Abstract
Purpose
In juvenile tree shrews that have developed minus lens-induced myopia, if lens treatment is discontinued, refractive recovery (REC) occurs. However, in age-matched juvenile animals, plus-lens wear (PLW) produces little refractive change, although the visual stimulus (myopia) is similar (an “IGNORE” response). Because the sclera controls axial elongation and refractive error, we examined gene expression in the sclera produced by PLW and compared it with the gene expression signature produced by REC to learn whether these similar refractive conditions produce similar, or differing, scleral responses.
Methods
Eight groups of tree shrews (n = 7 per group) were examined. Four groups wore a monocular −5 D lens for 11 days until 35 days of visual experience (DVE). Lens wear was then discontinued, and the animals recovered for 0 h (REC-0), 2 h (REC-2h), 1 day (REC-1d), or 4 days (REC-4d). Starting at 35 DVE, three groups wore a monocular +5 D lens for 2 h (PLW-2h), 1 day (PLW-1d), or 4 days (PLW-4d). A normal group (PLW-0) was examined at 38 DVE to provide baseline measures. Using quantitative real-time PCR (qPCR), we examined scleral mRNA levels in recovering, plus-lens treated, and untreated control eyes for 55 candidate genes whose protein products included signaling molecules, metallopeptidases (MPs) and their inhibitors (tissue inhibitors of metallopeptidases [TIMPs]), and extracellular matrix proteins.
Results
No refractive recovery was measured in the REC-2h group. The scleral mRNA expression pattern for recovering versus untreated control eyes after 2 h of recovery was similar to that found for the group (REC-0) that had no recovery time. Many genes in both groups still had downregulated expression in the treated eyes versus the control eyes. The REC-1d group showed little refractive recovery (0.1 ± 0.1 D, mean ± standard error of the mean [SEM]), and the mRNA expression pattern was similar to that of the REC-2h group, but had fewer statistically significantly downregulated genes in the recovering eyes. The REC-4d group recovered refractively by 2.6 ± 0.4 D, and displayed a “STOP” gene expression signature of mostly upregulated mRNA expression in the recovering eyes compared with the untreated control eyes. The PLW-0 (normal) group and the PLW-2h group showed no statistically significant differential gene expression. The PLW-1d group showed a small hyperopic shift (0.1 ± 0.2 D). Two genes were differentially expressed: NPR3 was upregulated in the plus lens-wearing eyes, and IGF1 was downregulated. The PLW-4d group showed a similar hyperopic shift (0.3 ± 0.4 D), confirming that the plus lens-induced 5 D of myopia produced little refractive change. In the sclera, there was an IGNORE pattern of general differential upregulation of genes in the treated eyes (22 upregulated, one downregulated) that was distinct from the STOP signature found in recovery. Ten genes were upregulated in the REC-4d group and the PLW-4d group. However, ten other genes were differentially expressed in recovery, but not in plus-lens wear, while 12 genes were differentially expressed in plus-lens wear but not in recovery.
Conclusions
One day of recovery is not long enough for the emmetropization mechanism to produce significant gene expression changes in the sclera or refractive recovery. After 4 days, recovery and plus-lens wear produced altered scleral gene expression, but the patterns (“signatures”) differed as to which genes showed altered expression, and whether the gene expression was up- or downregulated. Thus, myopia produced altered scleral mRNA expression in recovery and plus-lens wear, confirming that signals initiated by the retina reached the sclera, but the sclera in the elongated recovering eye responded differently from a normal sclera. This might have occurred because the recovering-eye sclera had remodeled during minus-lens compensation, making the sclera respond differently to the signals initiated by the retina. However, the myopia-produced retinal signals in plus lens-wearing animals also may have differed from those in the recovering eyes by the time the signals passed through the RPE and choroid to reach the sclera.
Introduction
Refractive error occurs when the axial length of the eye (and therefore, the location of the retina) does not match the location of the focal plane, produced by the cornea and the crystalline lens. A visually guided emmetropization feedback mechanism has been found to operate in children and in animal models (fish, chicks, mice, guinea pigs, tree shrews, monkeys, and other species) [1-10]. Postnatally, the emmetropization mechanism uses refractive error cues to modulate the elongation of the eye so that the retina comes to be located near the focal plane without accommodation (near emmetropia). If the axial length initially is shorter than the focal plane, the eye is hyperopic; the emmetropization mechanism increases the elongation rate to achieve emmetropia. If the axial length is longer than the focal plane, the eye is myopic; the emmetropization mechanism slows the axial elongation rate to achieve emmetropia.
When the emmetropization mechanism detects refractive error, the mechanism generates retinal signals that pass in a signaling cascade through the RPE and choroid to the sclera where they alter gene expression of the scleral fibroblasts [1,11]. This produces remodeling of the scleral extracellular matrix (ECM) that alters the axial elongation rate [12-16]. The signaling cascade is direct within the eye, as has been demonstrated in animal studies by severing or blocking the retinal output to the brain [17-20].
The emmetropization mechanism can be manipulated to increase or decrease the axial elongation rate of juvenile eyes. Placing a minus lens, held in a goggle frame, in front of an emmetropic eye moves the focal plane away from the cornea, producing refractive hyperopia while the lens is worn. This stimulates retinal responses that have been described as GO signals [21] that are communicated through the signaling cascade, producing an increase in the axial elongation rate and moving the retina to the shifted focal plane. When the retina has reached the shifted focal plane, the eye has “compensated” for the minus lens.
After compensation, if minus-lens wear is discontinued, the elongated eye is optically myopic. The emmetropization mechanism detects the myopic refractive state and produces retinal STOP signals that pass through the signaling cascade and slow the axial elongation rate. Over time, the focal plane moves away from the cornea, because the cornea flattens, and the lens power decreases; therefore, the refractive myopia decreases. This refractive recovery continues until the refraction and axial length of the recovering eye once again match that of untreated normal eyes [3,14,16,22-26].
Scleral remodeling is the mechanism that underlies the increase and decrease in the axial elongation rate of the eye. During minus-lens compensation, the GO signals initiated by the retina reach the sclera and produce altered gene expression that results in changes in protein levels. There is a small loss of scleral ECM that includes a reduction in the amount of type I collagen and other ECM proteins [12,14,27-29], and an increase in matrix metallopeptidase levels [30]. There also is a reduction in sulfated and un-sulfated glycosaminoglycan levels [14,15]. The remodeling increases the viscoelasticity of the sclera (measured by the creep rate) [16,31] and alters the collagen crimp angle [32]. These changes allow the globe to expand axially under normal intraocular pressure.
During recovery from induced myopia, STOP signals initiated by the retina arrive at the sclera and produce remodeling that is not a mirror image of the pattern produced by GO signals. The expression levels of many genes that were decreased during myopia development return to normal, or slightly above normal, and additional genes, unaffected during myopia development, show upregulation: a “scleral STOP signature” [33]. Similar changes have been found in the levels of glycosaminoglycans and proteins [12,14,34]. The result is decreased viscoelasticity in the sclera that slows axial elongation [16].
A myopic refractive state can also be produced by placing a plus-power (convex) lens in front of an emmetropic eye. Light rays from distant objects then are focused in front of the retina, producing refractive myopia while the lens is worn. In infantile tree shrews, plus-lens wear slows the axial elongation rate until the eyes become nearly emmetropic while wearing the lens [35]. However, in older, juvenile tree shrews, plus-lens wear has little effect on the axial elongation rate or on refraction [35]. The eyes remain myopic while wearing the lens, generally ignoring the myopic refractive error.
Optically, it would appear that plus-lens wear produces a myopia similar to that in eyes at the start of recovery from lens-induced myopia. Why does myopia produce different refractive responses in plus-lens wear versus recovery? One possibility is that no STOP signals arrive at the sclera in normal eyes. Another is that signals reach the sclera during plus-lens wear, but the response in the normal sclera differs from that in the remodeled, elongated sclera of recovering eyes, so that in normal sclera, the arriving signals do not cause the remodeling that is necessary to slow the axial elongation rate. A third possibility is that the STOP signals initiated by the retina in the plus lens-wearing animals are altered as they pass through the normal RPE and choroid, producing altered mRNA expression in the sclera that does not result in slowed axial elongation. The purpose of this study was to investigate these possibilities by examining gene expression in the sclera in juvenile tree shrews produced by plus-lens wear and comparing the expression to the scleral STOP signature found in recovery, as assessed with a set of 55 genes used previously [33].
Methods
Experimental groups
All of the juvenile northern tree shrews (Tupaia belangeri) used in this study were produced in the UAB Tree Shrew Core and raised by their mothers under a 14 h:10 h light-dark cycle. Tree shrew pups are born with their eyes closed, and open them about 3 weeks after birth. We assume that the emmetropization mechanism is inactive until both eyes are open, which we describe as day 1 of visual experience (DVE). All procedures complied with the ARVO Statement for the Use of Animals in Ophthalmic and Visual Research and were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham. Experimental groups were balanced to include males and females, and avoided pups from the same parents wherever possible. In the recovery and plus lens-wearing groups, the treated eye was selected randomly, and the untreated fellow eye served as a within-animal control.
Recovery groups
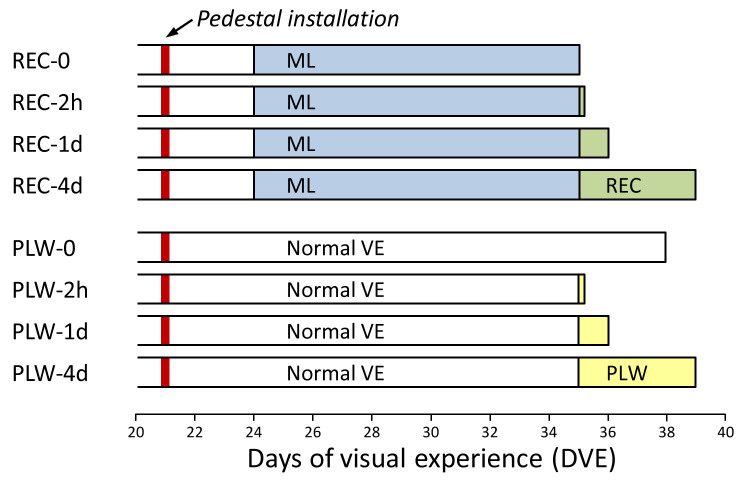
Four groups of animals (n = 7 per group) were used to examine the effect of recovery (REC) from lens-induced myopia on patterns of mRNA expression. As shown in Figure 1, all animals in the recovery groups received 11 days of –5 D lens treatment (12 mm diameter PMMA contact lens; Conforma Contact Lenses, Norfolk, VA) starting at 24 ± 1 DVE. Recovery began after 11 days by discontinuing lens wear at 35 ± 1 DVE. The REC-0 group experienced no recovery; this group served as a baseline for the other recovery groups. The REC-2h group recovered for 2 h, the REC-1d group recovered for 1 day, and the REC-4d group recovered for 4 days. The REC-0 group was needed, because although we expected that full refractive compensation would occur by 11 days of lens wear (thus, removing the retinal GO signals that triggered elongation), we found in previous studies [33,34] that the scleral mRNA levels do not return to normal at this time point. The mRNA results from two of the REC groups (REC-0 and REC-4d) were reported previously [33] as the ML-11 and REC-4 groups. The REC-2h and REC-1d groups were added to provide earlier time points on the development of the scleral STOP signature.
Figure 1.
Experimental groups and duration of treatments. The red vertical bars indicate the point when a dental acrylic pedestal was attached to the skull under anesthesia. Filled regions indicate the type and duration of the visual treatment. The right end of each bar indicates the time point when mRNA levels were measured. ML, minus-lens wear; VE, visual experience.
Plus-lens wear groups
Four additional groups of animals (n = 7 per group) were used to examine the effect of plus-lens wear (PLW) on the refractive state of the eyes and on mRNA expression at the same time points as for the REC groups (Figure 1). Starting at 35 ± 1 DVE, the PLW-2h, PLW-1d, and PLW-4d groups, which had normal visual experience until this point, began to wear a monocular +5 D lens. The PLW-2h group wore the lens for 2 h, the PLW-1d group wore the lens for 1 day, and the PLW-4d group wore the lens for 4 days. These animals experienced approximately the same amount of refractive myopia as was initially experienced by the animals in the recovery groups. The fourth group (PLW-0) comprised normal animals and provided baseline refractive and mRNA data. These animals were studied at 38 ± 1 DVE, a time point in between the shortest (PLW-2h) and longest (PLW-4d) groups. Data from this group were reported previously as the 38N group [33]. A single intermediate time point seemed sufficient because the time span between the PLW-2h and PLW-4d groups was brief (4 days), and at an age when the normal refractive state has nearly stabilized, such that little developmental alteration in scleral mRNA levels was expected. Importantly, no significant differential (left eye versus right eye) mRNA expression was found in tree shrew sclera in our previous studies [12-16,33,34].
Goggle installation
At 21 ± 1 DVE, animals in all groups were anesthetized (17.5 mg ketamine, 1.2 mg xylazine, supplemented with 0.5–2.0% isoflurane as needed; all, MWI, Boise, ID) and received a dental acrylic pedestal following procedures described by Siegwart and Norton [36]. After pedestal installation, all animals were placed in individual cages with standard colony fluorescent lighting, 100–300 lux on the floor of the cage. Three days later, in the REC-2h, REC-1d, and REC-4d groups, a goggle frame holding a monocular –5 D lens was clipped to the pedestal, firmly holding the –5 D lens in front of the treated eye. The control eye had unrestricted vision through an open goggle frame. The goggle was removed after 11 days (35 ± 1 DVE) to begin recovery.
Three PLW groups (PLW-2h, PLW-1d, and PLW-4d) had normal visual experience until 35 ± 1 DVE when a goggle frame containing a monocular +5 D lens was clipped to the pedestal. The control eye had unrestricted vision through an open goggle frame. The PLW-0 (normal) group received a pedestal at 21 DVE but did not wear a goggle.
During lens wear, the goggles were briefly (<3 min) removed twice daily (approximately 9:30 AM and 4:30 PM), under dim illumination, to clean the lens. During lens cleaning, animals were kept in a darkened nest box to minimize exposure to visual stimuli.
Refractive and axial measures
Non-cycloplegic refractive measures were made, in awake animals, with a Nidek ARK-700A infrared autorefractor (Marco Ophthalmic, Jacksonville, FL) [37]. Recovery animals were measured without the lens to show the amount of myopia that existed in the treated eyes relative to their control eyes. The REC-0 group was measured after 11 days of –5 D lens wear, with no recovery period. The REC-2h group was also measured at the end of 11 days of –5 D lens wear, and was not measured a second time after 2 h of recovery, because no significant refractive change was expected. The REC-1d and REC-4d groups were measured at the end of lens wear, and again at the end of their recovery period. The amount of myopia was measured as the refraction in the treated eye minus the control eye, measured with the minus lens removed, and averaged across the group. Recovery (compensation for the plus lens) was any reduction in the treated eye minus the control eye value between the start and end of treatment.
The PLW-0 (normal group) was measured just before euthanasia. The refractive measures in the treated and control eyes of the other PLW groups were made while the animals were wearing the +5 D lens. This provided a measure of the amount of refractive myopia experienced by the treated eyes relative to their fellow control eyes. The PLW-2h group was measured only at the start of lens wear. The PLW-1d and PLW-4d groups were measured at the start, and again at the end, of the plus-lens wear period. The amount of myopia was measured as the refraction in the treated eye minus the control eye, measured while wearing the plus lens, and averaged across the group. Refractive recovery was the reduction in the treated eye minus control eye value between the start and end of treatment.
Cycloplegic refractive measures were omitted to prevent any interference by atropine on retinoscleral signaling [38]. However, previous studies have shown that non-cycloplegic measures provide a valid estimate of the refractive state and of induced myopia in tree shrews. Cycloplegic refractions are approximately 0.8 D hyperopic when compared with non-cycloplegic refractions in myopic, control, and normal eyes [37,39]. Further, treated-eye versus control-eye differences are essentially identical between non-cycloplegic and cycloplegic measures [40]. All refractive values were corrected for the small eye artifact [41], previously shown to be approximately +4 D in tree shrews [37].
At the time the pedestal was attached, the ocular component dimensions were measured with A-scan ultrasound while under anesthesia, as described by Norton and McBrien [42], to ensure that the treated, control, and normal eyes did not differ statistically significantly in axial length before treatment began. Post-treatment A-scan measures were not made, to eliminate any possibility that the delay under anesthesia required for the A-scan procedure might alter scleral gene expression.
Gene expression analysis
Animals were euthanized (17.5 mg ketamine and 1.2 mg xylazine, followed by 50 mg xylazine) at approximately the same time of day (approximately 10 AM to 12 PM), and the scleral tissue collected in RNAlater (Life Technologies, Carlsbad, CA) according to published procedures [13] before the tissue was frozen in liquid nitrogen. The frozen sclera was pulverized to a fine powder in a chilled Teflon freezer mill (Sartorius Stedim, Bohemia, NY) from which total RNA was isolated using a RiboPure kit (Life Technologies) according to the manufacturer’s instructions, with the addition of an on-filter DNase treatment. The purified RNA was quantified (NanoDrop Technologies, Wilmington, DE), and the quality confirmed with denaturing gel electrophoresis (RNA FlashGel; Lonza, Rockland, ME). cDNA was synthesized from 1 µg of total RNA in a final reaction volume of 20 µl using a Superscript III RT kit (Life Technologies) with minor modifications (2.5 µM anchored oligo (dT)20 primers and DTT omitted). The resultant cDNA was diluted fivefold and stored at –20 °C until use.
To facilitate comparison with our previous studies that characterized the scleral GO, STAY, and STOP signatures [13,33], we used tree shrew–specific quantitative PCR (qPCR) primers for the same 55 genes used in those studies (Table 1) and the reference gene RNA polymerase II (POLR2A; Gene ID 5430, OMIM 180660). None of the treatment conditions affected the expression of the reference gene. Primer sequences are listed in Appendix 1. The selected genes included representatives of three major groupings: signaling, metallopeptidases (MPs) and tissue inhibitors of metallopeptidases (TIMPs), and extracellular matrix (ECM) proteins. All primers were designed to work under the same cycling conditions. All amplicons were located within the coding region, and most spanned at least one intron; amplicon identity was verified with gel electrophoresis and sequencing.
Table 1.
| Gene symbol | Protein name | Location |
|---|---|---|
| Signaling − Cell surface receptors | ||
| ACVRL1 | Activin A receptor 2-like 1 | Cell surface |
| FGFR2 | FGF receptor 2 | Cell surface |
| NPR3 | Atrial natriuretic peptide receptor 3 | Cell surface |
| SDC2 | Syndecan 2 | Cell surface |
| TGFBR3 | TGFβ receptor III | Cell surface |
| TRPV4 | Transient receptor potential cation channel V4 | Cell surface |
| UNC5B | Netrin receptor UNC5B | Cell surface |
| EFNA1 | Ephrin A1 | Cell surface |
| Signaling − Cytoskeleton related | ||
|---|---|---|
| ANXA1 | Annexin A1 | Cell surface |
| ANXA2 | Annexin A2 | Cell surface |
| CAPN2 | Calpain 2 | Cell surface |
| CAPNS1 | Calpain small subunit 1 | Cell surface |
| GJA1 | Connexin 43 | Cell surface |
| ACTA2 | Smooth muscle actin | Intracellular |
| NGEF | Neuronal guanine nucleotide exchange factor | Intracellular |
| Signaling − Transcription regulators | ||
|---|---|---|
| HIF1A | Hypoxia-inducible factor 1α | Intracellular |
| RARB | Retinoic acid receptor β | Intracellular |
| RXRB | Retinoid X receptor β | Intracellular |
| VDR | Vitamin D receptor | Intracellular |
| Signaling − Secreted | ||
|---|---|---|
| ANGPTL7 | Angiopoietin-related protein 7 | Extracellular |
| IGF1 | Insulin-like growth factor 1 | Extracellular |
| IGF2 | Insulin-like growth factor 2 | Extracellular |
| IL18 | Interleukin 18 | Extracellular |
| PENK | Proenkephalin A | Extracellular |
| TGFB1 | Transforming growth factor β1 | Extracellular |
| TGFB2 | Transforming growth factor β2 | Extracellular |
| TGFBI | TGFβ-induced protein | Extracellular |
| Signaling − Matricellular | ||
|---|---|---|
| CTGF | Connective tissue growth factor | Extracellular |
| CYR61 | Protein CYR61 | Extracellular |
| FBLN1 | Fibulin 1 | Extracellular |
| NOV | Nephroblastoma overexpressed gene | Extracellular |
| SPARC | Secreted protein acidic and rich in cysteine | Extracellular |
| SPP1 | Osteopontin | Extracellular |
| THBS1 | Thrombospondin 1 | Extracellular |
| THBS2 | Thrombospondin 2 | Extracellular |
| TNC | Tenascin C | Extracellular |
| WISP1 | WNT1 inducible signaling pathway protein 1 | Extracellular |
| MPs / TIMPs | ||
|---|---|---|
| ADAMTS5 | ADAM metallopeptidase with thrombospondin motif, 5 | Extracellular |
| MMP2 | Matrix metallopeptidase 2 | Extracellular |
| MMP14 | Matrix metallopeptidase 14 | Cell surface |
| TIMP1 | TIMP metallopeptidase inhibitor 1 | Extracellular |
| TIMP2 | TIMP metallopeptidase inhibitor 2 | Extracellular |
| TIMP3 | TIMP metallopeptidase inhibitor 3 | Extracellular |
| Extracellular matrix − Collagens | ||
|---|---|---|
| COL1A1 | Collagen type I, α1 | Extracellular |
| COL12A1 | Collagen type XII, α1 | Extracellular |
| COL14A1 | Collagen type XIV, α1 | Extracellular |
| Extracellular matrix − Proteoglycans | ||
|---|---|---|
| ACAN | Aggrecan | Extracellular |
| DCN | Decorin | Extracellular |
| FMOD | Fibromodulin | Extracellular |
| KERA | Keratocan | Extracellular |
| NYX | Nyctalopin | Extracellular |
| OGN | Mimecan | Extracellular |
| PRELP | Prolargin | Extracellular |
| Extracellular matrix-Other | ||
|---|---|---|
| HS6ST1 | Heparan-sulfate 6-O-sulfotransferase 1 | Cell surface |
| SERPINH1 | Serpin H1 | Intracellular |
Relative gene expression was measured with qPCR on a StepOnePlus Real-Time PCR System using Power SYBR Green PCR Master Mix (both, Life Technologies). Reactions were performed in triplicate in a 15 µl volume containing 300 nM each primer and 0.4 µl cDNA template. The cycling parameters were the same for all assays: initial denaturation at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 62 °C for 60 s. Single gene products were obtained for all reactions, as assessed with melt curve analysis. Relative gene expression was calculated using the ∆∆Ct method [43] to first normalize the expression level of the target gene to that of the reference gene, and then to compare the relative expression of the target gene for treated versus control eyes. The geometric group mean (for the seven biologic replicates) of these expression ratios was used to calculate the fold change in gene expression for each of the target genes.
Statistical analysis
One-way ANOVA (Statistica, StatSoft, Tulsa, OK) was used to compare control and normal eye refractive data across groups of animals; paired t tests were used to determine whether statistically significant myopia (treated eye versus control eye) or recovery had occurred. For gene expression data, paired t tests were used to assess treated-eye versus control-eye differences; unpaired t tests were used to test for gene expression differences between all independent groups. In all cases, p<0.05 was considered statistically significant, and no adjustment was applied for a possible false discovery rate. Linear regressions between all expression differences were made in SigmaPlot (Systat Software, San Jose, CA).
Results
Refraction
Recovery groups
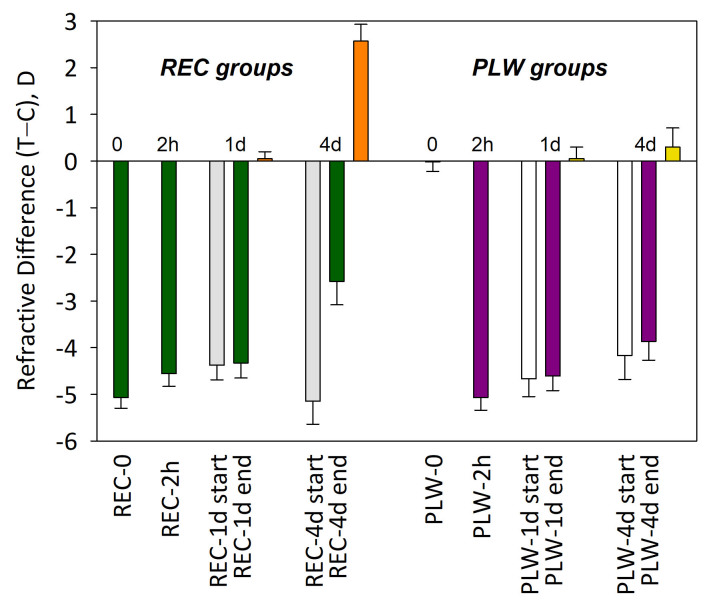
At the start of recovery, the treated eyes in all REC groups had fully compensated to the minus lens. Measured with the lens in place, the refractive difference between the treated eyes and the control eyes across groups was 0.1 ± 0.2 D (mean ± standard error of the mean [SEM]). Thus, the refractive hyperopia present at the start of lens wear had dissipated. With the –5 D lens removed, the treated eyes were all myopic compared with the fellow untreated control eyes (Figure 2). The relative myopia in the REC-0 treated eyes was –5.1 ± 0.2 D. The REC-2h treated eyes were –4.6 ± 0.3 D myopic at the start of recovery, and were not remeasured after 2 h of recovery, as no refractive change was expected. The REC-1d treated eyes were −4.4 ± 0.3 D myopic at the start of recovery. After 1 day, the myopia had recovered by only 0.1 ± 0.1 D. The REC-4d group treated eyes were –5.2 ± 0.5 D myopic at the start of recovery. After 4 days, the (treated – control eye) difference had recovered by 2.6 ± 0.4 D, compared with the start of recovery.
Figure 2.
Refractive difference between treated and control eyes for each group (mean ± standard error of the mean [SEM]). For the recovery (REC) groups, the dark green bars show the amount of myopia at the end of recovery. For the groups that recovered for 1 day (REC-1d) and 4 days (REC-4d), the amount of myopia at the start of recovery is shown by the gray bars; the orange bar shows the amount of refractive recovery. For the plus-lens wear (PLW) groups, the dark purple bars show the myopia present, while the lens was in place, at the end of PLW. For the groups that wore a monocular +5 D lens for 1 day (PLW-1d) and 4 days (PLW-4d), the amount of myopia of the start of PLW is shown by the open bars. The yellow bars show the refractive change (reduction in myopia while wearing the +5 D lens) between the start and end of PLW.
PLW groups
The PLW-0 (normal) group did not experience plus-lens wear, and the refractive difference between right eyes and left eyes was negligible (–0.02 ± 0.2 D, right eyes – left eyes). The other three groups also had little refractive difference between eyes at the start of PLW (0.04 ± 0.17 D). Plus-lens wear groups produced refractive myopia that was comparable in magnitude to the myopia experienced by the treated eyes in the recovery groups at the start of their recovery (Figure 2). Measured with the +5 D lens in place, the PLW-2h group had relative myopia of –5.1 ± 0.3 D; the eyes were not remeasured after 2 h. In the PLW-1d group, the plus lens produced a myopia of –4.7 ± 0.4 D. After 1 day, the myopia was slightly less, −4.6 ± 0.3 D, a hyperopic shift of 0.1 ± 0.2 D. In the PLW-4d group, the +5 D lens initially produced a myopia of –4.2 ± 0.5 D. After 4 days, the myopia decreased slightly to −3.9 ± 0.4 D, a hyperopic shift of 0.3 ± 0.4 D. Thus, as reported previously [35], plus-lens wear in juvenile tree shrews had little refractive effect. Any STOP signals produced by the refractive myopia were essentially ignored by the plus-lens wearing eyes, as measured by the lack of statistically significant change in the refractive state.
Differential gene expression
REC groups
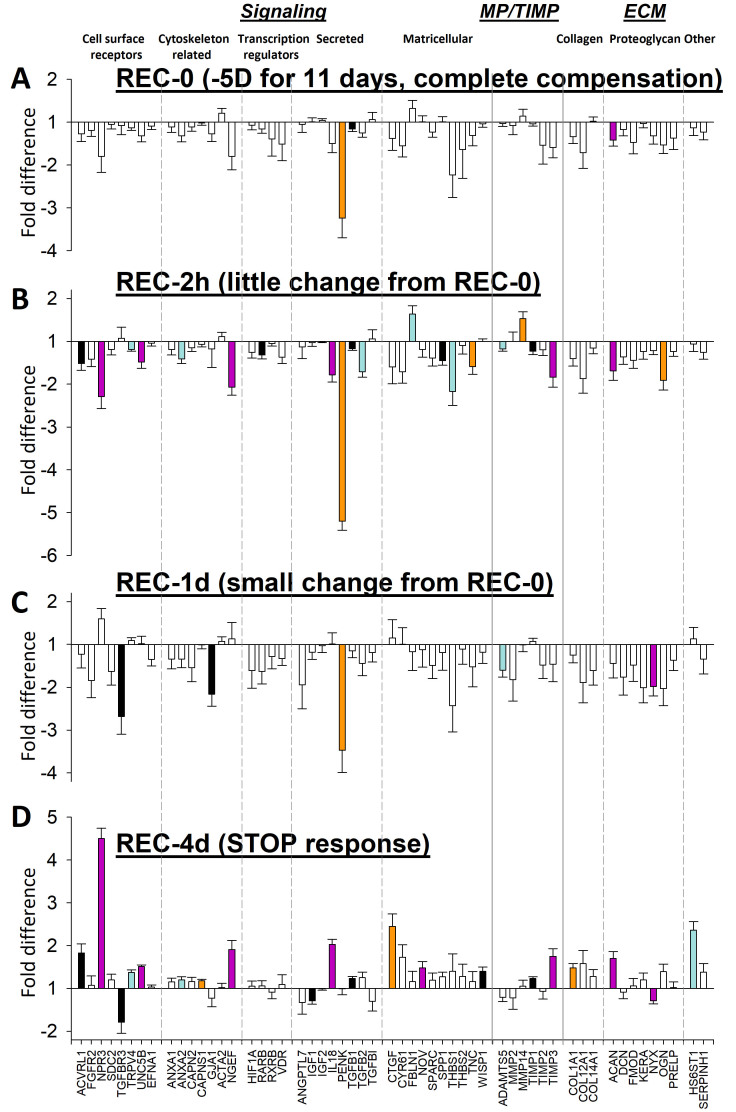
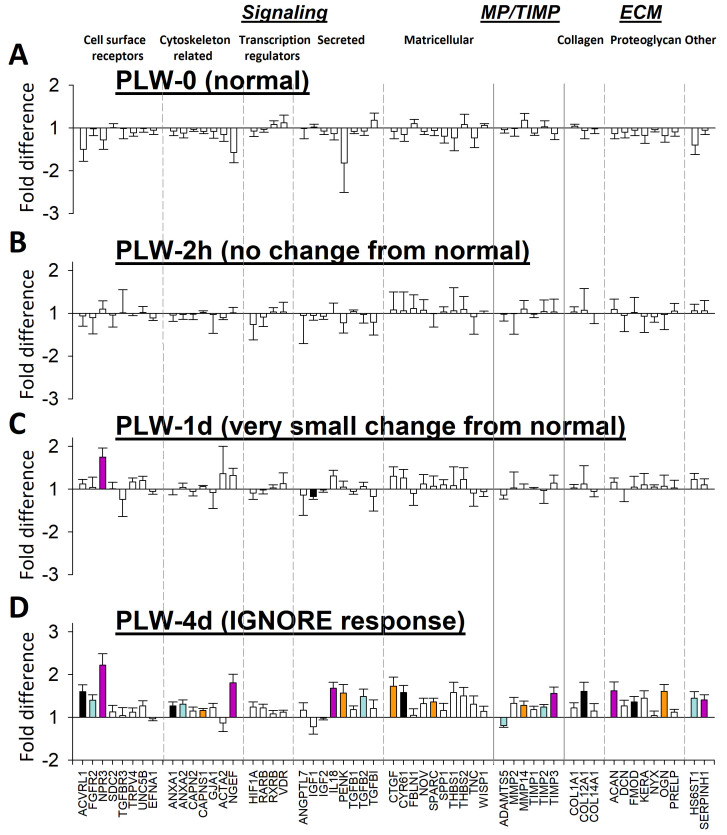
Figure 3 shows the gene expression differences between the treated eyes and the control eyes in all recovery groups. The expression fold differences are listed in Figure 4. The overall pattern of differential gene expression in the REC-0 and REC-2h groups was similar: mRNA levels generally were lower in the recovering eyes than in the control eyes; both groups of eyes showed the STAY responses of the elongated sclera described previously when eyes have compensated for the minus lens, so that the refractive hyperopia has dissipated, but the eye remains elongated [33].
Figure 3.
Gene expression fold differences (treated eyes versus control eyes). A: Recovery for 0 h (REC-0). B: Recovery for 2 h. C: Recovery for 1 day. D: Recovery for 4 days. Filled bars represent statistically significant differences between the treated and control eyes (p<0.05). The bar color is arbitrary, and is intended to help in comparing the same gene in the different conditions. Error bars = standard error of the mean (SEM).
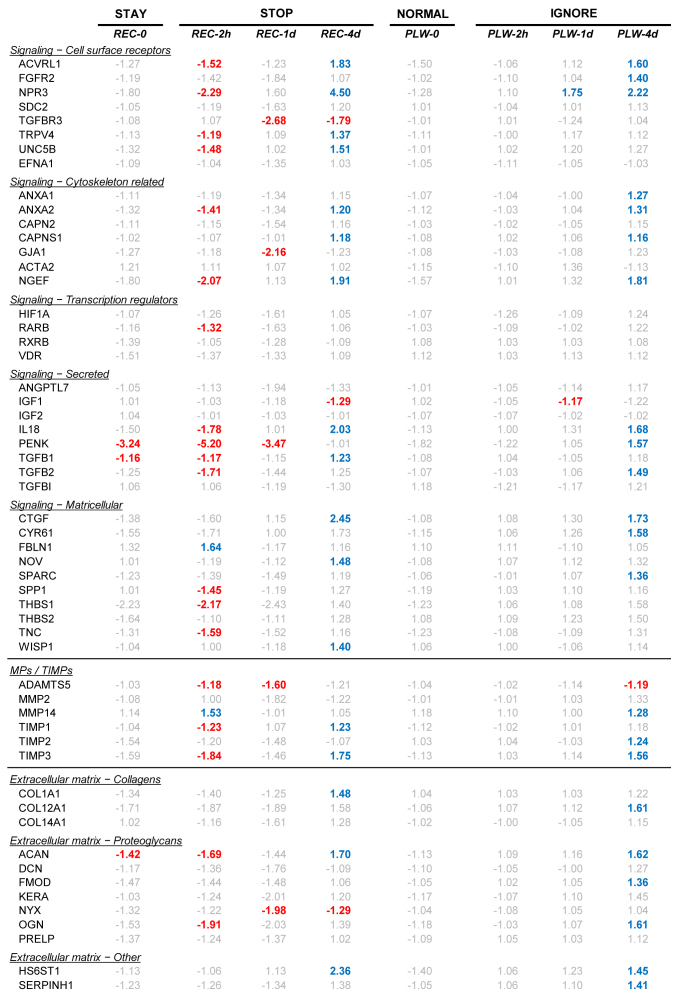
Figure 4.
Gene expression differences comparing treated versus control eyes. Red text = significant down-regulation, blue = significant up-regulation, grey = expression difference not statistically significant.
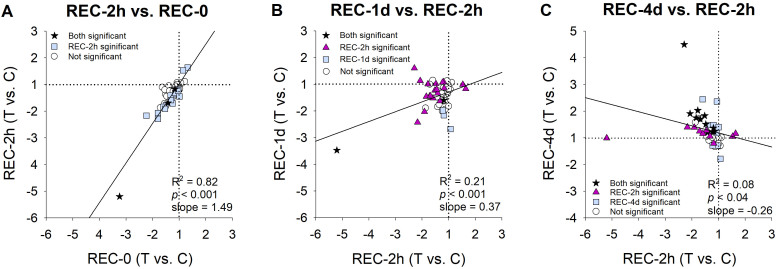
The similarity of the overall patterns is shown in Figure 5A which shows the high correlation (R2 = 0.82) between the REC-0 pattern (Figure 3A) and the REC-2h pattern (Figure 3B). The primary difference was that more of the mRNA expression differences were statistically significant in the REC-2h group than in the REC-0 group.
Figure 5.
Change in gene expression patterns over time during recovery from minus-lens induced myopia. Negative values indicate downregulation in the treated eyes. A: The pattern at the start of recovery (Figure 3A) compared with that observed after 2 h of recovery (Figure 3B). B: The differential expression after 2 h of recovery (Figure 3B) compared with that after 1 day (Figure 3C). C: The pattern after 2 h (Figure 3B) compared with the pattern after 4 days (Figure 3D).
After 1 day of recovery, the REC-1d gene expression pattern (Figure 3C) still resembled the STAY pattern found at the start of recovery; most mRNA levels were lower in the recovering-eye sclera, but the size of the fold differences was lower, and fewer genes showed statistically significant downregulation. The change from the pattern at 2 h is shown more clearly in Table 2 and in Figure 5B, where the correlation between the REC-2h group and the REC-1d group is much lower (R2 = 0.21).
After 4 days of recovery, a STOP gene expression signature was well-established (Figure 3D). As reported previously [33], this pattern was different from that at the start of recovery (Figure 5C). Many genes whose expression was downregulated at the start of recovery now showed upregulation in the recovering eye after 4 days of recovery. Other genes that did not show differential regulation at REC-0 or REC-2h now showed statistically significant upregulation.
PLW groups
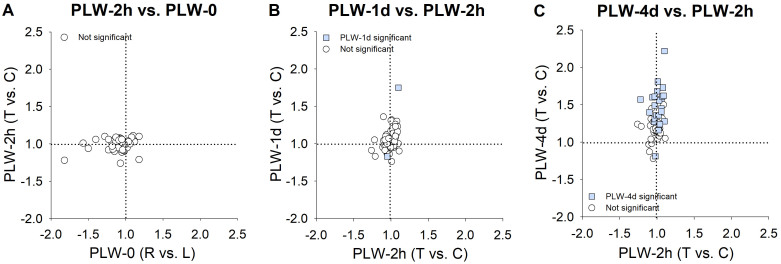
Figure 6 shows the expression differences between the treated eyes and the control eyes in the groups that wore a +5 D lens. The expression values are listed in Table 2. In the PLW-0 group (normal animals), none of the mRNA levels for any candidate gene differed statistically significantly between the right and left eyes (Figure 6A). This was also the case for the PLW-2h group (Figure 6B). In Figure 7A, the correlation between the PLW-0 pattern (Figure 6A) and the PLW-2h pattern (Figure 6B) is plotted. Most of the fold differences are clustered around 1.0, and the low correlation between the expression differences at the two time points (R2 = 0.04, p = 0.15) was not statistically significant.
Figure 6.
Gene expression fold differences. A: Normal animals with 38 days of visual experience (DVE; right eyes versus left eyes). B-D show treated eye versus control eye differences. B: Plus-lens wear for 2 h at 35 DVE. C: Plus-lens wear for 1 day from 35 DVE. D: Plus-lens wear for 4 days from 35 DVE. Filled bars represent statistically significant differences between the treated and control eyes (p<0.05). The bar color is arbitrary, and is intended to help in comparing the same gene in the different conditions. Error bars = standard error of the mean (SEM).
Figure 7.
Change in gene expression patterns over time during plus-lens wear. A: The pattern before plus-lens wear (Figure 6A) compared with that observed after 2 h of plus-lens wear (PLW-2h; Figure 6B). B: The change in the differential expression compared between the PLW-2h group (Figure 6B) and the PLW-1d group (Figure 6C). C: The change from the PLW-2h group (Figure 6B) compared to the PLW-4d group (Figure 6D).
After 1 day of PLW, the overall expression pattern (Figure 6C) still resembled the pattern at the start of PLW. The exception was that two genes now showed statistically significant differential expression: NPR3 (Gene ID 4833, OMIM 108962) was upregulated, and IGF1 (Gene ID 3479, OMIM 147440) was downregulated. However, comparison of the differential expression of all genes at PLW-1d with PLW-2h, including fold differences that were not statistically significantly different (Figure 7B), suggested that the gene expression pattern at PLW-1d was not identical to the pattern at PLW-2h. After 1 day of PLW, more genes showed slightly higher expression in the plus-lens wearing eye versus the control eye than was the case after 2 h of PLW. By 4 days of PLW (Figure 6D, Figure 7C), the gene expression pattern was different from the pattern at the start of PLW. Twenty-two genes were statistically significantly upregulated, and a single gene, ADAMTS5 (Gene ID 11096, OMIM 605007), was downregulated. The scleral mRNA response pattern at REC-4d occurred in the sclera of treated eyes that refractively changed only slightly in response to wearing a plus lens. We refer to this as a scleral “IGNORE” signature, because the pattern occurred in the sclera of eyes that were essentially ignoring the lens-induced myopia.
Comparison of STOP and IGNORE signatures
After 4 days of recovery or of PLW, the overall pattern of differential mRNA expression in the candidate genes in the REC-4d (STOP) group and the PLW-4d (IGNORE) group was upregulation. The two patterns are compared in Figure 8. Similar numbers of genes in the sample of 55 genes were statistically significantly upregulated in the REC-4d group (17) and in the PLW-4d group (22); ten genes were up-regulated in both groups: ACVRL1 (Gene ID 94, OMIM 601284), NPR3, ANXA2 (Gene ID 302, OMIM 151740), CAPNS1 (Gene ID 826, OMIM 114170), NGEF (Gene ID 25791, OMIM 605991), IL18 (Gene ID 3606, OMIM 600953), CTGF (Gene ID 1490, OMIM 121009), TIMP3 (Gene ID 7078, OMIM 188826), ACAN (Gene ID 176, OMIM 155760), and HS6ST1 (Gene ID 9394, OMIM 604846). However, an additional seven genes showed statistically significant upregulation in the REC-4d group that did not show differential expression in the PLW-4d group. Twelve other genes whose expression was statistically significantly upregulated in the PLW-4d group did not have statistically significant expression differences in the REC-4d group. Moreover, the magnitude of the statistically significant fold differences was larger in the REC-4d group than in the PLW-4d group, as indicated by the low slope (0.33) of the regression line.
Figure 8.
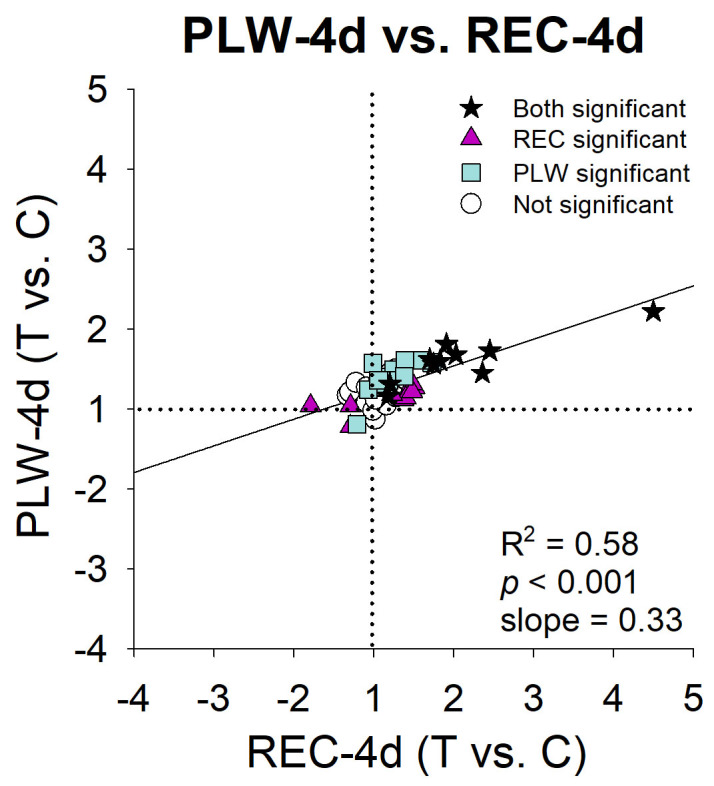
Comparison of the treated eye versus control eye gene expression differences in PLW and REC: PLW-4d (Figure 6D) versus REC-4d (Figure 3D). Stars = fold differences statistically significant for both treatments, triangles = fold differences statistically significant only for the Recovery (REC) group, squares = fold differences statistically significant only for the plus-lens wear (PLW) group, circles = fold differences not statistically significant for either treatment.
Discussion
In juvenile tree shrews, the refractive response of the eyes differed during recovery from myopia versus plus-lens wear. Removal of a monocular minus lens, after the eyes had compensated for the lens, produced refractive myopia that is apparently similar to the myopia produced by placing a monocular plus lens in front of a normal eye (Figure 2). However, in recovery, the myopia decreased over time whereas the myopia produced by plus-lens wear changed little over the same time period.
The similar myopia produced differential expression of many genes in the sclera of the treated versus the control eyes in both conditions, indicating that signals initiated by the retina reached the sclera. However, the differential gene expression patterns (“signatures”) in the sclera differed between the recovery and plus lens-wear groups in terms of which genes were upregulated, downregulated, or unaffected, and the magnitude of the differential gene expression.
Because a limited number of genes were examined in this study, the STOP and IGNORE gene expression signatures are incomplete. The 55 genes examined in this study are a subset of a presumably much larger group of genes that show differential expression in recovery from induced myopia. A preliminary whole-transcriptome (RNA-Seq) analysis of treated and control eyes from three of the REC-4d animals suggested that perhaps almost 400 transcripts (from the nearly 15,000 transcripts found to be expressed in tree shrew sclera) may be up- or downregulated by at least 1.20-fold (data not shown). Thus, the sampled genes were not intended to represent the whole scleral expression profile. Instead, the 55 candidate genes were sufficient to show that there are different scleral gene expression patterns in response to a similar refractive myopia. In addition, the myopia-produced mRNA responses in the sclera appear to be complex, and are unlikely to depend on the regulation of a single gene, or even a small number of genes, earlier in the signaling cascade from the retina.
We used altered levels of mRNA as a way to detect altered responses in the scleral fibroblasts. Changes in mRNA levels may, or may not, produce changes in protein levels, and proteins are the “effectors” that actually produce scleral remodeling. We previously compared changes in protein expression versus mRNA levels after 4 days of minus-lens wear and after 4 days of recovery, and found a low correlation between statistically significant differences in protein abundance and mRNA expression [13]. Other studies have reported a similarly low correlation [44-46]. This low correlation occurs because mRNA levels reflect the activity of cells at the time the sample is collected, whereas protein levels reflect the cumulative activity of synthetic and degradative processes over time, influenced by a large repertoire of systems that enhance or repress the synthesis of proteins from a specific copy number of mRNA transcripts. In this study, we focused on learning whether the scleral fibroblasts respond differently to the myopic refractive state produced by two different visual conditions: recovery from induced myopia and plus-lens wear. How these changes may translate into altered protein levels is undetermined.
STOP signature
Gao et al. [34] and Guo et al. [33] found that the gene expression pattern at the onset of recovery was similar to the GO signature that developed during minus-lens wear, although the eyes had compensated for the minus lens, thus removing the hyperopia that caused the scleral remodeling and axial elongation. Guo et al. [33] characterized this as a “STAY” response, and suggested that it was involved in maintaining the eyes in the elongated state; the gene expression categorized as a STAY signature was needed to keep the lens-wearing eye emmetropic with the lens. With the inclusion of more time points, the present study confirmed that this STAY pattern was still present after 2 h of recovery (the REC-2h group). Evidently, 2 h was too short a time for STOP signals initiated by the retina to pass through the RPE and choroid to begin the process of changing the STAY signature to a STOP gene expression pattern.
After 1 day of recovery, the recovering eye showed little refractive change. The mRNA levels showed a reduced form of the STAY signature (similar pattern, fewer statistically significant fold differences; Figure 3C, Figure 4), suggesting that the scleral fibroblasts were beginning to respond to STOP signals initiated by the retina arriving from the choroid. mRNA levels for ten “early response” genes were different at REC-1d versus REC-2h, that were predictive of the differential expression at REC-4d. Eight of these encode proteins involved in signaling. Four genes (ACVRL1, NPR3, TRPV4 [Gene ID 59341, OMIM 605427], and UNC5B [Gene ID 219699, OMIM 607870]) that encode cell-surface receptors were statistically significantly downregulated at REC-2h, but not at REC-1d, and were statistically significantly upregulated at REC-4d. In contrast, the mRNA for TGFBR3 (Gene ID 7049, OMIM 600742), not statistically significantly affected at REC-2h, was statistically significantly downregulated at REC-1d, and remained downregulated at REC-4d. Two genes for cytoskeleton-related proteins (ANXA2 and NGEF) also were downregulated at REC-2h, but not at REC-1d, and upregulated at REC-4d, as was mRNA for the signaling protein IL18. In addition, the mRNA for TIMP1 (Gene ID 7076, OMIM 305370), downregulated at REC-2h, was not statistically significantly different in recovering-eye versus control-eye sclera at REC-1d, and was upregulated at REC-4d. mRNA for NYX (Gene ID 60506, OMIM 300278) showed an opposite pattern: no statistically significant differential expression at REC-2h, but downregulation at REC-1d and REC-4d.
The transformation of the scleral response from STAY to STOP continued. After 2 days of recovery, Guo et al. [33] found that there was 1.3 ± 0.3 D of refractive recovery, and that the STAY signature was replaced by an early STOP signature. By 4 days, refractive recovery was approximately half completed (Figure 2), and the STOP signature was well-established in the sclera (Figure 3D, Figure 4). The delay in changing from STAY to STOP signatures may reflect the time taken for retinal signals, which presumably occur rapidly, to produce changes in the RPE [11], which then cause changes in the choroid [1] that reach the sclera where they cause scleral fibroblasts to change their gene expression from the generally downregulated STAY condition to the generally upregulated condition that characterizes the STOP signature. As described by Guo et al. [33] and Gao et al. [34], the observed upregulation involved a broad array of genes involved in signaling, including mRNAs for cell-surface receptors, cytoskeletal, transcription-related, secreted, and matricellular proteins, along with TIMP1 and HS6ST1, suggestive of increased formation of extracellular matrix.
IGNORE signature
The development of an IGNORE signature in the sclera confirms that retinal signals reach the sclera and produce altered mRNA levels. However, these changes in gene expression were slower to develop and did not appear to cause slowed axial elongation, as measured by the lack of refractive response to the plus-lens wear. The scleral IGNORE gene expression signature, like the STOP signature, developed over a period of days, but the gene expression signature produced in a normal sclera was different from either the STAY or STOP signature. In the normal (PLW-0) group, as expected, there were no statistically significant differences in gene expression between the two eyes. After 2 h of PLW, no genes showed statistically significant differential expression. After 1 day of plus-lens wear, most of the sampled genes still did not have statistically significant differential expression; mRNA levels for only two genes (NPR3, upregulated; IGF1, downregulated) were statistically significantly different between the treated and control eyes. In contrast, at REC-1d, ten early response genes had different differential expression from that found at REC-2h. At PLW-4d, the expression of 22 genes was statistically significantly upregulated in the plus-lens wearing eyes; expression of one gene (ADAMTS5) was downregulated (Figure 4).
The IGNORE signature shared similarities to, and differences from, the STOP signature. A similar number of genes in the sample showed differential expression in STOP (20 genes) and in IGNORE (23 genes), including genes in the signaling, MPs/TIMPs, and ECM categories. In both signatures, the majority of genes were upregulated (17 in STOP, 22 in IGNORE) in the treated-eye sclera. However, the two signatures differed in which genes were statistically significantly expressed. Although ten genes were statistically significantly upregulated in both conditions, the other genes whose expression was statistically significantly up- or downregulated were different between the two signatures (Figure 4), and the magnitude of the fold differences was lower in the IGNORE signature (Figure 7). It would appear that the upregulation of these ten genes in recovery and plus-lens wear (Figure 4) might be necessary for the scleral remodeling found in recovery, but evidently was not sufficient to produce a substantial change in refractive state in plus-lens wear, perhaps because other genes responded differently.
Different response, or different signal?
As noted in the Introduction, one aim of this study was to learn whether the lack of a refractive effect of the plus lens-induced myopia in normal animals occurred because the myopia-produced retinal signals did not pass through the signaling cascade to reach the sclera. The development of an IGNORE mRNA signature in the sclera over the 4 days of plus-lens wear confirmed that signals initiated by the retina produced by the myopic refractive state of the treated eyes reach the sclera and produce a response over a similar time-course as in recovery.
That these signals produced different gene expression signatures in the normal (PLW) versus myopic (REC) sclera may be due to differences in the state of the sclera, and may underlie the presence of a refractive effect in recovery and the lack thereof in plus-lens wear. The STOP and IGNORE scleral response patterns involve many genes, and it is not possible to attribute the different refractive responses to any one gene. Nonetheless, it may be of interest to compare the responses of some genes that seem likely, based on previous studies, to participate in the decreased scleral extensibility that occurs in recovery and is responsible for the slowed axial elongation rate that underlies recovery. For instance, in recovery, there is a change in mRNA expression of COL1A1 (Gene ID 1277, OMIM 120150) from downregulated to upregulated [29], but in this study, there was no statistically significant upregulation in the IGNORE pattern. mRNA levels for ACAN and OGN (Gene ID 4969, OMIM 602383), two proteoglycan core proteins, were downregulated at the start of recovery and became upregulated (OGN not statistically significantly) in STOP. Both genes were upregulated in IGNORE, but had not been downregulated at the start of plus-lens wear. Similarly, mRNA expression for TIMP3 changed from downregulated to upregulated in STOP, and was upregulated only in IGNORE.
As in previous studies, the sclera in the recovering animals had altered mRNA levels (Figure 3), protein levels [12], and biomechanical properties [16,32]. The sclera in the PLW groups was normal at the start of lens wear. If the same signals arrived at the sclera, they produced differing effects: remodeling and a reduced creep rate in the remodeled, elongated sclera that resulted in refractive recovery, but a negligible refractive effect in normal sclera. Possibly, the signals could not, in normal sclera, produce the specific changes needed to slow the axial elongation rate and produce refractive compensation to the plus lens. For instance, all but three (CAPNS1, CTGF, and HS6ST1) of the ten genes whose expression levels were upregulated in both recovery and plus-lens wear were downregulated in the recovery groups at the end of minus-lens wear (REC-0 or REC-2h), but not at the start of plus-lens wear. Perhaps the transition from downregulation to upregulation in those other seven genes during recovery was important in producing the scleral alterations that produced refractive recovery, a process that did not occur in the plus-lens wear groups.
However, normal versus remodeled sclera cannot explain why, in a previous study, younger, infantile tree shrews (11 DVE), also with a normal sclera, responded to a plus lens, becoming nearly emmetropic while wearing the lens and hyperopic with the lens removed [35]. This response to plus-lens wear in young animals may be related to the presence of more active growth in normal sclera. At 11 DVE, rapid growth is occurring in the sclera that is absent at the older age (35 DVE) used in the present study [27,47,48]. At 11 DVE, collagen synthesis is active, the scleral lamellae are increasing in thickness, and there is little susceptibility to minus-lens wear [24,26,27,47]. Siegwart and Norton [35] suggested that at this age, the PLW-induced myopia produced the refractive effect by slowing scleral growth, similar to the slowed growth that occurs in chick sclera in response to plus-lens wear [49]. In contrast, at the older (35 DVE) age of the PLW animals in the present study, collagen accumulation had stabilized [27], and normal scleral extensibility was low [16]. Thus, the presence of rapid growth in the sclera of younger animals, absent in older animals, might account for the different refractive response to plus-lens wear [50].
Different signals arriving at the sclera?
The difference in the refractive response, and in the scleral response signatures (STOP versus IGNORE), also might have occurred because the signals arriving at the sclera in plus-lens wear differed from those that occurred in recovery. The refractive myopia produced by a plus lens may have not generated the same retinal signals as did the myopia that occurred at the start of recovery. Recently, Ashby and Karouta [51] reported that retinal expression of the early-response gene EGR1 (Gene ID 1958, OMIM 128990) is upregulated in chicks during recovery, but downregulated in plus-lens wear. Perhaps plus lenses produce aberrations or distortions in the visual field that differ from those in eyes with an induced myopia that, in turn, produce differing retinal signals.
Even if the myopia in recovery and PLW produces identical retinal responses, signals in the direct retinoscleral signaling cascade must travel through the RPE and choroid to reach the sclera. Cells in these structures respond to minus-lens wear with altered gene expression [11,52]. The signals communicated through these compartments in the direct pathway may have modified signals initiated by the retina on the way to the sclera in ways that produced slowed axial elongation in minus-lens wear and not in plus-lens wear.
The lack of refractive response in the PLW groups at 35 DVE might indicate that the sclera of juvenile tree shrews cannot respond to visual stimuli with slowed axial elongation. However, there are visual stimuli that can produce slowed axial elongation and hyperopia in normal tree shrews at 35 DVE, and even at 95 DVE. Gawne et al. [53] found that housing tree shrews in narrow-band long wavelength (red) light causes normal eyes to respond with slowed axial elongation, producing a hyperopic refractive shift at ages when a plus lens is ineffective. Thus, a normal juvenile sclera can react to signals initiated by the retina with slowed axial elongation; the lack of a response to a plus lens must not depend solely on the sclera. Indeed, the rate of hyperopia development (hyperopic shift after 4 days in red light) was nearly the same as the hyperopic change in the REC-4d group, and much greater than the small hyperopic shift seen in the PLW-4d group.
Thus, although the present study showed that myopia-induced changes in mRNA levels occur for many scleral genes in PLW, we could not resolve the question of whether the lack of refractive response to PLW is due to the way the normal sclera in juvenile animals responds to the arriving signals or to alterations in the myopia-related signals that occur before they reach the sclera in normal animals. Resolving this question will require additional studies that examine growth, gene expression, and biomechanical properties in the sclera, and signaling in the RPE and choroid in response to PLW in young tree shrews, as well as similar studies in adolescent animals exposed to narrow-band red light.
Acknowledgments
This study was supported by NIH grants R01EY005922 and P30EY003039. Lin Guo was supported in part by funds from the School of Optometry. This work was performed in partial fulfillment of the requirements for the degree of Doctor of Philosophy at the University of Alabama at Birmingham (Lin Guo). Preliminary results were presented in abstract form.
Appendix 1.
To access the data, click or select the words “Appendix 1.”
References
- 1.He L, Frost MR, Siegwart JT, Jr, Norton TT. Gene expression signatures in tree shrew choroid in response to three myopiagenic conditions. Vision Res. 2014;102:52–63. doi: 10.1016/j.visres.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mutti DO, Mitchell GL, Jones LA, Friedman NE, Frane SL, Lin WK, Moeschberger ML, Zadnik K. Axial growth and changes in lenticular and corneal power during emmetropization in infants. Invest Ophthalmol Vis Sci. 2005;46:3074–80. doi: 10.1167/iovs.04-1040. [DOI] [PubMed] [Google Scholar]
- 3.Norton TT. Animal models of myopia: Learning how vision controls the size of the eye. ILAR J. 1999;40:59–77. doi: 10.1093/ilar.40.2.59. [DOI] [PubMed] [Google Scholar]
- 4.Schaeffel F, Burkhardt E, Howland HC, Williams RW. Measurement of refractive state and deprivation myopia in two strains of mice. Optom Vis Sci. 2004;81:99–110. doi: 10.1097/00006324-200402000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Schaeffel F, Glasser A, Howland HC. Accommodation, refractive error and eye growth in chickens. Vision Res. 1988;28:639–57. doi: 10.1016/0042-6989(88)90113-7. [DOI] [PubMed] [Google Scholar]
- 6.Schaeffel F, Howland HC. Mathematical model of emmetropization in the chicken. J Opt Soc Am A Opt Image Sci Vis. 1988;5:2080–6. doi: 10.1364/josaa.5.002080. [DOI] [PubMed] [Google Scholar]
- 7.Shen W, Sivak JG. Eyes of a lower vertebrate are susceptible to the visual environment. Invest Ophthalmol Vis Sci. 2007;48:4829–37. doi: 10.1167/iovs.06-1273. [DOI] [PubMed] [Google Scholar]
- 8.Smith EL, III, Hung L-F. The role of optical defocus in regulating refractive development in infant monkeys. Vision Res. 1999;39:1415–35. doi: 10.1016/s0042-6989(98)00229-6. [DOI] [PubMed] [Google Scholar]
- 9.Troilo D, Nickla DL, Wildsoet CF. Form deprivation myopia in mature common marmosets (Callithrix jacchus). Invest Ophthalmol Vis Sci. 2000;41:2043–9. [PubMed] [Google Scholar]
- 10.Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–68. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 11.He L, Frost MR, Siegwart JT, Jr, Norton TT. Altered gene expression in tree shrew retina and retinal pigment epithelium produced by short periods of minus-lens wear. Exp Eye Res. 2018;168:77–88. doi: 10.1016/j.exer.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frost MR, Norton TT. Alterations in protein expression in tree shrew sclera during development of lens-induced myopia and recovery. Invest Ophthalmol Vis Sci. 2012;53:322–36. doi: 10.1167/iovs.11-8354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo L, Frost MR, He L, Siegwart JT, Jr, Norton TT. Gene expression signatures in tree shrew sclera in response to three myopiagenic conditions. Invest Ophthalmol Vis Sci. 2013;54:6806–19. doi: 10.1167/iovs.13-12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moring AG, Baker JR, Norton TT. Modulation of glycosaminoglycan levels in tree shrew sclera during lens-induced myopia development and recovery. Invest Ophthalmol Vis Sci. 2007;48:2947–56. doi: 10.1167/iovs.06-0906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norton TT, Rada JA. Reduced extracellular matrix in mammalian sclera with induced myopia. Vision Res. 1995;35:1271–81. doi: 10.1016/0042-6989(94)00243-f. [DOI] [PubMed] [Google Scholar]
- 16.Siegwart JT, Jr, Norton TT. Regulation of the mechanical properties of tree shrew sclera by the visual environment. Vision Res. 1999;39:387–407. doi: 10.1016/s0042-6989(98)00150-3. [DOI] [PubMed] [Google Scholar]
- 17.McBrien NA, Moghaddam HO, Cottriall CL, Leech EM, Cornell LM. The effects of blockade of retinal cell action potentials on ocular growth, emmetropization and form deprivation myopia in chicks. Vision Res. 1995;35:1141–52. doi: 10.1016/0042-6989(94)00237-g. [DOI] [PubMed] [Google Scholar]
- 18.Norton TT, Essinger JA, McBrien NA. Lid-suture myopia in tree shrews with retinal ganglion cell blockade. Vis Neurosci. 1994;11:143–53. doi: 10.1017/s0952523800011184. [DOI] [PubMed] [Google Scholar]
- 19.Troilo D, Gottlieb MD, Wallman J. Visual deprivation causes myopia in chicks with optic nerve section. Curr Eye Res. 1987;6:993–9. doi: 10.3109/02713688709034870. [DOI] [PubMed] [Google Scholar]
- 20.Wildsoet CF, McFadden SA. Optic nerve section does not prevent form deprivation-induced myopia or recovery from it in the mammalian eye. Invest Ophthalmol Vis Sci. 2010;51:1737. [Google Scholar]
- 21.Rohrer B, Stell WK. Basic fibroblast growth factor (bFGF) and transforming growth factor beta (TGF-β) act as stop and go signals to modulate postnatal ocular growth in the chick. Exp Eye Res. 1994;58:553–61. doi: 10.1006/exer.1994.1049. [DOI] [PubMed] [Google Scholar]
- 22.Amedo AO, Norton TT. Visual guidance of recovery from lens-induced myopia in tree shrews (Tupaia glis belangeri). Oph Phys Optics. 2012;32:89–99. doi: 10.1111/j.1475-1313.2011.00875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McBrien NA, Gentle A, Cottriall C. Optical correction of induced axial myopia in the tree shrew: Implications for emmetropization. Optom Vis Sci. 1999;76:419–27. doi: 10.1097/00006324-199906000-00022. [DOI] [PubMed] [Google Scholar]
- 24.Norton TT, Amedo AO, Siegwart JT., Jr The effect of age on compensation for a negative lens and recovery from lens-induced myopia in tree shrews (Tupaia glis belangeri). Vision Res. 2010;50:564–76. doi: 10.1016/j.visres.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaikh AW, Siegwart JT, Jr, Norton TT. Effect of interrupted lens wear on compensation for a minus lens in tree shrews. Optom Vis Sci. 1999;76:308–15. doi: 10.1097/00006324-199905000-00019. [DOI] [PubMed] [Google Scholar]
- 26.Siegwart JT, Jr, Norton TT. The susceptible period for deprivation-induced myopia in tree shrew. Vision Res. 1998;38:3505–15. doi: 10.1016/s0042-6989(98)00053-4. [DOI] [PubMed] [Google Scholar]
- 27.Norton TT, Miller EJ. Collagen and protein levels in sclera during normal development, induced myopia and recovery in tree shrews. Invest Ophthalmol Vis Sci. 1995;36:3517. [Google Scholar]
- 28.Gentle A, McBrien NA. Retinoscleral control of scleral remodelling in refractive development: A role for endogenous FGF-2? Cytokine. 2002;18:344–8. doi: 10.1006/cyto.2002.1046. [DOI] [PubMed] [Google Scholar]
- 29.Siegwart JT, Jr, Norton TT. The time course of changes in mRNA levels in tree shrew sclera during induced myopia and recovery. Invest Ophthalmol Vis Sci. 2002;43:2067–75. [PMC free article] [PubMed] [Google Scholar]
- 30.Guggenheim JA, McBrien NA. Form-deprivation myopia induces activation of scleral matrix metalloproteinase-2 in tree shrew. Invest Ophthalmol Vis Sci. 1996;37:1380–95. [PubMed] [Google Scholar]
- 31.Phillips JR, Khalaj M, McBrien NA. Induced myopia associated with increased scleral creep in chick and tree shrew eyes. Invest Ophthalmol Vis Sci. 2000;41:2028–34. [PubMed] [Google Scholar]
- 32.Grytz R, Siegwart JT., Jr Changing material properties of the tree shrew sclera during minus lens compensation and recovery. Invest Ophthalmol Vis Sci. 2015;56:2065–78. doi: 10.1167/iovs.14-15352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo L, Frost MR, Siegwart JT, Jr, Norton TT. Scleral gene expression during recovery from myopia compared with expression during myopia development in tree shrew. Mol Vis. 2014;20:1643–59. [PMC free article] [PubMed] [Google Scholar]
- 34.Gao H, Frost MR, Siegwart JT, Jr, Norton TT. Patterns of mRNA and protein expression during minus-lens compensation and recovery in tree shrew sclera. Mol Vis. 2011;17:903–19. [PMC free article] [PubMed] [Google Scholar]
- 35.Siegwart JT, Jr, Norton TT. Binocular lens treatment in tree shrews: Effect of age and comparison of plus lens wear with recovery from minus lens-induced myopia. Exp Eye Res. 2010;91:660–9. doi: 10.1016/j.exer.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siegwart JT, Jr, Norton TT. Goggles for controlling the visual environment of small animals. Lab Anim Sci. 1994;44:292–4. [PubMed] [Google Scholar]
- 37.Norton TT, Wu WW, Siegwart JT., Jr Refractive state of tree shrew eyes measured with cortical visual evoked potentials. Optom Vis Sci. 2003;80:623–31. doi: 10.1097/00006324-200309000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKanna JA, Casagrande VA. Atropine affects lid suture myopic development. Doc Ophthalmol. 1981;28:187–92. [Google Scholar]
- 39.Norton TT, Siegwart JT, Jr, German AJ, Robertson J, Wu WW. Comparison of cycloplegic streak retinoscopy with autorefractor measures in tree shrew eyes with, and without, myopia. Invest Ophthalmol Vis Sci. 2000;41:2990. [Google Scholar]
- 40.Norton TT, Siegwart JT, Jr, Amedo AO. Effectiveness of hyperopic defocus, minimal defocus, or myopic defocus in competition with a myopiagenic stimulus in tree shrew eyes. Invest Ophthalmol Vis Sci. 2006;47:4687–99. doi: 10.1167/iovs.05-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glickstein M, Millodot M. Retinoscopy and eye size. Science. 1970;168:605–6. doi: 10.1126/science.168.3931.605. [DOI] [PubMed] [Google Scholar]
- 42.Norton TT, McBrien NA. Normal development of refractive state and ocular component dimensions in the tree shrew (Tupaia belangeri). Vision Res. 1992;32:833–42. doi: 10.1016/0042-6989(92)90026-f. [DOI] [PubMed] [Google Scholar]
- 43.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 44.Maier T, Güell M, Serrano L. Correlation of mRNA and protein in complex biological samples. FEBS Lett. 2009;583:3966–73. doi: 10.1016/j.febslet.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 45.Nie L, Wu G, Zhang W. Correlation between mRNA and protein abundance in Desulfovibrio vulgaris: A multiple regression to identify sources of variations. Biochem Biophys Res Commun. 2006;339:603–10. doi: 10.1016/j.bbrc.2005.11.055. [DOI] [PubMed] [Google Scholar]
- 46.Lee PS, Shaw LB, Choe LH, Mehra A, Hatzimanikatis V, Lee KH. Insights into the relation between mRNA and protein expression patterns: II. Experimental observations in Escherichia coli. Biotechnol Bioeng. 2003;84:834–41. doi: 10.1002/bit.10841. [DOI] [PubMed] [Google Scholar]
- 47.Kang RN, Norton TT. Alteration of scleral morphology in tree shrews with induced myopia. Invest Ophthalmol Vis Sci. 1993;34:2484. [Google Scholar]
- 48.Kang RN, Norton TT. Electron-microscopic examination of tree shrew sclera during normal development, induced myopia, and recovery. Invest Ophthalmol Vis Sci. 1996;37:1491. [Google Scholar]
- 49.Rada JA, Thoft RA, Hassell JR. Increased aggrecan (cartilage proteoglycan) production in the sclera of myopic chicks. Dev Biol. 1991;147:303–12. doi: 10.1016/0012-1606(91)90288-e. [DOI] [PubMed] [Google Scholar]
- 50.Grytz R, El Hamdaoui M. Multi-scale modeling of vision-guided remodeling and age-dependent growth of the tree shrew sclera during eye development and lens-induced myopia. J Elast. 2017;129:171–95. doi: 10.1007/s10659-016-9603-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ashby R, Karouta C. Egr-1 mRNA expression in response to myopic defocus. Invest Ophthalmol Vis Sci. 2017;58:5640. [Google Scholar]
- 52.He L, Frost MR, Siegwart JT, Jr, Norton TT. Gene expression signatures in tree shrew choroid during lens-induced myopia and recovery. Exp Eye Res. 2014;123:56–71. doi: 10.1016/j.exer.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gawne TJ, Ward AH, Norton TT. Long-wavelength (red) light produces hyperopia in juvenile and adolescent tree shrews. Vision Res. 2017;140:55–65. doi: 10.1016/j.visres.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]