 Concentrations of PCDD/Fs and DL-PCBs were determined to evaluate the human body burden of dioxin-like compounds.
Concentrations of PCDD/Fs and DL-PCBs were determined to evaluate the human body burden of dioxin-like compounds.
Abstract
Human milk samples were collected from 179 mothers in 2017 and 2018 in six counties of Guangdong province, China. Concentrations of polychlorinated dibenzo-p-dioxins/furans (PCDD/Fs) and dioxin-like polychlorinated biphenyls (DL-PCBs) were determined to evaluate the human body burden of dioxin-like compounds on the general population of South China. Samples were analyzed using high-resolution gas chromatography/high-resolution mass spectrometry in six pools, according to the subjects’ resident county. The mean ∑PCDD/Fs and ∑DL-PCBs concentrations in human milk samples were 323.10 pg g–1 lipid and 2166.58 pg g–1 lipid, respectively, and the corresponding WHO toxicity equivalent (TEQ) values calculated with Toxic Equivalent Factors (TEFs) established by the WHO in 2005 (TEFWHO 2005) were 6.96 and 2.13 pg g–1 lipid. The concentrations of samples collected in Guangzhou, the capital city of Guangdong Province, were higher than those taken in the other five investigation regions. The levels of PCDD/Fs and DL-PCBs in human milk and the estimated daily intake doses of breast-fed infants were still high when compared with some non-exposure areas in mainland China. TEQ levels of PCDD/Fs and DL-PCBs in the present study have been compared to data obtained from a reported national study conducted in 2011. The mean TEQ (calculated with TEFWHO 2005) of ∑(DL-PCBs + PCDD/Fs) (8.4–9.0 pg g–1 lipid in 2011 vs. 9.09 pg g–1 lipid in 2018) seemed to be relatively stable during the period 2011–2018. These findings and continuing the surveillance of PCDD/Fs and DL-PCBs in human milk will be helpful in furthering our understanding of human exposure to dioxin-like compounds in the general population.
1. Introduction
Polychlorinated dibenzo-p-dioxins/furans (PCDD/Fs) and dioxin-like polychlorinated biphenyls (DL-PCBs) are ubiquitous persistent organic pollutants (POPs). They are extremely resistant to environmental decomposition and accumulate in fatty food of animal origin. Concerns regarding PCDD/Fs and DL-PCBs are mainly due to their toxic effects on a number of systems, including the endocrine and immune systems, the developing nervous system and their cancer-causing potential.1 2,3,7,8-Tetrachlorodibenzo-para-dioxin (TCDD), 2,3,4,7,8-pentachlorodibenzofuran (PeCDF) and all the twelve dioxin-like PCBs have been identified as human carcinogens.2 The United Nations Environment Programme (UNEP) initially listed twelve types of POPs of major concern under the Stockholm Convention,3 including the PCDD/Fs and DL-PCBs.
WHO/UNEP consider human milk to be the best matrix for biomonitoring of POPs, because it is easily available, collection is non invasive, and its high lipid content makes the extraction of POPs easy.4 PCDD/Fs and DL-PCBs in human milk are also a good reflection of the body burden on the mother, as human blood and adipose tissue concentrations are often similar.5,6 In addition, human milk is the main way of transporting PCDD/Fs and DL-PCBs from mothers to infants. The WHO has carried out global surveys on PCDD/Fs and PCBs in human milk since 1987. However, in China, the first and 2nd national survey of POPs in human milk was carried out as a part of the WHO global surveys later in 2007 and 2011, respectively.7 Due to the rapid industrialization and urbanization during the past thirty years, environmental issues related to dioxins and DL-PCBs are of increasing concern in China, but studies on PCDD/Fs and DL-PCBs in human milk are limited. In the current study, the aim was to determine levels of PCDD/Fs and DL-PCBs in human milk from Guangdong residents and evaluate the human body burden of dioxin-like compounds of the general population of Guangdong province, a typical region having a developed economy and high level of industrialization in South China.
2. Materials and methods
2.1. Donor selection and sample collection
The criteria for donor selection and human milk sample collection was based on the ‘Guideline for Developing a National Protocol’ of the Fourth WHO-coordinated survey of human milk for persistent organic pollutants in cooperation with UNEP.8 Some modifications were made for the special situation of Guangdong. The participators were all primiparas, less than 35 years old and had lived in non-directly POPs polluted areas of the investigation area for more than 10 years. All donors were informed of the objective of this study and signed the participant information and consent forms, and were willing to provide a minimum of 50 mL milk during 3–8 weeks after delivery.
A multistage random cluster sampling method was applied to select sampling sites. First, all of the 21 counties in Guangdong province were classified according to geographical distribution and economic condition. Then six counties were randomly sampled, including Guangzhou, Zhuhai, Foshan, Zhaoqing, Yangdong, Boluo. Breast milk samples from a total of 179 mothers were collected between August 2017 and February 2018. Human milk samples were collected by manual expression and frozen at –20 °C immediately after collection, until analyzed.
2.2. Samples preparation and analysis
Individual human milk samples were thawed and homogenised before pooling. The individual samples of 50 ml from 29 or 30 donors from the same region were divided into the same group, and six pooled samples were created. Information regarding the six pooled samples is listed in Table 1. In addition, 150 ml was taken from each pooled sample for the PCDD/Fs and DL-PCBs analysis.
Table 1. Summary of characteristics of the six pooled samples.
| Pool identification | County identification | Number of donors | Age, mean (range) | BMI, mean (kg m–2) | Pregnancy weeks, mean |
| Pool 1 | Guangzhou | 29 | 25 (20–35) | 20.8 | 39.5 |
| Pool 2 | Zhuhai | 30 | 28 (20–35) | 20.1 | 39.3 |
| Pool 3 | Foshan | 30 | 28 (22–35) | 20.7 | 39.4 |
| Pool 4 | Zhaoqing | 30 | 26 (19–34) | 20.9 | 39.3 |
| Pool 5 | Yangdong | 30 | 25 (16–31) | 19.9 | 39.2 |
| Pool 6 | Boluo | 30 | 25 (19–34) | 20.9 | 39.5 |
| Overall pool | All participating regions | 179 | 26 (16–35) | 20.5 | 39.4 |
After spiking with 13C-labeled internal standards, samples were extracted individually using an ASE system (Accelerate solvent extractor equipment, ASE300, USA) with a mixed reagent of dichloromethane and n-hexane (1 : 1) for 10 min × 2 times under temperature (150 °C) and pressure (2000 psi). Gravimetric lipid determination was performed after solvent evaporation. The concentrated sample extract was cleaned-up automatically using an automated system (Fluid Management Systems, Waltham, MA, USA). The fraction containing PCDD/Fs and DL-PCBs congeners was then concentrated by vacuum evaporation and further cleaned-up using an alumina column. The eluate was dried by nitrogen evaporation to nearly dryness, and the residue was further reconstituted in 20 μL 13C-labeled injection standard for instrumental analysis.
Concentrations of seventeen 2,3,7,8-substituted PCDD/F congeners and twelve dioxin-like PCBs were analyzed using high-resolution gas chromatography/high-resolution mass spectrometry (HRGC/HRMS) (MAT95XL Thermo Finnigan, Germany) with a DB-5MS capillary column (60 m × 0.25 mm i.d. × 0.25 μm). Further details on the analysis of PCDD/Fs and DL-PCBs are described elsewhere.9
2.3. QA and QC
All analyses and results were accredited to ISO/IEC 17025. Both method blank and quality control samples were included. Certified reference material was used as the quality control sample to validate the long determination process. Chicken samples purchased from the Norwegian Institute of Public Health, were measured to confirm the laboratory performance and the method validation. The Department of POPs laboratory of Shenzhen Center for Disease Control and Prevention has regularly and successfully participated in international interlaboratory comparison studies of PCDD/Fs and PCBs in human milk organized by the Norwegian Institute of Public Health since 2005.
2.4. Expression of concentration of PCDD/Fs and DL-PCBs and statistics
Both Toxic Equivalent Factors (TEFs) established by WHO in 2005 (TEFWHO 2005)10 and in 1998 (TEFWHO 1998)11 were used to calculate the WHO toxicity equivalent (TEQ) values of each congener for comparison with previous studies. All data were reported on lipid weight. Values below the detection limit of the analytical method were replaced by LOD (Limit of Detection) values prior to calculating the TEQ values. All statistical analyses were performed using SAS Enterprise Guide (SAS Institute Inc., Cary, NC, USA).
3. Results and discussion
3.1. Pollutant concentrations
A total of seventeen 2,3,7,8-substituted PCDD/Fs congeners and DL-PCBs in all pooled human milk samples from Guangdong Province were determined. The mass concentrations, ranges, TEQ values and percent contribution of each congener to the total TEQ value are shown in Table 2. By mass concentration, the ∑(DL-PCBs + PCDD/Fs) concentration in the human milk samples was in a range of 1586.39–3261.47 pg g–1 lipid (mean 2489.68 pg g–1 lipid). The mean concentration of ∑PCDD/Fs and ∑DL-PCBs was 323.10 pg g–1 lipid and 2166.58 pg g–1 lipid, respectively. ∑DL-PCBs had 87.02% of contribution to ∑(DL-PCBs + PCDD/Fs). PCB 118 was the most predominant congener in all samples with the mean percentage contribution of 50.25% to ∑DL-PCBs, followed by PCB 156 (16.19%) and PCB 105 (15.66%). For PCDD/Fs, OCDD was the most predominant congener in all samples with the mean percentage contribution of 80.77%, followed by 1,2,3,4,6,7,8-HpCDD (10.44%), 2,3,4,7,8-PeCDF (2.02%), and 1,2,3,6,7,8-HxCDD (1.58%). The congener profiles in human milk samples were in agreement with studies in Shanghai,12 Shenzhen, China9 and in Japan.13 The most toxic congener, 2,3,7,8-TCDD, was detected in all pooled samples, with the concentration ranging from 0.31 to 0.81 pg g–1 lipid.
Table 2. Mass concentrations and TEQs of PCDD/Fs and DL-PCBs and their constituent ratios in human milk samples from Guangdong Province, China.
| Compound | Mass concentration (pg g–1 lipid) |
TEQ (TEFWHO 2005, pg TEQ per g lipid) |
TEQ (TEFWHO1998, mean, pg TEQ per g lipid) | |||
| Mean | Range | Percent contribution a | Mean | Percent contribution b | ||
| 1,2,3,7,8-PeCDD | 2.55 | 1.75–3.31 | 0.79 | 2.55 | 28.05 | 2.55 |
| 2,3,7,8-TCDD | 0.58 | 0.31–0.81 | 0.18 | 0.58 | 6.38 | 0.58 |
| 1,2,3,6,7,8-HxCDD | 5.11 | 3.22–8.89 | 1.58 | 0.51 | 5.61 | 0.51 |
| 1,2,3,4,6,7,8-HpCDD | 33.73 | 15.9–70.17 | 10.44 | 0.34 | 3.74 | 0.34 |
| 1,2,3,4,7,8-HxCDD | 1.12 | 0.49–1.59 | 0.35 | 0.11 | 1.21 | 0.11 |
| 1,2,3,7,8,9-HxCDD | 1.02 | 0.74–1.55 | 0.32 | 0.10 | 1.10 | 0.10 |
| OCDD | 260.96 | 126.47–561.95 | 80.77 | 0.08 | 0.88 | 0.03 |
| 2,3,4,7,8-PeCDF | 6.54 | 4.44–10.35 | 2.02 | 1.96 | 21.56 | 3.27 |
| 1,2,3,4,7,8-HxCDF | 2.9 | 2.24–3.67 | 0.90 | 0.29 | 3.19 | 0.29 |
| 1,2,3,6,7,8-HxCDF | 2.49 | 2.05–3.51 | 0.77 | 0.25 | 2.75 | 0.25 |
| 2,3,7,8-TCDF | 0.71 | 0.56–0.86 | 0.22 | 0.07 | 0.77 | 0.07 |
| 2,3,4,6,7,8-HxCDF | 0.62 | 0.31–0.97 | 0.19 | 0.06 | 0.66 | 0.06 |
| 1,2,3,7,8-PeCDF | 0.72 | 0.52–0.98 | 0.22 | 0.02 | 0.22 | 0.04 |
| 1,2,3,4,6,7,8-HpCDF | 2.55 | 1.36–3.76 | 0.79 | 0.03 | 0.33 | 0.03 |
| 1,2,3,7,8,9-HxCDF | 0.03 | ND-0.083 | 0.01 | 0.003 | 0.03 | 0.003 |
| 1,2,3,4,7,8,9-HpCDF | 0.15 | 0–0.33 | 0.05 | 0.002 | 0.02 | 0.002 |
| OCDF | 1.35 | 0.31–2.92 | 0.42 | 0.0004 | 0.004 | 0.0001 |
| PCB 126 | 16.56 | 10.85–24.54 | 0.76 | 1.656 | 18.21 | 1.656 |
| PCB 169 | 13.63 | 6.92–18.7 | 0.63 | 0.409 | 4.50 | 0.136 |
| PCB 118 | 1088.73 | 710.65–1316.82 | 50.25 | 0.033 | 0.36 | 0.109 |
| PCB 77 | 8.74 | 4.43–13.84 | 0.40 | 0.001 | 0.01 | 0.001 |
| PCB 81 | 3.41 | 2.07–5.76 | 0.16 | 0.001 | 0.01 | 0.0003 |
| PCB 105 | 339.21 | 225.59–398.99 | 15.66 | 0.010 | 0.11 | 0.034 |
| PCB 114 | 81.94 | 51.67–95.22 | 3.78 | 0.002 | 0.03 | 0.041 |
| PCB 123 | 19.82 | 14.98–25.18 | 0.92 | 0.001 | 0.01 | 0.002 |
| PCB 156 | 350.84 | 239.02–409.36 | 16.19 | 0.011 | 0.12 | 0.175 |
| PCB 157 | 100.01 | 58.70–121.80 | 4.62 | 0.003 | 0.03 | 0.050 |
| PCB 167 | 103.22 | 66.24–122.71 | 4.76 | 0.003 | 0.03 | 0.001 |
| PCB 189 | 40.47 | 27.9–45.95 | 1.87 | 0.001 | 0.01 | 0.004 |
| ∑PCDDs | 305.06 | 150–647.72 | 94.42 | 4.27 | 46.97 | 4.22 |
| ∑PCDFs | 18.04 | 14.33–26.21 | 5.58 | 2.69 | 29.59 | 4.01 |
| ∑PCDDs/Fs | 323.10 | 167.37–662.6 | 12.98 | 6.96 | 76.57 | 8.23 |
| ∑DL-PCBs | 2166.58 | 1419.02–2598.87 | 87.02 | 2.13 | 23.43 | 2.21 |
| ∑(DL-PCBs + PCDDs/Fs) | 2489.68 | 1586.39–3261.47 | 100.00 | 9.09 | 100.00 | 10.44 |
aPercent contribution of each PCDD/F congener, ∑PCDDs and ∑PCDFs to ∑PCDD/Fs, and percent contribution of each DL-PCB congener to ∑DL-PCBs, and percent contribution of ∑PCDD/Fs and ∑DL-PCBs to ∑(PCDD/Fs + DL-PCBs);
bContribution of each PCDD/F and DL-PCB congener, ∑PCDDs, ∑PCDFs and ∑PCBs to ∑(DL-PCBs + PCDD/Fs).
By TEQ concentration, the average concentration of ∑(DL-PCBs + PCDD/Fs) (9.09 pg TEQ per g lipid) calculated using TEFWHO 2005 was about 10% lower compared to the value (10.44 pg TEQ per g lipid) calculated with TEFWHO 1998. Calculated using TEFWHO 2005, the dominant TEQ contributor was 1,2,3,7,8-PeCDD (mean 2.55 pg TEQ per g lipid) which accounted for 28.05% of the total TEQs of PCDD/Fs and DL-PCBs, followed by 2,3,4,7,8-PeCDF (21.56%), PCB-126 (18.21%) and 2,3,7,8-TCDD (6.38%). These congeners were reported as the major TEQ contributors from studies in China's national survey in 2007 and 2011,7,14 as well as in Japan.15 The mean TEQ (calculated with TEFWHO 2005) of the ∑PCDD/Fs was 6.96 pg TEQ per g lipid, accounting for 76.57% of the total TEQs of PCDD/Fs and DL-PCBs, which was much higher than that of DL-PCBs.
3.2. Level comparisons and time trends
Table 3 shows recent reported data on levels of PCDD/Fs and DL-PCBs in human breast milk in other areas. In the present study, it was indicated that the mean level of ∑(DL-PCBs + PCDD/Fs) in Guangdong province (10.44 pg TEQ per g lipid, calculated with TEFWHO 1998) was obviously higher than the mean level of 16 representative areas of China (6.7 pg TEQ per g lipid)7 and some non-exposed areas in mainland China such as Hebei and Shijiazhuang.16 The TEQ (calculated with TEFWHO 1998) of ∑(DL-PCBs + PCDD/Fs) was similar to that surveyed in Shanghai (9.15 pg TEQ per g lipid)12 and Hong Kong (11.27 pg TEQ per g lipid)17 and slightly lower than the level detected in Shenzhen.9 It was noted that the levels of PCDD/Fs and DL-PCBs in Guangdong province were similar to those in Shanghai and Hong Kong, which may be due to the similar geographical environment and economic level of these three regions. Additionally more animal origin foods, especially fish, are consumed by residents of these regions.7 The dietary habits may also be closely related to exposure. Compared with the data from other countries, the TEQ (calculated with TEFWHO 1998) of ∑(DL-PCBs + PCDD/Fs) in Guangdong province was similar to that detected in Ireland (9.66 pg g–1 lipid),18 but much lower than that in Germany (19.8 pg TEQ per g lipid),19 Tohoku in Japan (18.8 pg TEQ per g lipid)20 and Slovakia (18.0 pg TEQ per g lipid).21 The comparison with these countries further indicates that the difference in the levels of these contaminants might be caused by different levels of industrialization.
Table 3. Summary of the recent 10 years studies on PCDD/Fs and DL-PCBs in human breast milk from worldwide areas (pg WHO-TEQ per g lipid).
| Country | Pools | Year of sampling | ∑PCDD/Fs |
∑PCBs |
∑(DL-PCBs + PCDD/Fs) |
Reference | |||
| TEQ1998 | TEQ2005 | TEQ1998 | TEQ2005 | TEQ1998 | TEQ2005 | ||||
| China | 32 pools from 1760 samples | 2011 | 4.9 | 1.8 | 6.7 | Zhang et al., 2016 7 | |||
| China | 24 pools from 1320 samples | 2007 | 3.73 | 1.69 | 5.42 | Li et al., 2009 14 | |||
| China, Guangdong | 6 pools from 179 samples | 2017–2018 | 8.23 | 6.96 | 2.21 | 2.13 | 10.44 | 9.09 | Present study |
| China, Shenzhen | 60 a | 2007–2009 | 7.16 | 4.77 | 11.9 | Deng et al., 2012 9 | |||
| China, Shanghai | 150 a | 2011–2012 | 6 | 5.4 | 3.1 | 2.9 | 9.15 | 8.3 | Lu et al., 2015 12 |
| Hong Kong | 4 pools from 137 samples | 2009 | 7.48 | 3.79 | 11.27 | Wong et al., 2013 17 | |||
| Northern China (Shijiazhuang, Hebei) | 20 a | 2002 | 2.88 | 1.59 | 4.47 | Sun et al., 2011 16 | |||
| 20 a | 2007 | 3.95 | 2.29 | 6.24 | Sun et al., 2011 16 | ||||
| Northern China (Shijiazhuang, Tianjin and Yantai) b | 60 a | 2006–2007 | 3.37–5.03 | 2.14–2.63 | 6.24–7.54 | Sun et al., 2010 24 | |||
| Japan | 26 a | 2015 | 8.7 | Ae et al., 2018 25 | |||||
| Japan, Sapporo City | 97 a | 2002–2005 | 5.2 | 3.4 | 8.6 | Todaka et al., 2011 15 | |||
| Japan, Tohoku | 49 a | 2001–2003 | 11.1 | 7.8 | 18.8 | Nakamura et al., 2008 20 | |||
| Turkey | 51 a | 2007 | 4.9–12 | 3.9–10 | 6.8–16 | Cok et al., 2009 26 | |||
| Vietnam b | 16 a | 2008 | 2.7; 6.6 | Nhu et al., 2011 27 | |||||
| Vietnam | 59(Quang Tri);66(Ha Tinh) | 2002–2003 | 8.96; 4.04 | Tawara et al., 2011 13 | |||||
| Asia and the Pacific b | — | 2005–2010 | 4.5 | UNEP 2011 28 | |||||
| Europe b | — | 2005–2010 | 5.9;6 | UNEP 2011 28 | |||||
| Belgium | 1 pool of 84 samples | 2009–2010 | 8.4 | 5.9 | 14.3 | Croes et al., 2012 23 | |||
| Sweden | 8 pools from 79 samples | 2008–2011 | 3.0–4.3 | 2.5–3.6 | 2.6–4.6 | 1.2–2.7 | 5.6–8.9 | 3.7–6.2 | Fång et al., 2013 29 |
| Sweden | 325 a | 1996–2006 | 8.2 | 7 | 16 | 13 | Lignell et al., 2009 30 | ||
| Italy | 59 a | 2008–2009 | 3.78–4.7 | 4.87–6.28 | 8.65–10.98 | Ulaszewska et al., 2011 31 | |||
| Italy | 4 pools from 39 samples | 1998–2001 | 20.4–34.2 | Abballe et al., 2008 32 | |||||
| Italy | 94 a | 2007 | 8.6 | 8 | Rivezzi et al., 2013 33 | ||||
| Hungarian | 22 a | 2007 | 2.13 | 1.04 | 3.17 | É. Vigh, 2013 34 | |||
| Germany | 42 a | 2005 | 9.92 | 8.17 | 9.89 | 6.31 | 19.8 | 14.48 | Raab et al., 2008 19 |
| Spain | 15 a | 2007 | 7.6 | Schuhmacher et al., 2009 35 | |||||
| Slovakia | 33 a | 2006–2007 | 18 | Chovancova J, 2011 21 | |||||
| Ireland | 11 pools from 109 samples | 2010 | 6.32 | 9.66 | Pratt, 2012 18 | ||||
| Africa b | — | 2005–2010 | 3.6 | UNEP, 2011 28 | |||||
| New Zealand | 39 a | 2007–2010 | 3.54 | Mannetje, 2013 36 | |||||
aDetected as individual samples.
bMedian concentration, the others were mean concentration.
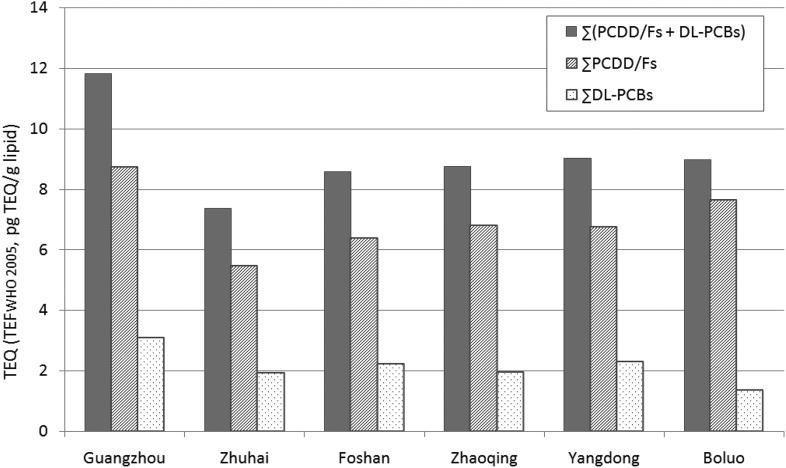
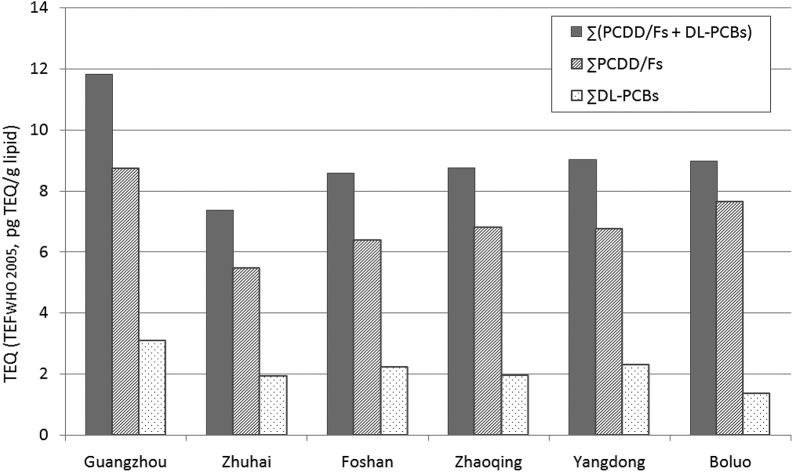
Moreover, comparison of TEQs in pooled human milk samples from six regions of Guangdong Province is shown in Fig. 1. The range of TEQ (calculated with TEFWHO 2005) in samples was from 5.45 to 8.74 pg TEQ per g lipid for ∑PCDD/Fs, from 1.34 to 3.09 pg TEQ per g lipid for ∑DL-PCBs, and from 7.37 to 11.83 pg TEQ per g lipid for ∑(DL-PCBs + PCDD/Fs). The TEQ levels of Guangzhou, the capital city of Guangdong Province, were notably higher than those from the other five regions, and the TEQ (calculated with TEFWHO 2005) of ∑(DL-PCBs + PCDD/Fs) (11.83 pg TEQ per g lipid) was similar to Shenzhen (11.9 pg TEQ per g lipid).9 Some previous studies have also shown the human body burden of PCDD/Fs and DL-PCBs in residents was higher in regions of high industrialization or developed economy.7,9
Fig. 1. Comparison of TEQs (calculated with TEFWHO 2005) in pooled human milk samples from six regions of Guangdong Province.
The information on concentrations of PCDD/Fs and DL-PCBs in human milk in Guangdong province was limited. The national survey in 2011 provided a baseline level for PCDD/Fs and DL-PCBs levels in human breast milk in Guangdong province for the first time. The TEQ (calculated with TEFWHO 1998) of ∑(DL-PCBs + PCDD/Fs) in urban and rural areas of Guangdong province in 2011 was 10.7 and 9.5 pg TEQ per g lipid, respectively,7 which is almost similar to the mean level of Guangdong province in the present study. Comparative data on PCDD/Fs and DL-PCBs levels in human milk from different regions of the world has been obtained from five rounds of human milk surveys by the WHO/UNEP during the period 1987–2011, indicating a consistent decline in the TEQ levels of PCDD/Fs and DL-PCBs due to global efforts and strict regulation on dioxin emissions worldwide.22 Conversely, in China, there was a significant increase of PCDD/Fs and some PCB congeners in human milk during the period 2007–2011, probably due to the increasing pollution of dioxin-like compounds.7 However, it seemed to be relatively stable for concentrations of PCDD/Fs and DL-PCBs in Guangdong province during the period 2011–2018.
3.3. Health risk assessment for breast-fed infants
A breast-fed infant may be subject to a much higher average daily intake of PCDD/Fs and DL-PCBs than adults, at least on a body weight basis. It was estimated that an infant's intake might be nearly 1–2 orders of magnitude greater than that of adults, albeit for only a short duration of expected life span.17 The WHO derived a Tolerable Daily Intake (TDI) for the dioxin-like compounds of 1–4 TEQ per kg bw per day.1 The TDI is meant for chronic life time exposure and is not applicable for the breast feeding situation, which covers a much shorter time period of life.4 However, scientifically sound arguments can be given to apply the TDI also to breast-fed infants. Thus, the estimated daily intake (EDI) was used to assess the health risk for infants who were exposed to PCDD/Fs and DL-PCBs via mother's milk. The traditional EDI calculation was used to acquire the EDI values, assuming an infant’s average daily milk consumption of 700 mL and average infant body weight of 5 kg.14 The mean EDI of breast-fed infants in Guangdong Province was 54.3 pg TEQ per kg bw per day based on the TEFWHO 1998 and 47.5 pg TEQ per kg bw per day based on the TEFWHO 2005, which was almost 2 times higher than the mean EDI of infants from the 12 representative areas of China in 2007 14 (28.0 pg TEQ per kg bw per day based on the TEFWHO 1998), but lower than those of countries reported recently.12,21,23 The mean EDI in the present study was also similar to that in Shenzhen and Shanghai.9,12 However, it was concluded by WHO/UNEP that the benefits of breast feeding far outweigh the toxicological disadvantages associated with certain POPs, based on present studies and knowledge.4
4. Conclusion
This was the only study investigating PCDD/Fs and DL-PCBs levels in residents’ human milk from Guangdong province since China's national survey in 2011, which firstly provided a baseline level for PCDD/Fs and DL-PCBs in human milk of the general population of Guangdong province. This study investigated concentrations of PCDD/Fs and DL-PCBs in 179 breast milk samples collected from mothers in six regions of Guangdong Province between 2017 and 2018. The TEQ levels of PCDD/Fs and DL-PCBs in human milk in Guangdong Province seemed to be relatively stable during the period 2011–2018. However, when compared with some non-exposed areas in mainland China, the PCDD/Fs and DL-PCBs levels in human milk and the EDI of breast-fed infants were still high in Guangdong Province. Furthermore, the TEQ levels of PCDD/Fs and DL-PCBs in Guangzhou, the capital city of Guangdong Province, were higher than those in the other five investigation regions. These findings and continuing the surveillance of PCDD/Fs and DL-PCBs in human milk will be helpful in furthering our understanding of human exposure to dioxin-like compounds in the general population. Since human exposure levels of PCDD/Fs and DL-PCBs are associated with rapid urbanization and industrialization in China, more studies are needed to focus on both the environmental pollution and the body burden of PCDD/Fs and DL-PCBs in the population from areas of high industrialization or developed economy.
Conflicts of interest
There are no conflicts of interest to declare.
Acknowledgments
We would like to express our gratitude to all of the participant mothers in this study. This study was supported by the Guangdong Provincial Natural Science Foundation (Grant No. 2018A030313230), Sanming Project of Medicine in Shenzhen (SZSM201811070) and the Guangdong Provincial Medical Research Foundation (Grant No. C2016008).
References
- WHO, Safety evaluation of certain food additives and contaminants polychlorinated dibenzodioxins, polychlorinated dibenzofurans, and coplanar polychlorinated biphenyls, WHO food additives series, 2002. <http://www.inchem.org/documents/jecfa/jecmono/v48je20.htm>. [Google Scholar]
- IARC, International Agency for Research on Cancer (IARC) Monographs on the Evaluation of carcinogenic Risks to Humans: agents Classified by the IARC Monographs, 2018, pp. 1–122. <https://monographs.iarc.fr/wp-content/uploads/2018/09/List_of_Classifications.pdf>. [PMC free article] [PubMed] [Google Scholar]
- Olsen M. A., Analysis of the Stockholm Convention on persistent organic pollutants, Oceana Publications, Inc., Dobbs Ferry, NY, 2003. [Google Scholar]
- Martin V. D. B., Kypke K., Kotz A., Tritscher A., Lee S. Y., Magulova K., Fiedler H., Malisch R. Arch. Toxicol. 2017;91:83–96. doi: 10.1007/s00204-016-1802-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham L. L., Grandjean P., Heinzow B., Jørgensen P. J., Nielsen F., Patterson D. G., Sjödin A., Turner W. E., Weihe P. Environ. Sci. Technol. 2011;45:1121–1126. doi: 10.1021/es1019614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaka T., Hirakawa H., Kajiwara J., Hori T., Tobiishi K., Yasutake D., Onozuka D., Sasaki S., Miyashita C., Yoshioka E., Yuasa M., Kishi R., Iida T., Furue M. Chemosphere. 2010;78:185–192. doi: 10.1016/j.chemosphere.2009.09.047. [DOI] [PubMed] [Google Scholar]
- Zhang L., Yin S., Li J., Zhao Y., Wu Y. Int. J. Hyg. Environ. Health. 2016;219:843–849. doi: 10.1016/j.ijheh.2016.07.013. [DOI] [PubMed] [Google Scholar]
- WHO, Fourth WHO-coordinated survey of human milk for persistent organic pollutants in cooperation with UNEP: guidelines for developing a national protocol, World Health Organization, 2007. [Google Scholar]
- Deng B., Zhang J., Zhang L., Jiang Y., Zhou J., Fang D., Zhang H., Huang H. Environ. Int. 2012;42:47–52. doi: 10.1016/j.envint.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Haws L. C., Su S. H., Harris M., Devito M. J., Walker N. J., Farland W. H., Finley B., Birnbaum L. S. Toxicol. Sci. 2006;89:4–30. doi: 10.1093/toxsci/kfi294. [DOI] [PubMed] [Google Scholar]
- Martin V. D. B., Birnbaum L., Bosveld A. T., Brunstrom B., Cook P., Feeley M., Giesy J. P., Hanberg A., Hasegawa R., Kennedy S. W., Kubiak T., Larsen J. C., van Leeuwen F. X., Liem A. K., Nolt C., Peterson R. E., Poellinger L., Safe S., Schrenk D., Tillitt D., Tysklind M., Younes M., Waern F., Zacharewski T. Environ. Health Perspect. 1998;106:775–792. doi: 10.1289/ehp.98106775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D., Lin Y., Feng C., Wang D., She J., Shen H., Wang G., Zhou Z. Chemosphere. 2015;137:14–24. doi: 10.1016/j.chemosphere.2015.04.043. [DOI] [PubMed] [Google Scholar]
- Tawara K., Nishijo M., Maruzeni S., Nakagawa H., Kido T., Naganuma R., Suzuki H., Nhu D. D., Hung N.N. N.N., Thomle T. H. Chemosphere. 2011;84:979–986. doi: 10.1016/j.chemosphere.2011.05.041. [DOI] [PubMed] [Google Scholar]
- Li J., Zhang L., Wu Y., Liu Y., Zhou P., Wen S., Liu J., Zhao Y., Li X. Chemosphere. 2009;75:1236–1242. doi: 10.1016/j.chemosphere.2009.01.073. [DOI] [PubMed] [Google Scholar]
- Todaka T., Hirakawa H., Kajiwara J., Onozuka D., Sasaki S., Miyashita C., Yoshioka E., Yuasa M., Kishi R., Iida T., Uchi H., Furue M. Chemosphere. 2011;85:1694–1700. doi: 10.1016/j.chemosphere.2011.09.014. [DOI] [PubMed] [Google Scholar]
- Sun S. J., Kayama F., Zhao J. H., Ge J., Yang Y. X., Fukatsu H., Iida T., Terada M., Liu D. W. Chemosphere. 2011;85:448–453. doi: 10.1016/j.chemosphere.2011.07.073. [DOI] [PubMed] [Google Scholar]
- Wong T. W., Wong A. H. S., Nelson E. A. S., Qiu H., Ku S. Y. K. Sci. Total Environ. 2013;463–464:1230–1238. doi: 10.1016/j.scitotenv.2012.07.097. [DOI] [PubMed] [Google Scholar]
- Pratt I. S., Anderson W. A., Crowley D., Daly S. F., Evans R. I., Fernandes A. R., Fitzgerald M., Geary M. P., Keane D. P., Malisch R., McBride J., Morrison J. J., Reilly A., Tlustos C. Chemosphere. 2012;88:865–872. doi: 10.1016/j.chemosphere.2012.03.095. [DOI] [PubMed] [Google Scholar]
- Raab U., Preiss U., Albrecht M., Shahin N., Parlar H., Fromme H. Chemosphere. 2008;72:87–94. doi: 10.1016/j.chemosphere.2008.01.053. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Nakai K., Matsumura T., Suzuki S., Saito Y., Satoh H. Sci. Total Environ. 2008;394:39–51. doi: 10.1016/j.scitotenv.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Chovancova J., Conka K., Kocan A., Sejakova Z. S. Chemosphere. 2011;83:1383–1390. doi: 10.1016/j.chemosphere.2011.02.070. [DOI] [PubMed] [Google Scholar]
- UNEP, Results of the Global Survey on Concentrations in Human Milk of Persistent Organic Pollutants by the United Nations Environment Programmeand (UNEP) the World Health Organization, Conference of the Parties to the Stockholm Convention on Persistent Organic Pollutants Sixth Meeting, Geneva, 2013. <http://www.who.int/foodsafety/chem/POPprotocol.pdf>.
- Croes K., Colles A., Koppen G., Govarts E., Bruckers L., Mieroop E. V. D., Nelen V., Covaci A., Dirtu A. C., Thomsen C., Haug L. S., Becher G., Mampaey M., Schoeters G., Van Larebeke N., Baeyens W. Chemosphere. 2012;89:988–994. doi: 10.1016/j.chemosphere.2012.06.058. [DOI] [PubMed] [Google Scholar]
- Sun S. J., Zhao J. H., Leng J. H., Wang P. Y., Wang Y., Fukatsu H., Liu D., Liu X., Kayama F. Chemosphere. 2010;80:1151–1159. doi: 10.1016/j.chemosphere.2010.06.021. [DOI] [PubMed] [Google Scholar]
- Ae R., Nakamura Y., Tada H., Kono Y., Matsui E., Itabashi K., Ogawa M., Sasahara T., Matsubara Y., Kojo T., Kotani K., Makino N., Aoyama Y., Sano T., Kosami K., Yamashita M., Oka A. J. Epidemiol. 2018:28. doi: 10.2188/jea.JE20170032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çok I., Donmez M. K., Kajiwara J., Uner M., Demirkaya E., Henkelmann B., Shen H. Q., Kotalik J., Schramm K. W. Chemosphere. 2009;76:1563–1571. doi: 10.1016/j.chemosphere.2009.05.032. [DOI] [PubMed] [Google Scholar]
- Nhu D. D., Kido T., Hung N. N., Thom L. T. H., Naganuma R., Son L. K., Honma S., Maruzeni S., Nishijo M., Nakagawa H. Toxicol. Environ. Chem. 2011;93:824–838. [Google Scholar]
- UNEP, Regional monitoring reports under the global monitoring plan for effectiveness evaluation: additional tissue data from the human milk survey, 2011. <http://chm.pops.int/Convention/ConferenceoftheParties%28COP%29/Meetings/COP5/COP5Documents/tabid/1268/Default.aspx>.
- Fång J., Nyberg E., Bignert A., Bergman Å. Environ. Int. 2013;60:224–231. doi: 10.1016/j.envint.2013.08.019. [DOI] [PubMed] [Google Scholar]
- Lignell S., Aune M., Darnerud P. O., Cnattingius S., Glynn A. Environ. Res. 2009;109:760–767. doi: 10.1016/j.envres.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Ulaszewska M. M., Zuccato E., Capri E., Iovine R., Colombo A., Rotella G., Generoso G., Grassi P., Melis M., Fanelli R. Chemosphere. 2011;82:1–8. doi: 10.1016/j.chemosphere.2010.10.044. [DOI] [PubMed] [Google Scholar]
- Abballe A., Ballard T. J., Dellatte E., Domenico A., Ferri F., Fulgenzi A. R., Grisanti G., Iacovella N., Ingelido A. M., Malisch R., Miniero R., Porpora M. G., Risica S., Ziemacki G., Felip E. D. Chemosphere. 2008;73:S220–S227. doi: 10.1016/j.chemosphere.2007.12.036. [DOI] [PubMed] [Google Scholar]
- Rivezzi G., Piscitelli P., Scortichini G., Giovannini A., Diletti G., Migliorati G., Ceci R., Rivezzi G., Cirasino L., Carideo P., Black D. M., Garzillo C., Giani U. Int. J. Environ. Res. Public Health. 2013;10:5953–5970. doi: 10.3390/ijerph10115953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigh É., Colombo A., Benfenati E., Håkansson H., Berglund M., Bódis J., Garai J. Sci. Total Environ. 2013;449:336–344. doi: 10.1016/j.scitotenv.2013.01.024. [DOI] [PubMed] [Google Scholar]
- Schuhmacher M., Kiviranta H., Ruokojarvi P., Nadal M., Domingo J. L. Environ. Int. 2009;35:607–613. doi: 10.1016/j.envint.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Mannetje A., Coakley J., Bridgen P., Brooks C., Harrad S., Smith A. H., Pearce N., Douwes J. Sci. Total Environ. 2013;458:399–407. doi: 10.1016/j.scitotenv.2013.04.055. [DOI] [PubMed] [Google Scholar]