregnancy.
Table 1 |.
Sociodemographic, pregnancy, and smoking characteristics of study participants (N = 48)
| Analytic sample receiving intervention (n = 30) | Excluded sample not receiving intervention (n = 18) | ||||
|---|---|---|---|---|---|
| Characteristics | n (%) | Mean (SD) | n (%) | Mean (SD) | p Valuea |
| Age, years | 28.3 (5.8) | 25.6 (4.2) | .088 | ||
| ≤24 | 9 (30.0) | 9 (50.0) | |||
| 25–29 | 10 (33.3) | 5 (27.8) | .420 | ||
| ≥30 | 11 (36.7) | 4 (22.2) | |||
| Race/ethnicity | |||||
| Non-Hispanic, White | 9 (30.0) | 2 (11.1) | |||
| Non-Hispanic, African American | 18 (60.0) | 10 (55.6) | .093 | ||
| Hispanic or American Indian | 3 (10.0) | 6 (33.3) | |||
| Education level | |||||
| High school or lower | 12 (40.0) | 11 (61.1) | |||
| Some college or vocational training | 13 (43.3) | 4 (22.2) | .379 | ||
| 2-year or 4-year college degree | 5 (16.7) | 3 (16.7) | |||
| Marital status | |||||
| Single or divorced | 19 (63.3) | 13 (72.2) | .753 | ||
| Married | 11 (36.7) | 5 (27.8) | |||
| Employment status | |||||
| Employed | 8 (26.7) | 7 (38.9) | |||
| Unemployed | 12 (40.0) | 8 (44.4) | .672 | ||
| Housewife | 7 (23.3) | 2 (11.1) | |||
| Student | 3 (10.0) | 1 (5.6) | |||
| Individual annual income, U.S. dollars | |||||
| <5,000 | 12 (40.0) | 12 (66.7) | |||
| 5,000–15,999 | 12 (40.0) | 3 (16.7) | .160 | ||
| ≥16,000 | 6 (20.0) | 3 (16.7) | |||
| Household annual income, U.S. dollars | |||||
| <5,000 | 8 (26.7) | 8 (44.4) | |||
| 5,000–11,999 | 8 (26.7) | 4 (22.2) | |||
| 12,000–24,999 | 8 (26.7) | 3 (16.7) | .679 | ||
| ≥25,000 | 6 (20.0) | 3 (16.7) | |||
| Gestational age at enrollment, weeks | 14.8 (5.9) | 11.7 (5.9) | .084 | ||
| ≤13 (first trimester) | 13 (43.3) | 13 (72.2) | |||
| 14–27 (second trimester) | 16 (53.3) | 5 (27.8) | .101 | ||
| ≥28 (third trimester) | 1 (3.3) | 0 (0.0) | |||
| Number of live births | |||||
| 0 | 9 (30.0) | 5 (27.8) | |||
| 1–2 | 9 (30.0) | 9 (50.0) | .316 | ||
| ≥3 | 12 (40.0) | 4 (22.2) | |||
| Having a child ≤12 months old | 6 (20.0) | 3 (16.7) | 1.000 | ||
| Number of cigarettes smoked, per day | 8.4 (4.5) | 7.1 (3.7) | .292 | ||
| 1–4 (light) | 4 (13.3) | 5 (27.8) | |||
| 5–9 (moderate) | 15 (50.0) | 7 (38.9) | .460 | ||
| ≥10 (heavy) | 11 (36.7) | 6 (33.3) | |||
| Current use of alcohol | 2 (6.7) | 0 (0.0) | .521 | ||
| Marijuana use during this pregnancy | 14 (46.7) | 6 (33.3) | .546 | ||
| Partner’s smoking status | |||||
| A smoking partner | 17 (56.7) | 9 (50.0) | |||
| A nonsmoking partner | 7 (23.3) | 4 (22.2) | .921 | ||
| No partner | 6 (20.0) | 5 (27.8) | |||
aFisher’s exact tests for categorical variables and t-tests for continuous variables.
Changes in smoking outcomes after intervention
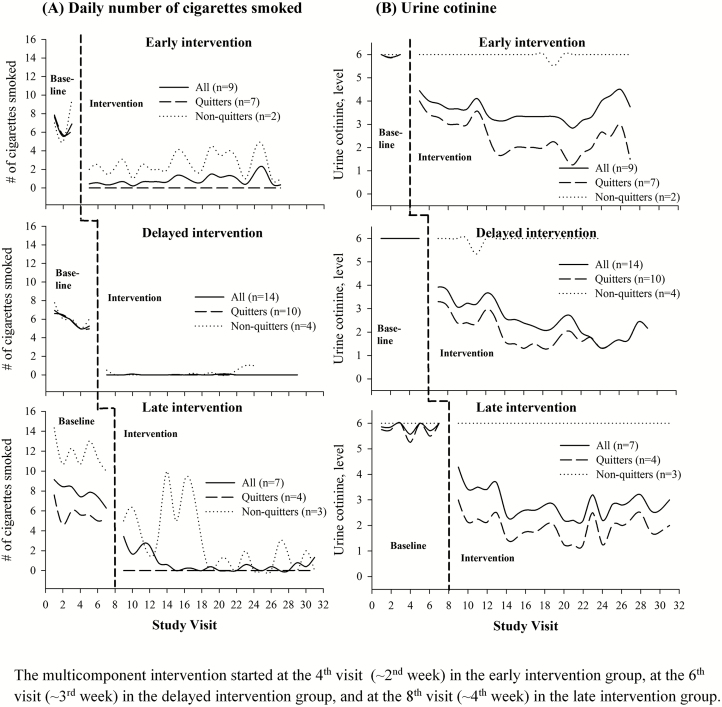
Among the 30 participants who received the intervention, smoking decreased moderately right after enrollment and then stabilized without further decline before intervention (Fig. 2A). Twenty-one of them quit smoking (verified by urine cotinine) after their quit date or at the beginning of daily intervention visits. The estimated smoking cessation rate was 70.0% (21/30) at the end of the second week of intervention, and 63.3% (19/30) at the post-test assuming the dropouts as smoking relapse or continuous smoking. The trajectories of mean urine cotinine levels among quitters were similar across three multiple-baseline groups: stably high levels (5–6) before the intervention followed by a rapid decline to the nonsmoking range (below Level 3) after intervention (Fig. 2B). The considerable variation in postquitting urine cotinine levels was mainly due to heavy secondhand smoke exposure among some participants.
Fig 2 |.
Cigarette smoking and urine cotinine trajectories pre- and postintervention, by groups and quitting status: (A) Daily number of cigarettes smoked. (B) Urine cotinine. Vertical dash line for start of daily intervention visits; top panel: early intervention group with three pre-intervention visits (1 week); middle panel: delayed intervention group with five pre-intervention visits (2 weeks); bottom panel: late intervention group with seven pre-intervention visits (3 weeks). Interpretation for urine cotinine readings of NicAlert strip: Level 0 (0–10 ng/mL of cotinine, no or minimal tobacco exposure), Level 1 (11–30 ng/mL, low secondhand smoke exposure), Level 2 (31–99 ng/mL, moderate secondhand smoke exposure), Level 3 (100–200 ng/mL, heavy secondhand smoke exposure or light smoking), Level 4 (201–500 ng/mL, light smoking), Level 5 (501–1,000 ng/mL, moderate smoking), and Level 6 (1,001+ ng/mL, heavy smoking). The multicomponent intervention started at the fourth visit (~2nd week) in the early intervention group, at the sixth visit (~3rd week) in the delayed intervention group, and at the eighth visit (~4th week) in the late intervention group.
Table 2 shows the results for interrupted time series analysis (ARIMA model). Across three multiple-baseline groups, all coefficients pertaining to the start of daily intervention visits were negative and statistically significant (p < .001) for the 21 quitters, indicating that both the daily number of cigarettes smoked and urine cotinine levels significantly decreased after intervention. The decline in the mean daily number of cigarettes smoked for quitters was 6.52, 5.34, and 4.67 cigarettes a day among early, delayed, and late intervention groups, respectively. The corresponding decline in the mean urine cotinine level for quitters was 2.07, 2.76, and 3.75, respectively. The other nine participants were classified as nonquitters based on urine cotinine results. Although their urine cotinine levels did not decrease (Fig. 2B), there was also a considerable reduction in the daily number of cigarettes smoked among nonquitters (Fig. 2A and Table 2).
Table 2 |.
Best-fit ARIMA models for interrupted time series analysis on the effect of multicomponent intervention on smoking and urine cotinine trajectories, by quitting status
| Mean daily no of cigarettes | Mean urine cotinine level (range, 0–6) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Best-fit ARIMA model | Postintervention change | Best-fit ARIMA model | Postintervention change | |||||||
| Model (p, d, q)a | p for residual autocorrelationb | Mean change | SE | p | Model (p, d, q) a | p for residual autocorrelationb | Mean change | SE | p | |
| All (n = 30) | ||||||||||
| Early intervention (n = 9) | (1,0,0) | .732 | –5.88 | 0.36 | <.001 | (1,0,0) | .552 | –1.91 | 0.28 | <.001 |
| Delayed intervention (n = 14) | (1,0,0) | .357 | –5.22 | 0.25 | <.001 | (1,0,0) | .486 | –2.08 | 0.35 | <.001 |
| Late intervention (n = 7) | (1,0,1) | .113 | –4.04 | 0.59 | <.001 | (1,0,0) | .831 | –2.73 | 0.30 | <.001 |
| Quitters (n = 21) | ||||||||||
| Early intervention (n = 7) | (1,0,0) | .585 | –6.52 | 0.16 | <.001 | (1,0,1) | .450 | –2.07 | 0.53 | <.001 |
| Delayed intervention (n = 10) | (1,0,1) | .498 | –5.34 | 0.08 | <.001 | (1,0,1) | .435 | –2.76 | 0.37 | <.001 |
| Late intervention (n = 4) | (1,0,2) | .238 | –4.67 | 0.28 | <.001 | (1,0,1) | .798 | –3.75 | 0.23 | <.001 |
| Nonquitters (n = 9) | ||||||||||
| Early intervention (n = 2) | (1,0,0) | .504 | –4.52 | 0.76 | <.001 | (0,0,1) | .988 | –0.02 | 0.06 | .715 |
| Delayed intervention (n = 4) | (1,0,0) | .697 | –6.07 | 0.31 | <.001 | (0,0,1) | .999 | –0.04 | 0.07 | .562 |
| Late intervention (n = 3) | (1,0,0) | .920 | –8.50 | 1.73 | <.001 | N/Ac | ||||
ARIMA, autoregressive integrated moving average; SE, standard error.
aARIMA model terms: p is the order of the autoregressive part; d is the order of the differencing; q is the order of the moving-average process.
b p Value for autocorrelation of residuals to lag 6.
cARIMA model not applicable because of the constant mean urine cotinine Level 6 throughout the 8-week intervention period.
Process evaluation
After the quit date, 545 (82.6%) out of 660 scheduled subsequent intervention visits (22 visits for each of 30 participants) were completed, with an average of 18.2 completed subsequent intervention visits per participant (20.0 among quitters and 13.9 among nonquitters). Twenty-two out of 30 participants (73.3%) completed 18 or more of the 22 scheduled intervention visits. Seventeen participants (56.7%) nominated a family supporter (11 partners, 4 parents, 1 sister, and 1 cousin) to receive family support training. The means of total monetary earnings including contingent incentives and quiz rewards were $583 (SD, $370) among all participants, $764 (SD, $285) among quitters, and $158 (SD, $65) among nonquitters, respectively.
Participation satisfaction
Among participants who completed the post-test survey (n = 21), 71.4% of them reported that the intervention was not at all burdensome, 85.7% met or exceeded their expectations, 100% would recommend our intervention program to others (Table 3). Most of them rated the four intervention components very or extremely useful: 90.5% for smoking monitoring and feedback, 90.5% for contingent financial incentives, 90.5% for education and counseling, and 76.2% for family support.
Table 3 |.
Participants’ satisfaction with the multicomponent intervention at the post-test survey (n = 21)
| Satisfaction item | n (%) |
|---|---|
| Perceived intervention intensity | |
| Extremely intensive | 2 (9.5) |
| Intensive | 6 (28.6) |
| Somewhat intensive | 4 (19.0) |
| Slightly intensive | 4 (19.0) |
| Not at all intensive | 4 (19.0) |
| Uncertain | 1 (4.8) |
| Perceived intervention burdensomeness | |
| Extremely burdensome | 1 (4.8) |
| Somewhat burdensome | 1 (4.8) |
| Slightly burdensome | 3 (14.3) |
| Not at all burdensome | 15 (71.4) |
| Uncertain | 1 (4.8) |
| Extent of expectations being met | |
| Below expectations | 3 (14.3) |
| Met expectations | 15 (71.4) |
| Above expectations | 3 (14.3) |
| Would recommend the program to others | 21 (100.0) |
| Usefulness of each intervention component | |
| 1. Stage-tailored education and counseling | |
| Extremely useful | 10 (47.6) |
| Very useful | 9 (42.9) |
| Slightly useful | 1 (4.8) |
| Not at all useful | 1 (4.8) |
| 2. Smoking monitoring and feedback | |
| Extremely useful | 13 (61.9) |
| Very useful | 6 (28.6) |
| Somewhat useful | 1 (4.8) |
| Uncertain | 1 (4.8) |
| 3. Contingent financial incentives | |
| Extremely useful | 11 (52.4) |
| Very useful | 8 (38.1) |
| Somewhat useful | 2 (9.5) |
| 4. Family support | |
| Extremely useful | 8 (38.1) |
| Very useful | 8 (38.1) |
| Somewhat useful | 4 (19.0) |
| Uncertain | 1 (4.8) |
DISCUSSION
Effect of intervention
The success of our pilot multicomponent intervention was supported by a relatively high smoking cessation rate and high participation satisfaction. As hypothesized, the smoking cessation rate in this study (63%) was relatively higher than the rates in our previous trials using contingent incentives only (34%) [10, 11] and other trials using nonincentive approaches (~6% overall) [8]. Two factors might contribute to this improvement. First, we added three other evidence-based intervention components, that are, education and counseling, monitoring and feedback, and family support. Second, we used a higher level of initial incentives ($25 vs. $6.25/visit) and faster escalation in incentives ($5 vs. $1.25/visit) than before, although the maximum amount remained similar ($50 vs. $45/visit).
While preliminary, these encouraging results support the promise of integrating multiple evidence-based intervention strategies to enhance smoking cessation rate. In order to maximize the total effect of an intervention package like the one pilot tested here, the process of integrating different components requires careful design and thoughtful considerations of their order, consistency, and reinforcement. In terms of order, we recommend intervention components should be logically introduced one by one to match the participant’s readiness and also not to make them feel overwhelmed. In this study, we started with smoking monitoring to know more about the participant’s smoking pattern. Then, we introduced education and counseling to help the participant to fully understand the benefits of smoking cessation, while practice and master skills for behavior change. Once the participant was ready, she chose a quit date and signed a smoke-free pledge and a quitting contract. During their quitting process, counselors provided timely feedback as well as rewarded their quitting effort with contingent incentives. The component of family support was not introduced until the patient stopped smoking or made significant progress, based on our assumption that initial smoking cessation with contingent financial incentives could strengthen her self-efficacy and confidence in our intervention, while later introduction of skillful family support could help maintain her motivation to stay abstinent. Consistency is another important consideration to ensure different intervention components do not contradict with each other. For example, the family supporter was trained to use positive behaviors such as encouraging and appreciating quitting effort while to avoid negative behaviors such as judging and criticizing smoking, which was consistent with the counselors’ styles for counseling the pregnant smoker, including open-minded attitudes, positive comments, encouraging feedback, and motivational interviewing without judgment and coercion. In addition, if used appropriately, the different intervention components can reinforce each other in addition to being complementary. In this multicomponent intervention, the major decline in smoking occurred right after the quit date (up to 2 weeks within the initial intervention visit) by which the participants had started receiving contingent incentives and a small amount of education/counseling and monitoring/feedback. Therefore, it is reasonable to believe the contingent incentives were the major driver of the initiation of smoking cessation. However, new knowledge and skills learned through education and counseling may have helped to strengthen motivation to quit and enhance self-efficacy to maintain smoking abstinence. The rule that the family supporter’s incentives were dependent on the pregnant woman’s performance could reinforce the family supporter’s effort to create a smoke-free home environment, which could potentially reduce recent quitters’ smoking cravings and secondhand smoke exposure. It could also motivate the family supporter, especially if it was the partner, to help on household chores such as childcare, a significant barrier for pregnant smokers to adhere to study visits.
Challenges during intervention
In this study, we encountered two main challenges that could suggest the improvements for future intervention. The first one was the frequent secondhand smoke exposure among smoking pregnant women. We had tried various strategies including distributing educational materials on the importance of a smoke-free home, hanging no-smoking signs, using air purifier, and wearing a mask, but none of them seemed to be very effective. Because partner smoking is the main source of maternal secondhand smoke exposure, one thing that can be considered would be to combine nicotine replacement therapy (e.g., free nicotine patch and gum from New York State Smokers Quitline [36]) with financial incentives for the partner based on negative test results on breath CO, a toxic chemical generated from cigarette smoking but not from using nicotine replacement therapy. The second challenge was the popular co-use of marijuana with cigarettes. We did not exclude marijuana users to ensure the representativeness of our eligible sample (>40% used marijuana during pregnancy). Most of these participants smoked marijuana with a cigar wrapper and binder (i.e., blunt wrap) that are usually made of tobacco leaves. With this manner of smoking, they were using both marijuana and tobacco simultaneously. This could substantially interfere with the urine cotinine test and reduce their earnings of financial incentives after they stopped smoking cigarettes, which could subsequently jeopardize their motivation to maintain abstinence from cigarette smoking. Future research should focus on helping those cigarette quitters to stop using marijuana too. If that is not possible, a harm reduction approach would be to switch to alternative nontobacco-involved ways of consuming marijuana, such as joints with rolling papers, bongs, pipes, and other devices.
Limitations
This study had several limitations. First, the relatively small sample size could threaten the reliability of our results including the estimated smoking cessation rate. Second, the generalizability of our findings might be limited because all participants were recruited from only the Buffalo area. In addition, our findings might not be generalizable to other age groups (<18 or ≥40 years). However, it should be noted that the mean maternal age (28.3 years) in our analytic sample was close to the national average (e.g., ~28 years in 2014) in the USA [41]. Third, we did not have a control group to fully control for other factors that might affect smoking cessation such as participation attention and time (progress in pregnancy). However, our multiple-baseline design could partially reduce this concern. Fourth, it is challenging to separate out the individual effect of each component of our multicomponent intervention, although our main goal was to enhance the total efficacy of intervention on smoking cessation. Fifth, a considerable proportion of participants did not complete all the scheduled repeated baseline visits due to spontaneous quitting, pre-term delivery, miscarriage, or unknown reasons. However, we did not find any substantial differences in sociodemographic, pregnancy, and smoking characteristics between participants who received intervention and others who were eligible but did not receive intervention (Table 1). Nevertheless, it is important to identify the possible reasons for dropouts, which can help to improve participant retention in future research. The intervention was designed to show proof of concept and was relatively intensive. Participants who were eligible but did not participate or complete scheduled visits might have substantial barriers, including long transportation duration with bus transfer despite our provided daily pass for public transportation, lack of childcare support despite our offered child sitting, health conditions, and/or limited available time due to employment schedule. It would be critical in future studies to evaluate how much the intervention intensity can be reduced while maintaining strong intervention effects. Possible modifications could include shortening the duration of daily visits from 2 weeks to 1 week and reducing the frequency of the weekly visits from twice a week to once a week, so that the total number of visits will decrease by half (11 vs. 22). This can reduce participant burden and may increase acceptability among less motivated individuals. Sixth, while patient satisfaction at the post-test was very positive, it needs to be interpreted with caution, given a substantial proportion (30%) did not respond to the satisfaction evaluation questions. Selection bias may have occurred, for example, participants who completed the post-test visit tended to have more positive experience than those who dropped out in the middle of intervention.
In summary, this multicomponent intervention was feasible and acceptable to racially diverse and mostly low–socioeconomic status pregnant smokers in our pilot study. It could achieve high smoking cessation rate (63%) among pregnant smokers who were unlikely to quit spontaneously. Novel intervention is needed to overcome challenges of secondhand smoke exposure and co-use of marijuana. An immediate next step would be to further validate our intervention package in a larger randomized controlled trial. After that, an experimental dismantling of the intervention package is needed to identify the contribution of each intervention component, which can help to allocate resources to the most powerful component. This can be accomplished through a factorial design, in which both individual and interactive effects of intervention components can be assessed.
Supplementary Material
Acknowledgments
We appreciate the administrative support from Teresa Quattrin, MD, the Chair of Department of Pediatrics, State University of New York at Buffalo; Vanessa M. Barnabei, MD, the Chair of Department of Obstetrics and Gynecology, State University of New York at Buffalo; and Aimée C. Gomlak, FACHE, MBA, BS, the Vice President of Women’s Services of Catholic Health System, as well as the assistance on recruitment by the staff in the Women and Children’s Hospital of Buffalo, the Sisters of Charity Hospital of Buffalo, the Buffalo Prenatal-Perinatal Network, and other local obstetrics and gynecology clinics. The authors also thank the study participants for their time and efforts, and Drs. Katelyn A. Carr and Tinuke Oluyomi Daniel for their assistance on training counselors. This work was supported by a Clinical and Translational Science Award Pilot funding by National Center for Advancing Translational Sciences of National Institutes of Health under grant number UL1TR001412, and a seed funding from Department of Pediatrics, State University of New York at Buffalo (both awarded to Xiaozhong Wen). S. T. Higgins received salary support from R01HD075669, R01HD078332, and P20GM103644 while assisting with this study. This work is solely the responsibility of the authors and does not represent official views of the sponsors. The funders had no role in writing the manuscript or the decision to submit it for publication.
Compliance with Ethical Standards
Conflicts of Interest: No financial disclosures were reported by the authors of this study.
Primary Data: Xiaozhong Wen had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. The findings reported in this manuscript have not been previously published, and the manuscript is not being simultaneously submitted elsewhere. A part of preliminary data of this study was only reported as a poster at the 30th New York State Perinatal Association Annual Perinatal Conference in Albany, NY, USA on June 2, 2016.
Ethical Approval: All study procedures were conducted in accordance with the ethical standards of the responsible committee on social and behavioral science research and with the Helsinki Declaration of 1975, as revised in 2000. This article does not contain any studies with animals performed by any of the authors.
Informed Consent: All participants signed consent form, and this study was approved by University at Buffalo Institutional Review Board.
References
- 1. Tong VT, Dietz PM, Morrow B, et al. ; Centers for Disease Control and Prevention (CDC). Trends in smoking before, during, and after pregnancy–Pregnancy Risk Assessment Monitoring System, United States, 40 sites, 2000–2010. mmwr Surveill Summ. 2013;62(6):1–19. [PubMed] [Google Scholar]
- 2. McBride CM, Emmons KM, Lipkus IM. Understanding the potential of teachable moments: the case of smoking cessation. Health Educ Res. 2003;18(2):156–170. [DOI] [PubMed] [Google Scholar]
- 3. Heil SH, Herrmann ES, Badger GJ, Solomon LJ, Bernstein IM, Higgins ST. Examining the timing of changes in cigarette smoking upon learning of pregnancy. Prev Med. 2014;68:58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Knopik VS, Maccani MA, Francazio S, McGeary JE. The epigenetics of maternal cigarette smoking during pregnancy and effects on child development. Dev Psychopathol. 2012;24(4):1377–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Horta BL, Victora CG, Menezes AM, Halpern R, Barros FC. Low birthweight, preterm births and intrauterine growth retardation in relation to maternal smoking. Paediatr Perinat Epidemiol. 1997;11(2):140–151. [DOI] [PubMed] [Google Scholar]
- 6. Chen A, Pennell ML, Klebanoff MA, Rogan WJ, Longnecker MP. Maternal smoking during pregnancy in relation to child overweight: follow-up to age 8 years. Int J Epidemiol. 2006;35(1):121–130. [DOI] [PubMed] [Google Scholar]
- 7. Ino T. Maternal smoking during pregnancy and offspring obesity: meta-analysis. Pediatr Int. 2010;52(1):94–99. [DOI] [PubMed] [Google Scholar]
- 8. Lumley J, Chamberlain C, Dowswell T, Oliver S, Oakley L, Watson L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2009(3):CD001055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davis DR, Kurti AN, Skelly JM, Redner R, White TJ, Higgins ST. A review of the literature on contingency management in the treatment of substance use disorders, 2009–2014. Prev Med. 2016;92:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Higgins ST, Washio Y, Heil SH, et al. Financial incentives for smoking cessation among pregnant and newly postpartum women. Prev Med. 2012;55(suppl):S33–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Higgins ST, Bernstein IM, Washio Y, et al. Effects of smoking cessation with voucher-based contingency management on birth outcomes. Addiction. 2010;105(11):2023–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Donatelle R, Hudson D, Dobie S, Goodall A, Hunsberger M, Oswald K. Incentives in smoking cessation: status of the field and implications for research and practice with pregnant smokers. Nicotine Tob Res. 2004;6(suppl 2):S163–S179. [DOI] [PubMed] [Google Scholar]
- 13. Donatelle RJ, Prows SL, Champeau D, Hudson D. Randomised controlled trial using social support and financial incentives for high risk pregnant smokers: significant other supporter (SOS) program. Tob Control. 2000;9(suppl 3):III67–III69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101(2):192–203. [DOI] [PubMed] [Google Scholar]
- 15. Heil SH, Higgins ST, Bernstein IM, et al. Effects of voucher-based incentives on abstinence from cigarette smoking and fetal growth among pregnant women. Addiction. 2008;103(6):1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mullen PD, Carbonari JP, Tabak ER, Glenday MC. Improving disclosure of smoking by pregnant women. Am J Obstet Gynecol. 1991;165(2):409–413. [DOI] [PubMed] [Google Scholar]
- 17. Dempsey D, Jacob P III, Benowitz NL. Accelerated metabolism of nicotine and cotinine in pregnant smokers. J Pharmacol Exp Ther. 2002;301(2):594–598. [DOI] [PubMed] [Google Scholar]
- 18. Jarvis MJ, Russell MA, Benowitz NL, Feyerabend C. Elimination of cotinine from body fluids: implications for noninvasive measurement of tobacco smoke exposure. Am J Public Health. 1988;78(6):696–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ierfino D, Mantzari E, Hirst J, Jones T, Aveyard P, Marteau TM. Financial incentives for smoking cessation in pregnancy: a single-arm intervention study assessing cessation and gaming. Addiction. 2015;110(4):680–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dallery J, Raiff BR. Optimizing behavioral health interventions with single-case designs: from development to dissemination. Transl Behav Med. 2014;4(3):290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McDonald S, Quinn F, Vieira R, et al. The state of the art and future opportunities for using longitudinal n-of-1 methods in health behaviour research: a systematic literature overview. Health Psychol Rev. 2017;11(4):307–323. [DOI] [PubMed] [Google Scholar]
- 22. Medical Research Council. Developing and evaluating complex interventions: New guidance Available at www.mrc.ac.uk/documents/pdf/complex-interventions-guidance/. Accessibility verified May 23, 2017.
- 23. Turner SA, Daniels JL, Hollandsworth JG. The effects of a multicomponent smoking cessation program with chronic obstructive pulmonary disease outpatients. Addict Behav. 1985;10(1):87–90. [DOI] [PubMed] [Google Scholar]
- 24. Feldner MT, Smith RC, Monson CM, Zvolensky MJ. Initial evaluation of an integrated treatment for comorbid PTSD and smoking using a nonconcurrent, multiple-baseline design. Behav Ther. 2013;44(3):514–528. [DOI] [PubMed] [Google Scholar]
- 25. Stasiewicz PR, Schlauch RC, Bradizza CM, Bole CW, Coffey SF. Pretreatment changes in drinking: relationship to treatment outcomes. Psychol Addict Behav. 2013;27(4):1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weinfeld C, Hall AB.. Need Help Putting Out That Cigarette? National Partnership for Smoke Free Families; 2001. Available at http://www.tobacco-cessation.org/. Accessibility verified February 21, 2016. [Google Scholar]
- 27. Peters A, Szeglowski A, Soni S, Haghdel A, Bozyk N, Wen X.. Participant Manual for Quitting Smoking in Pregnancy. Buffalo, NY: UB Maternal and Child Health Research Program; 2016. Available at www.surveymonkey.com/r/ubsmkStep4Intervention. Accessibility verified September 22, 2016. [Google Scholar]
- 28. Brandon TH, Simmons VN, Meade CD, et al. Self-help booklets for preventing postpartum smoking relapse: a randomized trial. Am J Public Health. 2012;102(11):2109–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Office on Women’s Health of U.S. Department of Health and Human Services. Your Guide to Breastfeeding. Washington, DC: Office on Women’s Health of U.S. Department of Health and Human Services; 2011. [Google Scholar]
- 30. Shisler S, Homish GG, Molnar DS, Schuetze P, Colder CR, Eiden RD. Predictors of changes in smoking from third trimester to 9 months postpartum. Nicotine Tob Res. 2016;18(1):84–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kendzor DE, Businelle MS, Costello TJ, et al. Breast feeding is associated with postpartum smoking abstinence among women who quit smoking due to pregnancy. Nicotine Tob Res. 2010;12(10):983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ratner PA, Johnson JL, Bottorff JL, Dahinten S, Hall W. Twelve-month follow-up of a smoking relapse prevention intervention for postpartum women. Addict Behav. 2000;25(1):81–92. [DOI] [PubMed] [Google Scholar]
- 33. Brown RA, Burgess ES, Sales SD, Whitley JA, Evans DM, Miller IW. Reliability and validity of a smoking timeline follow-back interview. Psychol Addict Behav. 1998;12(2):101–112. [Google Scholar]
- 34. Miller WR, Rollnick S.. Motivational Interviewing, Third Edition: Helping People Change. New York, NY: Guilford Publications; 2012. [Google Scholar]
- 35. Cohen S, Lichtenstein E. Partner behaviors that support quitting smoking. j Consult Clin Psychol. 1990;58(3):304–309. [DOI] [PubMed] [Google Scholar]
- 36. The NY State Department of Health Tobacco Control Program. The New York State Smokers’ Quitline Available at https://www.nysmokefree.com/SpecialPages/AboutUs.aspx?lang=E. Accessibility verified September 11, 2016.
- 37. Nymox Pharmaceutical Corporation. NicAlert®: Product Insert (Urine Samples) 2017. Available at http://www.nymox.com/default.action?itemid=47 Accessibility verified December 13, 2017.
- 38. Gaalema DE, Higgins ST, Bradstreet MP, Heil SH, Bernstein IM. Using NicAlert strips to verify smoking status among pregnant cigarette smokers. Drug Alcohol Depend. 2011;119(1–2):130–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Biglan A, Ary D, Wagenaar AC. The value of interrupted time-series experiments for community intervention research. Prev Sci. 2000;1(1):31–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Box GEP, Jenkins GM, Reinsel GC, Ljung GM.. Time Series Analysis: Forecasting and Control. 5th ed. Hoboken, NJ: John Wiley & Sons; 2015. [Google Scholar]
- 41. Mathews TJ, Hamilton BE. Mean age of mothers is on the rise: United States, 2000–2014. NCHS Data Brief. 2016;(232):1–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.