Abstract
Prions use cellular machineries for autocatalytic propagation by conformational conversion of the cellular prion protein into the pathological isoform PrPSc. Autophagy is a basic cellular degradation and recycling machinery that delivers cargo to lysosomes. Increase of autophagic flux in cells results in enhanced delivery of PrPSc in late endosomes to lysosomal degradation, providing a therapeutic target for prion diseases. Application of chemical enhancers of autophagy to cell or mouse models of prion infection provided a solid experimental proof-of-concept for this anti-prion strategy. In addition, increasing autophagy also reduces exosomal release of prions and transfer of prion infectivity between cells. Taken together, pharmacological induction of autophagy is a promising target for containing prion diseases, and ideal candidate for future combination therapies.
Introduction
Prion diseases are fatal infectious neurodegenerative disorders of man and animals which are characterized by spongiform degeneration, neuronal loss, PrPSc plaque formation, and gliosis in the central nervous system [1]. The autocatalytic conversion of the cellular prion protein (PrPC) into the pathologic isoform PrPSc is a key feature in prion propagation and prion disease pathogenesis. Prions propagate in the endocytic pathway of cells and endosomal and lysosomal compartments are involved in trafficking, re-cycling and final degradation of PrPSc [2,3]. In persistently prion-infected cells there is equilibrium between prion propagation and lysosomal clearance of prions. Shifting this equilibrium towards clearance reduces the cellular load of prions [4]. Consequently, chemical induction of the autophagic flux in cells is an example of how the cellular clearance of prions can be increased [5].
Macro-autophagy (here referred to as autophagy) is a basic cellular process for the degradation and recycling of organelles and cytoplasmic proteins, and nutrient supply under starvation [6]. Beyond these classical roles , autophagy contributes to various physiological processes such as intracellular cleansing, differentiation, longevity, elimination of invading pathogens, antigen transport to innate and adaptive immune systems, or counteracting endoplasmic reticulum stress [7**]. Moreover, autophagy is also directly implicated in patho-physiology and disease, interestingly both in promoting and protective functions. Autophagy plays a role in cancer, infectious and inflammatory diseases, and protein misfolding diseases [7**]. A role of autophagy in the pathogenesis of prion diseases was suggested early, e.g. by findings of autophagic vacuoles in the brains of CJD patients, tissues of experimentally prion-infected rodents, and in prion-infected cultured cells [8–11]. The concept was developed that small molecule enhancers of autophagy can be used for targeting neurodegenerative disorders [12], and work from various groups has provided experimental proof-of-concept for this [13*,14].
As the majority of PrPSc in persistently prion-infected cells resides within endocytic vesicles, access of autophagy to this PrPSc population is mostly of indirect nature [4, 5]. When autophagosomes fuse with late endosomes/multivesicular bodies (MVB) or lysosomes for final degradation of cargo in autophagolysosomes, PrPSc present in endosomal-lysosomal compartments can be subject to changes in the activity of autophagy [5]. Besides this involvement in lysosomal degradation of prions, we postulated that autophagy could be implicated in prion biogenesis and recycling [5]. Furthermore, we have recently showed that autophagic activity modulates exosomal release of prions [15**].
In this review we focus on the roles of autophagy in lysosomal clearance and exosomal release of prions, and how these can be exploited as therapeutic targets.
The interplay between autophagy, exosomes and prion disease
Autophagy is a highly conserved homeostatic process for isolation and degradation of misfolded proteins and damaged organelles upon fusion of autophagosomes with late endosomes or lysosomes[6]. Autophagosomes undergo a series of controlled maturation steps, before they fuse with lysosomes or with multivesicular bodies (MVBs) for lysosomal degradation of cargo [16,17]. MVBs are derived from endosomes by inward budding of their limiting membrane [18]. They are subjected to either fusion with the plasma membrane and secretion of intraluminal vesicles as exosomes or to maturation into lysosomes for degradation (Fig. 1). In addition, MVBs can fuse with autophagosomes to give rise to amphisomes [19], thereby linking the endosomal-lysosomal pathway with the autophagic machinery. The crosstalk between the endosomal and autophagosomal pathways has been addressed recently [20]. Upon autophagy stimulation by starvation or rapamycin treatment, MVBs are preferentially directed to the autophagic pathway for autophagic/lysosomal degradation, which results in reduced exosomal release [21]. In contrast, pharmacologic or genetic blocking of autophagy usually increases exosomal release [22], which can have implications in amyloid diseases. Indeed, blocking the autophagy/lysosomal pathway via silencing of ATG5 resulted in increased exosomal release of α-synuclein aggregates which are associated with Parkinson’s disease [23**].
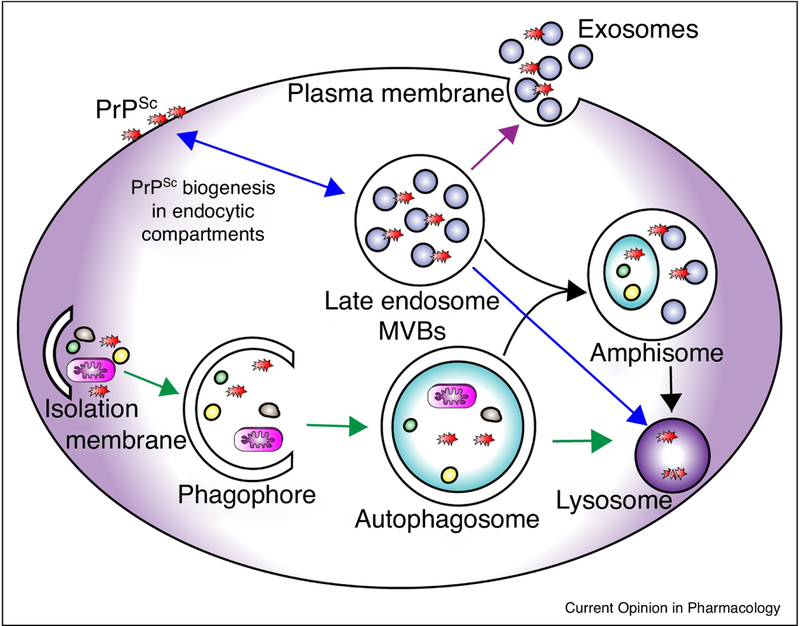
Figure 1: Overview of PrPSc fates through endocytic/exosomal pathway and/or autophagic pathway.
PrPSc is synthesized at the plasma membrane and along the endocytic compartment. PrPSc is either returned to the cell membrane via recycling endosomes or goes to late endosomes/MVBs (blue arrows). Fusion of late endosomes with the plasma membrane results in the release of exosomes that contain PrPSc into the extracellular space (purple arrow). Macroautophagy starts with the nucleation step by forming an isolation membrane followed by expansion of phagophores, which engulf misfolded proteins and damaged organelles. Sealing of the double-membraned phagophore results in autophagosome formation, which subsequently fuses with lysosomes for degradation (green arrows). In lieu, autophagosomes can fuse with late endosomes/MVBs to form hybrid multivesicular organelles termed amphisomes, which are subsequently submitted to lysosomal degradation (black arrows). Since the vast majority of cellular PrPSc is contained within endosomal vesicles, access of autophagy is mostly indirect. Increase of autophagic flux results in more fusion events of autophagosomes with PrPSc-containing MVBs or lysosomes, resulting in less exosomal release and more lysosomal degradation.
In this context, prion-infected cell cultures are unique models to delineate the link between autophagy and the biogenesis and release of exosomes. Cell-to-cell transfer of prions, involving direct cell contact or cell-free mechanisms, plays a pivotal role in the spreading of prions through infected tissues [11,24*]. PrPSc is associated with exosomes, and stimulating exosomal release by treatment with the ionophore monensin increases the intercellular transfer of prions [25]. Exosomal prions were also detected in blood using immunochemistry technique [26]. Recently, we reported that exosomes isolated from prion-infected cultured mouse cells of central nervous system (ScCAD5) and peripheral nervous system origin (ScN2a) contain prions. Autophagy stimulation with rapamycin hindered the exosomal release of prions. Conversely, inhibition of autophagy by wortmannin treatment or CRISPR/Cas9-mediated knock-out of autophagy related protein-5 (Atg5) elevated the release of exosomes and exosome-associated prions. Our results demonstrate that autophagy modulation can control lateral transfer of prions by interfering with their exosomal release [15**].
It is important to note that the crosstalk between autophagy and exosomal release is context dependent. In rotenone-treated cancer stem cells the release of exosomes is accompanied by mitochondrial damage and increased autophagy. These data support the association between mitochondrial damage, autophagy induction, and the release of exosomes in certain disease scenarios [27]. In the context of neurodegenerative disorders, compromised autophagy or lysosomal function in neuronal cell lines increases the secretion of amyloid proteins in exosomes, which is believed to enhance the transfer of amyloid proteins to neighbouring cells [15**,28,29].
Of note, the malfunction of basal autophagy results in neurodegeneration. Mice deficient for Atg5 in neuronal cells develop progressive deficits in their motor function, accompanied by the accumulation of cytoplasmic inclusion bodies in neurons [30]. In prion infection, on the one hand siRNA-mediated knock-down of Atg5 in prion-infected cultured neuronal cells increases the level of PrPSc. This is explained by more lateral infection due to enhanced exosomal release of prions and less intracellular PrPSc clearance in lysosomes [15**, 31]. On the other hand, autophagy stimulation using chemical compounds reduces prion levels by facilitating their lysosomal clearance [32**–34, 35**].
Taken together, increasing evidence indicates that autophagy modulation plays a role in prion disease and is a promising therapeutic target for prion and prion-like diseases.
Pharmacological induction of autophagy reduces prions and mitigates prion disease
Various studies in vitro and in vivo showed that induction of autophagy interferes with prion infection (Table 1) [5,32**]. Several pharmacological inducers of autophagy were studied in persistently prion-infected cells or mice [33,36–38]. Some of these compounds induce autophagy through inhibition of mTOR, which negatively regulates autophagy [39]. Others work in mTOR-independent pathways.
Table 1. Therapeutic agents proven to alleviate prion infection via autophagy induction.
: decrease in PrPSc level; (−): completely clears PrPSc; i.p: intraperitoneally; i.c: intracerebrally; dpi: days post inoculation.
| Therapeutic agent | In vitro | In vivo | References |
|---|---|---|---|
| Imatinib mesylate, also known as Gleevec or STI571 | ↓ RML- and 22L-N2a cells | Injection of imatinib mesylate (i.p) for 30 days delayed neuro-invasion and prolonged survival of RML-infected mice. | [33,40,41] |
| Lithium | ↓ RML-N2a and 22L-L929 cells | Oral lithium treatment started at 100 dpi in 139A i.c-infected mice did not prolong the survival significantly (some trend). | [34] |
| Rapamycin | ↓ RML-N2a cells and Fukuka-1 infected N2a cells. | Oral rapamycin treatment started at 100 dpi prolonged survival of 139A i.c-infected mice. Injection of rapamycin (i.p) 3 times per week, beginning at 6 weeks of age, prolonged survival of Tg(PrP-A116V) mice (GSS model). |
[31,34,38,42] |
| Trehalose | ↓ RML-N2a cells. | Trehalose in drinking water had no effect on the survival of 139A i.p-infected mice. However, it decreased PrPSc load in spleen after 30 and 60 dpi. | [31] |
| FK506 (Tacrolimus) | ↓ Fukuoka-1 -infected N2a58 cells and RML- and 22L-PK1 cells. | Injection of FK506 (i.p) prolonged survival of Fukuoka-1 i.c-infected CD1 mice. | [37,47] |
| Celecoxib derivatives (AR-12), also known as OSU-03012 |
↓ 22L-N2a, 22L-CAD5, and 22L-, RML, and Me7-MEF cells (−) on long term treatment (2 weeks) |
Injection of AR-12 (i.p) prolonged survival of RML i.c-infected mice (B. Abdulraham & KM. Schatzl, personal communication). | [32] |
| Spermine | ↓ SMB.sl5 and 22L-CAD5 cells. | Not reported. | [35] |
| Astemizole | ↓ RML- and 22L-PK1 cells. | Injection of astimizole (i.p.) for 30 days prolonged survival of RML i.c- infected mice, however it was not statistically significant (p=0.06). | [47] |
| Resveratrol | (−)SMB-S15 cells. Blocked neurotoxicity induced by PrP peptide (106–126) – in SH-SY5Y and SK-K-SH cells. |
Oral resveratrol had no effect on survival of Me7 i.c-infected mice (J. Braun and H.M. Schatzl, personal communication). | [36,44] |
| Ginsenoside-Rg3 | Blocked neurotoxicity induced by PrP peptide (106–126) in primary neurons and SK-K-SH cells. | Not reported. | [45] |
| Hinokitiol | Attenuated neurotoxicity induced by PrP peptide (106–126) in primary neurons and SK-K-SH cells. | Not reported | [46] |
The first compound for which induction of autophagy was described as underlying anti-prion mechanism is imatinib mesylate (Gleevec®, STI571), an inhibitor of the tyrosine kinase c-Abl. Initial studies found increased lysosomal clearance of PrPSc in cultured prion-infected cells when treated with low micromolar range concentrations of imatinib [33]. Follow-up studies revealed that imatinib dose-dependently activates the cellular autophagy machinery in mammalian cells, independent of their tissue type, species origin or immortalization status [40]. Time course studies of intraperitoneally (i.p) prion-infected mice showed that imatinib treatment for 30 days at an early phase of infection strongly reduced the PrPSc loads in the spleen and, to a lesser extent, in the spinal cord. It also delayed PrPSc accumulation in the brain and prolonged the survival time of treated mice (mean survival of treatment group was 263 days versus 246 days for the control) [41]. Imatinib does not efficiently cross the blood-brain-barrier and effects on prion infection were less pronounced when neuroinvasion was already accomplished [41].
Rapamycin, the classical mammalian target of rapamycin (mTOR) inhibitor and well recognized autophagy inducer, prolonged the survival of intracerebrally prion-infected mice (strain 139A) by averaging 13 days, when administered orally starting at 100 days p.i. [34]. In another study, rapamycin-treated Tg(PrP-A116V) mice, a model of Gerstmann-Sträussler-Scheinker syndrome (GSS), showed delayed disease onset and prolonged survival on average by 16 days compared to the vehicle treated controls [42]. Whereas imatinib mainly exerted its anti-prion effects outside the central nervous system, these two studies indicate a potential for rapamycin to cross the blood-brain-barrier and act within the brain. Interestingly, the anti-prion effect of rapamycin is strain-dependent in vitro. Rapamycin treatment reduced PrPSc in mouse neuroblastoma cells (ScN2a) persistently infected with the Fukuoka-1 strain but had no effect in ScN2a cells infected with 22L or Chandler/RML scrapie strains. This indicates a more efficient degradation of Fukuoka-1 prions by the autophagy pathway compared to other prion strains [38]. Other drugs induced autophagy and reduced PrPSc in prion-infected cells but were less efficient in in vivo experiments. Lithium chloride (LiCl) used to treat manic depressive illnesses and mood disorders, induces autophagy by mTOR-independent pathways. Despite having no statistically significant effect on the survival of 139A-infected mice, LiCl treatment cleared PrPSc in ScN2a cells after 10 days of treatment [34]. The non-reducing disaccharide trehalose reduced PrPSc in persistently prion-infected cultured cells [31]. It up-regulated autophagy markers in treated cells and its antiprion effect was inhibited by simultaneous application of autophagy inhibitors like 3-MA or silencing Atg5 expression using siRNA. Trehalose is another mTOR-independent autophagy inducer which was able to lower the PrPSc load in the spleen of intraperitoneally prion infected mice, however it did not prolong the survival significantly when applied orally [31].
A recent study published by our group demonstrated the potent anti-prion effect of AR-12, a celecoxib derivative also known as OSU-03012. AR-12 completely eradicated prion infection in persistently prion-infected neuronal (ScN2a) and non-neuronal (ScMEF) cultured cells after 2 weeks of treatment. No prion conversion activity was detectable by real-time quaking induced conversion (RT-QuIC), a highly sensitive in vitro prion amplification assay [32**]. Interestingly, intraperitoneal application of AR12 for 4 weeks to intracerebrally prion-infected FVB mice (scrapie prion strain RML) extended their survival on average by 11 days compared to that of the control group (manuscript in preparation), demonstrating that AR-12 has in vivo anti-prion effects. The effect of AR12 on PrPSc levels in vitro was less pronounced in ScN2a cells with Atg-5 knock-out, and the autophagy marker LC3-II was up-regulated in treated Atg5 wild-type cells. These findings indicate that induction of autophagy is an underlying mechanism for the observed anti-prion effects, although the exact mechanism by which AR-12 is inducing autophagy is not fully understood. Hitherto, AR-12 was shown to activate PERK, a key regulatory kinase for unfolded protein response in eukaryotic cells, which in turn phosphorylates eIF2α and induces the formation of the Atg5-Atg12 complex [43].
Recently, the natural polyamine spermine was found to clear PrPSc in prion-infected CAD5 and SMB.s15 cultured cells by induction of autophagy. Mass spectroscopy studies revealed that spermine induced hyperacetylation of tubulins, especially Tubb6, which in turn enhances the retrograde transport of autophagosomes to lysosomes [35**].
Resveratrol, a natural phenol and phytoalexin enriched in grapes, cured prion infection in SMB-s15 cells which was confirmed by inoculation of CD1 mice with resveratrol-treated cells [44]. In addition, resveratrol blocked the neurotoxicity caused by prion protein peptide (106–126) in cultured cells [36]. Oral application of resveratrol had no effect on survival or onset of prion disease in intracerebrally Me7-infected mice (J. Braun and H.M. Schatzl, personal communication). The anti-prion and neuroprotective effects of resveratrol were attributed to induction of autophagy through activation of Sirt1, a class III histone deacetylase [36]. Other natural compounds like hinokitiol and Ginsenoside-RG3, an active ingredient in ginseng, attenuated the neurotoxicity induced by prion protein peptide (106–126) in primary neurons and SK-K-SH cells through induction of autophagy [45,46]. Hinokitiol induced autophagy by stabilization of HIF-1α (Hypoxia inducible factor-1α), but the mechanism by which Ginsenoside-RG3 activates autophagy is to be determined [46]. There are no reports on whether these compounds have anti-prion effects in vitro or in vivo.
FK506 (tacrolimus), an immunosuppressant drug, decreased PrPSc in three different models of prion-infected neuroblastoma cells [37,47]. When administered intraperitoneally to prion-infected CD1 mice (strain Fukuoka-1), the mice survived 16 days longer than the controls [37]. FK506 treatment up-regulated autophagic markers in cell culture and in brain tissues of treated mice [37]. Like rapamycin, FK506 is a ligand of FK506 binding proteins (FKBPs), of which FKBP12 is the prototype. Although the rapamycin/FKBP12 complex inhibits mTOR, the FK506/FKBP12 complex interacts with the phosphatase calcineurin [48], and it needs to be determined whether activation of autophagy depends on inhibition of calcineurin [37].
In the high-throughput drug screening study which identified FK506, the antihistaminic astemizole was found to have anti-prion effects in vitro and in vivo [47]. In vitro, astemizole inhibited the replication of RML and 22L prions in neuroblastoma cells, without reappearance of the signal after treatment cessation. Additionally, the autophagy marker LC3II/I ratio was increased in astemizole-treated cells [47]. In vivo, the intraperitoneal injection of astemizole increased the survival of RML-infected mice by 6 days compared to that of the control [47].
Conclusion
Strong evidence suggests that autophagy plays a role in prion disease, with autophagosomes being observed in brains of patients and experimentally inoculated mice. However, this level of autophagy is not sufficient to resolve prion infection. Enhancing autophagy using chemical compounds has proven to increase PrPSc degradation, to limit its exosomal release, and to confer neuroprotection. While these multiple ways to interfere with prion propagation through autophagy are highly efficient in vitro, only a limited number of compounds also were efficient in extending the survival of prion-infected mice, calling for new approaches to strengthen these findings. A possible way to improve the encouraging in vivo anti-prion effects can be treatment with a combination of drugs. For example, a combination treatment with inducers of autophagy and compounds that inhibit de novo prion conversion can result in additive or even synergistic anti-prion effects. Some of the most promising drugs in vitro, such as imatinib, only poorly penetrate the blood brain barrier. Therefore, another strategy can be to pharmacologically improve existing drugs in order to facilitate efficient delivery into the brain. Nevertheless, extensive research is necessary to gain a full mechanistic understanding of the role of autophagy in prion infection in order to move those promising approaches from bench to bedside.
Highlights:
Prions are misfolded proteins causing fatal neurodegenerative diseases
Autophagy dictates the fate of PrPSc in various contexts in prion infection
Autophagy facilitates delivery of prions to lysosomal degradation
Increase in autophagy reduces the exosomal release of prion infectivity
Pharmacological stimulation of autophagy is beneficial in prion infection.
Achnowledgement
This work was supported by grants from Alberta Innovates/Alberta Prion Research Institute (APRI) (201300020), the National Institute of Health (R01 NS076853-01A1), and was performed within the framework of the Calgary Prion Research Unit (APRI grant 201600010). SG is supported by the Canada Research Chairs program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Prusiner SB: Novel proteinaceous infectious particles cause scrapie. Science 1982,216:136–44. [DOI] [PubMed] [Google Scholar]
- 2.Nunziante M, Gilch S, Schätzl HM: Prion Diseases: From Molecular Biology to Intervention Strategies. ChemBioChem 2003, 4:1268–1284. [DOI] [PubMed] [Google Scholar]
- 3.Krammer C, Vorberg I, Schätzl HM, Gilch S: Therapy in prion diseases: from molecular and cellular biology to therapeutic targets. Infect Disord Drug Targets 2009, 9:3–14. [DOI] [PubMed] [Google Scholar]
- 4.Gilch S, Krammer C, Schätzl HM: Targeting prion proteins in neurodegenerative disease. Expert Opin Biol Ther 2008, 8:923–940. [DOI] [PubMed] [Google Scholar]
- 5.Heiseke A, Aguib Y, Schatzl HM: Autophagy, prion infection and their mutual interactions. Curr Issues Mol Biol 2010, 12:87–97. [PubMed] [Google Scholar]
- 6.Klionsky DJ, Codogno P: The Mechanism and Physiological Function of Macroautophagy. J Innate Immun 2013, 5:427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7**.Levine B, Kroemer G: Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176:11–42. [DOI] [PMC free article] [PubMed] [Google Scholar]; A comprehensive and up-to-date review of the biological functions of autophagy genes in different pathways other than autophay. In addition, this review discusses the role of autophagy gene mutations in the pathophysiology of human diseases.
- 8.Liberski PP: Prion diseases: a riddle wrapped in a mystery inside an enigma. Folia Neuropathol 2008, 46:93–116. [PubMed] [Google Scholar]
- 9.Xu Y, Tian C, Wang S-B, Xie W-L, Guo Y, Zhang J, Shi Q, Chen C, Dong X-P: Activation of the macroautophagic system in scrapie-infected experimental animals and human genetic prion diseases. Autophagy 2012, 8:1604–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joshi-Barr S, Bett C, Chiang W-C, Trejo M, Goebel HH, Sikorska B, Liberski P, Raeber A, Lin JH, Masliah E, et al. : De Novo Prion Aggregates Trigger Autophagy in Skeletal Muscle. J Virol 2014, 88:2071–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schätzl HM, Laszlo L, Holtzman DM, Tatzelt J, DeArmond SJ, Weiner RI, Mobley WC, Prusiner SB: A hypothalamic neuronal cell line persistently infected with scrapie prions exhibits apoptosis. J Virol 1997, 71:8821–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubinsztein DC: Autophagy Induction Rescues Toxicity Mediated by Proteasome Inhibition. Neuron 2007, 54:854–856. [DOI] [PubMed] [Google Scholar]
- 13*.Boland B, Yu WH, Corti O, Mollereau B, Henriques A, Bezard E, Pastores GM, Rubinsztein DC, Nixon RA, Duchen MR, et al. : Promoting the clearance of neurotoxic proteins in neurodegenerative disorders of ageing. Nat Rev Drug Discov 2018, 17:660–688. [DOI] [PMC free article] [PubMed] [Google Scholar]; An up-to-date review of the emerging mechanisms for promoting the clearance of neurotoxic proteins; it includes the autophagic–lysosomal network, chaperone-mediated autophagy, and the ubiquitin–proteasome system.
- 14.Menzies FM, Fleming A, Caricasole A, Bento CF, Andrews SP, Ashkenazi A, Füllgrabe J, Jackson A, Jimenez Sanchez M, Karabiyik C, et al. : Autophagy and Neurodegeneration: Pathogenic Mechanisms and Therapeutic Opportunities. Neuron 2017, 93:1015–1034. [DOI] [PubMed] [Google Scholar]
- 15**.Abdulrahman BA, Abdelaziz DH, Schatzl HM: Autophagy regulates exosomal release of prions in neuronal cells. J Biol Chem 2018, 293. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first study which showed that induction of autophagy decreases the exosomal release of prions, and consequently reduces lateral infection between cells. On the other hand, Atg5-KO cells showed higher exosomal release than their wild-type counterparts, demonstrating that the level of autophagy affects the cellular release of prions
- 16.Lamb CA, Yoshimori T, Tooze SA: The autophagosome: origins unknown, biogenesis complex. Nat Rev Mol Cell Biol 2013, 14:759–74. [DOI] [PubMed] [Google Scholar]
- 17.Yin Z, Pascual C, Klionsky DJ: Autophagy: machinery and regulation. Microb cell (Graz, Austria) 2016, 3:588–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murk JLAN, Humbel BM, Ziese U, Griffith JM, Posthuma G, Slot JW, Koster AJ, Verkleij AJ, Geuze HJ, Kleijmeer MJ: Endosomal compartmentalization in three dimensions: implications for membrane fusion. Proc Natl Acad Sci U S A 2003, 100:13332–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hessvik NP, Llorente A: Current knowledge on exosome biogenesis and release. Cell Mol Life Sci 2018, 75:193–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tooze SA, Abada A, Elazar Z: Endocytosis and autophagy: exploitation or cooperation? Cold Spring Harb Perspect Biol 2014, 6:a018358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fader CM, Sánchez D, Furlán M, Colombo MI: Induction of autophagy promotes fusion of multivesicular bodies with autophagic vacuoles in k562 cells. Traffic 2008, 9:230–50. [DOI] [PubMed] [Google Scholar]
- 22.Guo JL, Lee VMY: Cell-to-cell transmission of pathogenic proteins in neurodegenerative diseases. Nat Med 2014, 20:130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23**.Fussi N, Höllerhage M, Chakroun T, Nykänen N-P, Rösler TW, Koeglsperger T, Wurst W, Behrends C, Höglinger GU: Exosomal secretion of α-synuclein as protective mechanism after upstream blockage of macroautophagy. Cell Death Dis 2018, 9:757. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors demonstrate that inhibition of autophagy at early stages by ATG5 silencing enhances the exosomal secretion of α-Syn, as a compensatory mechanism to get rid of α-Syn. The study showed a synergistic detrimental effect of compromised autophagy and impaired exosomal secretion
- 24*.Vilette D, Courte J, Peyrin JM, Coudert L, Schaeffer L, Andréoletti O, Leblanc P: Cellular mechanisms responsible for cell-to-cell spreading of prions. Cell Mol Life Sci 2018, 75:2557–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review discusses different mechanisms by which prions spread from cell to cell across the infected tissue, including release of infectious extracellular vesicles and intercellular transfer of PrPSc-containing organelles through tunneling nanotubes.
- 25.Guo BB, Bellingham SA, Hill AF: Stimulating the Release of Exosomes Increases the Intercellular Transfer of Prions. J Biol Chem 2016, 291:5128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Properzi F, Logozzi M, Abdel-Haq H, Federici C, Lugini L, Azzarito T, Cristofaro I, di Sevo D, Ferroni E, Cardone F, et al. : Detection of exosomal prions in blood by immunochemistry techniques. J Gen Virol 2015, 96:1969–74. [DOI] [PubMed] [Google Scholar]
- 27.Kumar D, Gupta D, Shankar S, Srivastava RK: Biomolecular characterization of exosomes released from cancer stem cells: Possible implications for biomarker and treatment of cancer. Oncotarget 2015, 6:3280–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poehler A-M, Xiang W, Spitzer P, May VEL, Meixner H, Rockenstein E, Chutna O, Outeiro TF, Winkler J, Masliah E, et al. : Autophagy modulates SNCA/α-synuclein release, thereby generating a hostile microenvironment. Autophagy 2014, 10:2171–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alvarez-Erviti L, Seow Y, Schapira AH, Gardiner C, Sargent IL, Wood MJA, Cooper JM: Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol Dis 2011, 42:360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, et al. : Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 2006, 441:885–889. [DOI] [PubMed] [Google Scholar]
- 31.Aguib Y, Heiseke A, Gilch S, Riemer C, Baier M, Schätzl HM, Ertmer A: Autophagy induction by trehalose counteracts cellular prion infection. Autophagy 2009, 5:361–9. [DOI] [PubMed] [Google Scholar]
- 32**.Abdulrahman BA, Abdelaziz D, Thapa S, Lu L, Jain S, Gilch S, Proniuk S, Zukiwski A, Schatzl HM: The celecoxib derivatives AR-12 and AR-14 induce autophagy and clear prion-infected cells from prions. Sci Rep 2017, 7:17565. [DOI] [PMC free article] [PubMed] [Google Scholar]; The first study to show that the autophagy stimulator AR-12 and its analogue AR-14 induce the degradation of PrPSc and erradicate prion seeding activity in three cell line models infected with various prion strains.
- 33.Ertmer A, Gilch S, Yun S-W, Flechsig E, Klebl B, Stein-Gerlach M, Klein MA, Schatzl HM: The Tyrosine Kinase Inhibitor STI571 Induces Cellular Clearance of PrPSc in Prion-infected Cells. J Biol Chem 2004, 279:41918–41927. [DOI] [PubMed] [Google Scholar]
- 34.Heiseke A, Aguib Y, Riemer C, Baier M, Schätzl HM: Lithium induces clearance of protease resistant prion protein in prion-infected cells by induction of autophagy. J Neurochem 2009, 109:25–34. [DOI] [PubMed] [Google Scholar]
- 35**.Phadwal K, Kurian D, Salamat MKF, MacRae VE, Diack AB, Manson JC: Spermine increases acetylation of tubulins and facilitates autophagic degradation of prion aggregates. Sci Rep 2018, 8:10004. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that spermine treatment in vitro submits prion aggregates to autophagy-based degradation by enhancing colocalisation of Tubb6 with PrPSc aggregates.
- 36.Jeong J-K, Moon M-H, Bae B-C, Lee Y-J, Seol J-W, Kang H-S, Kim J-S, Kang S-J, Park S-Y: Autophagy induced by resveratrol prevents human prion protein-mediated neurotoxicity. Neurosci Res 2012, 73:99–105. [DOI] [PubMed] [Google Scholar]
- 37.Nakagaki T, Satoh K, Ishibashi D, Fuse T, Sano K, Kamatari YO, Kuwata K, Shigematsu K, Iwamaru Y, Takenouchi T, et al. : FK506 reduces abnormal prion protein through the activation of autolysosomal degradation and prolongs survival in prion-infected mice. Autophagy 2013, 9:1386–94. [DOI] [PubMed] [Google Scholar]
- 38.Ishibashi D, Homma T, Nakagaki T, Fuse T, Sano K, Takatsuki H, Atarashi R, Nishida N: Strain-Dependent Effect of Macroautophagy on Abnormally Folded Prion Protein Degradation in Infected Neuronal Cells. PLoS One 2015, 10:e0137958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo F, Liu X, Cai H, Le W: Autophagy in neurodegenerative diseases: pathogenesis and therapy. Brain Pathol 2018, 28:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ertmer A, Huber V, Gilch S, Yoshimori T, Erfle V, Duyster J, Elsässer H-P, Schätzl HM: The anticancer drug imatinib induces cellular autophagy. Leukemia 2007, 21:936–942. [DOI] [PubMed] [Google Scholar]
- 41.Yun S-W, Ertmer A, Flechsig E, Gilch S, Riederer P, Gerlach M, Schätzl HM, Klein MA: The tyrosine kinase inhibitor imatinib mesylate delays prion neuroinvasion by inhibiting prion propagation in the periphery. J Neurovirol 2007, 13:328–337. [DOI] [PubMed] [Google Scholar]
- 42.Cortes CJ, Qin K, Cook J, Solanki A, Mastrianni JA: Rapamycin delays disease onset and prevents PrP plaque deposition in a mouse model of Gerstmann-Sträussler-Scheinker disease. J Neurosci 2012, 32:12396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park MA, Yacoub A, Rahmani M, Zhang G, Hart L, Hagan MP, Calderwood SK, Sherman MY, Koumenis C, Spiegel S, et al. : OSU-03012 stimulates PKR-like endoplasmic reticulum-dependent increases in 70-kDa heat shock protein expression, attenuating its lethal actions in transformed cells. Mol Pharmacol 2008, 73:1168–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J, Zhang B-Y, Zhang J, Xiao K, Chen L-N, Wang H, Sun J, Shi Q, Dong X-P: Treatment of SMB-S15 Cells with Resveratrol Efficiently Removes the PrP(Sc) Accumulation In Vitro and Prion Infectivity In Vivo. Mol Neurobiol 2016, 53:5367–76. [DOI] [PubMed] [Google Scholar]
- 45.Moon J-H, Lee J-H, Lee Y-J, Park S-Y: Autophagy flux induced by ginsenoside-Rg3 attenuates human prion protein-mediated neurotoxicity and mitochondrial dysfunction. Oncotarget 2016, 7:85697–85708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moon J-H, Lee J-H, Lee Y-J, Park S-Y: Hinokitiol protects primary neuron cells against prion peptide-induced toxicity via autophagy flux regulated by hypoxia inducing factor-1. Oncotarget 2016, 7:29944–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karapetyan YE, Sferrazza GF, Zhou M, Ottenberg G, Spicer T, Chase P, Fallahi M, Hodder P, Weissmann C, Lasmézas CI: Unique drug screening approach for prion diseases identifies tacrolimus and astemizole as antiprion agents. Proc Natl Acad Sci U S A 2013, 110:7044–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Romano S, Di Pace A, Sorrentino A, Bisogni R, Sivero L, Romano MF: FK506 binding proteins as targets in anticancer therapy. Anticancer Agents Med Chem 2010, 10:651–6. [DOI] [PubMed] [Google Scholar]