Abstract
Background
Microwave ablation (MWA) has recently become an established treatment option for topical therapy of lung cancer patients. In this study, we evaluated whether MWA combined with chemotherapy could improve progression‐free survival (PFS) of patients with stage IV lung adenocarcinoma compared with chemotherapy alone.
Methods
A total of 49 patients were enrolled into the study; 21 patients accepted MWA therapy combined with chemotherapy, 28 patients accepted only chemotherapy. Enumeration data were analyzed using χ2 test or Fisher's exact probability test and univariate analysis was analyzed using Kaplan–Meier survival curves. Multivariate analysis was carried out with the Cox proportional hazard model.
Results
The treatment regimen was not correlated with clinical features of the patients, which included gender, age, smoking history, tumor site, tumor size and Eastern Cooperative Oncology Group (ECOG). The patients’ 3‐year overall survival (OS) was 12.5%, and median survival time was 19.3 months. The median PFS was 6.1 months and the 1‐year PFS was 0.0%. The PFS was significantly associated with tumor size (P < 0.05), ECOG (P < 0.01) and treatment regimen (P < 0.01). The median time to local progression (TTLP) was 8.4 months and the 3‐year TTLP was 2.0%. The TTLP was significantly associated with tumor size (P < 0.05) and treatment regimen (P < 0.01). Cox multivariate regression demonstrated that MWA combined with chemotherapy was the independent factor for both the PFS and TTLP.
Conclusion
MWA, as a topical treatment method, when combined with chemotherapy improved the PFS and TTLP of patients with stage IV lung adenocarcinoma.
Keywords: Chemotherapy, lung adenocarcinoma, microwave ablation, progression‐free survival
Introduction
Carcinoma of the lung is the most common cause of tumor‐related mortality worldwide. More than 85% of lung neoplasia is non‐small cell lung cancer (NSCLC).1 Nearly 75% of NSCLC patients present with advanced disease at their first visit2 and the 5‐year survival rate is reported as 15%.3 For patients with advanced NSCLC, platinum‐based, doublet chemotherapy is used as the first line treatment prescription. According to previous reports, the overall survival (OS) of the patients with advanced NSCLC ranged from 7.9 to 10.3 months and progression‐free survival (PFS) was from 3.6 to 4.8 months.4, 5 A recent meta‐analysis of seven trials has reported that concomitant radiochemotherapy could improve survival of patients with locally advanced NSCLC, primarily because of better locoregional control, but at the cost of manageable increased acute esophageal toxicity.6
New alternatives to standard external beam radiation therapy are now entering clinical practice for the treatment of lung cancer or limited pulmonary metastases in medically inoperable patients. Several reports have demonstrated that topical therapies such as 125I seed implantation, or radiofrequency ablation (RFA) when combined with chemotherapy might relieve symptoms and improve the objective response rate.7, 8
Microwave ablation (MWA) has recently been applied as topical therapy for lung cancer patients and has been shown to enlarge ablative regions and shorten the treatment time when compared with RFA.9, 10 In this study, we evaluate whether MWA combined with chemotherapy could improve PFS of patients with stage IV lung adenocarcinoma compared with chemotherapy alone.
Methods
Patients
A total of 49 patients were enrolled into this retrospective study at the Department of Thoracic Surgery and Oncology, Jinan Central Hospital and the Department of Respiration and Oncology, Qilu Hospital of Shandong University between January 2013 and December 2015. The inclusion criteria were as follows: (i) Patients were confirmed to have lung adenocarcinoma following biopsy pathology; (ii) Only stage IV patients were included, and the clinical TNM staging system was summarized by the Union for International Cancer Control (UICC, 8th)11; (iii) Patients had not accepted any anticancer treatments such as surgery, chemotherapy, radiotherapy and targeted therapy before enrollment into the study. Patients had EGFR‐insensitive mutations or could not accept EGFR‐TKIs as the first‐line therapy; (iv) Patients scored 0–2 according to Eastern Cooperative Oncology Group (ECOG). Exclusion criteria were as follows: (i) patients had a life expectancy of no more than 3 months, or (ii) patients had symptomatic brain metastases. Table 1 shows the clinicopathological features of the patients.
Table 1.
Correlation between treatment regimen and clinical features of the patients
| Clinical features | Patients | MWA plus chemotherapy | Chemotherapy | P‐valuea |
|---|---|---|---|---|
| 49 | 21 | 28 | ||
| Gender | 0.779 | |||
| Male | 22 | 10 | 12 | |
| Female | 27 | 11 | 16 | |
| Age, year | 0.549* | |||
| ≤60 | 17 | 6 | 11 | |
| >60 | 32 | 15 | 17 | |
| Smoking | 0.750* | |||
| No | 35 | 16 | 19 | |
| Yes | 14 | 5 | 9 | |
| Tumor site | 1.000* | |||
| Left lung | 22 | 9 | 13 | |
| Right lung | 27 | 12 | 15 | |
| Tumor site of lung | 0.393* | |||
| Upper and middle lobe | 25 | 9 | 16 | |
| Lower lobe | 24 | 12 | 12 | |
| Tumor size | 0.076* | |||
| ≤3cm | 10 | 7 | 3 | |
| >3cm | 39 | 14 | 25 | |
| ECOG | 0.750* | |||
| 0 | 7 | 3 | 4 | |
| 1 | 38 | 17 | 21 | |
| 2 | 4 | 1 | 3 | |
| Stage | 1.000 | |||
| IVA | 42 | 18 | 24 | |
| IVB | 7 | 4 | 3 |
Fisher's exact probability test.
P‐value: χ2 test.
ECOG, Eastern Cooperative Oncology Group; MWA, microwave ablation.
Treatment regimen
A total of 21 patients accepted the combined MWA with chemotherapy and chemotherapy was administered after the MWA therapy, while 28 patients accepted chemotherapy alone. The same chemotherapy regimen was given to all patients and they were treated with pemetrexed 500 mg/m2 on the first day, followed by cisplatin 75 mg/m2 on the first and second days. Chemotherapy was given every three weeks and in 4–6 cycles and was terminated when the disease progressed.
Anesthesia
Food and water was withheld from patients for four hours prior to the MWA procedure. Local anesthetic was applied at the puncture site of the patient using 1% lidocaine and intravenous anesthesia (propofol 1.5–2mg/kg) was used when the ablation commenced.
MWA procedure
The patients were treated with MWA under computed tomography guidance (CT). An ECO‐100C MWA (ECO Microwave Electronic Institute, Nanjing, China; registration standard: YZB/country 3388–2011; China: SFDA certified No.20113251473) system was used. The microwave emission frequency was 2,450 ± 50 MHz, and output level adjustable continuous wave ranged from 0 to 150 W. The microwave antenna had a single slot, an effective length of 10–18 cm and an outside diameter of 14–20 G, with a 15 mm active tip. The ablation power was selected as 60–70 W with 4–8 minutes duration. One antenna was applied for tumors less than 3.5 cm in diameter, and two were used for those more than 3.5 cm in diameter simultaneously. The antenna was modified and repositioned according to imaging changes until the tumor was covered completely (plus an ablative margin of at least 5 mm, and ideally 10 mm around the tumor). A CT scan was performed immediately after the MWA procedure to assess the treatment response as well as to demonstrate immediate complications, such as pneumothorax and hemorrhage. The procedure of MWA described above was the same as those reported in the previous studies.12, 13
Follow‐up
A chest CT examination was performed on patients on the 7th day, 1 month, and every 3‐month follow‐ up visits after the MWA. RThe response to chemotherapy was evaluated every two cycles. Disease progression was included in progression at ablative sites, distant metastases, and death of tumor.
Ethics statement
The study was approved by Jinan Central Hospital Affiliated to Shandong University (Jinan, People's Republic of China). Written informed consent was obtained from all the 49 patients.
Statistical analyses
Enumeration data were analyzed using χ2 test or Fisher's exact probability test. Univariate analysis was performed by modeling the data with Kaplan–Meier survival curves. The log‐rank test was used to calculate the survival rate. Multivariate analysis was carried out with the Cox proportional hazard model. All statistical data were analyzed using SPSS (version 13; SPSS, Inc., Chicago, IL, USA) and P < 0.05 was considered to indicate a statistically significant difference.
Results
A total of 49 patients were enrolled in the study and all had one tumor in the lung. Of these, 21 cases were enrolled in the MWA plus chemotherapy group and 28 in the chemotherapy group. There were 22 male patients, 32 cases were more than 60 years old, 14 had a smoking history, 27 cases had primary tumors located in the right lung and 25 in the upper and middle lobes. A total of 45 cases had ECOG PS of 0–1, and 39 had primary tumors with a diameter greater than 3 cm. A total of 42 cases were stage IVA and seven cases stage IVB. In the MWA with chemotherapy group, the mean diameter of primary tumors was 3.5 (range 2.5–4.7) cm. In the chemotherapy group, the mean diameter of primary tumors was 4.7 (range 2.6–7.0) cm. Clinical characteristics of the patients are shown in Table 1. The treatment regimen was not correlated with clinical features of the patients, which included gender, age, smoking history, tumor site, tumor size, ECOG and stage. MWA‐associated complications were found in three patients (14.3%) which were pneumothorax (two cases) and hemoptysis (one case). The complications resolved when the patients were discharged. Adverse reaction induced by chemotherapy was observed in 13(61.9%) and 19 (67.9%) patients in the MWA/chemotherapy and chemotherapy group, respectively (Fig 1).
Figure 1.
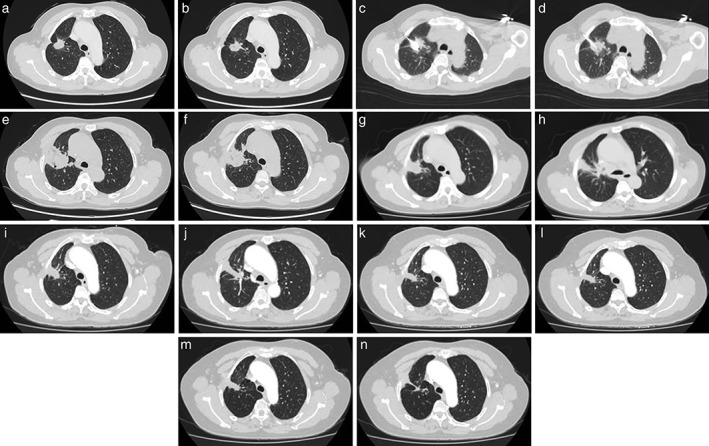
(a, b) A 48‐year‐old female was diagnosed with a right upper lobe lung mass and left renal mass. She was confirmed to have a primary lung adenocarcinoma with renal metastatic carcinoma on biopsy. CT showedithe primary lung tumor was 2.5 cm in diameter. (c, d) Puncture of the ablative antenna into the tumor. (e, f) One month after microwave ablation (MWA). (g, h) Four months after MWA. (i, j) Seven months after MWA. (k, l) Ten months after MWA. (m, n) Thirteen months after MWA. The tumor hasshrunk and fibrosis has developed, An irregular cavity has also formed.
The patients’ 3‐year OS was 12.5% (cutoff = 3 years), and median survival time was 19.3 months (range, 9–36 months). The median PFS was 6.1 months (range, 1.8–11.6 months) and the 1‐year PFS was 0.0%. The cases with primary tumors ≤3 cm in diameter had better PFS than those with tumors >3 cm in diameter (≤ 3 cm, 7.540 m vs. >3 cm, 5.731 m; P < 0.05). In addition, the PFS were significantly associated with ECOG (0 score, 6.814 m vs. 1 score, 6.276 m vs. 2 score, 3.175 m; P < 0.01). The cases with MWA plus chemotherapy had better PFS than those with chemotherapy alone (MWA plus chemotherapy, 8.029 m vs. chemotherapy, 4.654 m; P < 0.01) (Fig 2) (Table 2). The median time to local progression (TTLP) was 8.4 months (range, 1.8–36 months) and the 3‐year TTLP was 2.0%(cutoff = 3 years). The cases with primary tumors ≤ 3 cm in diameter had better TTLP than those with tumors >3 cm in diameter(≤ 3 cm, 13.240 m vs. >3 cm, 7.164 m; P < 0.05). The cases with MWA plus chemotherapy had better TTLP than those with chemotherapy alone (MWA plus chemotherapy, 13.105 m vs. chemotherapy, 4.879 m; P < 0.01) (Fig 2) (Table 2). No statistical difference was found in 3‐year OS between the treatment regimen (MWA plus chemotherapy, 21.057 m vs. chemotherapy, 17.843 m; P > 0.05). Cox regression analysis demonstrated that MWA combined with chemotherapy was the independent factor for the PFS and TTLP (Tables 3, 4).
Figure 2.
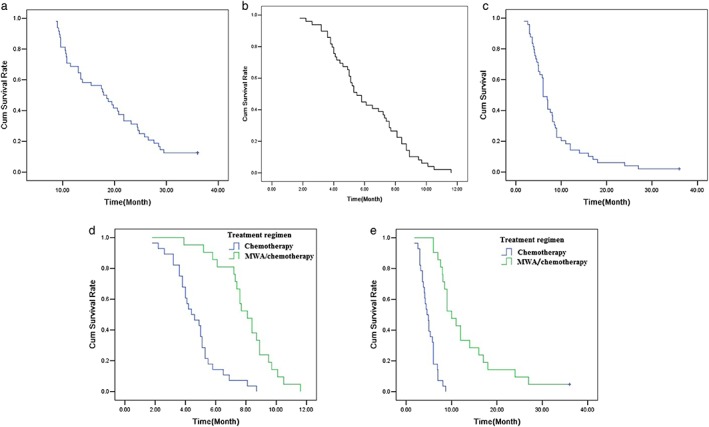
(a) A Kaplan‐Meier analysis of overall survival, (b) progression‐free survival (PFS), (c) median time to local progression (TTLP), (d) overall survival the progression‐free survival (PFS) in patients with treatment regimen, and (e) median time to local progression (TTLP) in patients with treatment regimen.
Table 2.
Univariate analysis with regard to PFS and TTLP
| 1‐year PFS (%) | 3‐year TTLP (%) | ||||||
|---|---|---|---|---|---|---|---|
| Clinical features | Patients | Patients0 | median time(m) | P‐valuea | Patients1 | median time(m) | P‐ valuea |
| 49 | 6.100 | 8.404 | |||||
| Gender | 0.319 | 0.792 | |||||
| Male | 22 | 0 | 6.532 | 0 | 8.000 | ||
| Female | 27 | 0 | 5.748 | 1 | 8.733 | ||
| Age, year | 0.826 | 0.750 | |||||
| ≤60 | 17 | 0 | 5.882 | 1 | 8.935 | ||
| >60 | 32 | 0 | 6.216 | 0 | 8.122 | ||
| Smoking | 0.708 | 0.124 | |||||
| No | 35 | 0 | 6.246 | 1 | 9.226 | ||
| Yes | 14 | 0 | 5.736 | 0 | 6.350 | ||
| Tumor site | 0.383 | 0.755 | |||||
| Right lung | 27 | 0 | 5.833 | 1 | 8.752 | ||
| Left lung | 22 | 0 | 6.427 | 0 | 7.977 | ||
| Tumor site of Lung | 0.432 | 0.781 | |||||
| Upper and middle lobe | 25 | 0 | 5.688 | 1 | 8.864 | ||
| Lower lobe | 24 | 0 | 6.529 | 0 | 7.925 | ||
| Tumor size | 0.027 | 0.019 | |||||
| ≤3 cm | 10 | 0 | 7.540 | 1 | 13 240 | ||
| >3 cm | 39 | 0 | 5.731 | 0 | 7.164 | ||
| ECOG | 0.006 | 0.540 | |||||
| 0 | 7 | 0 | 6.814 | 0 | 7.129 | ||
| 1 | 38 | 0 | 6.276 | 1 | 8.882 | ||
| 2 | 4 | 0 | 3.175 | 0 | 6.100 | ||
| Stage | 0.443 | 0.601 | |||||
| IVA | 42 | 0 | 6.224 | 1 | 8.600 | ||
| IVB | 7 | 0 | 5.357 | 0 | 7.229 | ||
| Treatment regimen | 0.001 | 0.001 | |||||
| MWA plus chemotherapy | 21 | 0 | 8.029 | 1 | 13.105 | ||
| Chemotherapy | 28 | 0 | 4.654 | 0 | 4.879 | ||
P‐value: Log‐rank test.
ECOG, Eastern Cooperative Oncology Group; m, month; MWA, microwave ablation; PFS, progression‐free survival; TTLP, time to local progression.
Table 3.
Results of Cox regression multivariate PFS analysis of the patients
| B | SE | Wald | P | HR | 95.0% CI for HR | |
|---|---|---|---|---|---|---|
| Gender | 0.237 | 0.386 | 0.376 | 0.540 | 1.267 | 0.595–2.700 |
| Age | −0.005 | 0.386 | 0.000 | 0.991 | 0.995 | 0.467–2.121 |
| Smoking | 0.299 | 0.521 | 0.329 | 0.566 | 1.348 | 0.485–3.746 |
| Tumor site | 0.571 | 0.363 | 2467 | 0.116 | 1.770 | 0.868–3.608 |
| Tumor site of lung | −0.290 | 0.400 | 0.528 | 0.468 | 0.748 | 0.342–1.637 |
| Tumor size | 0.308 | 0.480 | 0.412 | 0.521 | 1.361 | 0.531–3.485 |
| ECOG | 0.810 | 0.445 | 3.323 | 0.068 | 2.249 | 0.941–5.375 |
| Stage | 0.308 | 0.482 | 0.407 | 0.523 | 1.361 | 0.529–3.502 |
| Treatment regimen | −1.878 | 0.386 | 23.691 | 0.001 | 0.153 | 0.072–0.326 |
B, regression coefficient; SE, standard error; Wald, Wald value; HR, hazard ratio; CI, confidence interval; MWA, microwave ablation; PFS, progression‐free survival; HR, hazard ratio; CI, confidence interval. ECOG, Eastern Cooperative Oncology Group.
Table 4.
Results of Cox regression multivariate TTLP analysis of patients
| B | SE | Wald | P | HR | 95.0% CI for HR | |
|---|---|---|---|---|---|---|
| Gender | −0.063 | 0.388 | 0.027 | 0.870 | 0.939 | 0.439–2.009 |
| Age | −0.065 | 0.388 | 0.028 | 0.867 | 9.937 | 0.438–2.006 |
| Smoking | 0.697 | 0.502 | 1.926 | 0.165 | 2.007 | 0.750–5.367 |
| Tumor site | 0.196 | 0.361 | 0.294 | 0.587 | 1.216 | 0.600–2.467 |
| Tumor site of lung | 0.297 | 0.349 | 0.721 | 0.369 | 1.345 | 0.678–2.668 |
| Tumor size | 0.264 | 0.508 | 0.269 | 0.604 | 1.302 | 0.4981–3.527 |
| ECOG | 0.248 | 0.425 | 0.340 | 0.560 | 1.281 | 0.557–2.949 |
| Stage | 0.353 | 0.481 | 0.539 | 0.463 | 1.423 | 0.555–3.652 |
| Treatment regimen | −2.423 | 0.485 | 24.915 | 0.001 | 0.089 | 0.034–0.230 |
B, regression coefficient; SE, standard error; Wald, Wald value; HR, hazard ratio; CI, confidence interval; MWA, microwave ablation; time to local progression, TTLP; HR, hazard ratio; CI, confidence interval. ECOG, Eastern Cooperative Oncology Group.
Discussion
In 2000, Dupuy et al. firstly applied RFA to one lung cancer patient as topical therapy. From then on, some studies have showed the feasibility, safety, and efficacy of RFA for lung cancer. Lee et al.14 performed RFA on NSCLC patients and found that median survival times for patients treated with chemotherapy alone and RFA with chemotherapy for stage III to IV cancer were 29 and 42 months, respectively (P = 0.03). They concluded that RFA could play a role as adjuvant therapy with chemotherapy for patients with advanced lung cancer. Li reported that RFA was used as a supplemental treatment for 49 NSCLC patients after first‐line chemotherapy, and the PFS and OS were 16 weeks and 19 months, respectively.15 These studies showed RFA plus chemotherapy might be an effective therapeutic regimen for patients with advanced NSCLC.
Crabtree and colleagues compared the short‐term outcomes among three prospective clinical trials using stereotactic body radiotherapy (SBRT) (Radiation Therapy Oncology Group [RTOG] trial 0236), sublobar resection (American College of Surgeons Oncology Group [ACOSOG] trial Z4032), and radiofrequency ablation (ACOSOG trial Z4033) and they concluded that no difference was seen in early morbidity between sublobar resection, SBRT and RFA.16 RFA and SBRT have been demonstrated to be safe with reasonable efficacy in the treatment of NSCLC. RFA could be performed in one treatment session, whereas it now seems that SBRT is more effective if larger doses of radiation are given over two to three fractions. However, RFA is not recommended for centrally‐based tumors. In certain circumstances, a combined approach of RFA and SBRT may be beneficial.17, 18
MWA has been recently applied to deal with lung cancer as well as another local control treatment. When compared with RFA, MWA has some advantages. Firstly, MWA has higher energy and could ablate tumor necrosis in a shorter procedural time. Secondly, MWA creates less “heat sink” effect and consequently does not damage the perivascular tissue to the same extent. Moreover, the ablation zone could be maximized by simultaneously positioning multiple MWA antennae into a larger lesion simultaneously.17, 19 In the study by Wei, a total of 74 patients with stage IIIB and IV NSCLC were enrolled. Among them, 46 patients accepted MWA therapy combined with chemotherapy and 28 patients accepted only chemotherapy. The study demonstrated that the patients in the MWA with chemotherapy group had better PFS than that in the chemotherapy only group. However, there was no significant difference of median OS between the group treated by MWA with chemotherapy group and chemotherapy group only.20 In our study, a total of 49 patients were enrolled, and NSCLC stage IV patients were included. Our study showed the PFS was significantly associated with tumor size (≤ 3 cm, 7.540 m vs. >3 cm, 5.731 m; P < 0.05), ECOG (0 score, 6.814 m vs. 1 score, 6.276 m vs. 2 score, 3.175 m; P < 0.01) and treatment regimen (MWA plus chemotherapy, 8.029m vs. chemotherapy, 4.654 m; P < 0.01). The PFS of the patients in the MWA with chemotherapy group was higher than the chemotherapy only group. The TTLP was significantly associated with tumor size (≤3 cm, 13.240 m vs.>3 cm, 7.164 m; P < 0.05) and treatment regimen (MWA plus chemotherapy, 13.105 m vs. chemotherapy, 4.879 m; P < 0.01). The TTLP of the patients in the MWA with chemotherapy group was higher than the chemotherapy only group. However, no statistical difference was found in the 3‐year OS between the treatment regimens (MWA plus chemotherapy, 21.057 m vs. chemotherapy, 17.843 m; P > 0.05). Cox regression analysis demonstrated that MWA combined with chemotherapy was the independent factor for the PFS and TTLP, respectively.
With respect to the sequence of chemotherapy and thermal ablation, no conclusions have been reached. Previous studies applied RFA followed by chemotherapy and others applied chemotherapy followed by RFA, although the former was more common.21, 22 A preclinical study recommended administration of RFA first.23 In our study, all the cases underwent MWA first. We selected the lung adenocarcinoma patients with the same stage, and used the same chemotherapeutic drugs. To eliminate the impact of mixed factors correlated with PFS and TTLP on statistical analysis, we performed the Cox regression analysis to determine the independent factors. As a result, the comparability was increased and statistical bias was decreased, making the study results more objective. Nevertheless, our study has some potential limitations. Firstly, this is a retrospective study with a small sample size, which could limit the value of the findings. Secondly, in China, the indications for treatment depend not only on the advice of doctors, but also on patients’ willingness and their economic status. We recommended that patients with primary tumors ≤ 5 cm in diameter, especially ≤ 3 cm in diameter, accepted MWA with chemotherapy. In the study, the mean tumor size in the chemotherapy group is higher than the MWA with chemotherapy group. This may cause selection bias, although no statistical difference was found between the two groups. Lastly, in the study, we did not evaluate in detail whether MWA combined with chemotherapy could improve OS of patients with stage IV NSCLC compared with chemotherapy only. Because some patients had accepted second‐line chemotherapy, radiotherapy, or targeted therapy when their tumor progressed, these different therapeutic regimens could affect the OS of the patients.
In conclusion, MWA, as a topical treatment method, was able to improve both the PFS and TTLP of patients with stage IV lung adenocarcinoma when combined with chemotherapy. Our study suggests that MWA is an effective procedure for advanced lung adenocarcinoma. A randomized‐controlled prospective study with a larger sample size will be considered in further investigations.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
The present study was funded by The Second Group of Jinan Science and Technology Development Program (grant no. 201602204).
Contributor Information
Chunhai Li, Email: qlyylch2019@yeah.net.
Zhi‐Gang Sun, Email: sunszg@126.com.
References
- 1. Li Y, Wei S, Wang J, Hong L, Cui L, Wang C. Analysis of the factors associated with abnormal coagulation and prognosis in patients with non‐small cell lung cancer. Zhongguo Fei Ai Za Zhi 2014; 17: 789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sun W, Song L, Ai T, Zhang Y, Gao Y, Cui J. Prognostic value of MET,cyclin D1 and MET gene copy number in non‐small cell lung cancer. J Biomed Res 2012; 27: 220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non‐small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008; 83: 584–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schiller JH, Harrington D, Belani CP et al Comparison of four chemotherapy regimens for advanced non‐small‐cell lung cancer. N Engl J Med 2002; 346: 92–8. [DOI] [PubMed] [Google Scholar]
- 5. Scagliotti GV, Parikh P, von Pawel J et al Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy‐naive patients with advanced‐stage non‐small‐cell lung cancer. J Clin Oncol 2008; 26: 3543–51. [DOI] [PubMed] [Google Scholar]
- 6. Auperin A, Le Pechoux C, Rolland E et al Meta‐analysis of concomitant versus sequential radiochemotherapy in locally advanced non‐small‐cell lung cancer. J Clin Oncol 2010; 28: 2181e90. [DOI] [PubMed] [Google Scholar]
- 7. Duisters C, Beurskens H, Nijsten S et al Palliative chest irradiation in sitting position in patients with bulky advanced lung cancer. Radiother Oncol 2006; 79: 285–7. [DOI] [PubMed] [Google Scholar]
- 8. Zhang S, Zheng Y, Yu P et al The combined treatment of CT‐guided percutaneous 125I seed implantation and chemotherapy for non‐small‐cell lung cancer. J Cancer Res Clin Oncol 2011; 137: 1813–22. [DOI] [PubMed] [Google Scholar]
- 9. Jones C, Badger SA, Ellis G. The role of microwave ablation in the management of hepatic colorectal metastases. Surgeon 2011; 9: 33–7. [DOI] [PubMed] [Google Scholar]
- 10. Vogl TJ, Nour‐Eldin NA, Hammerstingl RM, Panahi B, Naguib NNN. Microwave ablation (MWA): Basics, technique and results in primary and metastatic liver neoplasms ‐ review article. Rofo 2017; 189: 1055–66. [DOI] [PubMed] [Google Scholar]
- 11. Lim W, Ridge CA, Nicholson AG, Mirsadraee S. The 8th lung cancer TNM classification and clinical staging system: Review of the changes and clinical implications. Quant Imaging Med Surg 2018; 8: 709–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang X, Ye X, Zhang L et al Microwave ablation for lung cancer patients with a single lung: Clinical evaluation of 11 cases. Thorac Cancer 2018; 9: 548–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang X, Ye X, Zheng A et al Percutaneous microwave ablation of stage I medically inoperable non‐small cell lung cancer: Clinical evaluation of 47 cases. J Surg Oncol 2014; 110: 758–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee H, Jin GY, Han YM et al Comparison of survival rate in primary non‐small‐cell lung cancer among elderly patients treated with radiofrequency ablation, surgery, or chemotherapy. Cardiovasc Intervent Radiol 2012; 35: 343–50. [DOI] [PubMed] [Google Scholar]
- 15. Li X, Zhao M, Wang J et al Percutaneous CT‐guided radiofrequency ablation as supplemental therapy after systemic chemotherapy for selected advanced non‐small cell lung cancers. Am J Roentgenol 2013; 201: 1362–7. [DOI] [PubMed] [Google Scholar]
- 16. Crabtree T, Puri V, Timmerman R et al Treatment of stage I lung cancer in high‐risk and inoperable patients: Comparison of prospective clinical trials using stereotactic body radiotherapy (RTOG 0236), sublobar resection (ACOSOG Z4032), and radiofrequency ablation (ACOSOG Z4033). J Thorac Cardiovasc Surg 2013; 145 (3): 692–9. [DOI] [PubMed] [Google Scholar]
- 17. Abbas G, Schuchert MJ, Pennathur A, Gilbert S, Luketich JD. Ablative treatments for lung tumors: Radiofrequency ablation, stereotactic radiosurgery, and microwave ablation. Thorac Surg Clin 2007; 17 (2): 261–71. [DOI] [PubMed] [Google Scholar]
- 18. Pennathur A, Luketich JD, Burton S et al Stereotactic radiosurgery for the treatment of lung neoplasm: Iinitial experience. Ann Thorac Surg 2007; 83: 1820–5. [DOI] [PubMed] [Google Scholar]
- 19. Yu J, Liang P, Yu X, Liu F, Chen L, Wang Y. A comparison of microwave ablation and bipolar radiofrequency ablation both with an internally cooled probe: Results in ex vivo and in vivo porcine livers. Eur J Radiol 2011; 79: 124–30. [DOI] [PubMed] [Google Scholar]
- 20. Wei Z, Ye X, Yang X et al Microwave ablation plus chemotherapy improved progression‐free survival of advanced non‐small cell lung cancer compared to chemotherapy alone. Med Oncol 2015; 32: 464. [DOI] [PubMed] [Google Scholar]
- 21. Li X, Zhao M, Wang J et al Percutaneous CT‐guided radiofrequency ablation as supplemental therapy after systemic chemotherapy for selected advanced non‐small cell lung cancer. Am J Roentgenol 2013; 201 (6): 1362–7. [DOI] [PubMed] [Google Scholar]
- 22. Wang SB, Chen JH, Cao W et al The observation of the clinical effect for combination therapy of RFA with GP on advanced stage lung cancer. Chin J Clin Oncol 2005; 32 (11): 628–30. [Google Scholar]
- 23. Kong G, Anyarambhatla G, Petros WP et al Efficacy of liposomes and hyperthermia in a human tumor xenograft model: Importance of triggered drug release. Cancer Res 2000; 60 (24): 6950–7. [PubMed] [Google Scholar]


