Abstract
Background
To investigate the prognostic impact of epidermal growth factor receptor (EGFR) mutation and anaplastic lymphoma kinase (ALK) rearrangement for the overall survival (OS) of patients with surgically treated lung adenocarcinomas.
Methods
A total of 689 patients with stage I–III lung adenocarcinomas (male:female = 334:355; median age, 64 years) underwent complete surgical resection between 2007 and 2013. The prognostic impact of EGFR mutation and ALK rearrangement on OS was analyzed using Cox regression analysis. Certain clinicopathological prognostic factors (i.e., age, sex, smoking status, nodule type, solid portion size, pathologic stage, adenocarcinoma subtype, and history of adjuvant chemotherapy) were included for adjustments of the hazard ratio (HR).
Results
EGFR mutation was observed in 438 patients (64%) and ALK rearrangement was seen in 28 patients (4%). Multivariable‐adjusted Cox regression demonstrated that the prognostic effect of EGFR mutation on OS differed by age (HR, exp.[−5.199 + 0.064*age]). The adjusted HR for EGFR mutation was 0.14 (95% CI: 0.05–0.36; P < 0.001) at 50 years, 0.26 (95% CI: 0.15–0.46; P < 0.001) at 60 years, and 0.50 (95% CI: 0.31–0.81; P = 0.005) at 70 years. However, the effect of ALK rearrangement on OS was without statistical significance (P > 0.05).
Conclusions
EGFR mutation was independently prognostic of the long‐term outcomes of patients with surgically treated lung adenocarcinomas. A more favorable prognostic effect was seen in younger than in older patients. ALK rearrangement was not associated with OS.
Keywords: Adenocarcinoma, anaplastic lymphoma kinase, epidermal growth factor receptor, prognosis, survival analysis
Introduction
Epidermal growth factor receptor (EGFR) mutation is a well‐known predictive marker of EGFR tyrosine kinase inhibitors (TKIs) in patients with advanced non‐small cell lung cancers (NSCLCs).1 Accordingly, EGFR TKI is generally the standard first‐line treatment administered to patients with activating mutations. Nevertheless, the prognostic value of EGFR mutation is controversial, particularly in patients with surgically resected NSCLCs. The prognostic role of other driver mutations, including anaplastic lymphoma kinase (ALK) rearrangement, is subject to the same ambiguity. Thus, solid evidence is presently lacking in this regard.
Performing an evaluation to determine the prognostic potential of driver mutations in a surgical cohort is vital, given the fact that this population differs from patients with advanced lung cancers who have not received prior treatment. Patients undergoing complete curative resection may experience postoperative recurrence, decreased pulmonary function, or poor performance due to lung surgery.2 Therefore, the survival dynamics of these patients are unique, and prognostic factors should be analyzed separately for this subset. It is thought that integrative prognostication using the anatomic elements of the tumor‐node‐metastasis staging system, as well as the non‐anatomical elements, i.e., genetic profiles (e.g., EGFR and ALK status), expedites accurate preoperative risk stratification and therapeutic planning.
Notably, there has been substantial heterogeneity in the methodologies of prognostic studies regarding driver mutations associated with resected lung cancer in terms of smoking status, cancer stage, histology, and imaging characteristics. Potential confounders require adjustment or stratification to determine the true prognostic effect of driver mutations. In addition, overall survival (OS) is considered to be an appropriate endpoint in the era of EGFR TKI, which prolonged survival in patients with metastatic lung cancers. Accordingly, this can lead to the decoupling between disease‐free survival and OS in patients with EGFR‐mutant lung cancers.
The aim of this study was to investigate the prognostic implications of EGFR mutation and ALK rearrangement for the OS of patients with surgically treated lung adenocarcinomas, having made adjustments to several important clinicopathologic prognostic factors.
Methods
This retrospective research was approved by the Institutional Review Board of Seoul National University Hospital. The requirement for written informed consent was waived.
Study population
Patients undergoing curative surgical resection without neoadjuvant chemo‐ and/or radiotherapy for lung adenocarcinoma were retrospectively identified at our tertiary referral hospital between October 2007 and December 2013 following a search of the electronic medical records (EMRs). Of 1466 patients, 1075 were subject to mutational analysis for both EGFR and ALK.
The following exclusion criteria were applied: (i) patients with synchronous or metachronous lung cancers (n = 145); (ii) a pathologic diagnosis of atypical adenomatous hyperplasia or adenocarcinoma in situ (n = 110); (iii) a pathologic diagnosis of invasive mucinous adenocarcinoma (n = 48); (iv) patients with pleural metastasis identified intraoperatively (n = 19); (v) adenocarcinoma subtypes not available (n = 57); (vi) the absence of a preoperative chest computed tomography (CT) scan (n = 1); and (vii) the absence of available preoperative clinical information (i.e., smoking history) (n = 2). Patients with both EGFR mutation and ALK rearrangement were also excluded (n = 4). A total of 689 patients participated in the study (Fig 1).
Figure 1.
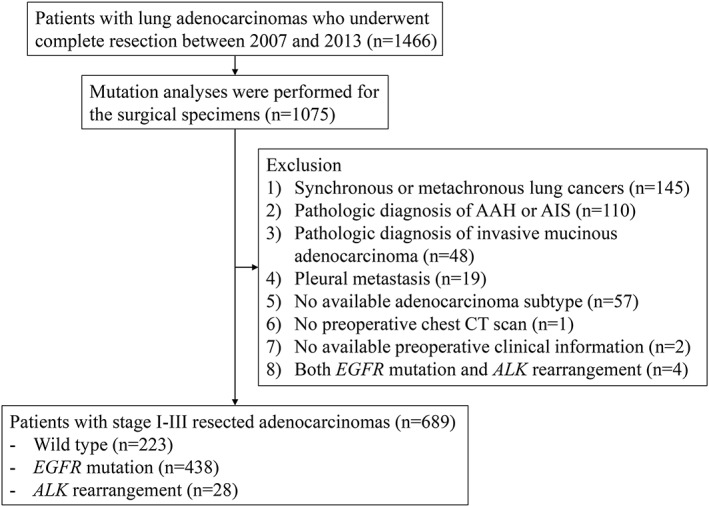
Flow diagram of patient inclusion and exclusion. AAH, atypical adenomatous hyperplasia; AIS, adenocarcinoma in situ; ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor.
Data collection
The patient characteristics (age and sex), date of surgery, surgical mode (lobectomy or sublobar resection), history of adjuvant therapy, smoking status (never smoker, ex‐ or current smoker), nodule location (upper lobe or other lobes), pathologic diagnosis, driver mutations (EGFR and ALK), pathologic stage based on the seventh edition of the American Joint Committee on Cancer staging system,3 and predominant adenocarcinoma subtype according to the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification were obtained from the EMRs.4 Pathologic diagnosis and adenocarcinoma subtype were confirmed by board‐certified pathologists at our hospital as part of routine clinical practice. The adenocarcinoma subtypes were categorized into three groups for the statistical analysis: (i) minimally invasive adenocarcinoma and lepidic predominant adenocarcinoma; (ii) acinar or papillary predominant adenocarcinoma; and (iii) micropapillary or solid predominant adenocarcinoma.5, 6
The nodule characteristics were evaluated using preoperative chest CT scans. Nodule type was determined according to one of the following patterns for each nodule: (i) pure ground‐glass (100% ground‐glass opacity [GGO]); (ii) GGO‐dominant (50% ≤ GGO < 100%); (iii) solid‐dominant (0% < GGO < 50%); or (iv) solid (0% GGO).7 The solid portion size (effective diameter) was obtained after manual volumetric segmentation of the entire nodule.7 Solid portion segmentation was achieved within the manually drawn regions‐of‐interest for the nodule using fuzzy C‐means clustering. The effective diameter (that of the sphere where the volume equals the solid portion volume) was then calculated.7 The solid portion was considered to be a substitute for the pathologic invasive component8, 9 not directly measured with regard to the surgical specimens in this study. The solid portion size was categorized as ≤3, >3 to ≤5 cm, >5 to ≤7 cm, or > 7 cm.8 Reviews of the images were performed by a single board‐certified thoracic radiologist (H.J.L. with 20 years of chest CT experience).
The primary endpoint was OS, measured from the date of surgery to the date of death from any cause. The last known surviving patients were censored at the date of the last outpatient visit.
Mutational analysis
The EGFR mutational status of the surgical specimen was analyzed using direct DNA sequencing, as previously described.10, 11 Following the extraction of genomic tumor DNA from the paraffin sections of the tumor block, EGFR exons 18–21 were amplified using polymerase chain reaction (PCR). Sequencing was performed on the PCR fragments in both the sense and anti‐sense directions. EGFR mutation was considered to be positive in the presence of activating mutations. ALK rearrangement was tested using fluorescence in situ hybridization (FISH). ALK FISH was deemed to be positive when ≥15% of the tumor cells counted showed a split signal of the fluorescent probes flanking the ALK locus.12 Wild‐type patients were neither EGFR‐mutant nor ALK‐positive.
Statistical analysis
The demographic and clinical characteristics of the patients were described as frequencies and percentages for the categorical variables, and as medians and interquartile ranges (IQR) for the continuous variables. Clinicopathological variables, including age, sex, smoking status, nodule type, location, surgical mode, solid portion size, pathologic stage, adenocarcinoma subtype, history of adjuvant chemotherapy, and driver mutation status, were assessed for their prognostic value.
An evaluation of the prognostic factors was performed using the Cox proportional hazard model. After univariable analysis, variables with a P‐value of ≤ 0.10 were considered for inclusion in the multivariable analysis. Interaction terms between driver mutation status and the other variables were also included in the multivariable analysis. The final multivariable model was determined using stepwise selection with an entry criterion of P‐value <0.10 and a removal criterion of P‐value ≥ 0.05. The proportional hazard assumption was confirmed using the Schoenfeld residuals. The P‐values were based on two‐sided tests. A P‐value of <0.05 was considered to represent statistical significance. Statistical analysis was conducted using SAS version 9.3 (SAS Institute, Cary, NC).
Results
The study population comprised 689 patients (334 males and 355 females; median age of 64 years; IQR of 56–70 years). EGFR mutation was seen in 438 patients (64%). ALK rearrangement was observed in 28 patients (4%). A total of 520 patients (76%) were categorized as pathologic stage I, 75 patients (11%) as stage II, and 92 patients (13%) as stage III. Solid portion size of ≤ 3 cm was attributed to 452 patients (66%), a size of >3 to ≤ 5 cm to 127 patients (18%), a size of >5 to ≤7 cm to 59 patients (9%), and a size of >7 cm to 51 patients (7%). Lung cancer manifested as pure ground‐glass nodules in 49 patients (7%), as GGO‐dominant nodules in 151 patients (22%), as solid‐dominant nodules in 247 patients (36%), and as solid nodules in 242 patients (35%). Of the adenocarcinoma histologic subtypes, 56 patients (8%) had minimally invasive adenocarcinomas, 82 (12%) had lepidic predominant adenocarcinomas, 351 (51%) had acinar predominant adenocarcinomas, 99 (14%) had papillary predominant adenocarcinomas, 16 (2%) had micropapillary predominant adenocarcinomas, and 85 (12%) had solid predominant adenocarcinomas. A total of 75 patients (11%) underwent sublobar resection and 115 (17%) were treated with adjuvant chemotherapy after surgery. Deaths occurred in 96 patients (14%). The median follow‐up interval was 2142 days (IQR of 1839–2505 days). The patient and lung nodule characteristics were described according to driver mutation status (Table 1).
Table 1.
Patient and tumor characteristics
| Variable | Wild‐type (n = 223) | EGFR mutation (n = 438) | ALK rearrangement (n = 28) | Total (n = 689) |
|---|---|---|---|---|
| Age (years)† | 65 (58, 71) | 63 (56, 70) | 59 (50, 69) | 64 (56, 70) |
| Sex | ||||
| Male | 143 (64.1) | 180 (41.1) | 11 (39.3) | 334 (48.5) |
| Female | 80 (35.9) | 258 (58.9) | 17 (60.7) | 355 (51.5) |
| Smoking history | ||||
| Never smoker | 95 (42.6) | 296 (67.6) | 21 (75.0) | 412 (59.8) |
| Ex‐ or current smoker | 128 (57.4) | 142 (32.4) | 7 (25.0) | 277 (40.2) |
| Nodule type | ||||
| Pure ground‐glass | 10 (4.5) | 39 (8.9) | 0 (0.0) | 49 (7.1) |
| GGO‐dominant | 36 (16.1) | 112 (25.6) | 3 (10.7) | 151 (21.9) |
| Solid‐dominant | 75 (33.6) | 168 (38.4) | 4 (14.3) | 247 (35.8) |
| Solid | 102 (45.7) | 119 (27.2) | 21 (75.0) | 242 (35.1) |
| Location | ||||
| Upper lobe | 123 (55.2) | 252 (57.5) | 11 (39.3) | 386 (56.0) |
| Other lobes | 100 (44.8) | 186 (42.5) | 17 (60.7) | 303 (44.0) |
| Surgical mode | ||||
| Sublobar resection | 35 (15.7) | 40 (9.1) | 0 (0.0) | 614 (89.1) |
| Lobectomy | 188 (84.3) | 398 (90.9) | 28 (100.0) | 75 (10.9) |
| Solid portion size | ||||
| ≤3 cm | 134 (60.1) | 297 (67.8) | 21 (75.0) | 452 (65.6) |
| >3 and ≤5 cm | 43 (19.3) | 81 (18.5) | 3 (10.7) | 127 (18.4) |
| >5 and ≤7 cm | 18 (8.1) | 38 (8.7) | 3 (10.7) | 59 (8.6) |
| >7 cm | 28 (12.6) | 22 (5.0) | 1 (3.6) | 51 (7.4) |
| Pathologic stage‡ | ||||
| I | 157 (70.4) | 350 (79.9) | 15 (53.6) | 522 (75.8) |
| II | 33 (14.8) | 39 (8.9) | 3 (10.7) | 75 (10.9) |
| III | 33 (14.8) | 49 (11.2) | 10 (35.7) | 92 (13.4) |
| Adenocarcinoma subtype | ||||
| MIA | 15 (6.7) | 41 (9.4) | 0 (0.0) | 56 (8.1) |
| Lepidic predominant | 24 (10.8) | 57 (13.0) | 1 (3.6) | 82 (11.8) |
| Acinar predominant | 107 (48.0) | 236 (53.9) | 8 (28.6) | 351 (50.6) |
| Papillary predominant | 31 (13.9) | 63 (14.4) | 5 (17.9) | 99 (14.3) |
| Micropapillary predominant | 4 (1.8) | 7 (1.6) | 5 (17.9) | 16 (2.3) |
| Solid predominant | 42 (18.8) | 34 (7.8) | 9 (32.1) | 85 (12.3) |
| Adjuvant chemotherapy | ||||
| Yes | 38 (17.0) | 64 (14.6) | 13 (46.4) | 115 (16.7) |
| No | 185 (83.0) | 374 (85.4) | 15 (53.6) | 574 (83.3) |
| Deaths | 54 (24.2) | 38 (8.7) | 4 (14.3) | 96 (13.9) |
| Follow‐up period (days)† | 1999 (1715, 2370) | 2239 (1897, 2549) | 2221 (1777, 2443) | 2142 (1839, 2505) |
Unless otherwise specified, the numbers in parentheses are percentages.
The data are expressed as medians with interquartile ranges in parentheses.
Pathologic stage according to the seventh edition staging system for lung cancer.
ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; GGO, ground‐glass opacity; MIA, minimally invasive adenocarcinoma.
Univariable Cox regression analysis for overall survival
The results of univariable Cox regression analysis for OS in lung adenocarcinomas are detailed in Table 2. EGFR mutation was significantly associated with OS (hazard ratio [HR] of 0.32; 95% confidence interval [CI]: 0.21–0.49; P < 0.001). By contrast, the effect of ALK rearrangement on OS was without statistical significance (HR of 0.54; 95% CI: 0.20–1.49; P = 0.232).
Table 2.
Univariable Cox regression analysis for overall survival in lung adenocarcinomas
| Variable | Subcategory | HR | 95% CI of HR | P‐value |
|---|---|---|---|---|
| Age (year) | 1.05 | 1.02, 1.07 | <0.001 | |
| Female sex | 0.51 | 0.33, 0.77 | 0.001 | |
| Ex‐ or current smoker (reference: never smoker) | 2.04 | 1.36, 3.05 | 0.001 | |
| Nodule type (reference: pure ground‐glass) | GGO‐dominant | 2.65 | 0.33, 21.18 | 0.358 |
| Solid‐dominant | 6.47 | 0.88, 47.34 | 0.066 | |
| Solid | 12.72 | 1.76, 91.80 | 0.012 | |
| Location at upper lobes (reference: other lung lobes) | 0.80 | 0.54, 1.19 | 0.271 | |
| Sublobar resection (reference: lobectomy) | 1.00 | 0.52, 1.92 | 0.994 | |
| Solid portion size (reference: ≤ 3 cm) | >3 cm and ≤5 cm | 2.69 | 1.63, 4.46 | <0.001 |
| >5 cm and ≤7 cm | 3.68 | 2.04, 6.63 | <0.001 | |
| >7 cm | 5.61 | 3.19, 9.88 | <0.001 | |
| Pathologic stage† (reference: stage I) | II | 2.95 | 1.69, 5.18 | <0.001 |
| III | 5.89 | 3.78, 9.18 | <0.001 | |
| Adenocarcinoma subtype (reference: MIA or lepidic predominant) | Acinar or papillary predominant | 22.42 | 3.12, 161.18 | 0.002 |
| Micropapillary or solid predominant | 42.58 | 5.80, 312.86 | <0.001 | |
| Adjuvant chemotherapy | 2.60 | 1.69, 3.99 | <0.001 | |
| Driver mutation (reference: wild‐type) | EGFR mutation | 0.32 | 0.21, 0.49 | <0.001 |
| ALK rearrangement | 0.54 | 0.20, 1.49 | 0.232 |
Pathologic stage followed the seventh edition staging system for lung cancer.
ALK, anaplastic lymphoma kinase; CI, confidence interval; EGFR, epidermal growth factor receptor; GGO, ground‐glass opacity; HR, hazard ratio; MIA, minimally invasive adenocarcinoma
A P‐value of <0.10 was reported for age, sex, smoking status, nodule type, solid portion size, pathologic stage, adenocarcinoma subtype, and history of adjuvant chemotherapy. Consequently, these were included in multivariable Cox regression analysis, along with the respective interaction terms. The Kaplan‐Meier survival curves stratified according to driver mutation status are shown in Fig 2.
Figure 2.
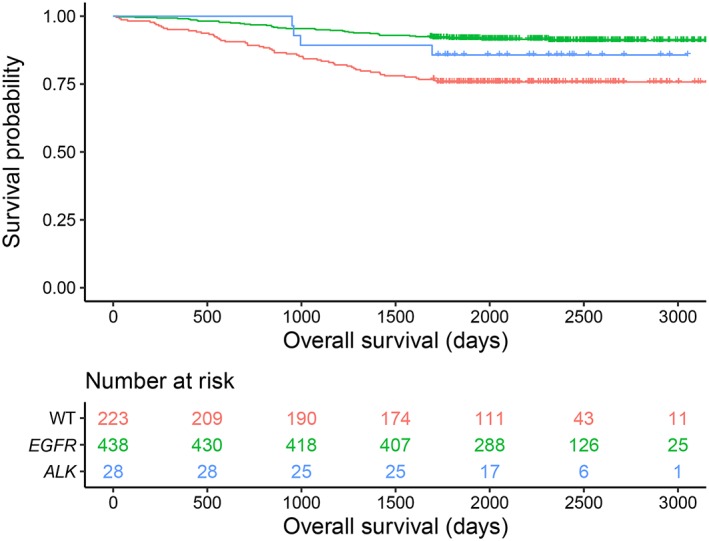
Kaplan–Meier curves of overall survival categorized according to driver mutation status ( , WT,
, WT,  EGFR mutation, and
EGFR mutation, and  ALK rearrangement) in patients with resected lung adenocarcinomas. ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; WT, wild‐type.
ALK rearrangement) in patients with resected lung adenocarcinomas. ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; WT, wild‐type.
Multivariable‐adjusted Cox regression analysis for overall survival
Multivariable‐adjusted Cox regression demonstrated that the prognostic effect of EGFR mutation on OS differed by age (Fig 3). Patients with EGFR mutation experienced significantly longer survival than those with wild‐type, and this relationship was dependent on the age of the patients (HR, exp.[−5.199 + 0.064*age]). Younger patients with EGFR mutation experienced significantly longer OS than those with wild‐type. However, with advancing age, the beneficial effect of EGFR mutation decreased, and the HR value became close to that of patients with wild‐type. For example, the adjusted HR for EGFR mutation was 0.14 (95% CI: 0.05–0.36; P < 0 0.001) at 50 years of age, 0.26 (95% CI: 0.15–0.46; P < 0.001) at 60 years, and 0.50 (95% CI: 0.31–0.81; P = 0.005) at 70 years. The effect of ALK rearrangement on OS was seen to have an inverse relationship with age. The HR value for patients who experienced ALK rearrangement decreased with advancing age (HR, exp.[2.194–0.049*age]), but this finding was without statistical significance (P > 0.05). Other independent predictors included in the Cox model were age, smoking status, solid portion size, pathologic stage, and adenocarcinoma subtype. The detailed results are outlined in Table 3.
Figure 3.
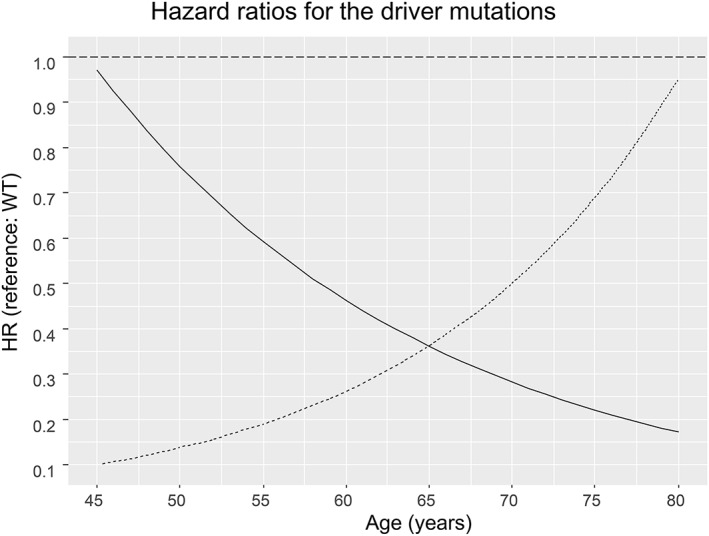
A plot of log‐hazard ratio (HR) for driver mutation status.
The favorable prognostic effect of EGFR mutation was seen to decrease gradually with advancing patient age (P < 0.050). An inverse relationship was found for ALK rearrangement, but the finding was not statistically significant (P > 0.05). ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; WT, wild‐type. Group ( ) ALK rearrangement, (
) ALK rearrangement, ( ) EGFR mutation
) EGFR mutation
Table 3.
Multivariable Cox regression analysis for overall survival in lung adenocarcinomas
| Variable | Subcategory | HR | 95% CI of HR | P‐value |
|---|---|---|---|---|
| Age (year) | Wild‐type | 1.02 | 0.99, 1.05 | 0.332 |
| EGFR mutation | 1.08 | 1.04, 1.13 | <0.001 | |
| ALK rearrangement | 0.97 | 0.89, 1.05 | 0.432 | |
| Ex‐ or current smoker (reference: never smoker) | 1.61 | 1.02, 2.53 | 0.040 | |
| Solid portion size (reference: ≤ 3 cm) | >3 cm and ≤ 5 cm | 1.62 | 0.96, 2.73 | 0.073 |
| >5 cm and ≤ 7 cm | 2.08 | 1.11, 3.88 | 0.022 | |
| >7 cm | 2.95 | 1.62, 5.37 | <0.001 | |
| Pathologic stage† (reference: stage I) | II | 1.48 | 0.80, 2.73 | 0.208 |
| III | 4.68 | 2.89, 7.58 | <0.001 | |
| Adenocarcinoma subtype (reference: MIA or lepidic predominant) | Acinar or papillary predominant | 8.13 | 1.54, 42.76 | 0.013 |
| Micropapillary or solid predominant | 7.93 | 1.43, 42.94 | 0.018 | |
| Driver mutation (reference: wild‐type) | EGFR mutation (age, 50 years) | 0.14 | 0.05, 0.36 | <0.001 |
| ALK rearrangement (age, 50 years) | 0.76 | 0.22, 2.57 | 0.657 | |
| EGFR mutation (age, 60 years) | 0.26 | 0.15, 0.46 | <0.001 | |
| ALK rearrangement (age, 60 years) | 0.46 | 0.17, 1.25 | 0.129 | |
| EGFR mutation (age, 70 years) | 0.50 | 0.31, 0.81 | 0.005 | |
| ALK rearrangement (age, 70 years) | 0.28 | 0.07, 1.22 | 0.090 |
Pathologic stage followed the seventh edition staging system for lung cancer.
ALK, anaplastic lymphoma kinase; CI, confidence interval; EGFR, epidermal growth factor receptor; HR, hazard ratio; MIA, minimally invasive adenocarcinoma.
Discussion
EGFR mutation was demonstrated to be a significant independent prognostic factor of the long‐term outcomes (OS) of patients with surgically treated lung adenocarcinomas in the current study. A more favorable prognostic effect was seen in younger than in elderly patients. However, ALK rearrangement was not associated with OS.
The prognostic implications of EGFR mutation in patients with resected lung cancers has previously been evaluated in a number of studies, and conflicting results have been reported.6, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 EGFR mutation was demonstrated to constitute a favorable prognostic factor in several studies,13, 19, 21 while others failed to show a significant association between EGFR mutation and patient survival.6, 14, 16, 17, 18, 20, 22 Controversially, even the results of two meta‐analyses were discordant.23, 24 EGFR mutations were not observed to be a prognostic factor in stage I–III resected NSCLCs (HR for OS of 0.85; 95% CI: 0.67–1.15; P = 0.210) in the meta‐analysis performed by Zhang et al.23 By contrast, these mutations were significantly associated with disease‐free survival and OS in patients with resected NSCLCs (HR for OS of 0.72; 95% CI: 0.66–0.80; P < 0.001) in a more recent meta‐analysis of 9635 patients from 32 studies.24
In the current study, EGFR mutation was an independent prognostic factor for OS after adjusting for multiple covariates, including age, sex, smoking status, pathologic stage, adenocarcinoma subtype, and solid portion size. The inclusion of multiple clinicopathological covariates in the Cox model was important because EGFR mutations have a close correlation with several well‐known beneficial prognostic factors, including female sex, having never smoked, and low or intermediate tumor grades.22, 25 Thus, it is important to determine the prognostic effect of EGFR mutation after adjusting for these variables in order to prevent false positive or false negative results. In this context, one of the strengths of the present study was that pathological stage, adenocarcinoma subtype, and solid portion size, all of which are powerful prognostic factors with a potential association with EGFR mutation, were included in the analysis.26, 27, 28 The substantial discrepancy between the current study findings and the reported results in the literature might be attributable to differences in the included variables. In addition, heterogeneity of the mutational status in the reference group (patients with wild‐type) and the proportion of EGFR‐mutant patients treated with EGFR TKIs after recurrence may have caused inconsistencies.
An intriguing finding of the present study was that the favorable prognostic effect of EGFR mutation was dependent on patient age. The difference in survival between EGFR‐mutant and wild‐type decreased with the advancing age of the patients. The rationale for this phenomenon was unclear. However, a possible hypothesis is that this occurred owing to the increased tumor mutational burden (TMB) in elderly patients.29 TMB is a measure of the number of somatic mutations within a tumor, and is defined as the total number of somatic mutations per coding area of a tumor genome.30 High TMB was associated with worse OS of patients with resected NSCLCs,31 and was also negatively associated with survival in patients with metastatic EGFR‐mutant lung cancers treated with EGFR TKIs.32 Thus, it is possible that TMB was a hidden confounder in the current study with respect to the relationship between the prognostic effect of EGFR mutation and patient age. This issue should be assessed further in future prospective studies.
ALK rearrangement was not seen to be an independent prognostic factor for OS in the current research, although the point estimation of HR for ALK rearrangement was smaller than 1. Previous study results on the prognostic effect of ALK rearrangement on resected NSCLCs have been controversial.33, 34, 35, 36, 37 The Lungscape project, a European multi‐institutional effort, reported that ALK FISH positivity was a predictor of enhanced OS in resected adenocarcinomas.33 However, Paik et al.36 indicated that ALK FISH positivity was not associated with disease recurrence nor OS, and Shin et al.37 reported that ALK rearrangement was associated with shorter disease‐free survival in resected stage IA adenocarcinomas. Kim et al.35 reported similar negative results for populations who had never smoked, and Chaft et al.34 revealed that ALK‐positive NSCLCs were associated with poorer outcomes than those of EGFR‐mutant NSCLCs, but not KRAS‐mutant NSCLCs. Interestingly, a recent meta‐analysis conducted by Wang et al.38 showed that ALK rearrangement was predictive of better survival in the general population with NSCLCs, but poorer survival in the non‐smoking population. However, an association between ALK rearrangement and smoking status was not established in the current study.
Our study had several limitations. Firstly, the prevalence of ALK rearrangement was low (4%) with only 28 ALK‐positive patients. Consequently, this limited the statistical power of the research. Analysis with a larger population and a more sensitive ALK testing schema (e.g., the combined use of immunohistochemistry and FISH) is warranted in future studies. Secondly, the results of treatments administered after locoregional recurrence or distant metastasis were not analyzed. Nevertheless, the current study objective was to investigate the role of driver mutation as a prognostic marker, not as a predictive marker. Thirdly, the eighth edition of the lung cancer staging system was not investigated. The study population comprised patients who had undergone surgery by the end of December 2013, prior to its implementation. Therefore, it was not feasible to obtain staging information based on this classification. Nevertheless, the solid portion size of adenocarcinomas was measured at CT as a substitute for the invasive component,9 a T factor in the eighth staging system. Fourthly, driver mutations other than EGFR and ALK were not included in this study. Patients with wild‐type constitute a heterogeneous group, and this includes true wild‐type and several other uninvestigated driver mutations (e.g., KRAS, MET, and ROS1). Fifthly, patient performance status (e.g., Zubrod or Eastern Cooperative Oncology Group scale) was not investigated, which is a well‐known prognostic indicator.39 Unfortunately, patient performance status was not recorded for a number of patients in the EMRs, and as a result could not be utilized in this study. However, we assume that the participants’ performance may have been acceptable to a certain level for general anesthesia and lung resection in this surgical cohort.
In conclusion, EGFR mutation was demonstrated to be an independent favorable prognostic factor for the long‐term outcomes (OS) of patients with resected adenocarcinomas in the present study. The favorable prognostic effect was observed to reduce gradually in elderly patients. By contrast, ALK rearrangement was not associated with survival.
Disclosure
Activities related to the present article: none. Activities not related to the present article: H.K. received a research grant from Lunit Inc. (Seoul, South Korea).
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant number: 2017R1D1A1B04032467). The funder had no role in the study design; in the collection, analysis, and interpretation of the data; in the writing of the report; and in the decision to submit the article for publication.
References
- 1. Lynch TJ, Bell DW, Sordella R et al Activating mutations in the epidermal growth factor receptor underlying responsiveness of non‐small‐cell lung cancer to gefitinib. N Engl J Med 2004; 350: 2129–39. [DOI] [PubMed] [Google Scholar]
- 2. Jeon JH, Kang CH, Kim HS, Seong YW, Park IK, Kim YT. Prognostic and predictive role of epidermal growth factor receptor mutation in recurrent pulmonary adenocarcinoma after curative resection. Eur J Cardiothorac Surg 2015; 47: 556–62. [DOI] [PubMed] [Google Scholar]
- 3. Goldstraw P, Crowley J, Chansky K et al The IASLC lung cancer staging project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2007; 2: 706–14. [DOI] [PubMed] [Google Scholar]
- 4. Travis WD, Brambilla E, Noguchi M et al International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011; 6: 244–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sica G, Yoshizawa A, Sima CS et al A grading system of lung adenocarcinomas based on histologic pattern is predictive of disease recurrence in stage I tumors. Am J Surg Pathol 2010; 34: 1155–62. [DOI] [PubMed] [Google Scholar]
- 6. Suh YJ, Lee HJ, Kim YT et al Added prognostic value of CT characteristics and IASLC/ATS/ERS histologic subtype in surgically resected lung adenocarcinomas. Lung Cancer 2018; 120: 130–6. [DOI] [PubMed] [Google Scholar]
- 7. Suh YJ, Lee HJ, Kim YJ et al Computed tomography characteristics of lung adenocarcinomas with epidermal growth factor receptor mutation: A propensity score matching study. Lung Cancer 2018; 123: 52–9. [DOI] [PubMed] [Google Scholar]
- 8. Travis WD, Asamura H, Bankier AA et al The IASLC lung cancer staging project: Proposals for coding T categories for subsolid nodules and assessment of tumor size in part‐solid tumors in the forthcoming eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2016; 11: 1204–23. [DOI] [PubMed] [Google Scholar]
- 9. Lee KH, Goo JM, Park SJ et al Correlation between the size of the solid component on thin‐section CT and the invasive component on pathology in small lung adenocarcinomas manifesting as ground‐glass nodules. J Thorac Oncol 2014; 9: 74–82. [DOI] [PubMed] [Google Scholar]
- 10. Keam B, Kim DW, Park JH et al Rare and complex mutations of epidermal growth factor receptor, and efficacy of tyrosine kinase inhibitor in patients with non‐small cell lung cancer. Int J Clin Oncol 2014; 19: 594–600. [DOI] [PubMed] [Google Scholar]
- 11. Kim YT, Kim TY, Lee DS et al Molecular changes of epidermal growth factor receptor (EGFR) and KRAS and their impact on the clinical outcomes in surgically resected adenocarcinoma of the lung. Lung Cancer 2008; 59: 111–8. [DOI] [PubMed] [Google Scholar]
- 12. Lee JO, Kim TM, Lee SH et al Anaplastic lymphoma kinase translocation: A predictive biomarker of pemetrexed in patients with non‐small cell lung cancer. J Thorac Oncol 2011; 6: 1474–80. [DOI] [PubMed] [Google Scholar]
- 13. Izar B, Sequist L, Lee M et al The impact of EGFR mutation status on outcomes in patients with resected stage I non‐small cell lung cancers. Ann Thorac Surg 2013; 96: 962–8. [DOI] [PubMed] [Google Scholar]
- 14. Kim YT, Seong YW, Jung YJ et al The presence of mutations in epidermal growth factor receptor gene is not a prognostic factor for long‐term outcome after surgical resection of non‐small‐cell lung cancer. J Thorac Oncol 2013; 8: 171–8. [DOI] [PubMed] [Google Scholar]
- 15. Lee YJ, Park IK, Park MS et al Activating mutations within the EGFR kinase domain: A molecular predictor of disease‐free survival in resected pulmonary adenocarcinoma. J Cancer Res Clin Oncol 2009; 135: 1647–54. [DOI] [PubMed] [Google Scholar]
- 16. Li R, Li Q, Lin S et al Prognostic implication of EGFR mutation status and subtype in resected lung adenocarcinoma patients irrespective of therapy. Clin Transl Oncol 2019; 21: 298–303. [DOI] [PubMed] [Google Scholar]
- 17. Lin CY, Wu YM, Hsieh MH et al Prognostic implication of EGFR gene mutations and histological classification in patients with resected stage I lung adenocarcinoma. PLoS One 2017; 12: e0186567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu WS, Zhao LJ, Pang QS, Yuan ZY, Li B, Wang P. Prognostic value of epidermal growth factor receptor mutations in resected lung adenocarcinomas. Med Oncol 2014; 31: 771. [DOI] [PubMed] [Google Scholar]
- 19. Park IK, Hyun K, Kim ER, Park S, Kang CH, Kim YT. The prognostic effect of the epidermal growth factor receptor gene mutation on recurrence dynamics of lung adenocarcinoma. Eur J Cardiothorac Surg 2018; 54: 1022–7. [DOI] [PubMed] [Google Scholar]
- 20. Sonobe M, Kobayashi M, Ishikawa M et al Impact of KRAS and EGFR gene mutations on recurrence and survival in patients with surgically resected lung adenocarcinomas. Ann Surg Oncol 2012; 19 (Suppl. 3): S347–54. [DOI] [PubMed] [Google Scholar]
- 21. Mizuno T, Yatabe Y, Kuroda H, Sakakura N, Sakao Y. Impact of the oncogenic status on the mode of recurrence in resected non‐small cell lung cancer. Jpn J Clin Oncol 2016; 46: 928–34. [DOI] [PubMed] [Google Scholar]
- 22. Kosaka T, Yatabe Y, Onozato R, Kuwano H, Mitsudomi T. Prognostic implication of EGFR, KRAS, and TP53 gene mutations in a large cohort of Japanese patients with surgically treated lung adenocarcinoma. J Thorac Oncol 2009; 4: 22–9. [DOI] [PubMed] [Google Scholar]
- 23. Zhang Z, Wang T, Zhang J et al Prognostic value of epidermal growth factor receptor mutations in resected non‐small cell lung cancer: A systematic review with meta‐analysis. PLoS One 2014; 9: e106053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang SM, Zhu QG, Ding XX et al Prognostic value of EGFR and KRAS in resected non‐small cell lung cancer: A systematic review and meta‐analysis. Cancer Manag Res 2018; 10: 3393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Isaka T, Yokose T, Ito H et al Correlations between the EGFR mutation status and clinicopathological features of clinical stage I lung adenocarcinoma. Medicine (Baltimore) 2015; 94: e1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Russell PA, Wainer Z, Wright GM, Daniels M, Conron M, Williams RA. Does lung adenocarcinoma subtype predict patient survival?: A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol 2011; 6: 1496–504. [DOI] [PubMed] [Google Scholar]
- 27. Sui X, Jiang W, Chen H, Yang F, Wang J, Wang Q. Validation of the stage groupings in the eighth edition of the TNM classification for lung cancer. J Thorac Oncol 2017; 12: 1679–86. [DOI] [PubMed] [Google Scholar]
- 28. Groome PA, Bolejack V, Crowley JJ et al The IASLC lung cancer staging project: Validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2007; 2: 694–705. [DOI] [PubMed] [Google Scholar]
- 29. Goodman AM, Kato S, Bazhenova L et al Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther 2017; 16: 2598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fenizia F, Pasquale R, Roma C, Bergantino F, Iannaccone A, Normanno N. Measuring tumor mutation burden in non‐small cell lung cancer: Tissue versus liquid biopsy. Transl Lung Cancer Res 2018; 7: 668–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Owada‐Ozaki Y, Muto S, Takagi H et al Prognostic impact of tumor mutation burden in patients with completely resected non‐small cell lung cancer: Brief report. J Thorac Oncol 2018; 13: 1217–21. [DOI] [PubMed] [Google Scholar]
- 32. Offin M, Rizvi H, Tenet M et al Tumor mutation burden and efficacy of EGFR‐tyrosine kinase inhibitors in patients with EGFR‐mutant lung cancers. Clin Cancer Res 2019; 25: 1063–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Blackhall FH, Peters S, Bubendorf L et al Prevalence and clinical outcomes for patients with ALK‐positive resected stage I to III adenocarcinoma: Results from the European thoracic oncology platform Lungscape project. J Clin Oncol 2014; 32: 2780–7. [DOI] [PubMed] [Google Scholar]
- 34. Chaft JE, Dagogo‐Jack I, Santini FC et al Clinical outcomes of patients with resected, early‐stage ALK‐positive lung cancer. Lung Cancer 2018; 122: 67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim MH, Shim HS, Kang DR et al Clinical and prognostic implications of ALK and ROS1 rearrangements in never‐smokers with surgically resected lung adenocarcinoma. Lung Cancer 2014; 83: 389–95. [DOI] [PubMed] [Google Scholar]
- 36. Paik JH, Choi CM, Kim H et al Clinicopathologic implication of ALK rearrangement in surgically resected lung cancer: A proposal of diagnostic algorithm for ALK‐rearranged adenocarcinoma. Lung Cancer 2012; 76: 403–9. [DOI] [PubMed] [Google Scholar]
- 37. Shin SH, Lee H, Jeong BH et al Anaplastic lymphoma kinase rearrangement in surgically resected stage IA lung adenocarcinoma. J Thorac Dis 2018; 10: 3460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang Z, Yang H, Luo S et al Anaplastic lymphoma kinase gene rearrangement predicts better prognosis in NSCLC patients: A meta‐analysis. Lung Cancer 2017; 112: 1–9. [DOI] [PubMed] [Google Scholar]
- 39. Buccheri G, Ferrigno D, Tamburini M. Karnofsky and ECOG performance status scoring in lung cancer: A prospective, longitudinal study of 536 patients from a single institution. Eur J Cancer 1996; 32A: 1135–41. [DOI] [PubMed] [Google Scholar]


