Abstract
Objectives
Multiple acyl-CoA dehydrogenase deficiency (MADD) is a severe inborn disorder of mitochondrial fatty acid oxidation. The only treatment option for MADD is the use of exogenous ketone bodies, like sodium β-hydroxybutyrate (NaβHB). However, the use of ketone body salts leads to a high intake of accompanying minerals, which can lead to additional side effects. The use of mineral-free formulations could improve tolerability.
Methods
In this report, the use of a βHB acid (βHBA) in a patient with MADD is described. The production of D/L-βHBA was carried out using ion exchange chromatography (IEX) and using a precipitation method. During two inpatient treatment intervals, the tolerability as well as clinical and metabolic effects were monitored. D-βHB in serum, blood gas analysis, and standard blood measurements (like minerals) were used as control parameters.
Results
Production of D/L-βHBA using the precipitation method was more effective than using IEX. The tube feed solution used had a minimum pH of 3.5. Capillary D-βHB measurements were between 0.1 and 0.4 mmol/L and venous were at 0.1 mmol/L or below. Minerals and serum pH were within the normal range. During application of D/L-βHBA, gastrointestinal discomfort occurred and no clinical improvement was observed.
Conclusions
The use of D/L-βHBA in the therapy of severe MADD could be a good addition to the use of classical ketone body salts. The observed gastrointestinal side effects were of a mild nature and could not be specifically attributed to the D/L-βHBA treatment. In short-term application, no clinical benefit and no substantial increase of D-βHB in serum were noted. No tendency towards acidosis or alkalosis was observed during the entire period of treatment.
Keywords: MADD, Ketone bodies, Ketone body salts, β-Hydroxybutyric acid, Ketone body therapy, Metabolic disease
1. Introduction
Multiple acyl-coenzyme A dehydrogenase deficiency (MADD; OMIM #231680), also known as glutaric aciduria type II (GAII), is a autosomal recessively inherited disorder of mitochondrial fatty acid oxidation [1,2]. The basis of the disorder is a functional impairment of the electron transfer flavoprotein with its two subunits ETFA and ETFB or of the ETFDH (synonym ETF-QO) [3]. Caused by the functional impairment of the flavoproteins, a deficit in the utilization of fatty acids and some amino acids occurs [4]. The phenotypes for MADD are named according to the severity of the enzymatic defect. The onset of the first type is neonatal with congenital anomalies, the second has the same onset but without anomalies, and the third one has a mild and/or later onset of symptoms [5]. Severe MADD is clinically characterized by severe myopathy, cardiomyopathy, and leukodystrophy [6,7].
Currently, apart from the riboflavin responsive variants of the disease, there is no reliable form of therapy for severe MADD [4,7]. A supplementation of riboflavin, l-carnitine, and a diet adapted to the defect is frequently used. The diet involves restriction of fat, low content of specific amino acids and high carbohydrate content [4]. Several case reports showed that the treatment with exogenous ketone bodies might be an effective additional therapy. In all published reports, a sodium β-hydroxybutyrate (βHB) salt was used for the treatment and showed no adverse effects [[8], [9], [10]]. Recent publications reported side effects such as dehydration, vomiting, nausea, diarrhoea, constipation, abdominal pain and recurring alkalosis [11,12]. A supplementation with βHB is also interesting for other diseases, like pyruvate dehydrogenase deficiency or hyperinsulinemic hypoglycaemia, giving the supplement a broad range of possible applications in medicine [13,14].
Apart from the treatment of rare diseases, the use of βHB is also interesting for the lifestyle supplement market. The postulated application areas are weight loss, increasing athletic performance, providing energy, and improving the mental performance. A critical point of the ingestion of βHB salts is the excessive intake of minerals, like sodium, potassium, magnesium, and calcium [15]. To avoid a high load of minerals, the use of alternative βHB products, like esters, is necessary [16]. Another option could be the use of the β-hydroxybutyric acid (βHBA). The utilization of βHBA has not been scientifically described so far. This is the first report of a short-term treatment with β-hydroxybutyric acid (βHBA) solution in severe MADD.
2. Material & methods
2.1. Production of D/L-β-hydroxybutyric acid
The production base of D/L-β-hydroxybutyric acid was a βHB calcium salt from a US supplier (Ketotech, USA; ≥ 98% CaβHB; 82.1% βHB, 16,8% calcium) in food quality.
2.1.1. Ion exchange (IEX)
50 g βHB calcium salt was dissolved in 500 mL demineralized water forming a 10% (0.406 mol/L) CaβHB solution. In each 100 mL, a total of 40 g cation exchange material Lewatit S 2568H (Lanxess, Germany) was added to remove the calcium ions. The total duration of the exchange process was 15 min. After incubation with ion exchange material, the solution was cleaned using activated carbon and subsequently filtered. The remaining calcium content was measured using a 0.01 M EDTA solution (≥99%; AppliChem, Germany) after dilution with demineralized water and previous sample preparation with 25% ammonia (Roth, Germany) in combination with an indicator buffer tablet (Merck, Germany). D-βHB content was measured by using an enzymatic test kit for food samples (r-biopharm, Germany). The colorimetric reaction is based on the reduction of iodnitrotetrazolium chloride (INT) to formazan by the enzyme diaphorase. The redox partner in the reaction is NADH from the oxidation of D-βHB to acetoacetate. Extinction was measured at 492 nm; the extinction is proportional to the D-βHB concentration in the sample. For the calculation of the racemate D/L-βHB, the measured D-βHB value was doubled. Microbial contamination of the solution was tested using a plate count agar (PCA; Applichem, Germany) and a violet red bile dextrose agar (VRBD; Applichem, Germany).
2.1.2. Precipitation with oxalic acid (OAP)
CaβHB (188 g/L; 0.763 mol/L) and oxalic acid dehydrate (90 g/L; Roth, Germany) were separately dissolved in scalding hot distilled water. After cooling, the oxalic acid solution was poured into the CaβHB solution while stirring continuously. The precipitation of calcium oxalate was left to take place overnight at a temperature of +4 °C. After centrifugation at 3000 U/min for 10 min the supernatant was filtered under sterile conditions (0.22 μm; Saerstedt, Germany) and transferred into single-use bottles (Medela, Switzerland). Storage temperature, after heating to 90 °C for 3 h, was −20 °C. The resulting solution had a concentration of 100 g ± 10 g D/L-βHBA/L (10% D/L-βHBA, 0.961 ± 0.096 mmol/L; assay kit, see Section 2.1.1). Approval analytics included visual control of clarity, sodium (70 ± 20 mM), potassium (70 ± 20 mM), calcium (15 ± 15 mM), pH (4 ± 0.5), osmolality (1200 ± 200 mOsmol/kg), and oxalic acid (20 ± 10 mg/L). A food chemist at the central laboratory of the University Hospital in Muenster performed all the routine analysis.
2.2. Blood analysis
2.2.1. D-βHB
Venous blood samples were used to determine D-βHB content using an enzymatic assay produced by Sigma-Aldrich (St. Louis, USA). The generated compound is colorimetric and a photometric measurement at a wavelength of 450 nm can determine its concentration which is proportional to the D-βHB concentration in the serum. Additionally, D-βHB was analyzed in capillary blood using a freestyle precision neo monitoring system and beta-ketone test strips from Abbott (Witney, Oxon, UK).
2.2.2. Blood gas analyses
The blood was measured directly after sampling using the fully automated blood gas analyer ABL 800 Flex produced by Radiometer (Krefeld, Germany). The blood gas analysis (BGA) includes parameters like pH, electrolytes and metabolites.
2.2.3. Minerals and standard analytic in serum
In addition to the BGA, all mineral values (Na, K, Ca, Mg) and the standard haemograms for routine control were determined using the analyzer Cobas 8000 manufactured by Roche Diagnostics (Mannheim, Germany).
2.3. Treatment with βHBA
2.3.1. Treatment 1
For the first test, the D/L-βHBA solution prepared using IEX was used. The intake of the βHBA was by a gastric tube. A combination of water and the D/L-βHBA was the liquid base of the tube feeding. The addition of other nutrients, like an amino acid composition specific for MADD, was possible. A regulated continuous increase of the βHBA solution was executed for a period of seven days during an inpatient stay at the hospital (see Table 1). Control of blood parameters included D-βHB, minerals (Na, K, Mg, Ca), and a blood gas analysis. D-βHB was carried out two times a day and the other blood values once a day by the central laboratory of the University Hospital in Muenster. In addition, the nurses and medical doctors documented all events, such as side effects or positive effects, during the stay. Special attention was given to gastrointestinal adverse effects, like vomiting or diarrhoea. The total duration of the treatment was seven days.
Table 1.
Pre-calculation of the D/L-βHBA intake in g/kg bw, g/day and the solution volume (mL) for the first seven-day treatment course.
| Day | D/L-βHBA (g/kg bw) | D/L-βHBA (g/day) | D/L-βHBA solution (mL/day) |
|---|---|---|---|
| 1 | 0.08 | 1.60 | 20 |
| 2 | 0.16 | 3.20 | 40 |
| 3 | 0.40 | 8.00 | 100 |
| 4 | 0.60 | 12.00 | 150 |
| 5 | 0.80 | 16.00 | 200 |
| 6 | 1.20 | 24.00 | 300 |
| 7 | 1.60 | 32.00 | 400 |
2.3.2. Treatment 2
In the second trial, the βHBA solution obtained through precipitation with oxalic acid was used. There was no difference in preparation and intake of the tube feeding. Total duration was five days with a continuous increase of βHBA, this time with a higher start and end concentration of βHBA in comparison to trial 1. The final intake of D/L-βHBA on day five was 2.50 g/kg bw, which adds up to 50 g per day (see Table 2). There was no difference in the intervals of blood measurement. In addition to blood sampling, the D/L-βHB and acetoacetate in urine were analyzed semi quantitatively on day one, two and five using gas chromatography. The treatment was performed during an in-patient stay of five days and the clinical staff documented all events.
Table 2.
Pre-calculation of the D/L-βHBA intake in g/kg bw, g/day and the solution volume (mL) for the second treatment course.
| Day | D/L-βHBA (g/kg bw) | D/L-βHBA (g/day) | D/L-βHBA solution (mL/day) |
|---|---|---|---|
| 1 | 0.75 | 15.00 | 150 |
| 2 | 1.00 | 20.00 | 200 |
| 3 | 1.50 | 30.00 | 300 |
| 4 | 2.00 | 40.00 | 400 |
| 5 | 2.50 | 50.00 | 500 |
3. Patient short history
The 12 year old boy is the first child of Turkish stock parents. In the first days of life, a metabolic crisis with massive hyperammonemia (1841 μmol/L; 3136 μg/dL) and lactic acidosis (5.2 mmol/L) occurred. The critical event was life threatening and caused cognitive deficits. In laboratory analyses, a pathological profile of acylcarnitines with an increase of short-, medium- and long-chain acylcarnitines (C4 – C16) was detected and the palmitate oxidation test in fibroblasts showed a severe deficiency. Genetic analyses showed a homozygous mutation for c.1141G>C (G381R) in the ETFDH gene which confirmed the diagnosis of MADD. After the diagnosis of MADD, different nutritional therapies and supplementations, for example riboflavin and coenzyme Q10, were administered. The first treatment with external ketone bodies started eight days after birth. A βHB sodium salt, purity ≥99,0%, manufactured by Sigma-Aldrich (St. Louis, USA) was administered and increased in small steps while monitoring metabolic parameters like D-βHB, minerals, and BGA. The salt is still in use today with a standard daily dose of 16 g (0.8 g/kg bw). However, the used amount of NaβHB did not lead to a measurable rise of βHB in serum and a further increase of the salt was not possible due to the development of side effects (alkalosis).
The development of the child was mildly delayed in the first two years. Within the second year, he learned to walk and spoke some words which seemed to be the positive effects of the ketone body treatment. However, one year later he lost the ability to walk and developed spasticity in lower extremities. Further clinical tests in the first three years presented a MADD typical leukodystrophy and a generalized disposition to seizures. The combination of antiepileptic drugs (levetiracetam, ethosuximid) and optimized nutrition enabled the patient to be completely seizure-free.
Today, the patient is wheelchair-bound and has cognitive restrictions. Simple conversations in German and his parent's native language are possible with ease. The general health condition is satisfactory, given the severity of the disease.
Nutritional treatment and medication are not part of this report. A detailed report has been published [12]. The report was done with the written consent of the patients parents.
4. Results
4.1. Production of βHBA
In both cases, the resulting solution was colourless and clear with a strong fruity sour taste without any unpleasant aftertaste. The production base CaβHB has a disagreeable bitter taste. The concentration of D/L-βHB in the solutions, calculated on the basis of the measured D-βHB, differed only slightly from the pre-calculations (see 2.3.1, 2.3.2; Table 3).
Table 3.
Administered daily dose of D/L-βHB in treatment 1 (IEX) and 2 (OAP) in g/kg bw. The calculation of D/L-βHBA was based on the enzymatic measurement of D-βHB.
| Day | Treatment 1 (IEX) D/L-βHBA (g/kg bw/day) | Treatment 2 (OAP) D/L-βHBA (g/kg bw/day) |
|---|---|---|
| 1 | 0.07 | 0.77 |
| 2 | 0.14 | 1.02 |
| 3 | 0.35 | 1.53 |
| 4 | 0.53 | 2.04 |
| 5 | 0.71 | 2.55 |
| 6 | 1.06 | |
| 7 | 1.41 |
The used ion chromatography material led to higher contents of residual calcium (65 vs. 15 mmol/L; Table 4) but at the same time decreased added quantity of D/L-CaβHB salt. Simultaneously, there was an increase in sodium with an unknown cause. In both cases, the pH was 4.0 ± 0.5 (Table 3). After the use of ion exchange material, it was not possible to achieve a sufficient regeneration for the reuse of the matrix. Free oxalic acid after precipitation was <30 mg/L.
Table 4.
Analyses of D-βHB (mmol/L; g/L), sodium (Na; mmol/L; g/L), calcium (Ca; mmol/L; g/L), potassium (K; mmol/L; g/L), and pH in βHB acid solutions produced with ion exchange chromatography (IEX) or oxalic acid precipitation (OAP).
| Method | D-βHB (mmol/L) | D-βHB (g/L) | Na (mmol/L) | Na (g/L) | Ca (mmol/L) | Ca (g/L) | K (mmol/L) | K (g/L) | pH |
|---|---|---|---|---|---|---|---|---|---|
| IEX | 342 | 35.3 | 193 | 4.44 | 65 | 2.61 | 43 | 1.68 | 4 |
| OAP | 495 | 51.0 | 72 | 1.66 | 15 | 0.60 | 71 | 2.78 | 4 |
4.2. Tube feed solution
Tube feeding was based on three components; a non-allergenic infant nutrition product (Neocate infant, Nutricia, Erlangen, Germany), a fat-free product (Milupa Basic F, Nutricia, Erlangen, Germany) and maltodextrin (Nutricia, Erlangen, Germany). The daily water intake by tube feeding was calculated at 550 mL. The macronutrient intake was 10.5% of daily energy (E%) in protein, 20.0 E% in fat, and 69.5 E% in carbohydrates. Total daily caloric intake by tube feed was 1126 kcal. Additional oral eating depended on the condition and personal preferences of the child.
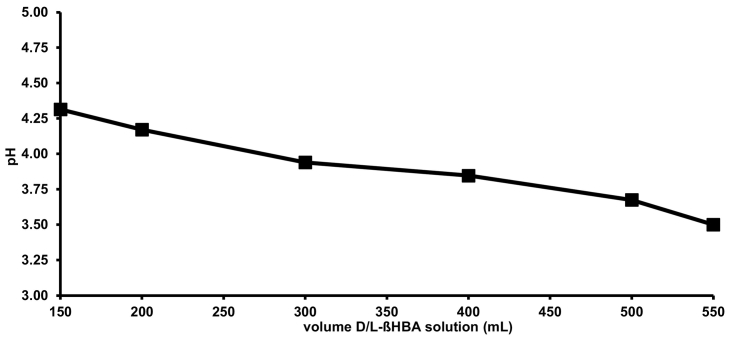
Replacing water with the D/L-βHBA solution led to a continuous decrease of the pH in the tube feed. The complete substitution of water with the D/L-βHBA solution led to a pH of 3.50 (see Fig. 1). All mixtures of D/L-βHBA and infant nutrition products were homogenous with no indication of ingredient precipitation.
Fig. 1.
Influence on pH by replacement of water with D/L-βHBA solution in tube feeding.
4.3. Treatment 1
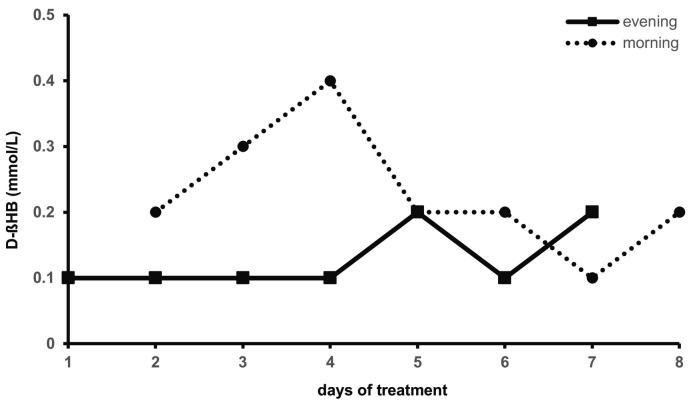
During the intervention with D/L-βHBA (IEX), the base excess (3.83 ± 2.47 mmol/L) and bicarbonate (26.11 ± 1.63) were partially increased with a continuous normal pH value (7.39 ± 0.05) in blood samples. Glucose (5.36 ± 1.45 mmol/L; 96.60 ± 26.06 mg/dL) and sodium (138.13 ± 1.73 mmol/L) were within the reference range. At treatment start, a D-βHB value of 0.1 mmol/L was determined. Measurements of D-βHB in the morning and evening presented a maximum increase to 0.4 mmol/L. In further course and at higher concentrations of D/L-βHBA the D-βHB in capillary blood was 0.2 mmol/L or lower (see Fig. 2). The measurement of D-βHB in venous blood was consistently lower than 0.10 mmol/L or around the detection limit (<0.05 mmol/L).
Fig. 2.
D-βHB (mmol/L) capillary blood measurement in the morning (8 a.m.) and evening (6 p.m.) during therapy with D/L-βHBA solution (IEX).
The daily increase of the D/L-βHBA dosage to a maximum of 1.41 g/kg bw had no adverse effects. However, by the short-term application of βHBA, no significant improvements of the health status were observed.
4.4. Treatment 2
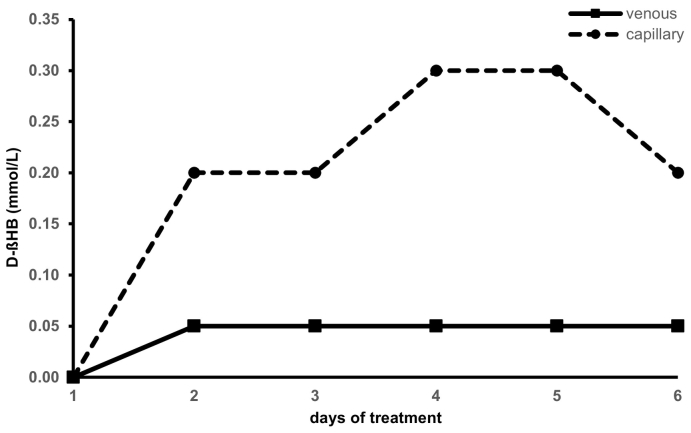
In the second treatment with D/L-βHBA (OAP), the base excess (2.53 ± 2.21 mmol/L) and the bicarbonate (25.77 ± 2.10 mmol/L) increased only slightly at a normal pH value (7.42 ± 0.04). All minerals, like sodium (141.50 ± 2.88 mmol/L), potassium (3.83 ± 0.43 mmol/L), calcium (2.24 ± 0.10 mmol/L), and magnesium (0.80 ± 0.06 mmol/L) were within the normal range. Analysis of D-βHB in serum was constant at 0.05 mmol/L or below. Measurement of D-βHB in capillary blood presented a maximum increase to 0.3 mmol/L (see Fig. 3). During the therapy, the creatine kinase (CK) level was reduced from 949 U/L at the start to 459 U/L after the fifth day of βHBA ingestion. Semi-quantitative urine analysis using GC showed a steady increase of D/L-βHB and acetoacetate excretion from day 1 to day 5.
Fig. 3.
D-βHB (mmol/L) in serum and capillary blood during a second therapeutic trial with D/L-βHBA solution (OAP). The blood samples were taken in the morning between 8 a.m. and 9 a.m.
The increase to a dosage of 2.55 g/kg bw was not free of side effects. On the fifth day, vomiting and diarrhoea occurred. For the child's safety and reduction of gastrointestinal discomfort, the treatment was stopped after the fifth day.
5. Discussion
In several case reports the use of βHB sodium salt is described as an effective therapy in MADD [[8], [9], [10]]. In one case an additional treatment with a βHB calcium salt was tested and had no clinical benefit. A critical part of the salt intake is the high value of cations, like sodium, calcium or others, which can lead to side effects like alkalosis [12]. It was already shown in the 1980s that ketone body salts can lead to an increase of blood pH [17,18]. In a recent study on healthy subjects, a ketone body salt increased, and a ketone ester decreased, the pH of the blood. In both cases the acid-base balance was influenced by the intake of ketone body supplements [16]. A possible reason could be a shift of the electrolyte balance according to the Stewart-theory [12]. The ingestion of the D/L-βHBA did not result in a pH change in short-term application. Temporary increase of BE, bicarbonate, and pCO2 with normal pH is an indication of compensated metabolic alkalosis. The tendency to acidosis was not present throughout the treatment.
The treatment with D/L-βHBA as an alternative to the salt intake resulted in an only mild increase of D-βHB in blood with a tendency to gastrointestinal discomfort. The higher intake of βHBA in the second intervention did not result in a higher concentration of D-βHB in blood. In both interventions the target concentration of 2–5 mmol/L, accordant to a ketogenic diet, was not reached. The high target concentration of D-βHB in blood and the increasing dosage of D/L-βHBA to this end in this study aims to cover a potentially existing energy deficit caused by the breakdown of fatty acid oxidation in MADD [12]. A minimal increase of D-βHB in blood caused by βHB supplements is a well-known fact in MADD and is probably caused by its use for energy production [8,10,12]. Also, in healthy subjects a relatively small increase of D-βHB was observed after the intake of a ketone body salt. One reason forthe use of a racemic mixture of βHBaffects the physiological use [16]. Overall, the bioavailability and the maximum intake of D/L-βHB salt seem to be limited [16,19]. More human studies, especially long-term studies are necessary to improve the understanding of the application of βHB-based products.
For this patient, frequent vomiting is common and cannot be attributed directly to the intake of βHBA. However, there was a higher rate of vomiting during the intervention with βHBA. A dosage of around 1.5 g/kg bw showed no side effects and at the same time no positive clinical result in short-term application. For both salts and esters, gastrointestinal side effects have already been reported. Symptoms include nausea, vomiting, abdominal pain, and diarrhoea [[20], [21], [22]]. The potential side effects should be observed for a treatment with βHB products to ensure that the application is as safe as possible for the patient.
A pH of less than pH 4.0 is not unusual in beverages. In a pH analyses of 379 beverages, 354 (93%) had a pH below 4.0. For example, apple juice has a pH of 3.6 and a classic coke 2.4 [23]. The measured minimum pH of the tube feed was 3.5 and the solution was administered continuously over the course of the day with a nutrition pump. By this handling, a fast intake of the βHBA solution was avoided. In accordance with the detected side effects, an intake as a bolus of βHBA is not recommendable because adverse effects could be intensified and the procedure is generally less physiological. A long-term application of sour solutions could lead to dental erosion or gastrointestinal discomfort, like triggering reflux [[24], [25], [26]]. Specific adverse effects of the weak acid βHBA are unknown and should be closely monitored during the clinical application.
The strong sour taste of βHBA is typical for organic acids. The human organism perceives organic acids as being more sour in taste than inorganic acids, such as phosphoric or hydrochloric acid, with the same pH value [27,28]. Justified by the sensory restriction, an oral intake is only possible in small amounts, for example as an acidifier in special food or beverages. An unpleasant aftertaste of the βHBA solution was not detected; on the contrary the solution was perceived to have a fruity sour taste.
Chemical analysis of the D/L-βHBA solution indicated that the precipitation method is more precise in pre-calculation and better suited to produce higher volumes of D/L-βHBA. An increased volume of the initial solution led to higher concentrations of calcium using the IEX method. The higher calcium level indicates a poorer purification performance in production of increased batch size. For better purification results using IEX, the testing of further IEX material and a method change is necessary. The precipitation with oxalic acid left only small amounts of free oxalic acid (<30 mg/L). Remaining quantity of oxalic acid can be assumed to be non-critical for ingestion. Foods like orange, tomato, potato, nuts and many more have a higher content of oxalate than the used βHBA solution [29,30].
At present, the manufacture of the βHBA is elaborate, time intensive, and the application is not extensively studied. In the present case, a 4 weeks storage time in the refrigerator and deep-frozen long-term storage (1 year) was regarded as unproblematic for the stability of the solution. However, further investigations on stability and method improvements are necessary.
A primary limitation of this report is the transfer to other cases or healthy subjects, as it is a description of the experience with a single patient.
6. Conclusion
The use of D/L-βHBA in severe MADD could be a good addition to the use of classical ketone body salts. In particular, a combination of βHBA and βHB-salts is of interest in reducing the extensive intake of cations, like sodium or calcium. Potential adverse effects of the intake of D/L-βHBA are gastric irritation, vomiting and diarrhoea. To ensure a safe application in severe MADD, further studies on tolerability and possible additional benefits are necessary.
Declaration of Competing Interest
None.
Acknowledgments
The individual medical treatment with D/L-βHBA and producing of D/L-βHBA by OAP were performed and funded by the department of congenital metabolic disorders of the University Hospital, Muenster. Manufacturing of D/L-βHBA by IEX was performed and funded by the department food, nutrition, facilities of the University of Applied Sciences, Muenster. The authors are grateful to Monika Brummel, Rita Wehmeyer, Hedwig Schindler, Tabea Pöhler and Ursula Bordewick-Dell of the University of Applied Sciences in Muenster for the manufacturing of the D/L-βHBA solution with IEX.
References
- 1.Schiff M., Froissart R., Olsen R.K.J. Electron transfer flavoprotein deficiency: functional and molecular aspects. Mol. Genet. Metab. 2006;88(2):153–158. doi: 10.1016/j.ymgme.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Przyrembel H., Wendel U., Becker K. Glutaric aciduria type II: report on a previously undescribed metabolic disorder. Clin. Chim. Acta. 1976;66(2):227–239. doi: 10.1016/0009-8981(76)90060-7. [DOI] [PubMed] [Google Scholar]
- 3.Frerman F.E., Goodman S.I. Deficiency of electron transfer flavoprotein or electron transfer flavoprotein:ubiquinone oxidoreductase in glutaric acidemia type II fibroblasts. Proc. Natl. Acad. Sci. U. S. A. 1985;82(13):4517–4520. doi: 10.1073/pnas.82.13.4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frerman F.E., Goodman S.I. Defects of electron transfer flavoprotein and electron transfer flavoprotein-ubiquinone oxidoreductase: glutaric acidemia type II. In: Scriver C.R., Childs B., Kinzler K.W., editors. The Metabolic & Molecular Bases of Inherited Disease. McGraw-Hill; New York, N.Y., London: 2004. [Google Scholar]
- 5.Goodman S.I., Frerman F.E. Glutaric acidaemia type II (multiple Acyl-Coa dehydrogenation deficiency) J. Inherit. Metab. Dis. 1984;7(S1):33–37. doi: 10.1007/BF03047371. [DOI] [PubMed] [Google Scholar]
- 6.al-Essa M.A., Rashed M.S., Bakheet S.M. Glutaric aciduria type II: observations in seven patients with neonatal- and late-onset disease. J Perinatol: official journal of the California Perinatal Association. 2000;20(2):120–128. doi: 10.1038/sj.jp.7200325. [DOI] [PubMed] [Google Scholar]
- 7.Uziel G., Garavaglia B., Ciceri E. Riboflavin-responsive glutaric aciduria type II presenting as a leukodystrophy. Pediatr. Neurol. 1995;13(4):333–335. doi: 10.1016/0887-8994(95)00187-5. [DOI] [PubMed] [Google Scholar]
- 8.van Hove J.L.K., Grünewald S., Jaeken J. D,L-3-hydroxybutyrate treatment of multiple acyl-CoA dehydrogenase deficiency (MADD) Lancet. 2003;361(9367):1433–1435. doi: 10.1016/S0140-6736(03)13105-4. [DOI] [PubMed] [Google Scholar]
- 9.Gautschi M., Weisstanner C., Slotboom J. Highly efficient ketone body treatment in multiple acyl-CoA dehydrogenase deficiency-related leukodystrophy. Pediatr. Res. 2015;77(1–1):91–98. doi: 10.1038/pr.2014.154. [DOI] [PubMed] [Google Scholar]
- 10.van Rijt W.J., Heiner-Fokkema M.R., du Marchie Sarvaas Gideon J. Favorable outcome after physiologic dose of sodium-D,L-3-hydroxybutyrate in severe MADD. Pediatrics. 2014;134(4):8. doi: 10.1542/peds.2013-4254. [DOI] [PubMed] [Google Scholar]
- 11.van Rijt W.J., Jager E.A., Cigdem Aktuglu Zeybek A. O-50: 3-hydroxybutyrate (3-HB) treatment in MADD: a systematic literature review and international retrospective cohort study. J. Inherit. Metab. Dis. 2018;41(S1):1–36. [Google Scholar]
- 12.Fischer T., Och U., Marquardt T. Long-term ketone body therapy of severe multiple acyl-CoA dehydrogenase deficiency: a case report. Nutrition. 2019;60:122–128. doi: 10.1016/j.nut.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Plecko B., Stoeckler-Ipsiroglu S., Schober E. Oral beta-hydroxybutyrate supplementation in two patients with hyperinsulinemic hypoglycemia: monitoring of beta-hydroxybutyrate levels in blood and cerebrospinal fluid, and in the brain by in vivo magnetic resonance spectroscopy. Pediatr. Res. 2002;52(2):301–306. doi: 10.1203/00006450-200208000-00025. [DOI] [PubMed] [Google Scholar]
- 14.Habarou F., Bahi-Buisson N., Lebigot E. Ketone bodies as a possible adjuvant to ketogenic diet in PDHc deficiency but not in GLUT1 deficiency. JIMD Rep. 2017;(58):53–59. doi: 10.1007/8904_2017_30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer T., Marquardt T. Dietary supplements based on the ketone body β-hydroxybutyrate: market analysis and evaluation of ingredients of supplements used in the USA. Ernährungs-Umschau. 2018;65(12):204–212. [Google Scholar]
- 16.Stubbs B.J., Cox P.J., Evans R.D. On the metabolism of exogenous ketones in humans. Front. Physiol. 2017;8:137. doi: 10.3389/fphys.2017.00848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Müller M.J., Paschen U., Seitz H.J. Effect of ketone bodies on glucose production and utilization in the miniature pig. J. Clin. Invest. 1984;74(1):249–261. doi: 10.1172/JCI111408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Féry F., Balasse E.O. Effect of exercise on the disposal of infused ketone bodies in humans. J. Clin. Endocrinol. Metab. 1988;67(2):245–250. doi: 10.1210/jcem-67-2-245. [DOI] [PubMed] [Google Scholar]
- 19.Fischer T., Och U., Klawon I. Effect of a sodium and calcium DL-β-Hydroxybutyrate salt in healthy adults. J. Nutr. Metab. 2018;2018 doi: 10.1155/2018/9812806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leckey J.J., Ross M.L., Quod M. Ketone diester ingestion impairs time-trial performance in professional cyclists. Front. Physiol. 2017;8 doi: 10.3389/fphys.2017.00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clarke K., Tchabanenko K., Pawlosky R. Kinetics, safety and tolerability of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate in healthy adult subjects. Regul. Toxicol. Pharmacol. 2012;63(3):401–408. doi: 10.1016/j.yrtph.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans M., Patchett E., Nally R. Effect of acute ingestion of β-hydroxybutyrate salts on the response to graded exercise in trained cyclists. Eur. J. Sport Sci. 2018;18(3):376–386. doi: 10.1080/17461391.2017.1421711. [DOI] [PubMed] [Google Scholar]
- 23.Reddy A., Norris D.F., Momeni S.S. The pH of beverages in the United States. J. Am. Dent. Assoc. 1939;147(4):255–263, 2016. doi: 10.1016/j.adaj.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larsen M.J., Nyvad B. Enamel erosion by some soft drinks and orange juices relative to their pH, buffering effect and contents of calcium phosphate. Caries Res. 1999;33(1):81–87. doi: 10.1159/000016499. [DOI] [PubMed] [Google Scholar]
- 25.Shen P., Walker G.D., Yuan Y. Food acid content and erosive potential of sugar-free confections. Aust. Dent. J. 2017;62(2):215–222. doi: 10.1111/adj.12498. [DOI] [PubMed] [Google Scholar]
- 26.Sethi S., Richter J.E. Diet and gastroesophageal reflux disease: role in pathogenesis and management. Curr. Opin. Gastroenterol. 2017;33(2):107–111. doi: 10.1097/MOG.0000000000000337. [DOI] [PubMed] [Google Scholar]
- 27.Roper S.D. Signal transduction and information processing in mammalian taste buds. Pflugers Arch. - Eur. J. Physiol. 2007;454(5):759–776. doi: 10.1007/s00424-007-0247-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramos Da Conceicao Neta E.R., Johanningsmeier S.D., McFeeters R.F. The chemistry and physiology of sour taste—a review. J. Food Sci. 2007;72(2):R33–R38. doi: 10.1111/j.1750-3841.2007.00282.x. [DOI] [PubMed] [Google Scholar]
- 29.Massey L.K. Food oxalate: factors affecting measurement, biological variation, and bioavailability. J. Am. Diet. Assoc. 2007;107(7):1191–1194. doi: 10.1016/j.jada.2007.04.007. quiz 1195-6. [DOI] [PubMed] [Google Scholar]
- 30.Attalla K., De S., Monga M. Oxalate content of food: a tangled web. Urology. 2014;84(3):555–560. doi: 10.1016/j.urology.2014.03.053. [DOI] [PubMed] [Google Scholar]